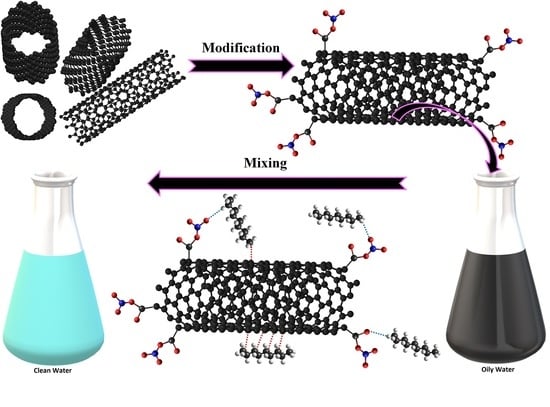
V2O5, CeO2 and Their MWCNTs Nanocomposites Modified for the Removal of Kerosene from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. Preparation of Metal Oxide Nanoparticles
2.2.1. Preparation of Vanadium Oxide Nanoparticles
2.2.2. Preparation of Cerium Oxide Nanoparticles
2.2.3. Preparation of Mixed V2O5:CeO2 Nanocomposites
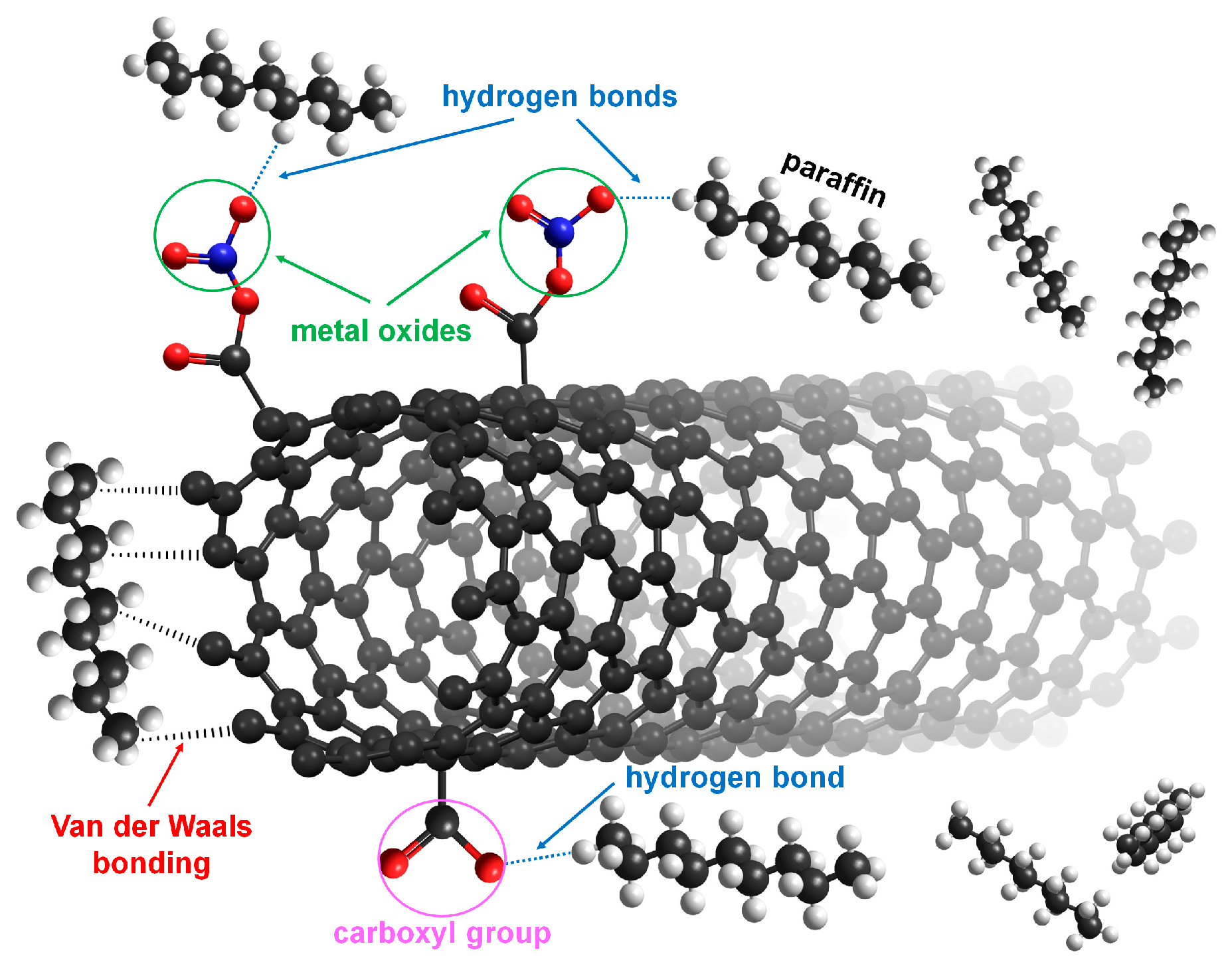
2.3. Functionalization of MWCNTs with Metal Oxide Composites
2.4. Characterization Methods
2.5. Adsorption Test
2.6. Kinetic Studies
3. Results
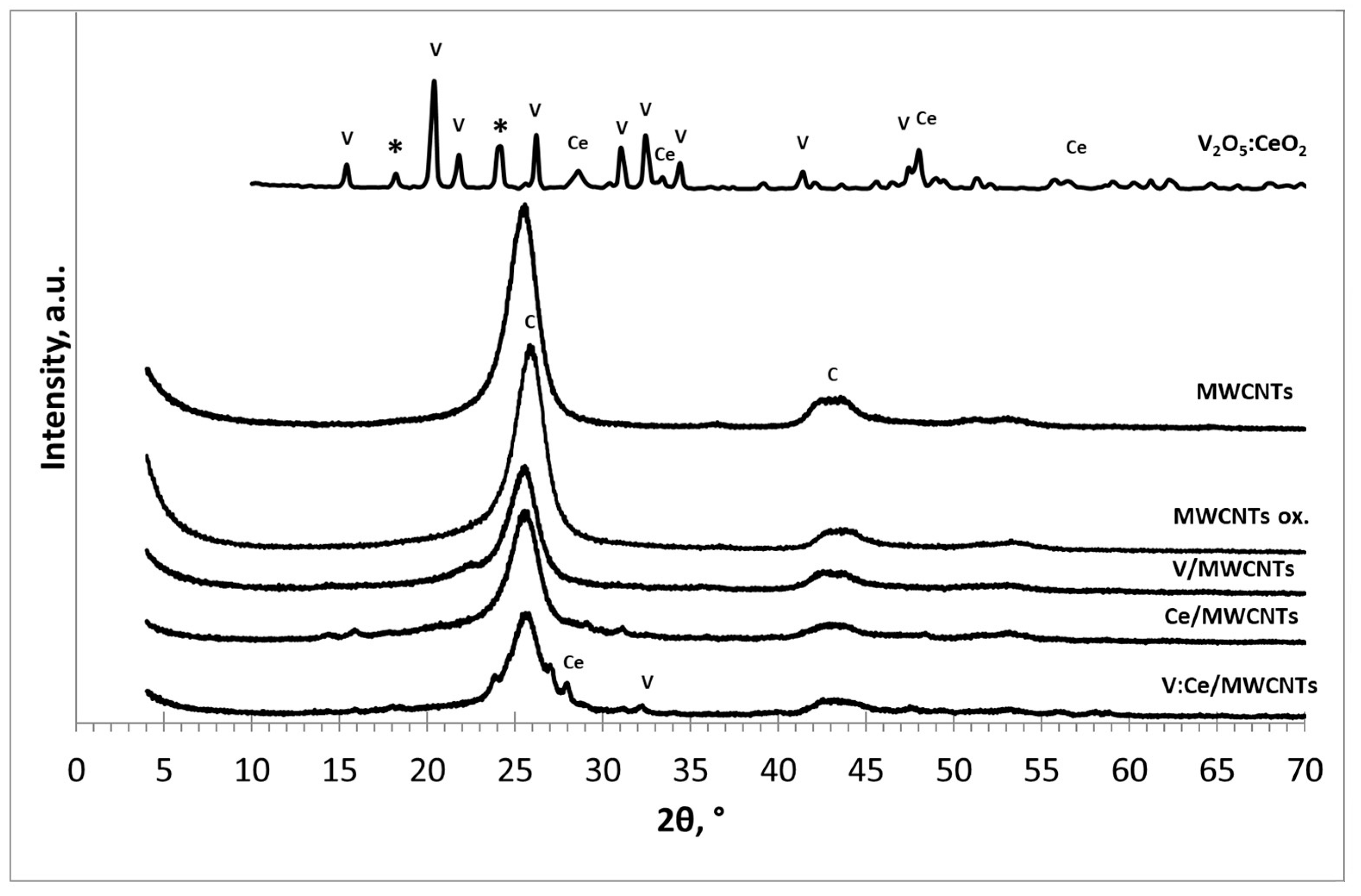
3.1. X-ray Diffraction
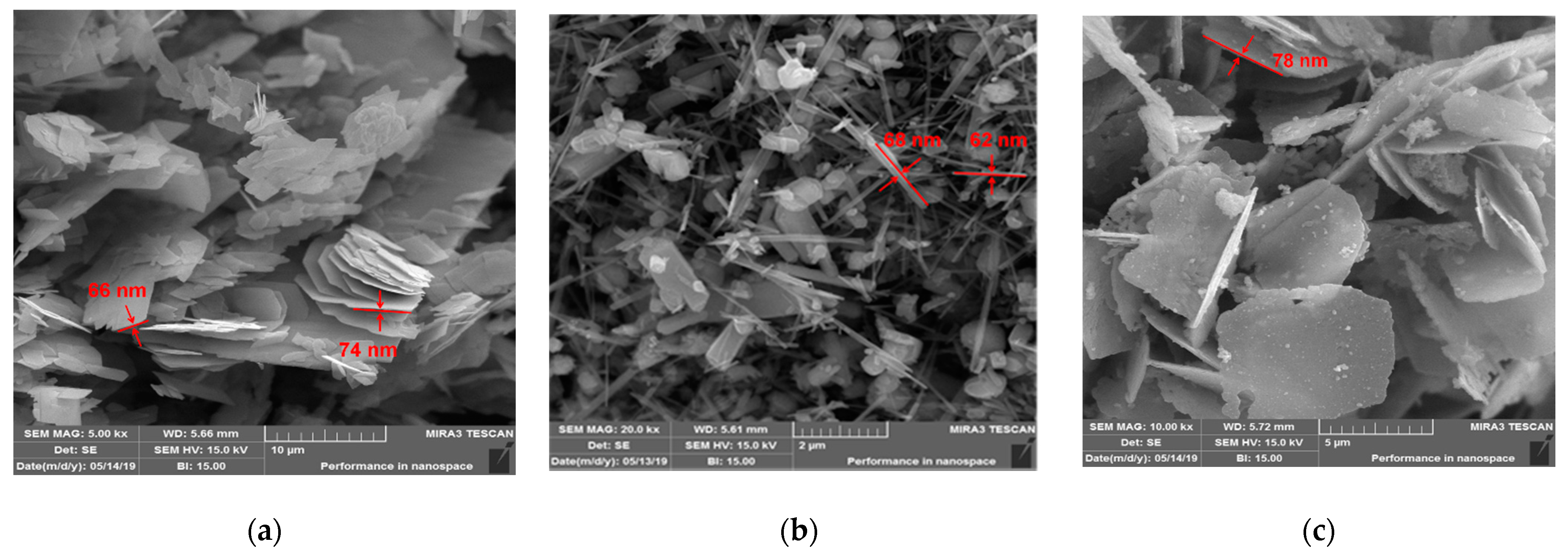
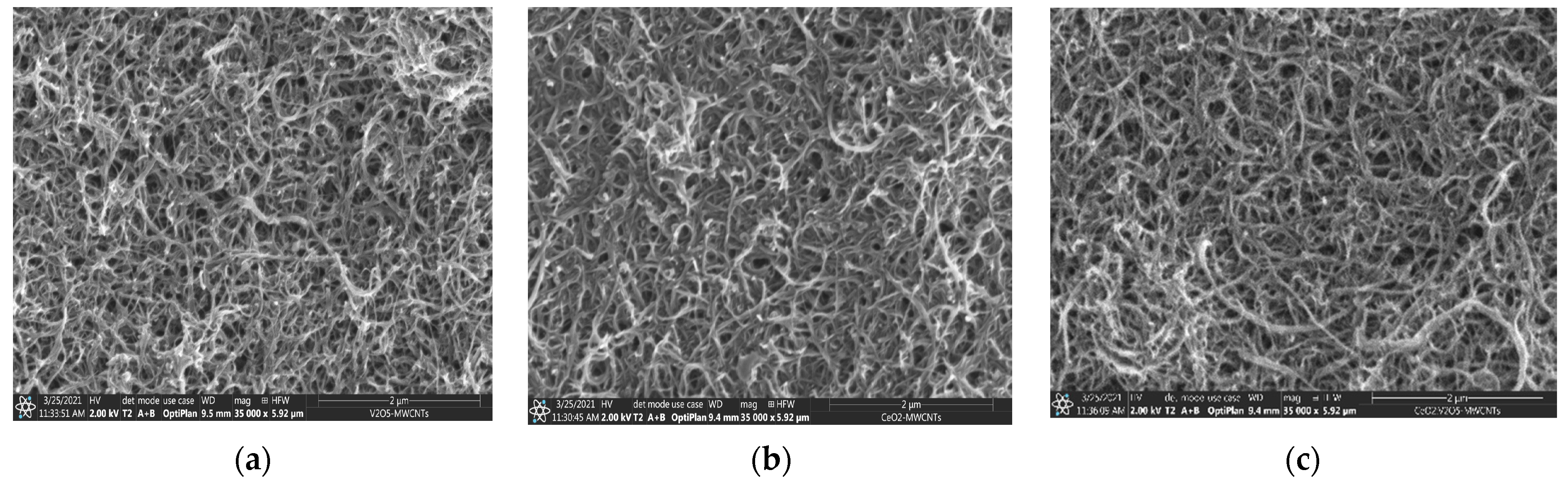
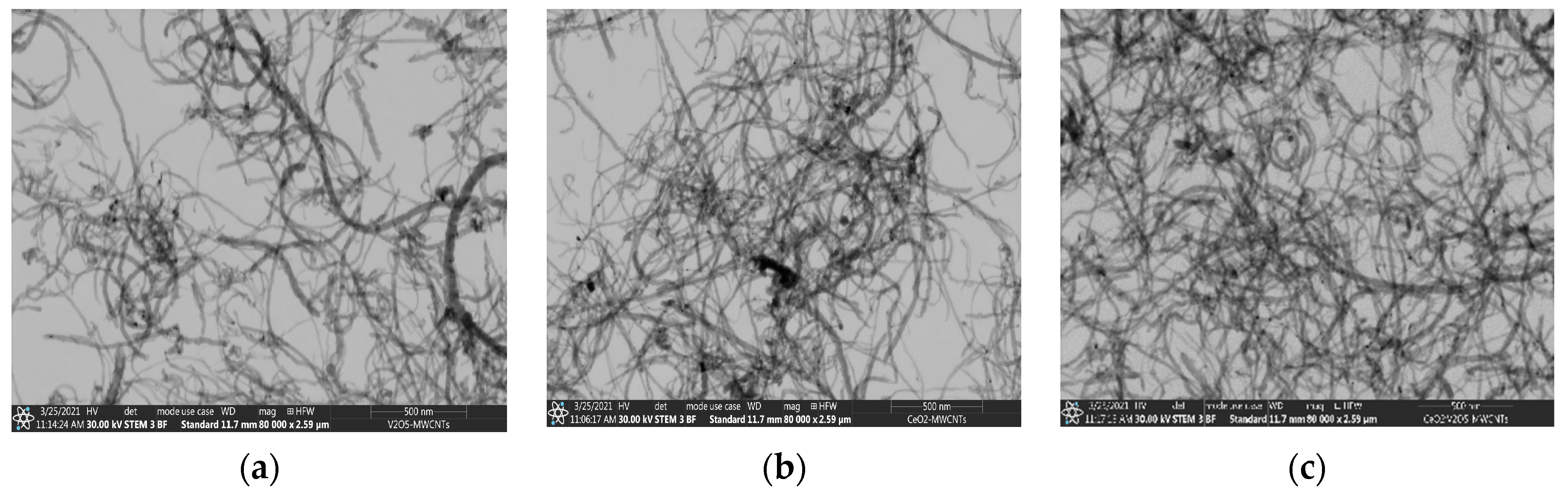
3.2. Electron Spectroscopy and Energy Dispersive X-ray Spectroscopy Results
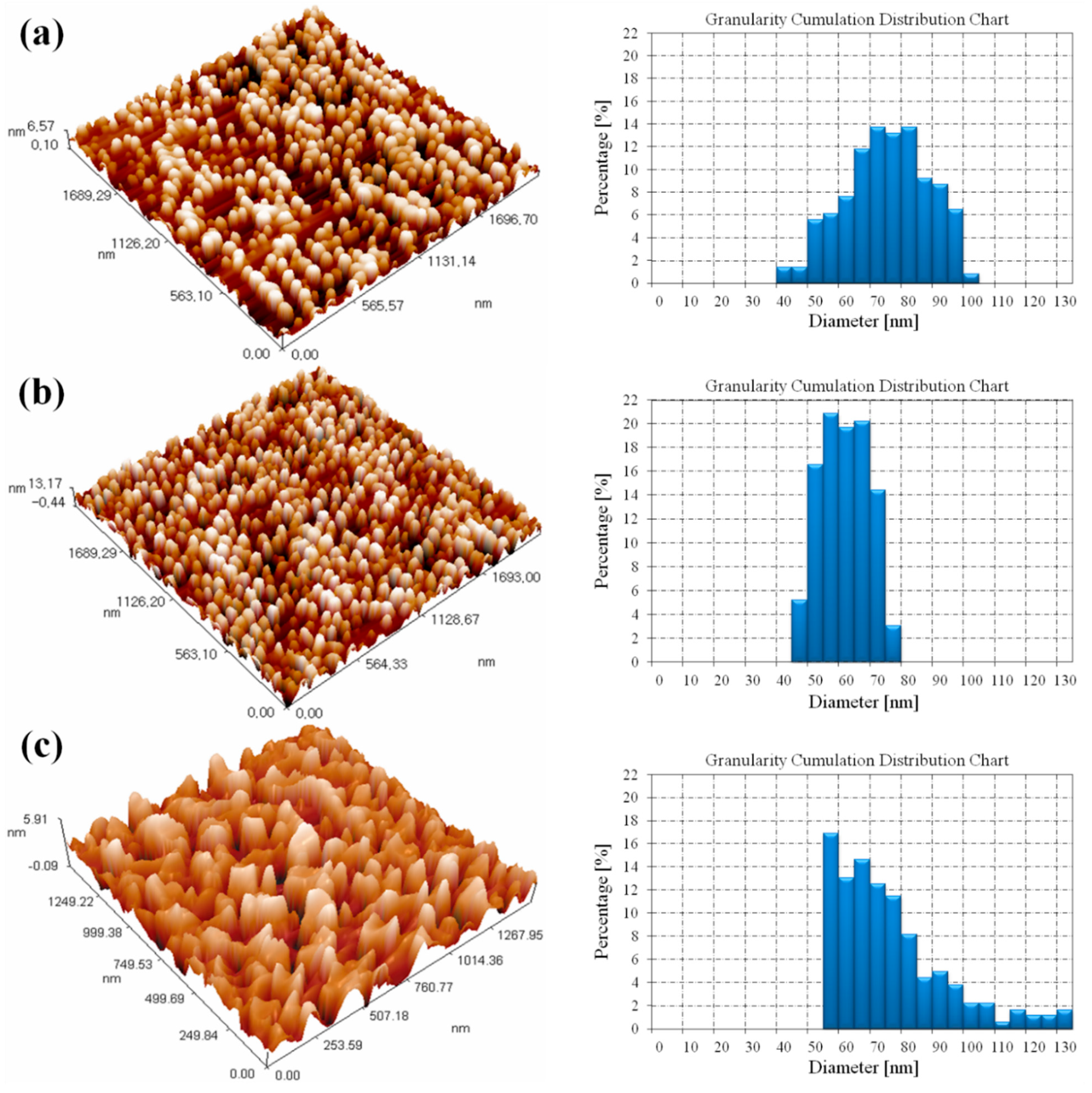
3.3. Atomic Force Microscopy Results
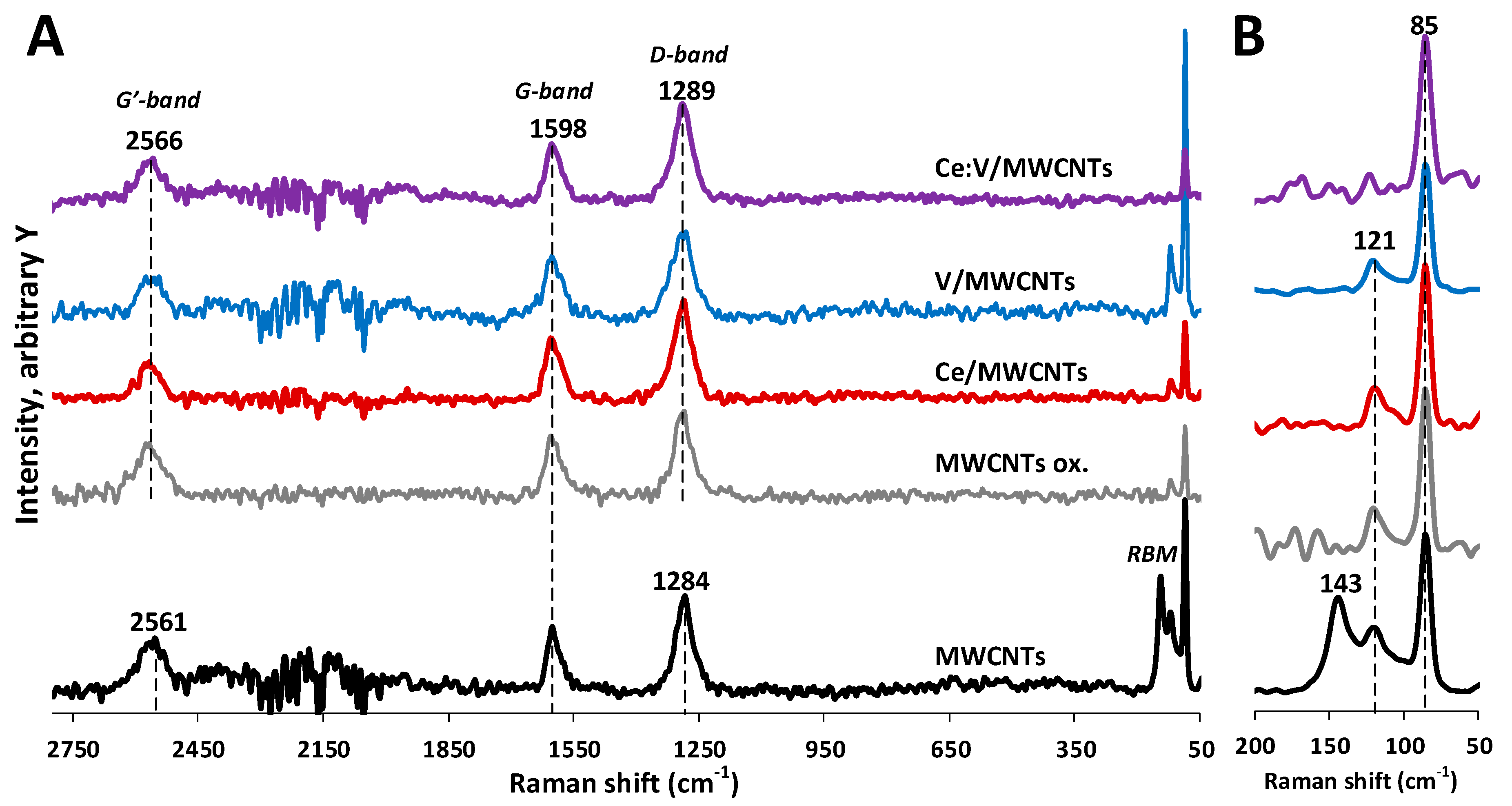
3.4. Raman Spectroscopy Results
3.5. Thermogravimetric Analysis Results
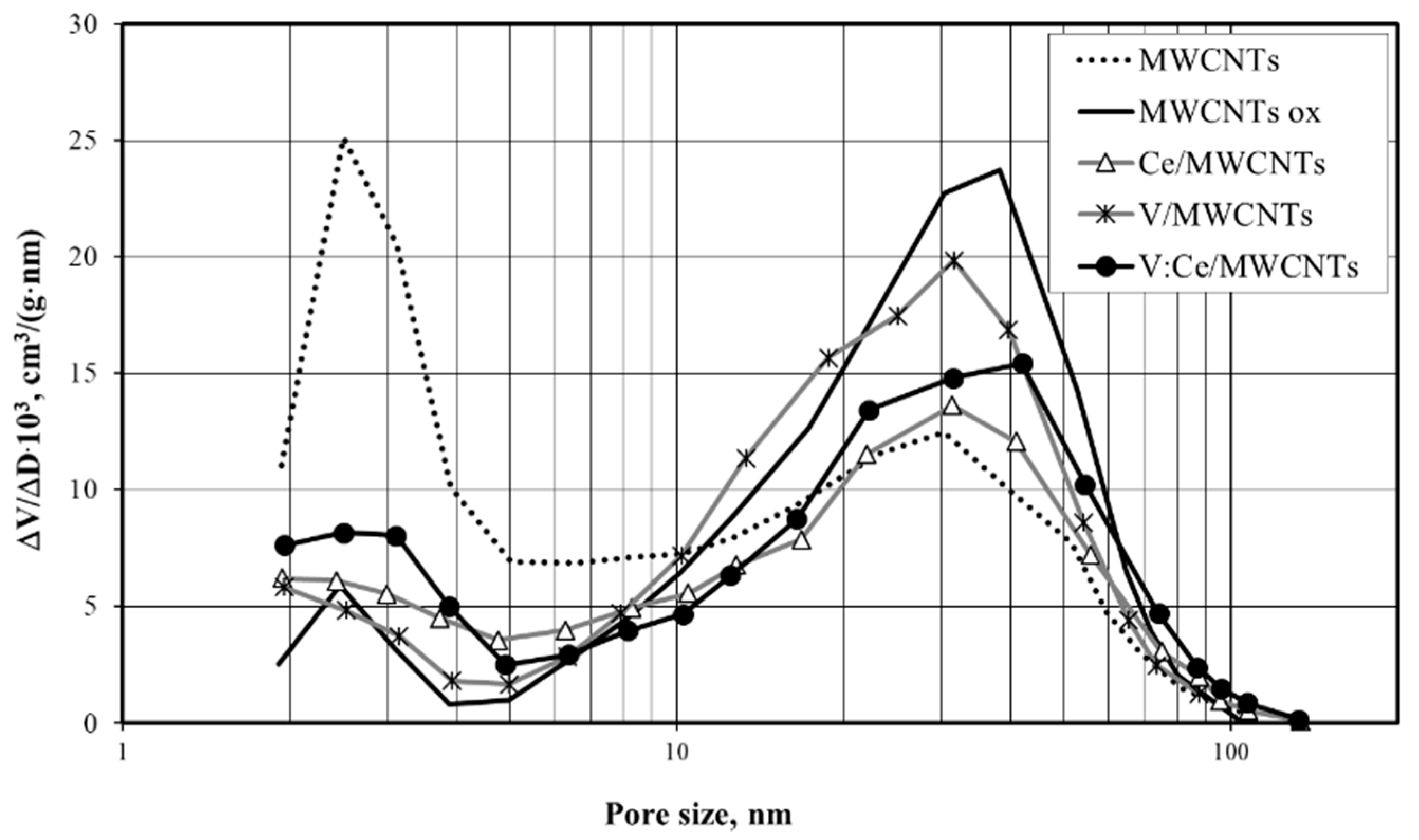
3.6. Low-Temperature Nitrogen Adsorption
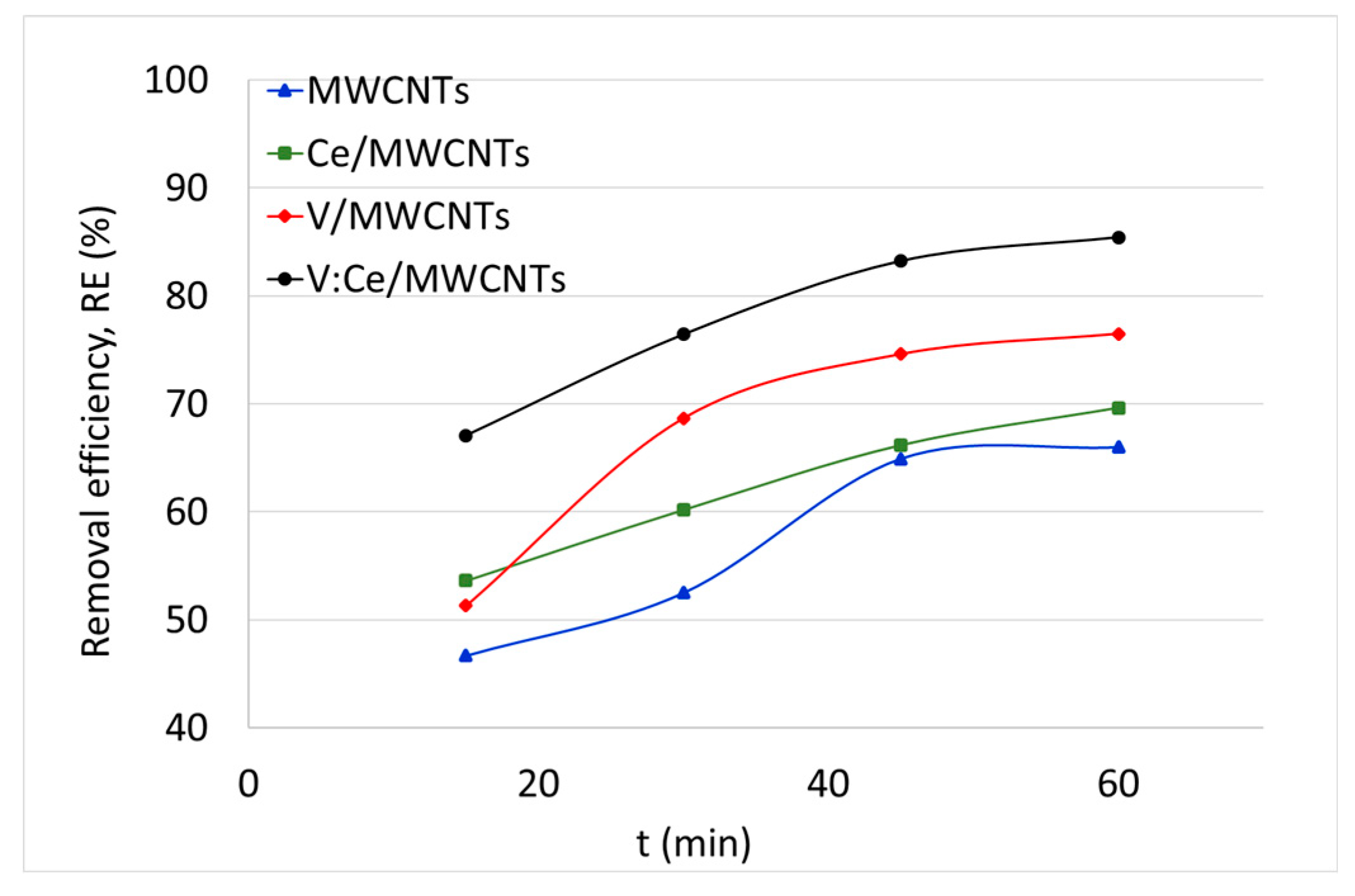
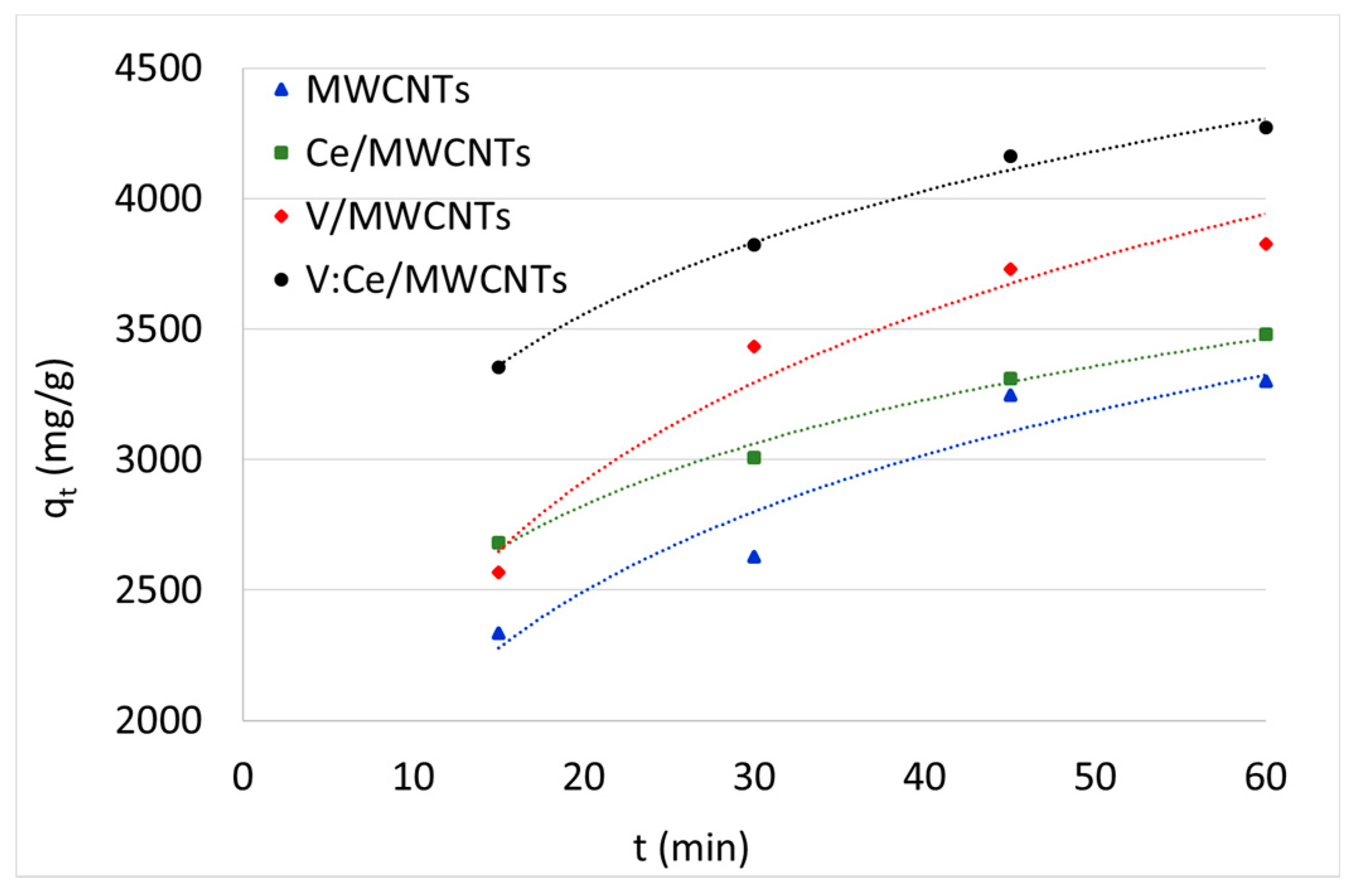
3.7. Kerosene Adsorption from Water Samples
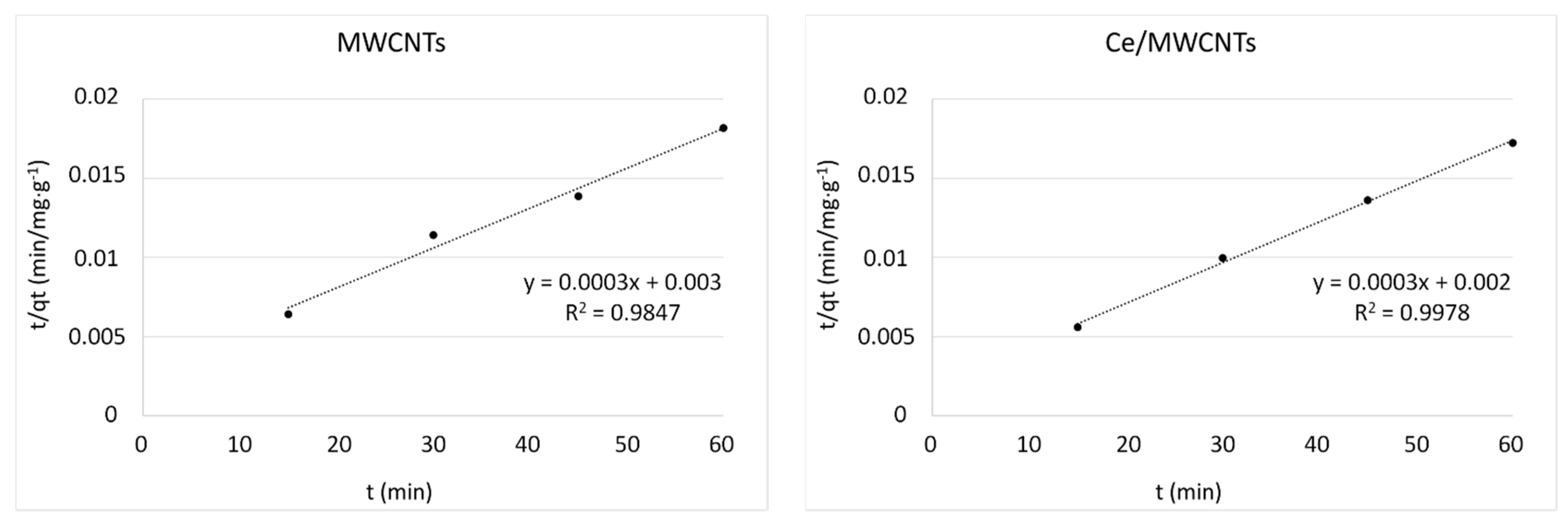
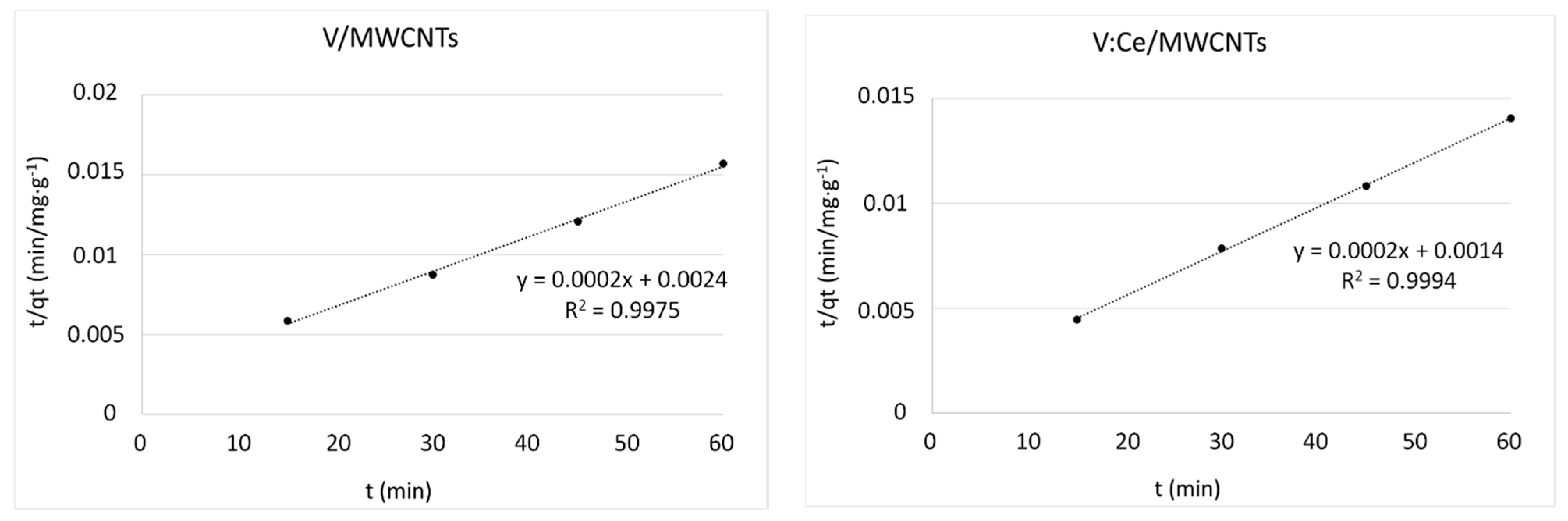
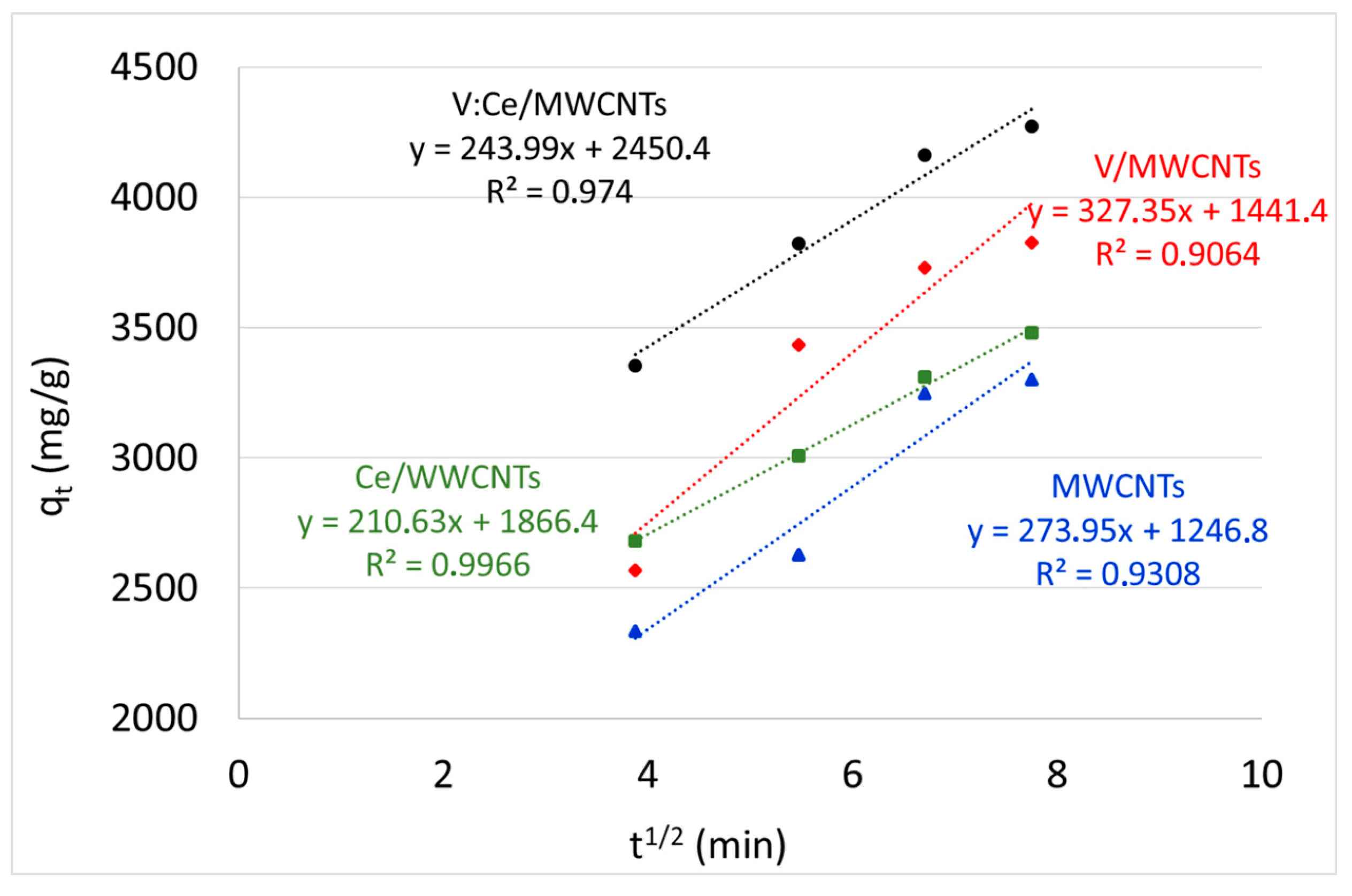
3.8. Kinetic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef]
- Medjor, W.O.; Akpoveta, V.O.; Adebowale, M.E. Remediation of hydrocarbons contaminated groundwater by silica encapsulation technique. Water Energy Nexus 2018, 1, 134–141. [Google Scholar] [CrossRef]
- Oliveira, L.M.; Saleem, J.; Bazargan, A.; Duarte, J.L.D.S.; McKay, G.; Meili, L. Sorption as a rapidly response for Oil Spill accidents: A material and mechanistic approach. J. Hazard. Mater. 2020, 124842. [Google Scholar] [CrossRef]
- Singh, H.; Bhardwaj, N.; Arya, S.K.; Khatri, M. Environmental impacts of oil spills and their remediation by magnetic nanomaterials. Environ. Nanotech. Monitor. Manag. 2020, 14, 100305. [Google Scholar] [CrossRef]
- Javadian, S.; Khalilifard, M.; Sadrpoor, S.M. Functionalized graphene oxide with core-shell of Fe3O4@oliec acid nanospheres as a recyclable demulsifier for effective removal of emulsified oil from oily wastewater. J. Water Process Eng. 2019, 32, 100961. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Emamy, F.H.; Bumajdad, A.; Lukaszewicz, J.P. Adsorption of Hexavalent Chromium and Divalent Lead Ions on the Nitrogen-Enriched Chitosan-Based Activated Carbon. Nanomaterials 2021, 11, 1907. [Google Scholar] [CrossRef]
- Rad, L.R.; Anbia, M. Zeolite-based composites for the adsorption of toxic matters from water: A review. J. Environ. Chem. Eng. 2021, 9, 106088. [Google Scholar] [CrossRef]
- Yang, W.; Hu, W.; Zhang, J.; Wang, W.; Cai, R.; Pan, M.; Huang, C.; Chen, X.; Yan, B.; Zeng, H. Tannic acid/Fe3+ functionalized magnetic graphene oxide nanocomposite with high loading of silver nanoparticles as ultra-efficient catalyst and disinfectant for wastewater treatmentt. Chem. Eng. J. 2021, 405, 126629. [Google Scholar] [CrossRef]
- Yang, W.; Pan, M.; Zhang, J.; Zhang, L.; Lin, F.; Liu, X.; Huang, C.; Chen, X.; Wang, J.; Yan, B.; et al. A universal strategy for constructing robust and antifouling cellulose nanocrystal coating. Adv. Funct. Mater. 2021, 2109989. [Google Scholar] [CrossRef]
- Zhang, R.; Tian, Y. Characteristics of natural biopolymers and their derivative as sorbents for chromium adsorption: A review. J. Leather Sci. Eng. 2020, 2, 24. [Google Scholar] [CrossRef]
- Su, R.; Xie, C.; Alhassan, S.I.; Huang, S.; Chen, R.; Xiang, S.; Wang, Z.; Huang, L. Oxygen Reduction Reaction in the Field of Water Environment for Application of Nanomaterials. Nanomaterials 2020, 10, 1719. [Google Scholar] [CrossRef]
- Abdullah, T.A.; Juzsakova, T.; Rasheed, R.T.; Salman, A.D.; Sebestyen, V.; Domokos, E.; Sluser, B.; Cretescu, I. Polystyrene-Fe3O4-MWCNTs nanocomposites for toluene removal from water. Materials 2021, 14, 5503. [Google Scholar] [CrossRef]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Rahman, M.M.; Adil, A.; Yusof, A.M.; Kamaruzzaman, Y.B.; Ansary, R.H. Removal of heavy metal ions with acid activated carbons derived from oil palm and coconut shells. Materials 2014, 7, 3634–3650. [Google Scholar] [CrossRef]
- Nino, A.D.; Olivito, F.; Algieri, V.; Costanzo, P.; Jiritano, A.; Tallarida, M.A.; Maiuolo, L. Efficient and fast removal of oils from water surfaces via highly oleophilic polyurethane composites. Toxics 2021, 9, 186. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J. Developments in the Application of Nanomaterials for Water Treatment and Their Impact on the Environment. Nanomaterials 2020, 10, 1764. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, T.A.; Juzsakova, T.; Mansoor, H.; Salman, A.D.; Rasheed, R.T.; AbdulhadI, S.H.; Mallah, M.A.; Domokos, E.; Cuong, N.X.; Nadda, A.K.; et al. Polyethylene over magnetite-multiwalled carbon nanotubes for kerosene removal from water. Chemosphere 2021, 287, 132310. [Google Scholar] [CrossRef]
- Al-Jammal, N.; Abdullah, T.A.; Juzsakova, T.; Zsirka, B.; Cretescu, I.; Vágvölgyi, V.; Sebestyén, V.; Le Phuoc, C.; Rasheed, R.T.; Domokos, E. Functionalized carbon nanotubes for hydrocarbon removal from water. J. Environ. Chem. Eng. 2020, 8, 103570. [Google Scholar] [CrossRef]
- Xu, H.; Jia, W.; Ren, S.; Wang, J. Magnetically responsive multi-wall carbon nanotubes as recyclable demulsifier for oil removal from crude oil-in-water emulsion with different pH levels. Carbon 2019, 145, 229–239. [Google Scholar] [CrossRef]
- Parmar, K.R.; Dora, D.T.K.; Pant, K.K.; Roy, S. An ultra-light flexible aerogel-based on methane derived CNTs as a reinforcing agent in silica-CMC matrix for efficient oil adsorption. J. Hazard. Mater. 2019, 375, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Lin, Z.; Wang, Y.; Li, Y.; Liu, S.; Gui, X.; Yang, X. Three-dimensional AlFe2O3/amino-functionalization carbon nanotube sponge for adsorption and oxidative removal of tetrabromobisphenol A. Sep. Purif. Technol. 2019, 211, 359–367. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, N.; Jing, Y.; Jiang, X.X.; Yu, L.; Yan, X.; Zhang, T.; Li, Z.Z.T.; Lü, Y.; Liu, Y.; et al. Facile one-step fabrication of highly hydrophobic, renewable and mechanically flexible sponge with dynamic coating for efficient oil/water separation. J. Taiwan Inst. Chem. Eng. 2019, 91, 571–579. [Google Scholar] [CrossRef]
- Tan, X.; Wang, H.-M.D.; Zang, D.; Wu, L.; Liu, F.; Cao, G.; Xu, Y.; Ho, S.-H. Superhydrophobic/superoleophilic corn straw as an eco-friendly oil sorbent for the removal of spilled oil. Clean Technol. Environ. Policy 2021, 23, 145–152. [Google Scholar] [CrossRef]
- Cao, Q.; Yu, Q.; Connell, D.W.; Yu, G. Titania/carbon nanotube composite (TiO2/CNT) and its application for removal of organic pollutants. Clean Technol. Environ. Policy 2013, 15, 871–880. [Google Scholar] [CrossRef]
- Zandipak, R.; Sobhanardakani, S. Novel mesoporous Fe3O4/SiO2/CTAB–SiO2 as an effective adsorbent for the removal of amoxicillin and tetracycline from water. Clean Technol. Environ. Policy 2018, 20, 871–885. [Google Scholar] [CrossRef]
- Kirti, S.; Bhandari, V.M.; Jena, J.; Sorokhaibam, L.G.; Bhattacharyya, A.S. Exploiting functionalities of biomass in nanocomposite development: Application in dye removal and disinfection along with process intensification. Clean Technol. Environ. Policy 2018, 20, 981–994. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Kamel, A.H.; Hassan, A.A.; Amr, A.E.-G.E.; El-Naby, H.A.; Elsayed, E.A. A SnO2/CeO2 Nano-Composite Catalyst for Alizarin Dye Removal from Aqueous Solutions. Nanomaterials 2020, 10, 254. [Google Scholar] [CrossRef]
- Abdullah, T.A.; Juzsakova, T.; Hafad, S.A.; Taleb, R.; Noor, R.; Jammal, A. Functionalized multi-walled carbon nanotubes for oil spill cleanup from water. Clean Tech. Environ. Polic. 2021. [Google Scholar] [CrossRef]
- Ouda, M.; Ibrahim, Y.; Banat, F.; Hasan, S.W. Oily wastewater treatment via phase-inverted polyethersulfone-maghemite (PES/γ-Fe2O3) composite membranes. J. Water Process Eng. 2020, 37, 101545. [Google Scholar] [CrossRef]
- Mottaghi, H.; Mohammadi, Z.; Abbasi, M.; Tahouni, N.; Panjeshahi, M.H. Experimental investigation of crude oil removal from water using polymer adsorbent. J. Water Process Eng. 2021, 40, 101959. [Google Scholar] [CrossRef]
- Lawal, A.T. Recent progress in graphene based polymer nanocomposites. Cogent Chem. 2020, 6, 1833476. [Google Scholar] [CrossRef]
- Javad Nazarahari, M.; Khaksar Manshad, A.; Moradi, S.; Shafiei, A.; Abdulazez Ali, J.; Sajadi, S.M.; Keshavarz, A. Synthesis, Characterization, and Assessment of a CeO2@Nanoclay Nanocomposite for Enhanced Oil Recovery. Nanomaterials 2020, 10, 2280. [Google Scholar] [CrossRef]
- Rasheed, R.T.; Mansoor, H.S.; Abdullah, T.A.; Juzsakova, T.; Al-Jammal, N.; Salman, A.D.; Al-Shaikhly, R.R.; Le, P.C.; Endre, D.; Abdulla, T.A. Synthesis, characterization of V2O5 nanoparticles and determination of catalase mimetic activity by new colorimetric method. J. Therm. Anal. Calorim. 2021, 145, 297–307. [Google Scholar] [CrossRef]
- Abdullah, A.T.; Nguyen, B.-S.; Juzsakova, T.; Rasheed, T.R.; Hafad, S.; Mansoor, H.; Al-Jammal, N.; Salman, D.A.; Awad, A.H.; Domokos, E.; et al. Promotional effect of metal oxides (MxOy = TiO2, V2O5) on multi-walled carbon nanotubes (MWCNTs) for kerosene removal from contaminated water. Mater. Lett. 2021, 292, 129612. [Google Scholar] [CrossRef]
- Rezvanpour, A.; Roostaazad, R.; Hesampour, M.; Nystr, M. Effective factors in the treatment of kerosene-water emulsion by using UF membranes. J. Hazard. Mater. 2009, 161, 1216–1224. [Google Scholar] [CrossRef]
- Wang, P.; Meng, F.; Gao, C.; Xie, W.; Wang, J.; Li, A. Structural, morphological and optical characteristics of fusiform Co-doped CeO2 via a facile hydrothermal method. J. Mater. Sci. Mater. Electron. 2018, 29, 11482–11488. [Google Scholar] [CrossRef]
- Ding, S.J.; Nan, F.; Liu, X.L.; Hao, Z.H.; Zhou, L.; Zeng, J.; Xu, H.X.; Zhang, W.; Wang, Q.Q. Plasmon-Modulated Excitation-Dependent Fluorescence from Activated CTAB Molecules Strongly Coupled to Gold Nanoparticles. Sci. Rep. 2017, 7, 43282. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghani, N.T.; El-Chaghaby, G.A.; Helal, F.S. Individual and competitive adsorption of phenol and nickel onto multiwalled carbon nanotubes. J. Adv. Res. 2015, 6, 405–415. [Google Scholar] [CrossRef]
- Costa, S.; Borowiak-Palen, E.; Kruszyñska, M.; Bachmatiuk, A.; Kaleńczuk, R.J. Characterization of carbon nanotubes by Raman spectroscopy. Mater. Sci. Pol. 2008, 26, 433–441. [Google Scholar]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Jorio, A.; Saito, R. Raman spectroscopy for carbon nanotube applications. J. Appl. Phys. 2021, 129, 021102. [Google Scholar] [CrossRef]
- Puech, P.; Kandara, M.; Paredes, G.; Moulin, L.; Weiss-Hortala, E.; Kundu, A.; Ratel-Ramond, N.; Plewa, J.M.; Pellenq, R.; Monthioux, M. Analyzing the Raman Spectra of Graphenic Carbon Materials from Kerogens to Nanotubes: What Type of Information Can Be Extracted from Defect Bands? C—J. Carbon Res. 2019, 5, 69. [Google Scholar] [CrossRef]
- Murphy, H.; Papakonstantinou, P.; Okpalugo, T.I.T. Raman study of multiwalled carbon nanotubes functionalized with oxygen groups. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2006, 24, 715. [Google Scholar] [CrossRef]
- Osswald, S.; Havel, M.; Gogotsi, Y. Monitoring oxidation of multiwalled carbon nanotubes by Raman spectroscopy. J. Raman Spectrosc. 2007, 38, 728–736. [Google Scholar] [CrossRef]
- DiLeo, R.A.; Landi, B.J.; Raffaelle, R.P. Purity assessment of multiwalled carbon nanotubes by Raman spectroscopy. J. Appl. Phys. 2007, 101, 064307. [Google Scholar] [CrossRef]
- Notthoff, C.; Winterer, M.; Beckel, A.; Geller, M.; Heindl, J. Spatial high resolution energy dispersive X-ray spectroscopy on thin lamellas. Ultramicroscopy 2013, 129, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Shvets, P.; Dikaya, O.; Maksimova, K.; Goikhman, A. A review of Raman spectroscopy of vanadium oxides. J. Raman Spectrosc. 2019, 50, 1226–1244. [Google Scholar] [CrossRef]
- Schilling, C.; Hofmann, A.; Hess, C.; Ganduglia-Pirovano, M.V. Raman Spectra of Polycrystalline CeO2: A Density Functional Theory Study. J. Phys. Chem. C 2017, 121, 20834–20849. [Google Scholar] [CrossRef]
- Shah, K.A.; Tali, B.A. Synthesis of carbon nanotubes by catalytic chemical vapour deposition: A review on carbon sources, catalysts and substrates. Mater. Sci. Semicond. Process. 2016, 41, 67–82. [Google Scholar] [CrossRef]
- Sezer, N.; Koç, M. Oxidative acid treatment of carbon nanotubes. Surf. Interfaces 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Bom, D.; Andrews, R.; Jacques, D.; Anthony, J.; Chen, B.; Meier, M.S.; Selegue, J.P. Thermogravimetric Analysis of the Oxidation of Multiwalled Carbon Nanotubes: Evidence for the Role of Defect Sites in Carbon Nanotube Chemistry. Nano Lett. 2002, 2, 615–619. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. (Eds.) CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2017; pp. 4–55. [Google Scholar]
- Trovarelli, A. Catalytic Properties of Ceria and CeO2-Containing Materials. Catal. Rev. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Vita, A. Catalytic Applications of CeO2-Based Materials. Catalysts 2020, 10, 576. [Google Scholar] [CrossRef]
- Wachs, I.E. Catalysis science of supported vanadium oxide catalysts. Dalton Trans. 2013, 42, 11762. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, S.A.; Ivanov, S.A.; Maslakov, K.I.; Egorov, A.V.; Shen, Z.; Savilov, S.S.; Luninab, V.V. Oxidation, defunctionalization and catalyst life cycle of carbon nanotubes: A Raman spectroscopy view. Phys. Chem. Chem. Phys. 2017, 19, 2276–2285. [Google Scholar] [CrossRef] [PubMed]
- Birch, M.E.; Ruda-Eberenz, T.A.; Chai, M.; Andrews, R.; Randal, L. Properties that Influence the Specific Surface Areas of Carbon Nanotubes and Nanofibers. Ann. Occup. Hyg. 2013, 57, 1148–1166. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aljeboree, A.M.; Alshirifi, A.N.; Alkaim, A.F. Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab. J. Chem. 2017, 10, S3381–S3393. [Google Scholar] [CrossRef]
- Nazal, M.K.; Khaled, M.; Atieh, M.A.; Aljundi, I.H.; Oweimreen, G.A.; Abulkibash, A.M. The nature and kinetics of the adsorption of dibenzothiophene in model diesel fuel on carbonaceous materials loaded with aluminum oxide particles. Arab. J. Chem. 2019, 12, 3678–3691. [Google Scholar] [CrossRef]
- Padmajan Sasikala, S.; Mahesh, K.V.; Prabhakaran, P.; Peer Mohammed, A.; Ananthakumar, S. Multiwall carbon nanotube reinforced teflon fibrils for oil spill clean up and its effective recycling as textile dye sorbent. J. Environ. Manag. 2018, 211, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.M.; Bagheri, M.G.; Zohuriaan, M.J. Nitrate removal from aqueous solutions by adsorption onto hydrogel—Rice husk biochar composite. Water Environ. Res. 2020, 92, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Ribas, M.C.; De Franco, M.A.; Adebayo, M.A.; Lima, E.C.; Parkes, G.M.; Feris, L.A. Adsorption of Procion Red MX-5B dye from aqueous solution using homemade peach and commercial activated carbons. Appl. Water Sci. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Dawodu, F.A.; Akpomie, K.G. Simultaneous adsorption of Ni (II) and Mn (II) ions from aqueous solution unto a Nigerian kaolinite clay. J. Mater. Res. Technol. 2014, 3, 129–141. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S. Rapid adsorption of toxic Pb(II) ions from aqueous solution using multiwall carbon nanotubes synthesized by microwave chemical vapor deposition technique. J. Environ. Sci. 2016, 45, 143–155. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Shuaib, D.T.; Ajala, A.O.; Mohammed, A.K. Application of TiO2 and ZnO nanoparticles immobilized on clay in wastewater treatment: A review. Appl. Water Sci. 2020, 10, 49. [Google Scholar] [CrossRef]

| Sample | Theoretical wt% | wt% by EDX |
|---|---|---|
| V/MWCNTs | 2.0/98.0 | 2.3/97.7 |
| Ce/MWCNTs | 2.0/98.0 | 7.4/92.6 |
| V:Ce/MWCNTs | 6.0:2.0/92.0 | 6.0:2.1/91.9 |
| Samples | Weight Loss (wt%) | SBET (m2/g) | Smicro (m2/g) | V1.7–300 nm (cm3/g) | Vmicro (cm3/g) | Dav (nm) |
|---|---|---|---|---|---|---|
| MWCNTs | 0.1 | 156 | 25.4 | 0.6577 | 0.0109 | 16.1 |
| MWCNTs ox. | 7.9 | 140 | 13.6 | 1.0384 | 0.0052 | 28.2 |
| V2O5 | 0.8 | 3.0 | 0 | 0.0073 | 0 | 13.4 |
| CeO2 | 0.5 | 69.4 | 15.5 | 0.1676 | 0.0069 | 13.4 |
| V2O5:CeO2 | 3.5 | 8.8 | 0.5 | 0.0368 | 0.0001 | 15.0 |
| V/MWCNTs | 1.3 | 135 | 6.9 | 0.8439 | 0.0018 | 24.5 |
| Ce/MWCNTs | 4.5 | 115 | 0 | 0.7002 | 0 | 24.1 |
| V:Ce/MWCNTs | 6.9 | 129 | 0 | 0.8545 | 0 | 25.8 |
| MWCNTs | Ce/MWCNTs | V/MWCNTs | V:Ce/MWCNTs | |||||
|---|---|---|---|---|---|---|---|---|
| Time (min) | RE (%) | qt (mg/g) | RE (%) | qt (mg/g) | RE (%) | qt (mg/g) | RE % | qt (mg/g) |
| 15 | 47 | 2335 | 54 | 2681 | 51 | 2567 | 67 | 3355 |
| 30 | 53 | 2626 | 60 | 3009 | 69 | 3435 | 76 | 3822 |
| 45 | 65 | 3247 | 66 | 3308 | 75 | 3731 | 83 | 4161 |
| 60 | 66 | 3300 | 70 | 3481 | 77 | 3825 | 85 | 4271 |
| Kinetic Models | Parameters | MWCNTs | Ce/MWCNTs | V/MWCNTs | V:Ce/MWCNTs |
|---|---|---|---|---|---|
| qt exp (mg/g) | 3300 | 3481 | 3825 | 4271 | |
| pseudo-first order | k1 (min−1) | 0.089 | 0.061 | 0.090 | 0.090 |
| qe cal (mg/g) | 4946 | 2394 | 5164 | 4674 | |
| R2 | 0.8969 | 0.9631 | 0.9962 | 0.9494 | |
| pseudo-second order | k2 (g/mg min) | 0.30 × 10−4 | 0.45 × 10−4 | 0.16 × 10−4 | 0.28 × 10−4 |
| qe cal (mg/g) | 3333 | 3333 | 5000 | 5000 | |
| R2 | 0.9847 | 0.9978 | 0.9975 | 0.9994 | |
| intra-particle diffusion | Kd (mg/g min1/2) | 274 | 2104 | 3274 | 244 |
| I | 1247 | 1866 | 1441 | 2450 | |
| R2 | 0.9308 | 0.9966 | 0.9064 | 0.9740 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, T.A.; Juzsakova, T.; Rasheed, R.T.; Mallah, M.A.; Salman, A.D.; Cuong, L.P.; Jakab, M.; Zsirka, B.; Kułacz, K.; Sebestyén, V. V2O5, CeO2 and Their MWCNTs Nanocomposites Modified for the Removal of Kerosene from Water. Nanomaterials 2022, 12, 189. https://doi.org/10.3390/nano12020189
Abdullah TA, Juzsakova T, Rasheed RT, Mallah MA, Salman AD, Cuong LP, Jakab M, Zsirka B, Kułacz K, Sebestyén V. V2O5, CeO2 and Their MWCNTs Nanocomposites Modified for the Removal of Kerosene from Water. Nanomaterials. 2022; 12(2):189. https://doi.org/10.3390/nano12020189
Chicago/Turabian StyleAbdullah, Thamer Adnan, Tatjána Juzsakova, Rashed Taleb Rasheed, Muhammad Ali Mallah, Ali Dawood Salman, Le Phuoc Cuong, Miklós Jakab, Balázs Zsirka, Karol Kułacz, and Viktor Sebestyén. 2022. "V2O5, CeO2 and Their MWCNTs Nanocomposites Modified for the Removal of Kerosene from Water" Nanomaterials 12, no. 2: 189. https://doi.org/10.3390/nano12020189
APA StyleAbdullah, T. A., Juzsakova, T., Rasheed, R. T., Mallah, M. A., Salman, A. D., Cuong, L. P., Jakab, M., Zsirka, B., Kułacz, K., & Sebestyén, V. (2022). V2O5, CeO2 and Their MWCNTs Nanocomposites Modified for the Removal of Kerosene from Water. Nanomaterials, 12(2), 189. https://doi.org/10.3390/nano12020189