Multiferroic and Magnetodielectric Effects in Multiferroic Pr2FeAlO6 Double Perovskite
Abstract
:1. Introduction
2. Materials and Methods
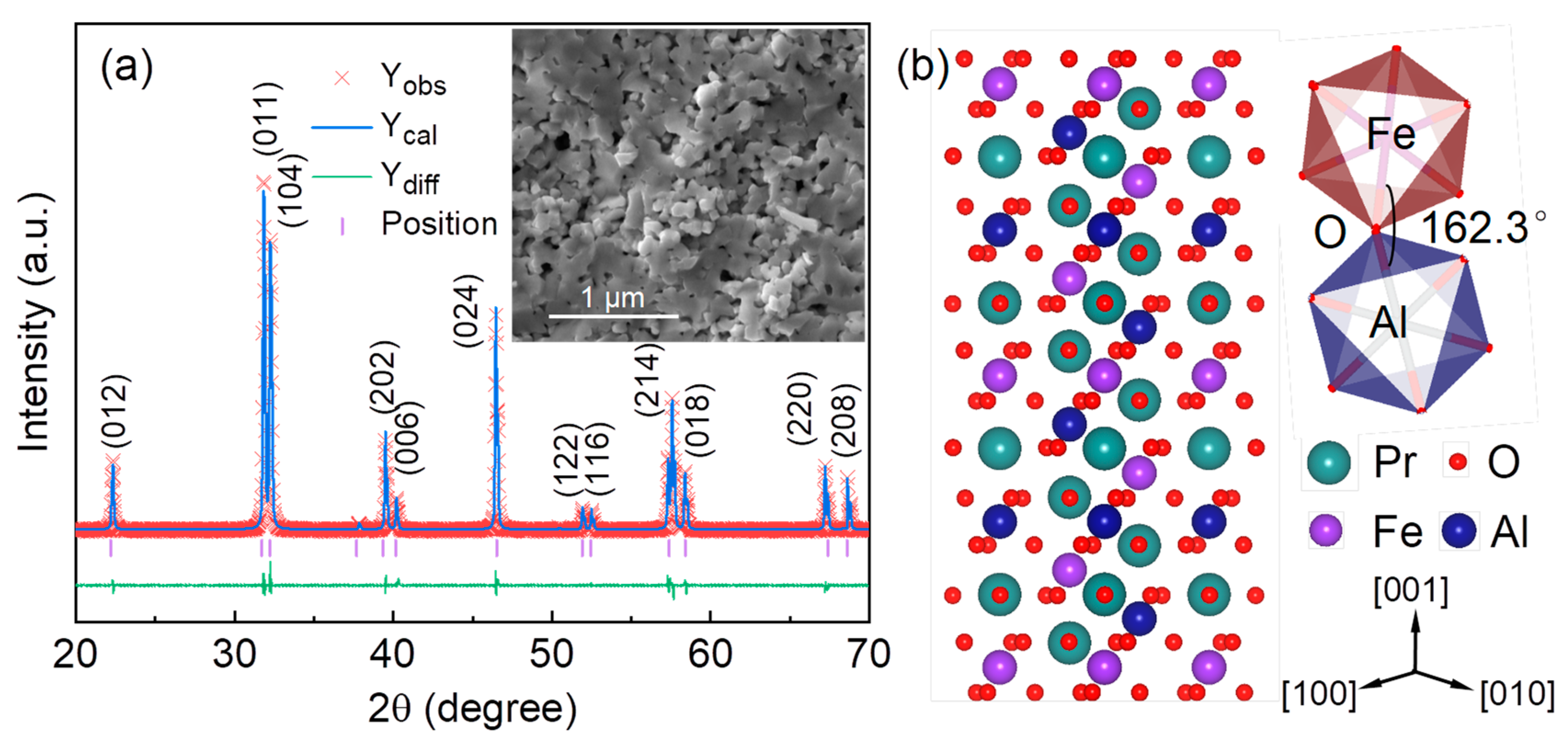
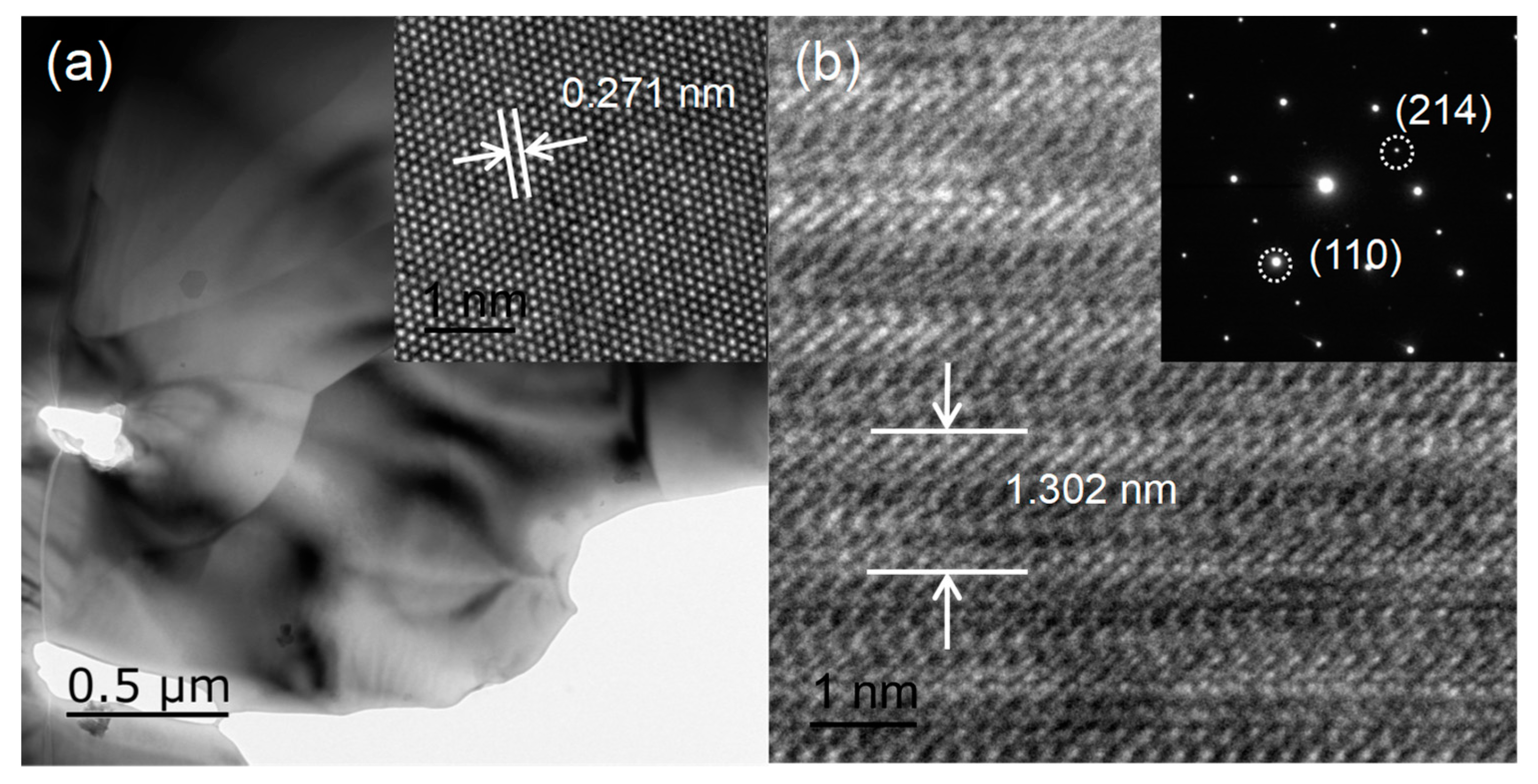
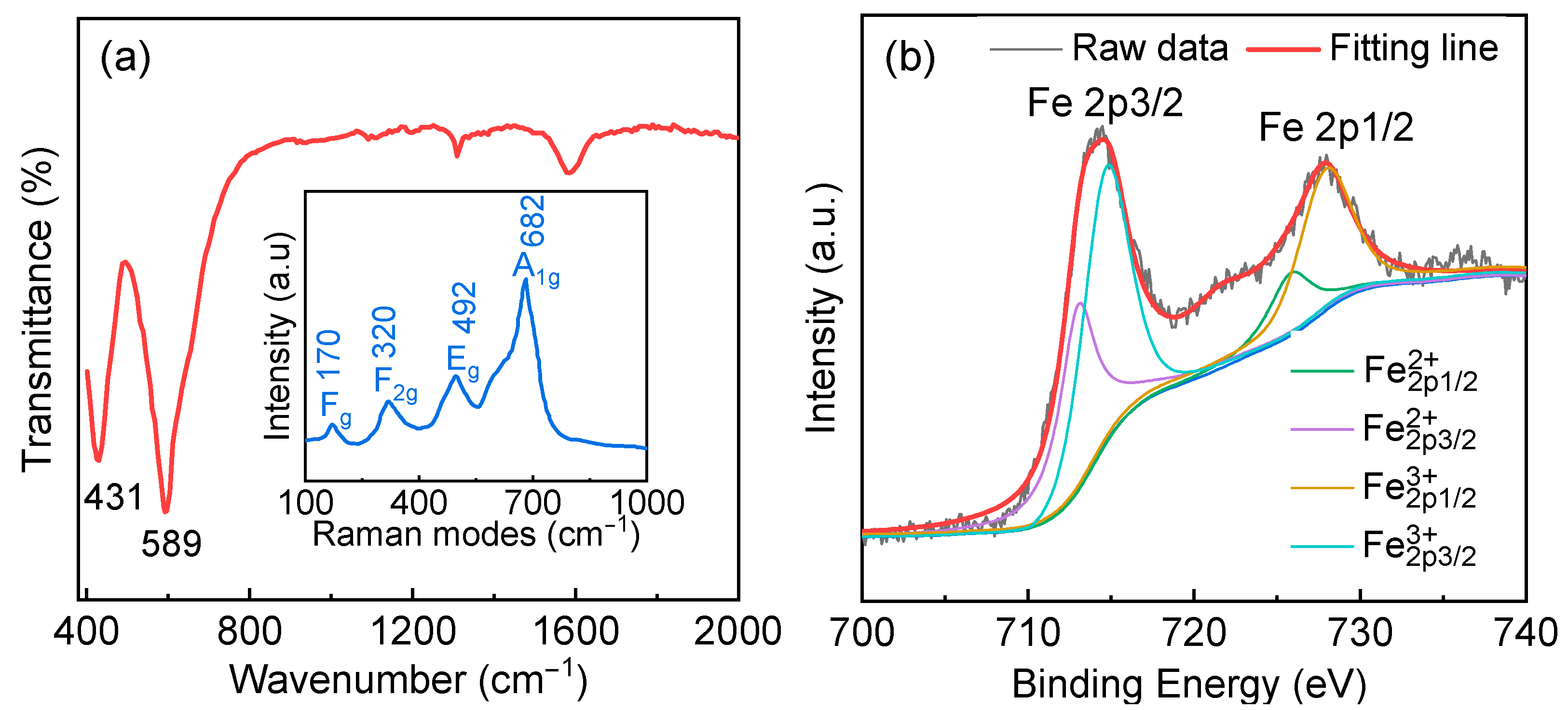
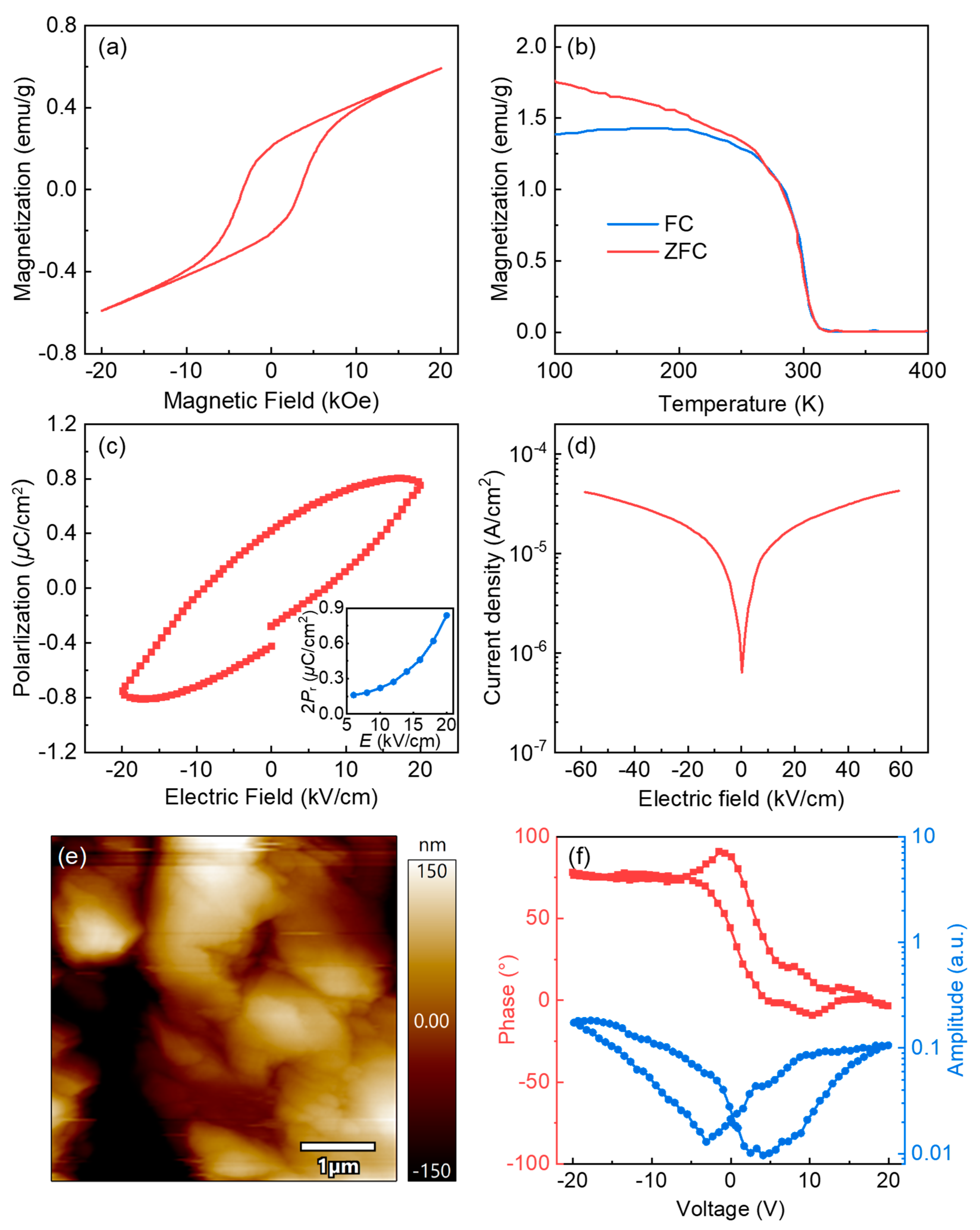
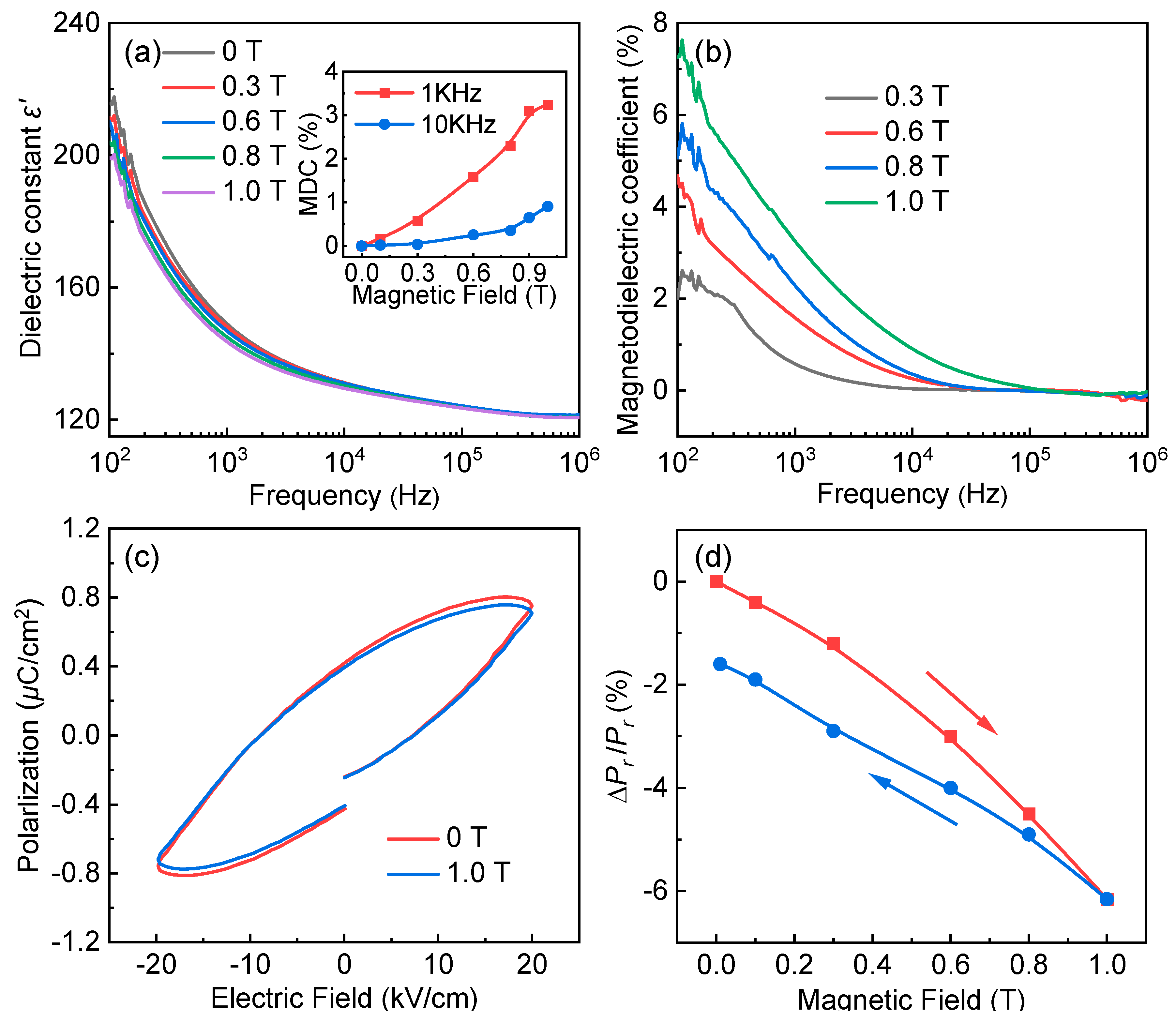
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spaldin, N.A.; Ramesh, R. Advances in magnetoelectric multiferroics. Nat. Mater. 2019, 18, 203–212. [Google Scholar] [CrossRef]
- Nan, C.-W.; Liu, J.-M. Multiferroics: A beautiful but challenging multi-polar world. Nat. Sci. Rev. 2019, 6, 620. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.K.; Kumari, S.; Rack, P.D. Magnetoelectric composites: Applications, coupling mechanisms, and future directions. Nanomaterials 2020, 10, 2072. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Chen, H.; Sun, N.X. Magnetoelectric materials and devices. APL Mater. 2021, 9, 041114. [Google Scholar] [CrossRef]
- Song, C.; Cui, B.; Li, F.; Zhou, X.; Pan, F. Recent progress in voltage control of magnetism: Materials, mechanisms, and performance. Prog. Mater. Sci. 2017, 87, 33–82. [Google Scholar] [CrossRef]
- Dong, S.; Liu, J.-M.; Cheong, S.-W.; Ren, Z. Multiferroic materials and magnetoelectric physics: Symmetry, entanglement, excitation, and topology. Adv. Phys. 2015, 64, 519–626. [Google Scholar] [CrossRef]
- Wu, H.; Li, L.; Liang, L.-Z.; Liang, S.; Zhu, Y.-Y.; Zhu, X.-H. Recent Progress on the structural characterizations of domain structures in ferroic and multiferroic perovskite oxides: A review. J. Eur. Ceram. Soc. 2015, 35, 411–441. [Google Scholar] [CrossRef]
- Serrate, D.; De Teresa, J.; Ibarra, M. Double perovskites with ferromagnetism above room temperature. J. Phys. Condens. Matter. 2006, 19, 023201. [Google Scholar] [CrossRef]
- Vasala, S.; Karppinen, M. A2B′B″O6 perovskites: A review. Prog. Solid. State. Chem. 2015, 43, 1–36. [Google Scholar] [CrossRef]
- Hossain, A.; Bandyopadhyay, P.; Roy, S. An overview of double perovskites A2B’B”O6 with small ions at a site: Synthesis, structure and magnetic properties. J. Alloys Compd. 2018, 740, 414–427. [Google Scholar] [CrossRef]
- Jin, X.-W.; Lu, L.; Mi, S.-B.; Liu, M.; Jia, C.-L. Phase stability and B-site ordering in La2NiMnO6 thin films. Appl. Phys. Lett. 2016, 109, 031904. [Google Scholar] [CrossRef]
- Wu, S.-Q.; Cheng, S.; Lu, L.; Liu, M.; Jin, X.-W.; Cheng, S.-D.; Mi, S.-B. B-Site ordering and strain-induced phase transition in double-perovskite La2NiMnO6 Films. Sci. Rep. 2018, 8, 2516. [Google Scholar] [CrossRef]
- Masud, M.G.; Dey, K.; Ghosh, A.; Majumdar, S.; Giri, S. Occurrence of magnetoelectric effect correlated to the Dy order in Dy2NiMnO6 double perovskite. J. Appl. Phys. 2015, 118, 064104. [Google Scholar] [CrossRef]
- Das, R.; Choudhary, R.N.P. Dielectric relaxation and magneto-electric characteristics of lead-free double perovskite: Sm2NiMnO6. J. Adv. Ceram. 2019, 8, 174–185. [Google Scholar] [CrossRef]
- Das, N.; Singh, S.; Joshi, A.G.; Thirumal, M.; Reddy, V.R.; Gupta, L.C.; Ganguli, A.K. Pr2FeCrO6: A type I multiferroic. Inorg. Chem. 2017, 56, 12712–12718. [Google Scholar] [CrossRef] [PubMed]
- King, G.; Woodward, P.M. Cation ordering in perovskites. J. Mater. Chem. 2010, 20, 5785–5796. [Google Scholar] [CrossRef]
- Rondinelli, J.M.; Fennie, C.J. Octahedral rotation-induced ferroelectricity in cation ordered perovskites. Adv. Mater. 2012, 24, 1961–1968. [Google Scholar] [CrossRef]
- Saha, S.; Cao, B.-C.; Motapothula, M.; Cong, C.-X.; Sarkar, T.; Srivastava, A.; Sarkar, S.; Patra, A.; Ghosh, S.; Ariando; et al. Magnetic modes in rare earth perovskites: A magnetic-field-dependent inelastic light scattering study. Sci. Rep. 2016, 6, 36859. [Google Scholar] [CrossRef]
- Tezuka, K.; Henmi, K.; Hinatsu, Y.; Masaki, N.M. Magnetic susceptibilities and mossbauer spectra of perovskites A2NbO6 (A = Sr, Ba). J. Solid State. Chem. 2000, 154, 591–597. [Google Scholar] [CrossRef]
- Ravi, S. Multiferroism in Pr2FeCrO6 perovskite. J. Rare. Earth. 2018, 36, 1175–1178. [Google Scholar] [CrossRef]
- Mazumdar, D.; Das, I. Structural, magnetic, and magnetocaloric properties of the multiferroic host double perovskite compound Pr2FeCrO6. Phys. Chem. Chem. Phys. 2021, 23, 5596–5606. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S. High curie temperature and room temperature magnetoresistance in Pr2FeCrO6 material for spintronics applications. Mater. Lett. 2020, 278, 128448. [Google Scholar] [CrossRef]
- Song, B.; Shen, J.; Zhao, H.; Kumar, A.; Xu, Q.; Zhai, Y.; Li, Q. A new room-temperature multiferroic material: Y2FeAlO6. Ceram. Int. 2021, 47, 10873–10879. [Google Scholar] [CrossRef]
- Farzin, Y.A.; Babaei, A.; Ataie, A. Low-temperature synthesis of Sr2femoo6 double perovskite; structure, morphology, and magnetic Properties. Ceram. Int. 2020, 46, 16867–16878. [Google Scholar] [CrossRef]
- Yadav, R.; Para, T.A.; Reshi, H.A.; Pillai, S.; Shelke, V. Easy synthesis and electric, magneto-transport and magnetic properties of double perovskite La2CoMnO6 compound. J. Mater. Sci. Mater. Electron. 2017, 28, 2970–2975. [Google Scholar] [CrossRef]
- Hu, Y.-C.; Cui, Y.-W.; Wang, X.-W.; Liu, Y.-P. Effect of quench treatment on Fe/Mo order and magnetic properties of double perovskite Sr2FeMoO6. Chin. Phys. Lett. 2016, 33, 026101. [Google Scholar]
- Kumar, A.; Yadav, K.L. A Systematic Study on magnetic, dielectric and magnetocapacitance properties of Ni doped bismuth ferrite. J. Phys. Chem. Solids 2011, 72, 1189–1194. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Bhuyan, M.D.I.; Das, S.; Basith, M.A. Sol-Gel synthesized double perovskite GdFeCrO6 nanoparticles: Structural, magnetic and optical properties. J. Alloys Compd. 2021, 878, 160389. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 1–408. [Google Scholar]
- Zhang, Y.; Yao, C.; Fan, Y.; Zhou, M. One-Step hydrothermal synthesis, characterization and magnetic properties of orthorhombic PrCrO3 cubic particles. Mater. Res. Bull. 2014, 59, 387–393. [Google Scholar] [CrossRef]
- Chandel, S.; Thakur, P.; Thakur, S.S.; Kanwar, V.; Tomar, M.; Gupta, V.; Thakur, A. Effect of non-magnetic Al3+ doping on structural, optical, electrical, dielectric and magnetic properties of BiFeO3 ceramics. Ceram. Int. 2018, 44, 4711–4718. [Google Scholar] [CrossRef]
- Gaikwad, V.M.; Brahma, M.; Borah, R.; Ravi, S. Structural, Optical and magnetic properties of Pr2FeCrO6 nanoparticles. J. Solid State. Chem. 2019, 278, 120903. [Google Scholar] [CrossRef]
- Hou, L.; Shi, L.; Zhao, J.; Zhou, S.; Pan, S.; Yuan, X.; Xin, Y. Room-temperature multiferroicity in CeFeO3 ceramics perovskite. J. Alloys Compd. 2019, 797, 363–369. [Google Scholar] [CrossRef]
- Wu, H.; Lu, Y.; Zhu, X.; Xia, W.; Leng, K.; Pei, Z. Structural, magnetic, dielectric and optical properties of double-perovskite Bi2FeCrO6 ceramics synthesized under high pressure. J. Alloys Compd. 2020, 819, 153007. [Google Scholar] [CrossRef]
- Ravi, S.; Senthilkumar, C. Room temperature multiferroicity in a new Ba2FeMnO6 double perovskite material. Ceram. Int. 2017, 43, 14441–14445. [Google Scholar] [CrossRef]
- Benedek, N.A.; Fennie, C.J. Hybrid improper ferroelectricity: A mechanism for controllable polarization-magnetization coupling. Phys. Rev. Lett. 2011, 106, 107204. [Google Scholar] [CrossRef] [PubMed]
- Catalan, G. Magnetocapacitance without magnetoelectric coupling. Appl. Phys. Lett. 2006, 88, 102902. [Google Scholar] [CrossRef]
- Liu, S.; Yan, S.; Luo, H.; Huang, S.; Liao, C.; Deng, L. Magnetic effects on polarization response in particulate magnetoelectric Bi0.5Na0.5TiO3-La0.67Sr0.33MnO3 composites. Mater. Lett. 2018, 212, 139–142. [Google Scholar] [CrossRef]
- Sreenivasulu, G.; Popov, M.; Chavez, F.A.; Hamilton, S.L.; Lehto, P.R.; Srinivasan, G. Controlled Self-assembly of multiferroic core-shell nanoparticles exhibiting strong magneto-electric effects. Appl. Phys. Lett. 2014, 104, 052901. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Xiang, F.; Cheng, Y.; Luo, Y.; Sun, J. Multiferroic and Magnetodielectric Effects in Multiferroic Pr2FeAlO6 Double Perovskite. Nanomaterials 2022, 12, 3011. https://doi.org/10.3390/nano12173011
Liu S, Xiang F, Cheng Y, Luo Y, Sun J. Multiferroic and Magnetodielectric Effects in Multiferroic Pr2FeAlO6 Double Perovskite. Nanomaterials. 2022; 12(17):3011. https://doi.org/10.3390/nano12173011
Chicago/Turabian StyleLiu, Sheng, Feng Xiang, Yulan Cheng, Yajun Luo, and Jing Sun. 2022. "Multiferroic and Magnetodielectric Effects in Multiferroic Pr2FeAlO6 Double Perovskite" Nanomaterials 12, no. 17: 3011. https://doi.org/10.3390/nano12173011
APA StyleLiu, S., Xiang, F., Cheng, Y., Luo, Y., & Sun, J. (2022). Multiferroic and Magnetodielectric Effects in Multiferroic Pr2FeAlO6 Double Perovskite. Nanomaterials, 12(17), 3011. https://doi.org/10.3390/nano12173011






