Biomineralized Manganese Oxide Nanoparticles Synergistically Relieve Tumor Hypoxia and Activate Immune Response with Radiotherapy in Non-Small Cell Lung Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bio-MnO2 NPs Preparation
2.2. Characterization
2.3. Detection of GSH
2.4. Detection of Oxygen Production
2.5. Cell Culture
2.6. Cellular Uptake
2.7. Measurement of Intracellular H2O2
2.8. ROS Detection In Vitro
2.9. Cytotoxicity In Vitro
2.10. Cell Apoptosis Assay
2.11. Colony Formation Assay
2.12. RNA Isolation and qRT-PCR
2.13. Immunoblotting
2.14. Immunofluorescence
2.15. Comet Assay
2.16. Enzyme-Linked Immunosorbent Assay (ELISA)
2.17. Mice Model
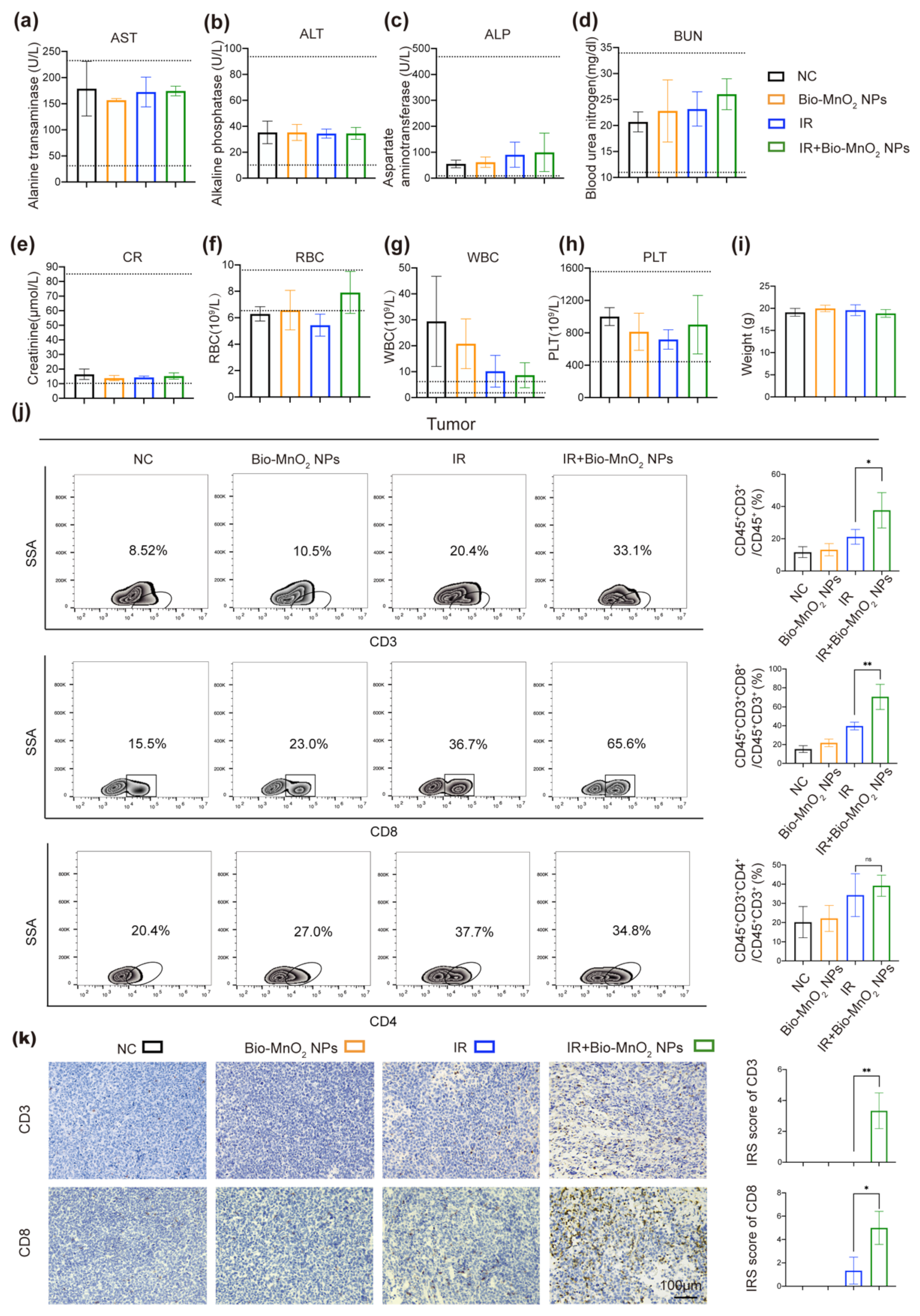
2.18. In Vivo Safety Analysis
2.19. In Vivo Antitumor Efficacy
2.20. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Bio-MnO2 NPs
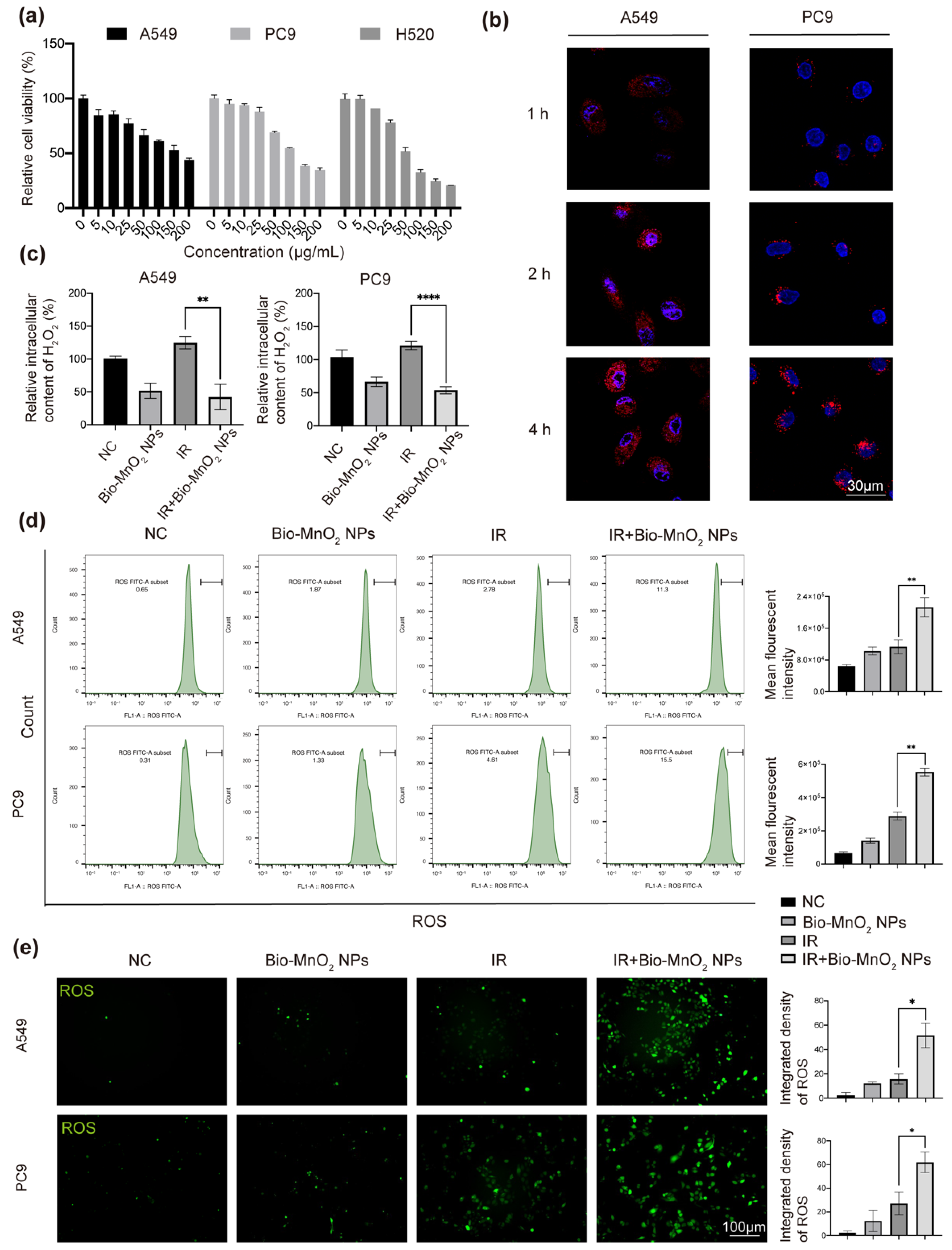
3.2. Bio-MnO2 NPs Eliminated H2O2 and Enhanced ROS Production in NSCLC Cells
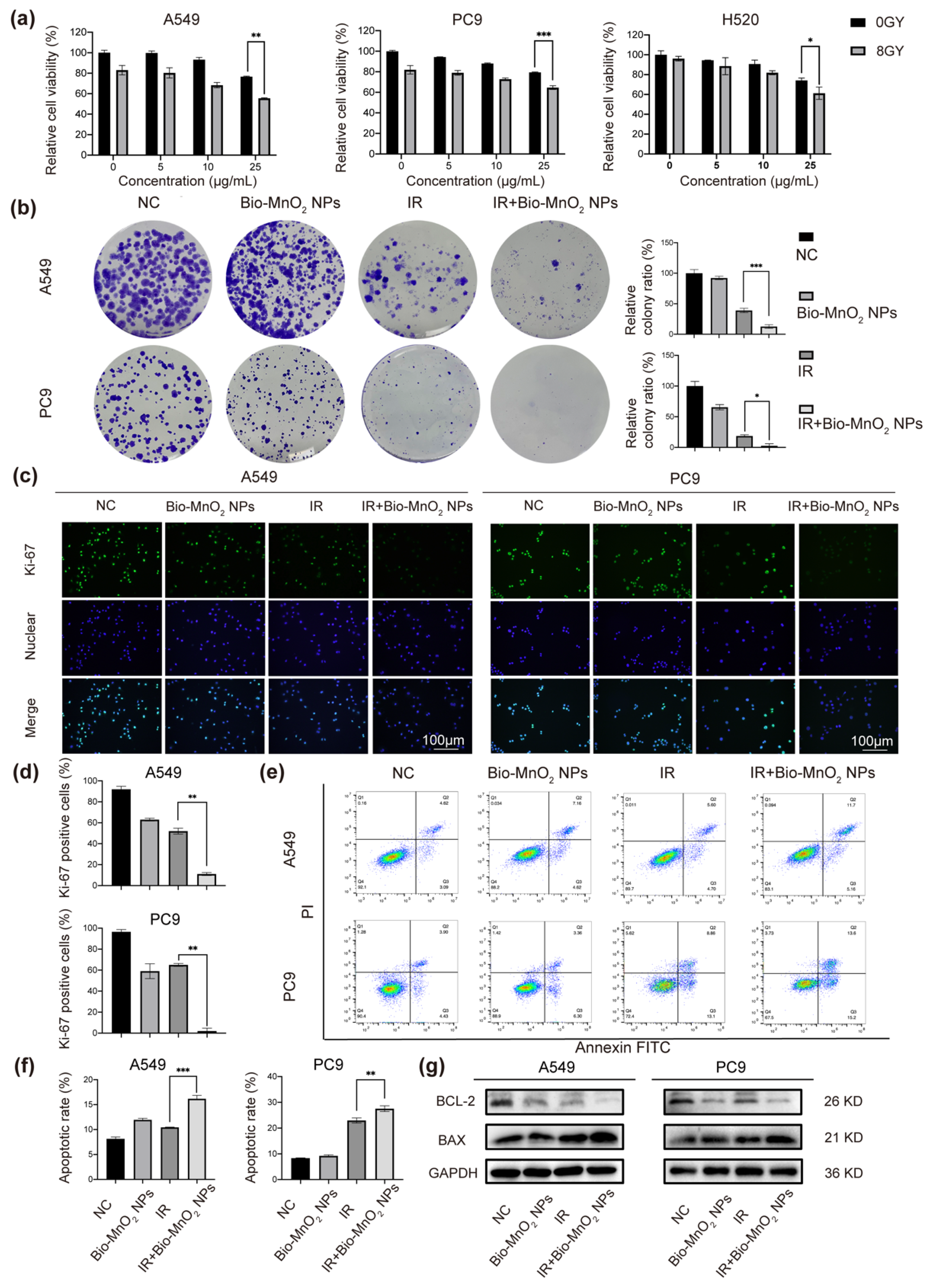
3.3. Bio-MnO2 NPs Enhanced Radiosensitivity of NSCLC Cells In Vitro
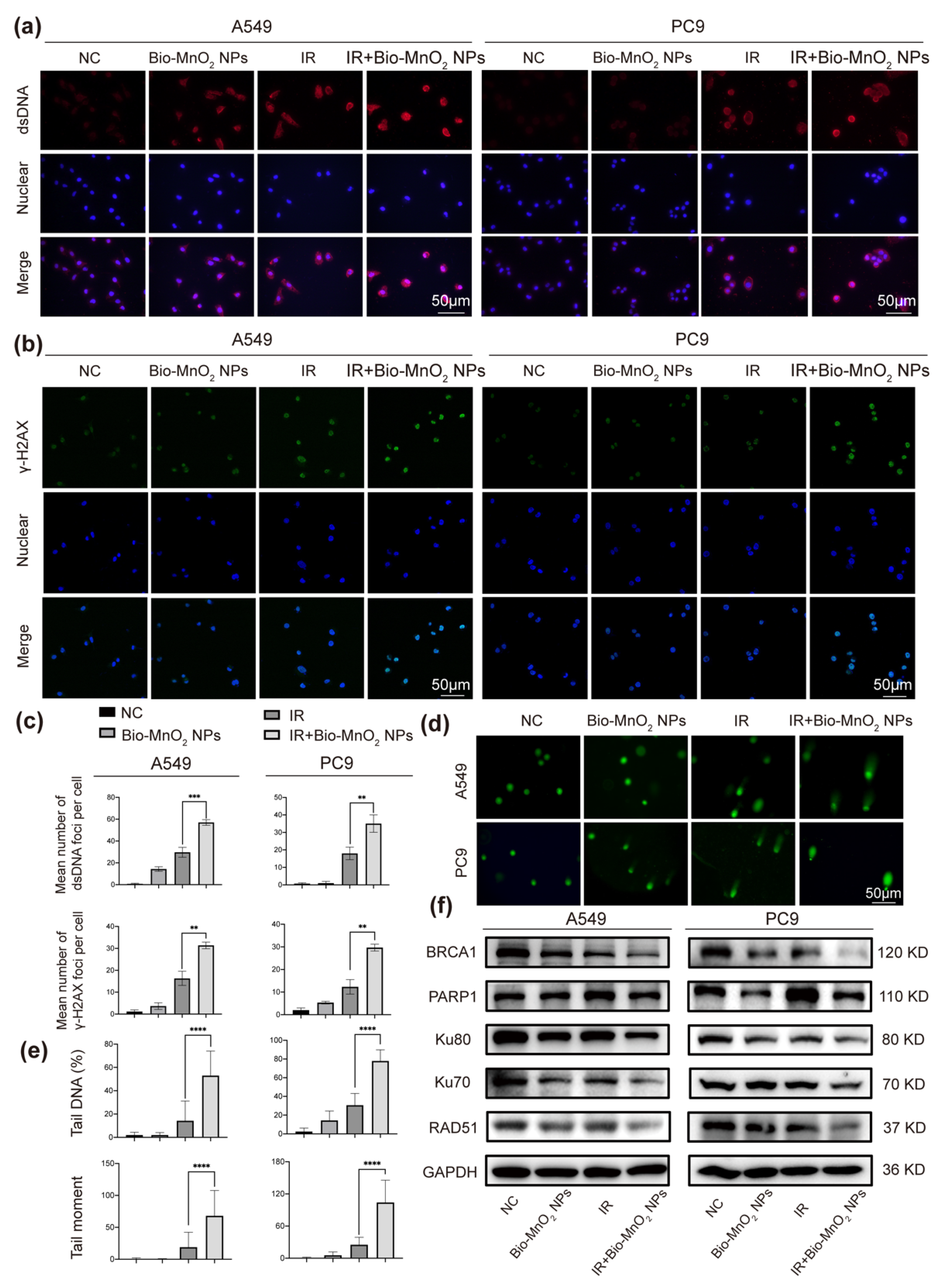
3.4. Bio-MnO2 NPs plus Radiation Increased DNA Damage in NSCLC Cells
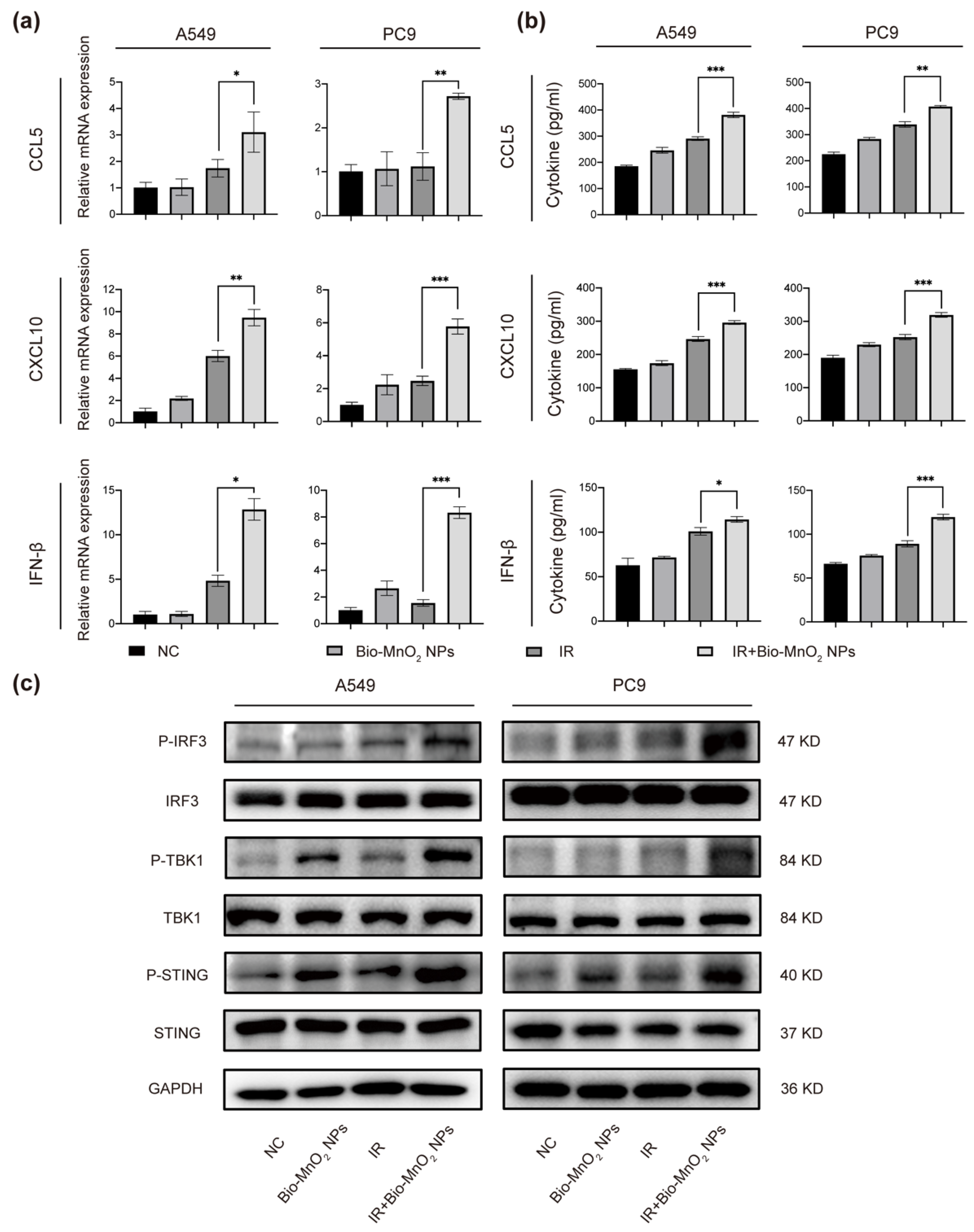
3.5. Bio-MnO2 NPs plus Radiation Enhanced the Activation of cGAS/STING Signaling Pathway
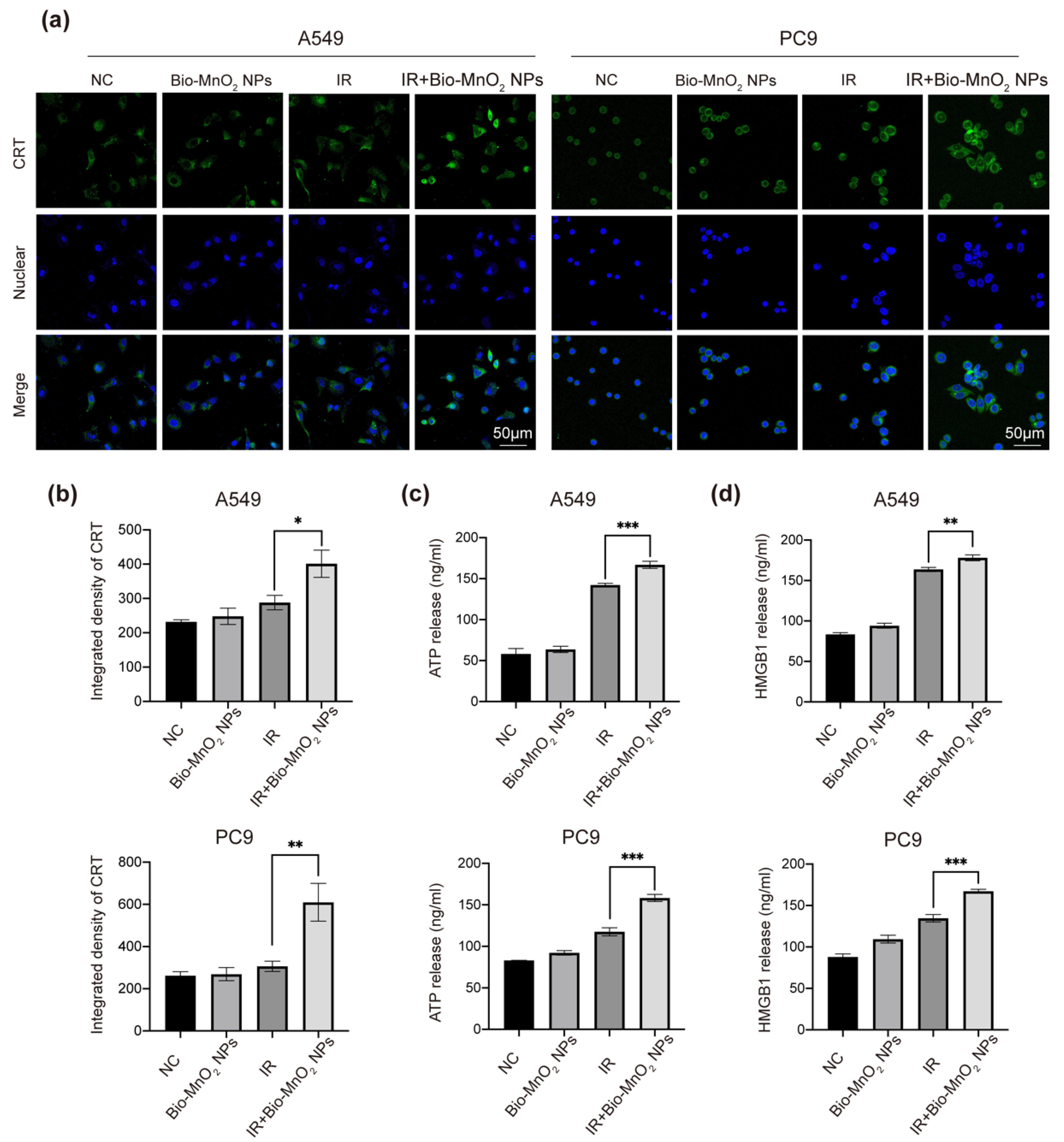
3.6. Bio-MnO2 NPs plus Radiation Induced ICD
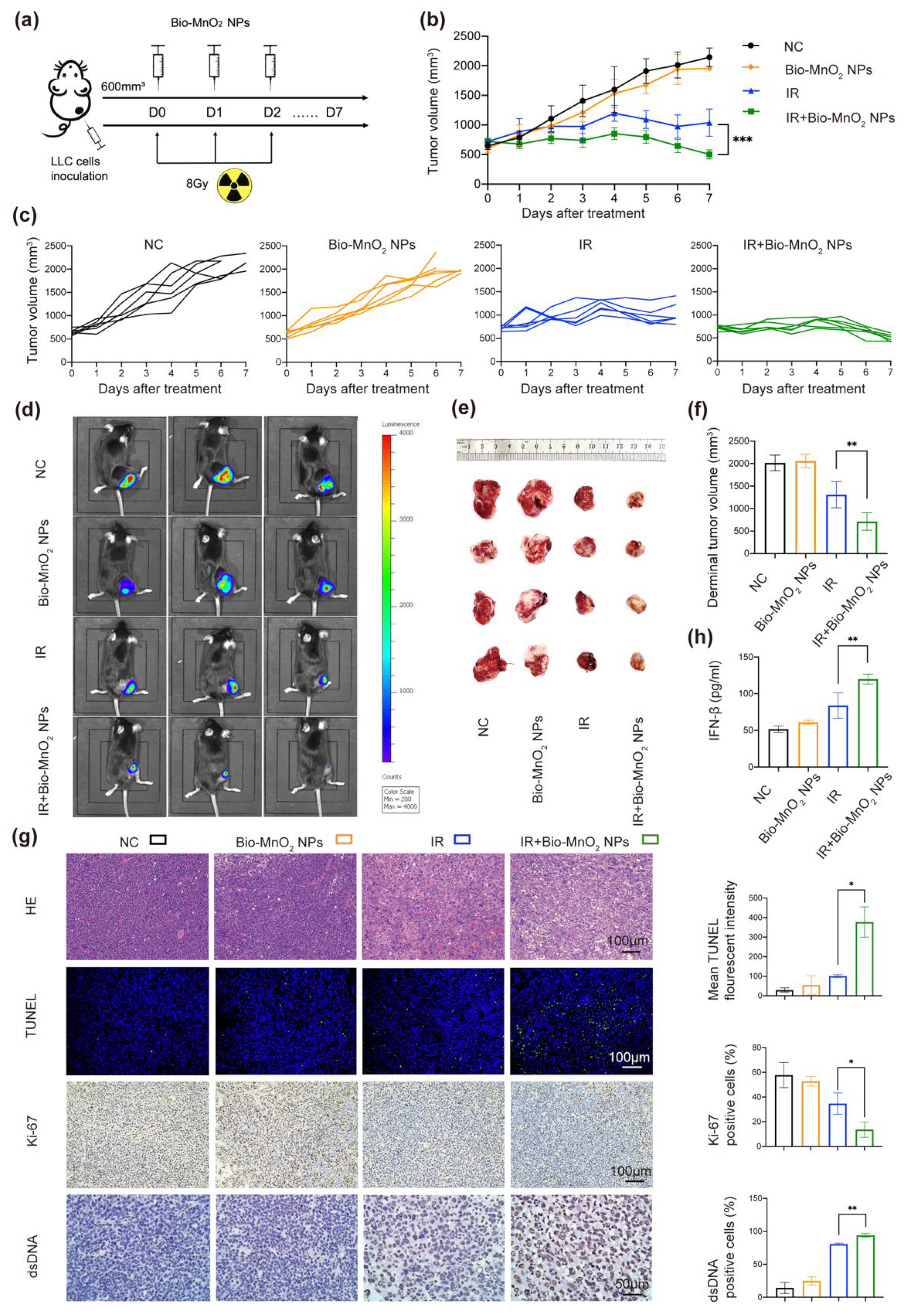
3.7. Bio-MnO2 NPs Enhanced RT In Vivo
3.8. Bio-MnO2 NPs plus Radiation Activated Immune Responses In Vivo
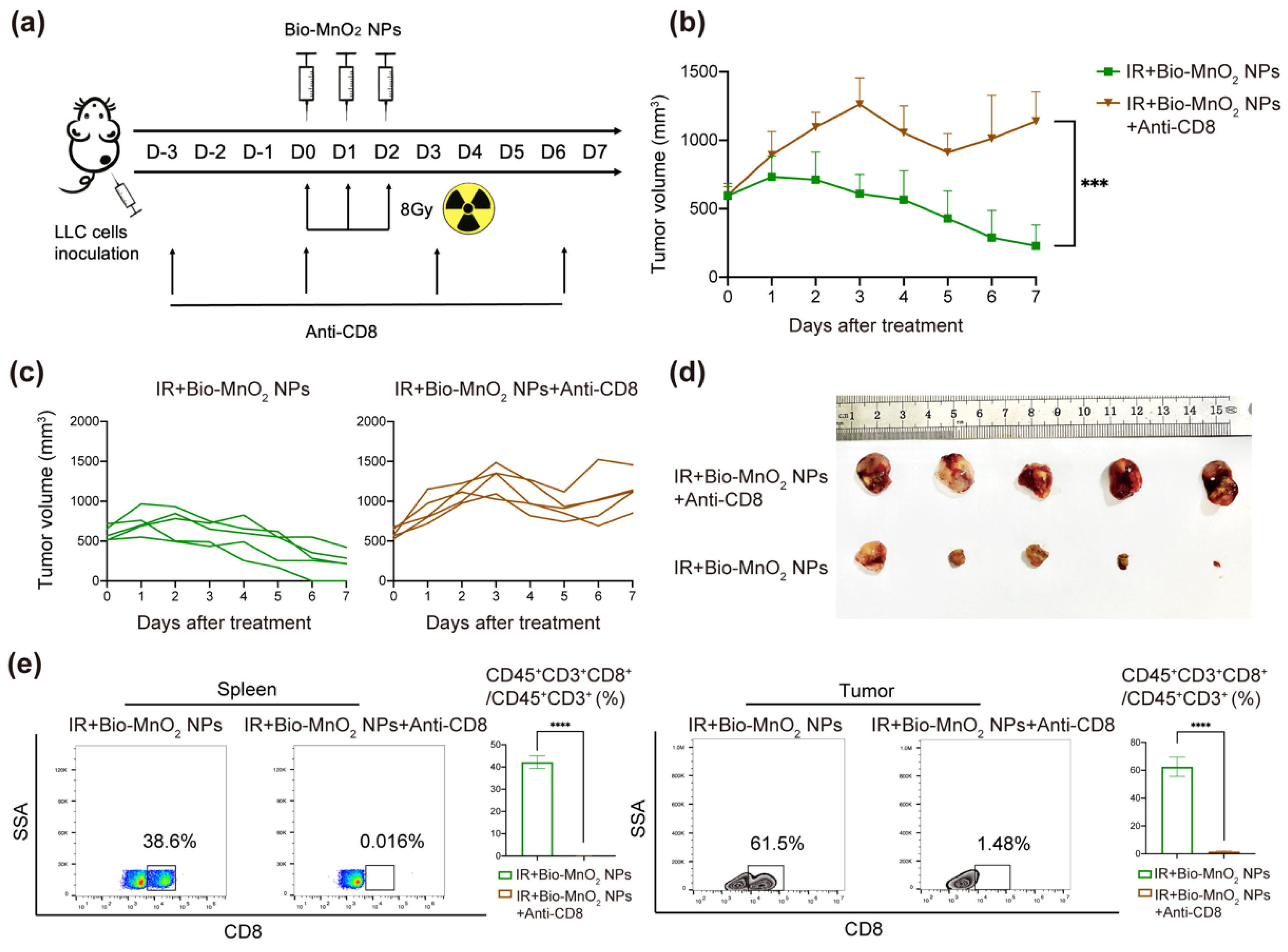
3.9. Cytotoxic T Cells Played an Important Role in Immune Responses Activated by Bio-MnO2 NPs Plus Radiation In Vivo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 world health organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef]
- Desai, S.; Kim, C.; Veytsman, I. Role of anti-egfr targeted therapies in stage iii locally advanced non-small cell lung cancer: Give or not to give? Curr. Oncol. Rep. 2019, 21, 84. [Google Scholar] [CrossRef] [PubMed]
- Van Schil, P.E.; Rami-Porta, R.; Asamura, H. The 8(th) tnm edition for lung cancer: A critical analysis. Ann. Transl. Med. 2018, 6, 87. [Google Scholar] [CrossRef]
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Bernier, J.; Hall, E.J.; Giaccia, A. Radiation oncology: A century of achievements. Nat. Rev. Cancer 2004, 4, 737–747. [Google Scholar] [CrossRef]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef]
- Li, X.; Shu, C.; Yi, G.; Chaton, C.T.; Shelton, C.L.; Diao, J.; Zuo, X.; Kao, C.C.; Herr, A.B.; Li, P. Cyclic gmp-amp synthase is activated by double-stranded DNA-induced oligomerization. Immunity 2013, 39, 1019–1031. [Google Scholar] [CrossRef]
- Storozynsky, Q.; Hitt, M.M. The impact of radiation-induced DNA damage on cgas-sting-mediated immune responses to cancer. Int. J. Mol. Sci. 2020, 21, 8877. [Google Scholar] [CrossRef]
- Hopfner, K.P.; Hornung, V. Molecular mechanisms and cellular functions of cgas-sting signalling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Omura, H.; Ishitani, R.; Nureki, O. Cyclic gmp-amp as an endogenous second messenger in innate immune signaling by cytosolic DNA. Annu. Rev. Biochem. 2017, 86, 541–566. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8, e000337. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Zhang, X.; Pan, W.; Li, N.; Tang, B. Immunogenic cell death inducers for enhanced cancer immunotherapy. Chem. Commun. 2021, 57, 12087–12097. [Google Scholar] [CrossRef]
- Weichselbaum, R.R.; Liang, H.; Deng, L.; Fu, Y.X. Radiotherapy and immunotherapy: A beneficial liaison? Nat. Rev. Clin. Oncol. 2017, 14, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.G.; Hill, R.P. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 2008, 8, 180–192. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Ding, Y.; Wu, J.; Hu, Y.; Yuan, A. Synergy of hypoxia relief and chromatin remodeling to overcome tumor radiation resistance. Biomater. Sci. 2020, 8, 4739–4749. [Google Scholar] [CrossRef]
- Abbasi, A.Z.; Gordijo, C.R.; Amini, M.A.; Maeda, A.; Rauth, A.M.; DaCosta, R.S.; Wu, X.Y. Hybrid manganese dioxide nanoparticles potentiate radiation therapy by modulating tumor hypoxia. Cancer Res. 2016, 76, 6643–6656. [Google Scholar] [CrossRef]
- Rey, S.; Schito, L.; Koritzinsky, M.; Wouters, B.G. Molecular targeting of hypoxia in radiotherapy. Adv. Drug Deliv. Rev. 2017, 109, 45–62. [Google Scholar] [CrossRef]
- Xiong, Y.; Xiao, C.; Li, Z.; Yang, X. Engineering nanomedicine for glutathione depletion-augmented cancer therapy. Chem. Soc. Rev. 2021, 50, 6013–6041. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Song, K.; Du, J.; Wang, X.; Liu, J.; Li, B.; Ouyang, R.; Miao, Y.; Sun, Y.; et al. Tumor microenvironment modulation platform based on composite biodegradable bismuth-manganese radiosensitizer for inhibiting radioresistant hypoxic tumors. Small 2021, 17, e2101015. [Google Scholar] [CrossRef]
- Yao, Y.; Li, P.; He, J.; Wang, D.; Hu, J.; Yang, X. Albumin-templated bi(2)se(3)-mno(2) nanocomposites with promoted catalase-like activity for enhanced radiotherapy of cancer. ACS Appl. Mater. Interfaces 2021, 13, 28650–28661. [Google Scholar] [CrossRef] [PubMed]
- Bilynsky, C.; Millot, N.; Papa, A.L. Radiation nanosensitizers in cancer therapy-from preclinical discoveries to the outcomes of early clinical trials. Bioeng. Transl. Med. 2022, 7, e10256. [Google Scholar] [CrossRef] [PubMed]
- Retif, P.; Pinel, S.; Toussaint, M.; Frochot, C.; Chouikrat, R.; Bastogne, T.; Barberi-Heyob, M. Nanoparticles for radiation therapy enhancement: The key parameters. Theranostics 2015, 5, 1030–1044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shao, S.; Yue, H.; Wang, X.; Zhang, W.; Chen, F.; Zheng, L.; Xing, J.; Qin, Y. High stability au nps: From design to application in nanomedicine. Int. J. Nanomed. 2021, 16, 6067–6094. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, Y. Nanostructured manganese dioxide for anticancer applications: Preparation, diagnosis, and therapy. Nanoscale 2020, 12, 17982–18003. [Google Scholar] [CrossRef]
- Ding, B.; Zheng, P.; Ma, P.; Lin, J. Manganese oxide nanomaterials: Synthesis, properties, and theranostic applications. Adv. Mater. 2020, 32, e1905823. [Google Scholar] [CrossRef]
- Yang, G.; Ji, J.; Liu, Z. Multifunctional mno(2) nanoparticles for tumor microenvironment modulation and cancer therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1720. [Google Scholar] [CrossRef]
- Prasad, P.; Gordijo, C.R.; Abbasi, A.Z.; Maeda, A.; Ip, A.; Rauth, A.M.; DaCosta, R.S.; Wu, X.Y. Multifunctional albumin-mno2 nanoparticles modulate solid tumor microenvironment by attenuating hypoxia, acidosis, vascular endothelial growth factor and enhance radiation response. ACS Nano 2014, 8, 3202–3212. [Google Scholar] [CrossRef]
- Hou, L.; Tian, C.; Yan, Y.; Zhang, L.; Zhang, H.; Zhang, Z. Manganese-based nanoactivator optimizes cancer immunotherapy via enhancing innate immunity. ACS Nano 2020, 14, 3927–3940. [Google Scholar] [CrossRef]
- Luo, X.L.; Xu, J.J.; Zhao, W.; Chen, H.Y. A novel glucose enfet based on the special reactivity of mno2 nanoparticles. Biosens. Bioelectron. 2004, 19, 1295–1300. [Google Scholar] [CrossRef]
- Wang, C.; Guan, Y.; Lv, M.; Zhang, R.; Guo, Z.; Wei, X.; Du, X.; Yang, J.; Li, T.; Wan, Y.; et al. Manganese increases the sensitivity of the cgas-sting pathway for double-stranded DNA and is required for the host defense against DNA viruses. Immunity 2018, 48, 675–687.e677. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Chen, M.; Zhang, R.; Zhang, W.; Wang, C.; Zhang, Y.; Wei, X.; Guan, Y.; Liu, J.; Feng, K.; et al. Manganese is critical for antitumor immune responses via cgas-sting and improves the efficacy of clinical immunotherapy. Cell Res. 2020, 30, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Yue, J.; Zheng, P.; Ma, P.; Lin, J. Manganese oxide nanomaterials boost cancer immunotherapy. J. Mater. Chem. B 2021, 9, 7117–7131. [Google Scholar] [CrossRef] [PubMed]
- Laoui, D.; Van Overmeire, E.; Di Conza, G.; Aldeni, C.; Keirsse, J.; Morias, Y.; Movahedi, K.; Houbracken, I.; Schouppe, E.; Elkrim, Y.; et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the m2-like macrophage population. Cancer Res. 2014, 74, 24–30. [Google Scholar] [CrossRef]
- Chen, Q.; Feng, L.; Liu, J.; Zhu, W.; Dong, Z.; Wu, Y.; Liu, Z. Intelligent albumin-mno2 nanoparticles as ph-/H2O2-responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy. Adv. Mater. 2016, 28, 7129–7136. [Google Scholar] [CrossRef]
- Yang, G.; Xu, L.; Chao, Y.; Xu, J.; Sun, X.; Wu, Y.; Peng, R.; Liu, Z. Hollow mno(2) as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017, 8, 902. [Google Scholar] [CrossRef]
- Lin, L.S.; Song, J.; Song, L.; Ke, K.; Liu, Y.; Zhou, Z.; Shen, Z.; Li, J.; Yang, Z.; Tang, W.; et al. Simultaneous fenton-like ion delivery and glutathione depletion by mno(2) -based nanoagent to enhance chemodynamic therapy. Angew. Chem. Int. Ed. Engl. 2018, 57, 4902–4906. [Google Scholar] [CrossRef]
- Liu, J.; Feng, L.; Wu, Y. Enzymatically synthesised mno(2) nanoparticles for efficient near-infrared photothermal therapy and dual-responsive magnetic resonance imaging. Nanoscale 2021, 13, 11093–11103. [Google Scholar] [CrossRef]
- Butterfield, C.N.; Soldatova, A.V.; Lee, S.W.; Spiro, T.G.; Tebo, B.M. Mn(ii,iii) oxidation and mno2 mineralization by an expressed bacterial multicopper oxidase. Proc. Natl. Acad. Sci. USA 2013, 110, 11731–11735. [Google Scholar] [CrossRef] [Green Version]
- Soldatova, A.V.; Tao, L.; Romano, C.A.; Stich, T.A.; Casey, W.H.; Britt, R.D.; Tebo, B.M.; Spiro, T.G. Mn(ii) oxidation by the multicopper oxidase complex mnx: A binuclear activation mechanism. J. Am. Chem. Soc. 2017, 139, 11369–11380. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Abdeen, A.A.; Wang, Y.; Shahi, P.K.; Robertson, S.; Xie, R.; Suzuki, M.; Pattnaik, B.R.; Saha, K.; Gong, S. A biodegradable nanocapsule delivers a cas9 ribonucleoprotein complex for in vivo genome editing. Nat. Nanotechnol. 2019, 14, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Feng, F.; Meng, F.; Deng, C.; Feijen, J.; Zhong, Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Control. Release 2011, 152, 2–12. [Google Scholar] [CrossRef]
- Cho, M.H.; Choi, E.S.; Kim, S.; Goh, S.H.; Choi, Y. Redox-responsive manganese dioxide nanoparticles for enhanced mr imaging and radiotherapy of lung cancer. Front. Chem. 2017, 5, 109. [Google Scholar] [CrossRef]
- Ma, N.; Jiang, Y.W.; Zhang, X.; Wu, H.; Myers, J.N.; Liu, P.; Jin, H.; Gu, N.; He, N.; Wu, F.G.; et al. Enhanced radiosensitization of gold nanospikes via hyperthermia in combined cancer radiation and photothermal therapy. ACS Appl. Mater. Interfaces 2016, 8, 28480–28494. [Google Scholar] [CrossRef]
- Ding, B.; Zheng, P.; Jiang, F.; Zhao, Y.; Wang, M.; Chang, M.; Ma, P.; Lin, J. Mno(x) nanospikes as nanoadjuvants and immunogenic cell death drugs with enhanced antitumor immunity and antimetastatic effect. Angew. Chem. Int. Ed. Engl. 2020, 59, 16381–16384. [Google Scholar] [CrossRef]
- Wang, S.; Li, F.; Qiao, R.; Hu, X.; Liao, H.; Chen, L.; Wu, J.; Wu, H.; Zhao, M.; Liu, J.; et al. Arginine-rich manganese silicate nanobubbles as a ferroptosis-inducing agent for tumor-targeted theranostics. ACS Nano 2018, 12, 12380–12392. [Google Scholar] [CrossRef]
- Zhang, X.D.; Wu, D.; Shen, X.; Chen, J.; Sun, Y.M.; Liu, P.X.; Liang, X.J. Size-dependent radiosensitization of peg-coated gold nanoparticles for cancer radiation therapy. Biomaterials 2012, 33, 6408–6419. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef] [PubMed]
- Sicard-Roselli, C.; Brun, E.; Gilles, M.; Baldacchino, G.; Kelsey, C.; McQuaid, H.; Polin, C.; Wardlow, N.; Currell, F. A new mechanism for hydroxyl radical production in irradiated nanoparticle solutions. Small 2014, 10, 3338–3346. [Google Scholar] [CrossRef] [PubMed]
- Morozov, K.V.; Kolyvanova, M.A.; Kartseva, M.E.; Shishmakova, E.M.; Dement’eva, O.V.; Isagulieva, A.K.; Salpagarov, M.H.; Belousov, A.V.; Rudoy, V.M.; Shtil, A.A.; et al. Radiosensitization by gold nanoparticles: Impact of the size, dose rate, and photon energy. Nanomaterials 2020, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.N.; Sørensen, J.B. Clinical impact of ki-67 labeling index in non-small cell lung cancer. Lung Cancer 2013, 79, 1–7. [Google Scholar] [CrossRef]
- Tubiana, M.; Courdi, A. Cell proliferation kinetics in human solid tumors: Relation to probability of metastatic dissemination and long-term survival. Radiother. Oncol. 1989, 15, 1–18. [Google Scholar] [CrossRef]
- Spitz, A.Z.; Gavathiotis, E. Physiological and pharmacological modulation of bax. Trends Pharm. Sci. 2022, 43, 206–220. [Google Scholar] [CrossRef]
- Dadsena, S.; King, L.E.; García-Sáez, A.J. Apoptosis regulation at the mitochondria membrane level. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183716. [Google Scholar] [CrossRef]
- Mladenov, E.; Magin, S.; Soni, A.; Iliakis, G. DNA double-strand break repair as determinant of cellular radiosensitivity to killing and target in radiation therapy. Front. Oncol. 2013, 3, 113. [Google Scholar] [CrossRef]
- Löbrich, M.; Shibata, A.; Beucher, A.; Fisher, A.; Ensminger, M.; Goodarzi, A.A.; Barton, O.; Jeggo, P.A. Gammah2ax foci analysis for monitoring DNA double-strand break repair: Strengths, limitations and optimization. Cell Cycle 2010, 9, 662–669. [Google Scholar] [CrossRef]
- Tatin, X.; Muggiolu, G.; Sauvaigo, S.; Breton, J. Evaluation of DNA double-strand break repair capacity in human cells: Critical overview of current functional methods. Mutat. Res. Rev. Mutat. Res. 2021, 788, 108388. [Google Scholar] [CrossRef]
- Civril, F.; Deimling, T.; de Oliveira Mann, C.C.; Ablasser, A.; Moldt, M.; Witte, G.; Hornung, V.; Hopfner, K.P. Structural mechanism of cytosolic DNA sensing by cgas. Nature 2013, 498, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and damps in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Q.; Huang, Q.; Liu, Z.; Zeng, L.; Zhang, L.; Chen, X.; Song, H.; Zhang, J. Photothermal mno(2) nanoparticles boost chemo-photothermal therapy-induced immunogenic cell death in tumor immunotherapy. Int. J. Pharm. 2022, 617, 121578. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yang, M.; Zhang, J.; Yin, Y.; Fan, X.; Zhang, Y.; Qin, S.; Zhang, H.; Yu, F. Immunogenic cell death induction by ionizing radiation. Front. Immunol. 2021, 12, 705361. [Google Scholar] [CrossRef]
- Xie, P.; Zhang, M.; He, S.; Lu, K.; Chen, Y.; Xing, G.; Lu, Y.; Liu, P.; Li, Y.; Wang, S.; et al. The covalent modifier nedd8 is critical for the activation of smurf1 ubiquitin ligase in tumorigenesis. Nat. Commun. 2014, 5, 3733. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Roche, K.C.; Tian, S.; Eblan, M.J.; McKinnon, K.P.; Caster, J.M.; Chai, S.; Herring, L.E.; Zhang, L.; Zhang, T.; et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol. 2017, 12, 877–882. [Google Scholar] [CrossRef]
- Awad, M.M.; Govindan, R.; Balogh, K.N.; Spigel, D.R.; Garon, E.B.; Bushway, M.E.; Poran, A.; Sheen, J.H.; Kohler, V.; Esaulova, E.; et al. Personalized neoantigen vaccine neo-pv-01 with chemotherapy and anti-pd-1 as first-line treatment for non-squamous non-small cell lung cancer. Cancer Cell 2022. [Google Scholar] [CrossRef]
- Yu, M.; Duan, X.; Cai, Y.; Zhang, F.; Jiang, S.; Han, S.; Shen, J.; Shuai, X. Multifunctional nanoregulator reshapes immune microenvironment and enhances immune memory for tumor immunotherapy. Adv. Sci. 2019, 6, 1900037. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Kifle, M.T.; Xie, H.; Xu, L.; Luo, M.; Li, Y.; Huang, Z.; Gong, Y.; Wu, Y.; Xie, C. Biomineralized Manganese Oxide Nanoparticles Synergistically Relieve Tumor Hypoxia and Activate Immune Response with Radiotherapy in Non-Small Cell Lung Cancer. Nanomaterials 2022, 12, 3138. https://doi.org/10.3390/nano12183138
Liu X, Kifle MT, Xie H, Xu L, Luo M, Li Y, Huang Z, Gong Y, Wu Y, Xie C. Biomineralized Manganese Oxide Nanoparticles Synergistically Relieve Tumor Hypoxia and Activate Immune Response with Radiotherapy in Non-Small Cell Lung Cancer. Nanomaterials. 2022; 12(18):3138. https://doi.org/10.3390/nano12183138
Chicago/Turabian StyleLiu, Xinyu, Meron Tsegay Kifle, Hongxin Xie, Liexi Xu, Maoling Luo, Yangyi Li, Zhengrong Huang, Yan Gong, Yuzhou Wu, and Conghua Xie. 2022. "Biomineralized Manganese Oxide Nanoparticles Synergistically Relieve Tumor Hypoxia and Activate Immune Response with Radiotherapy in Non-Small Cell Lung Cancer" Nanomaterials 12, no. 18: 3138. https://doi.org/10.3390/nano12183138
APA StyleLiu, X., Kifle, M. T., Xie, H., Xu, L., Luo, M., Li, Y., Huang, Z., Gong, Y., Wu, Y., & Xie, C. (2022). Biomineralized Manganese Oxide Nanoparticles Synergistically Relieve Tumor Hypoxia and Activate Immune Response with Radiotherapy in Non-Small Cell Lung Cancer. Nanomaterials, 12(18), 3138. https://doi.org/10.3390/nano12183138







