Mechanical Properties and Antibacterial Effect on Mono-Strain of Streptococcus mutans of Orthodontic Cements Reinforced with Chlorhexidine-Modified Nanotubes
Abstract
1. Introduction
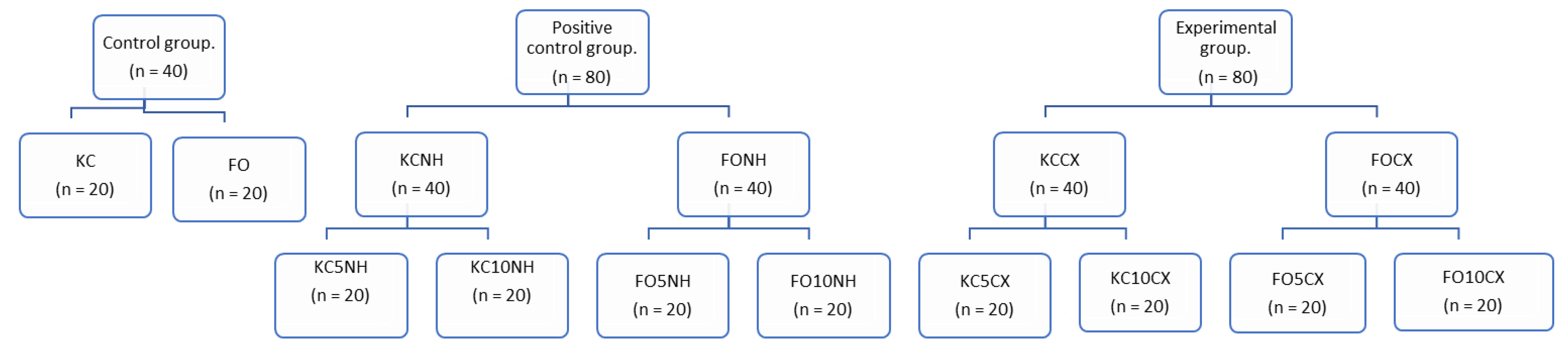
2. Materials and Methods
2.1. Modification of Halloysite Nanotubes with Chlorhexidine
2.2. Incorporation of Modified Nanotubes into Glass Ionomers
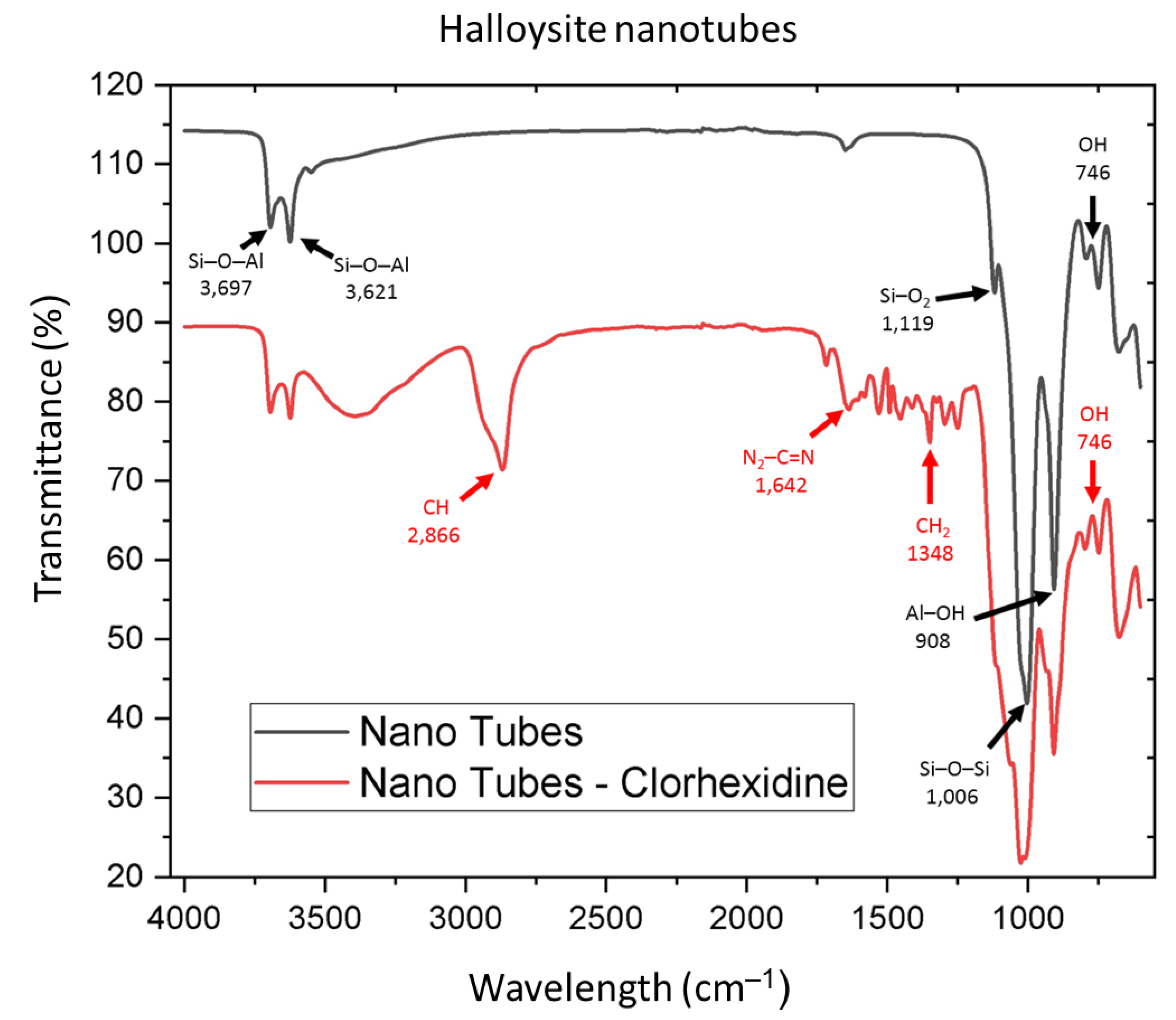
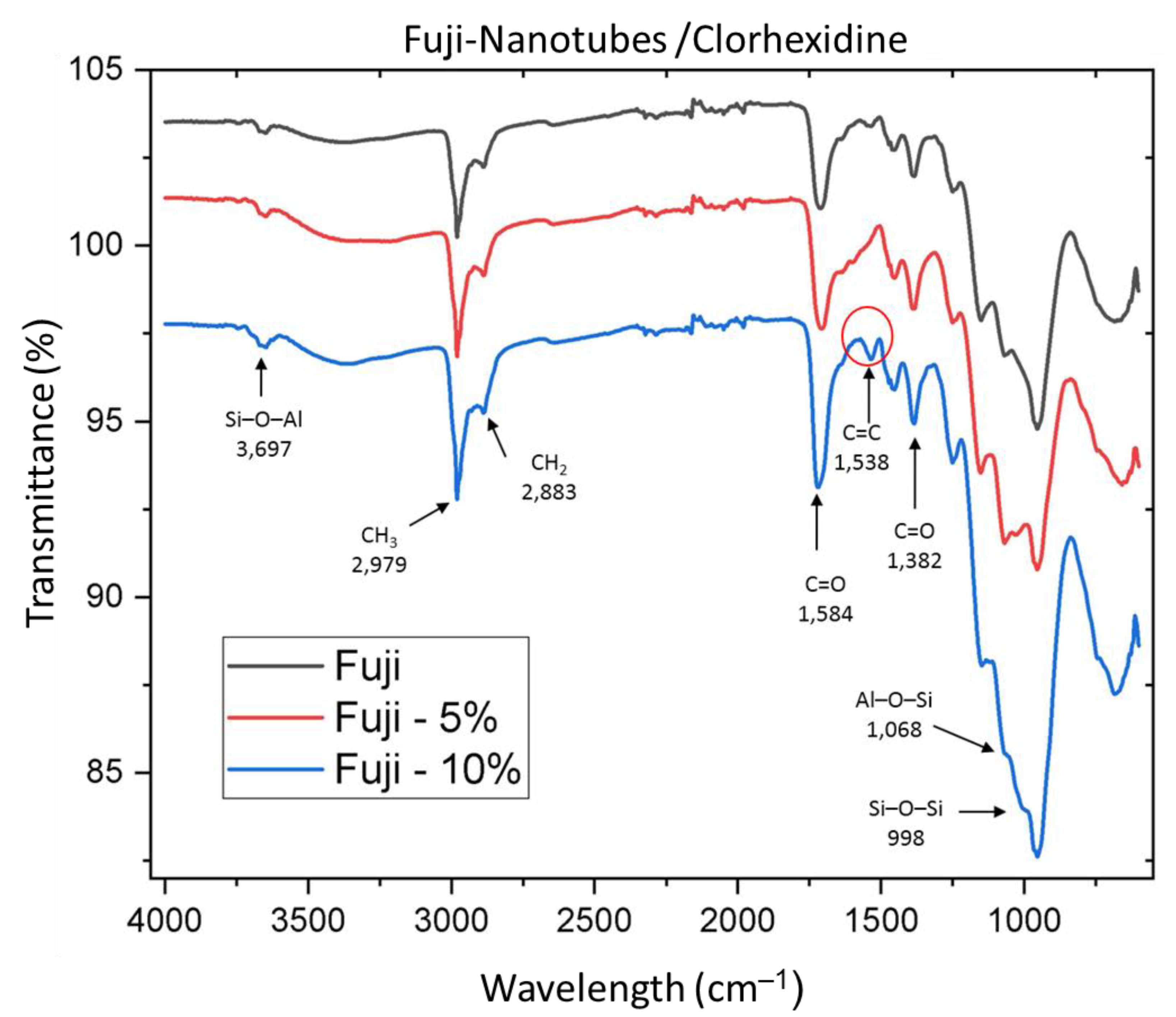
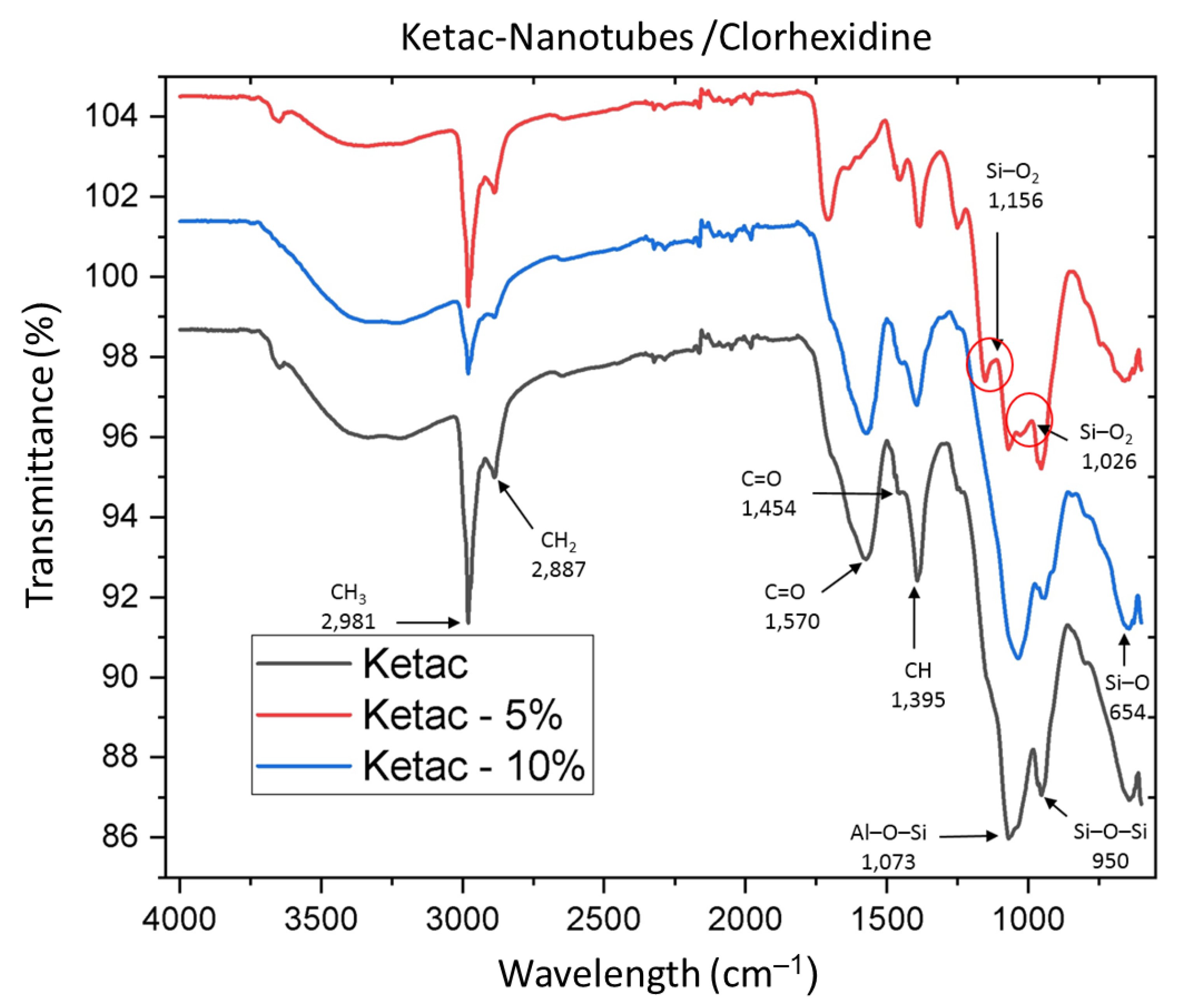
2.3. Sample Characterization with Fourier Transform Infrared Spectroscopy (FTIR)
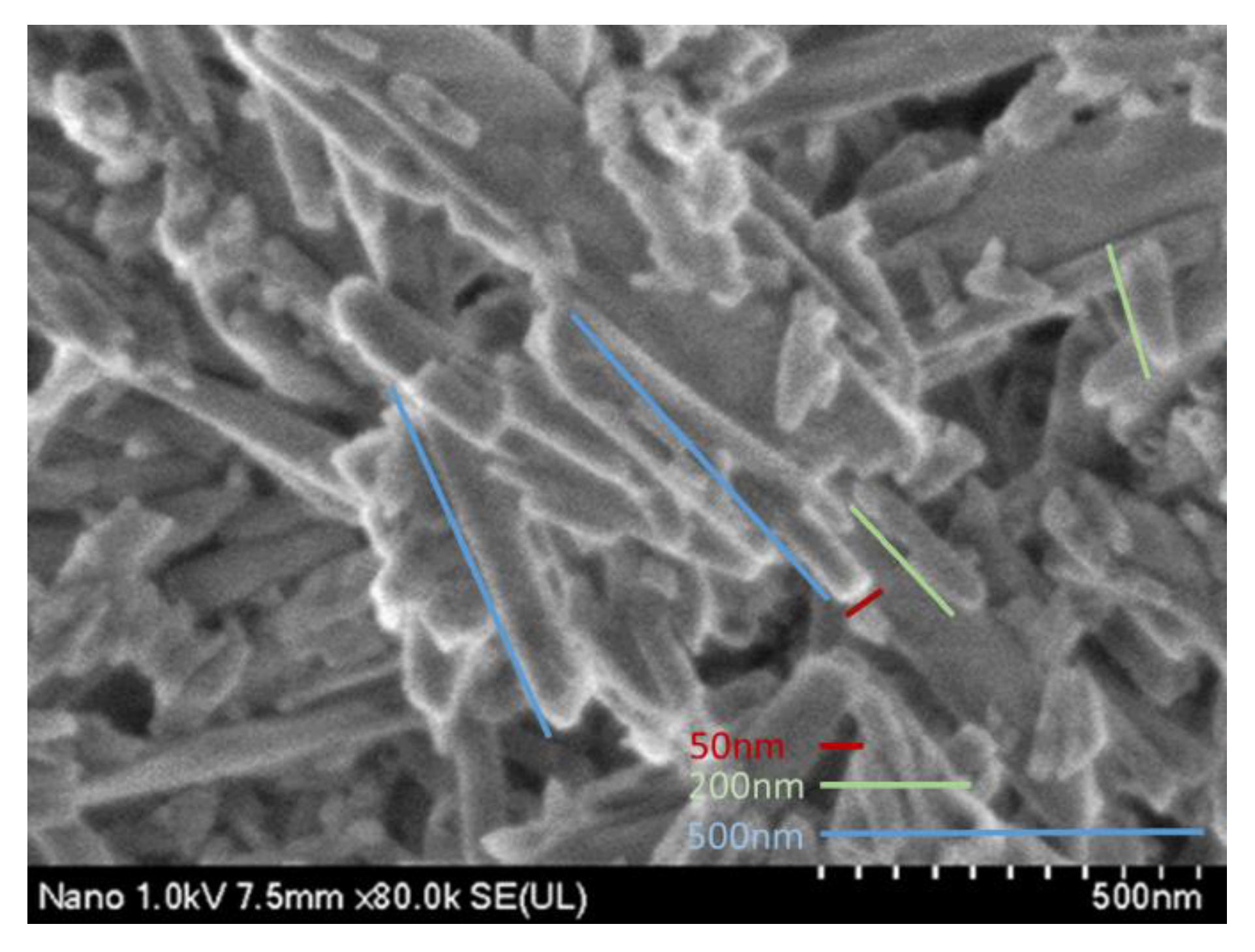
2.4. Scanning Electron Microscopy (SEM)
2.5. Microbiology Assay
2.6. Microhardness
2.7. Compression Strength
2.8. Setting Time
3. Results
3.1. IR Spectroscopy
3.2. SEM Results
3.3. Microbiology Assay
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, L.; Yan, Z.; Duan, Y.; Zhang, J.; Liu, B. Improvement of the mechanical, tribological and antibacterial properties of glass ionomer cements by fluorinated graphene. Dent. Mater. 2018, 34, e115–e127. [Google Scholar] [CrossRef] [PubMed]
- Heravi, F.; Bagheri, H.; Rangrazi, A. Evaluation of Microleakage of Orthodontic Bands Cemented With CPP-ACP-Modified Glass Ionomer Cement. J. Adv. Oral Res. 2019, 10, 128–131. [Google Scholar] [CrossRef]
- Araújo, J.L.D.S.; Alvim, M.M.A.; Campos, M.J.D.S.; Apolônio, A.C.M.; Carvalho, F.G.; Lacerda-Santos, R. Analysis of Chlorhexidine Modified Cement in Orthodontic Patients: A Double-Blinded, Randomized, Controlled Trial. Eur. J. Dent. 2021, 15, 639–646. [Google Scholar] [CrossRef]
- Zandi Karimi, A.; Rezabeigi, E.; Drew, R.A.L. Glass ionomer cements with enhanced mechanical and remineralizing properties containing 45S5 bioglass-ceramic particles. J. Mech. Behav. Biomed. Mater. 2019, 97, 396–405. [Google Scholar] [CrossRef]
- Heravi, F.; Omidkhoda, M.; Koohestanian, N.; Hooshmand, T.; Bagheri, H.; Ghaffari, N. Retentive Strength of Orthodontic Bands Cemented with Amorphous Calcium Phosphate-Modified Glass Ionomer Cement: An In-Vitro Study. Front. Dent. J. Dent. Tehran Univ. Med Sci. 2017, 14, 13–20. [Google Scholar]
- Tarasingh, P.; Sharada Reddy, J.; Suhasini, K.; Hemachandrika, I. Comparative evaluation of antimicrobial efficacy of resin-modified glass ionomers, compomers and giomers—An invitro study. J. Clin. Diagn. Res. 2015, 9, ZC85–ZC87. [Google Scholar] [CrossRef]
- Zayed, M.M.; Hassan, R.E.; Riad, M.I. Evaluation of the antibacterial efficacy of different bioactive lining and pulp capping agents. Tanta Dent. J. 2015, 12, 132–139. [Google Scholar] [CrossRef]
- Tüzüner, T.; Dimkov, A.; Nicholson, J.W. The effect of antimicrobial additives on the properties of dental glass-ionomer cements: A review. Acta Biomater. Odontol. Scand. 2019, 5, 9–21. [Google Scholar] [CrossRef]
- Kurt, A.; Tüzüner, T.; Baygın, Ö. Antibacterial characteristics of glass ionomer cements containing antibacterial agents: An in vitro study. Eur. Arch. Paediatr. Dent. 2021, 22, 49–56. [Google Scholar] [CrossRef]
- Tüzüner, T.; Ulusu, T. Effect of antibacterial agents on the surface hardness of a conventional glass-ionomer cement. J. Appl. Oral Sci. 2012, 20, 45–49. [Google Scholar] [CrossRef][Green Version]
- Soygun, K.; Soygun, A.; Dogan, M.C. The effects of chitosan addition to glass ionomer cement on microhardness and surface roughness. J. Appl. Biomater. Funct. Mater. 2021, 19, 2280800021989706. [Google Scholar] [CrossRef] [PubMed]
- Kalagi, S.; Feitosa, S.A.; Münchow, E.A.; Martins, V.M.; Karczewski, A.E.; Cook, N.B.; Diefenderfer, K.; Eckert, G.J.; Geraldeli, S.; Bottino, M.C. Chlorhexidine-modified nanotubes and their effects on the polymerization and bonding performance of a dental adhesive. Dent. Mater. 2020, 36, 687–697. [Google Scholar] [CrossRef]
- Feitosa, S.A.; Palasuk, J.; Geraldeli, S.; Windsor, L.J.; Bottino, M.C. Physicochemical and biological properties of novel chlorhexidine-loaded nanotube-modified dentin adhesive. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Degrazia, F.W.; Genari, B.; Leitune, V.C.B.; Arthur, R.A.; Luxan, S.A.; Samuel, S.M.W.; Collares, F.M.; Sauro, S. Polymerisation, antibacterial and bioactivity properties of experimental orthodontic adhesives containing triclosan-loaded halloysite nanotubes. J. Dent. 2018, 69, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Imazato, S.; Kaneshiro, A.V.; Ebisu, S.; Frencken, J.E.; Tay, F.R. Antibacterial effects and physical properties of glass-ionomer cements containing chlorhexidine for the ART approach. Dent. Mater. 2006, 22, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Cunha, D.A.; Rodrigues, N.S.; Souza, L.C.; Lomonaco, D.; Rodrigues, F.P.; Degrazia, F.W.; Collares, F.M.; Sauro, S.; Saboia, V.P.A. Physicochemical and microbiological assessment of an experimental composite doped with triclosan-loaded halloysite nanotubes. Materials 2018, 11, 1080. [Google Scholar] [CrossRef] [PubMed]
- Degrazia, F.W.; Leitune, V.C.B.; Takimi, A.S.; Collares, F.M.; Sauro, S. Physicochemical and bioactive properties of innovative resin-based materials containing functional halloysite-nanotubes fillers. Dent. Mater. 2016, 32, 1133–1143. [Google Scholar] [CrossRef]
- Massaro, M.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Covalently modified halloysite clay nanotubes: Synthesis, properties, biological and medical applications. J. Mater. Chem. B 2017, 5, 2867–2882. [Google Scholar] [CrossRef]
- Stavitskaya, A.; Batasheva, S.; Vinokurov, V.; Fakhrullina, G.; Sangarov, V.; Lvov, Y.; Fakhrullin, R. Antimicrobial applications of clay nanotube-based composites. Nanomaterials 2019, 9, 708. [Google Scholar] [CrossRef]
- Lvov, Y.M.; DeVilliers, M.M.; Fakhrullin, R.F. The application of halloysite tubule nanoclay in drug delivery. Expert Opin. Drug Deliv. 2016, 13, 977–986. [Google Scholar] [CrossRef]
- Suma, N.K. Effect of Dentin Disinfection with 2% Chlorhexidine Gluconate and 0.3% Iodine on Dentin Bond Strength: An in vitro Study. Int. J. Clin. Pediatr. Dent. 2017, 10, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Morales-Valenzuela, A.A.; Scougall-Vilchis, R.J.; Lara-Carrillo, E.; Garcia-Contreras, R.; Salmeron-Valdes, E.N.; Aguillón-Sol, L. Comparison of Fluoride release in conventional glass-Ionomer cements with a new mechanical mixing cement. Oral Health Prev. Dent. 2020, 18, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Kheur, M.; Kantharia, N.; Iakha, T.; Kheur, S.; Husain, N.A.H.; Özcan, M. Evaluation of mechanical and adhesion properties of glass ionomer cement incorporating nano-sized hydroxyapatite particles. Odontology 2020, 108, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.A.; Mulay, S.; Mahajan, P.; Raj, A.T. Assessing the effect of ceramic additives on the physical, rheological and mechanical properties of conventional glass ionomer luting cement—An in-vitro study. Heliyon 2019, 5, e02094. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.B. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2017; ISBN 1562388053. [Google Scholar]
- Teubner, S.; Schmidlin, P.R.; Menghini, G.; Attin, T.; Baumgartner, S. The Impact of Orthodontic Bands on the Marginal Periodontium of Maxillary First Molars: A Retrospective Cross-Sectional Radiographic Analysis. Open Dent. J. 2018, 12, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Bourouni, S.; Dritsas, K.; Kloukos, D.; Wierichs, R.J. Efficacy of resin infiltration to mask post-orthodontic or non-post-orthodontic white spot lesions or fluorosis—A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 4711–4719. [Google Scholar] [CrossRef]
- Tasios, T.; Papageorgiou, S.N.; Papadopoulos, M.A.; Tsapas, A.; Haidich, A.B. Prevention of orthodontic enamel demineralization: A systematic review with meta-analyses. Orthod. Craniofacial Res. 2019, 22, 225–235. [Google Scholar] [CrossRef]
- Kamber, R.; Meyer-Lückel, H.; Kloukos, D.; Tennert, C.; Wierichs, R.J. Efficacy of sealants and bonding materials during fixed orthodontic treatment to prevent enamel demineralization: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16556. [Google Scholar] [CrossRef]
- Greene, L.E.; Bearn, D.R. Reducing white spot lesion incidence during fixed appliance therapy. Dent. Update 2013, 40, 487–492. [Google Scholar] [CrossRef]
- Palenik, C.J.; Behnen, M.J.; Setcos, J.C.; Miller, C.H. Inhibition of microbial adherence and growth by various glass ionomers in vitro. Dent. Mater. 1992, 8, 16–20. [Google Scholar] [CrossRef]
- Pradiptama, Y.; Purwanta, M.; Notopuro, H. Antibacterial Effects of Fluoride in Streptococcus mutans Growth in Vitro. Biomol. Health Sci. J. 2019, 2, 1. [Google Scholar] [CrossRef]
- Prabhakar, A.R.; Balehosur, D.V.; Basappa, N. Comparative evaluation of shear bond strength and fluoride release of conventional glass Ionomer with 1% ethanolic extract of propolis incorporated glass Ionomer cement—Invitro study. J. Clin. Diagn. Res. 2016, 10, ZC88–ZC91. [Google Scholar] [CrossRef] [PubMed]
- Ching, H.S.; Luddin, N.; Kannan, T.P.; Ab Rahman, I.; Abdul Ghani, N.R.N. Modification of glass ionomer cements on their physical-mechanical and antimicrobial properties. J. Esthet. Restor. Dent. 2018, 30, 557–571. [Google Scholar] [CrossRef]
- Boaro, L.C.C.; Campos, L.M.; Varca, G.H.C.; dos Santos, T.M.R.; Marques, P.A.; Sugii, M.M.; Saldanha, N.R.; Cogo-Müller, K.; Brandt, W.C.; Braga, R.R.; et al. Antibacterial resin-based composite containing chlorhexidine for dental applications. Dent. Mater. 2019, 35, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, V.; Akhila, C. Streptococcus mutans: Has it become prime perpetrator for oral manifestations? J. Microbiol. Exp. 2019, 7, 207–213. [Google Scholar] [CrossRef]
- Noori, A.J.; Kareem, F.A. Setting time, mechanical and adhesive properties of magnesium oxide nanoparticles modified glass-ionomer cement. J. Mater. Res. Technol. 2020, 9, 1809–1818. [Google Scholar] [CrossRef]
- Farret, M.M.; de Lima, E.M.; Mota, E.G.; Mitsuo, H.S.O.; Maguilnik, G.; Scheid, P.A. Assessment of the mechanical properties of glass ionomer cements for orthodontic cementation. Dental Press J. Orthod. 2012, 17, 154–159. [Google Scholar] [CrossRef]
- Moshaverinia, M.; Navas, A.; Jahedmanesh, N.; Shah, K.C.; Moshaverinia, A.; Ansari, S. Comparative evaluation of the physical properties of a reinforced glass ionomer dental restorative material. J. Prosthet. Dent. 2019, 122, 154–159. [Google Scholar] [CrossRef]
- Sharafeddin, F.; Jowkar, Z.; Bahrani, S. Comparison between the effect of adding microhydroxyapatite and chitosan on surface roughness and Microhardness of resin modified and conventional glass ionomer cements. J. Clin. Exp. Dent. 2021, 13, 737–744. [Google Scholar] [CrossRef]
- Wajong, K.H.; Damiyanti, M.; Irawan, B. The effects of shelf life on the compressive strength of resin-modified glass ionomer cement. J. Phys. Conf. Ser. 2017, 884, 12101. [Google Scholar] [CrossRef]
- Aguiar, D.A.; Ritter, D.E.; Rocha, R.; Locks, A.; Borgatto, A.F. Evaluation of mechanical properties of five cements for orthodontic band cementation. Braz. Oral Res. 2013, 27, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Mallmann, A.; Oliveira Ataíde, J.C.; Amoeda, R.; Rocha, P.V.; Jacques, L.B. Compressive strength of glass ionomer cements using different specimen dimensions. Braz. Oral Res. 2007, 21, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Piwowarczyk, A.; Ottl, P.; Lauer, H.C. Laboratory strength of glass ionomer and zinc phosphate cements. J. Prosthodont. 2001, 10, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Pazourková, L.; Reli, M.; Hundáková, M.; Pazdziora, E.; Predoi, D.; Martynková, G.S.; Lafdi, K. Study of the structure and antimicrobial activity of Ca-deficient ceramics on chlorhexidine nanoclay substrate. Materials 2019, 12, 2996. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, C.; Song, H.; Bai, L.; Cheng, Y.; Ba, X.; Wu, Y. A facile one-step grafting of polyphosphonium onto halloysite nanotubes initiated by Ce(iv). Chem. Commun. 2019, 55, 1040–1043. [Google Scholar] [CrossRef]
- Fareed, M.A.; Stamboulis, A. Effect of nanoclay dispersion on the properties of a commercial glass ionomer cement. Int. J. Biomater. 2014, 2014, 685389. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Sahu, P.; Bhajiwala, H.; Mohanty, S.; Gupta, V.; Bhowmick, A.K. Synthesis, characterization and properties of self-healable ionomeric carboxylated styrene–butadiene polymer. J. Mater. Sci. 2019, 54, 14986–14999. [Google Scholar] [CrossRef]


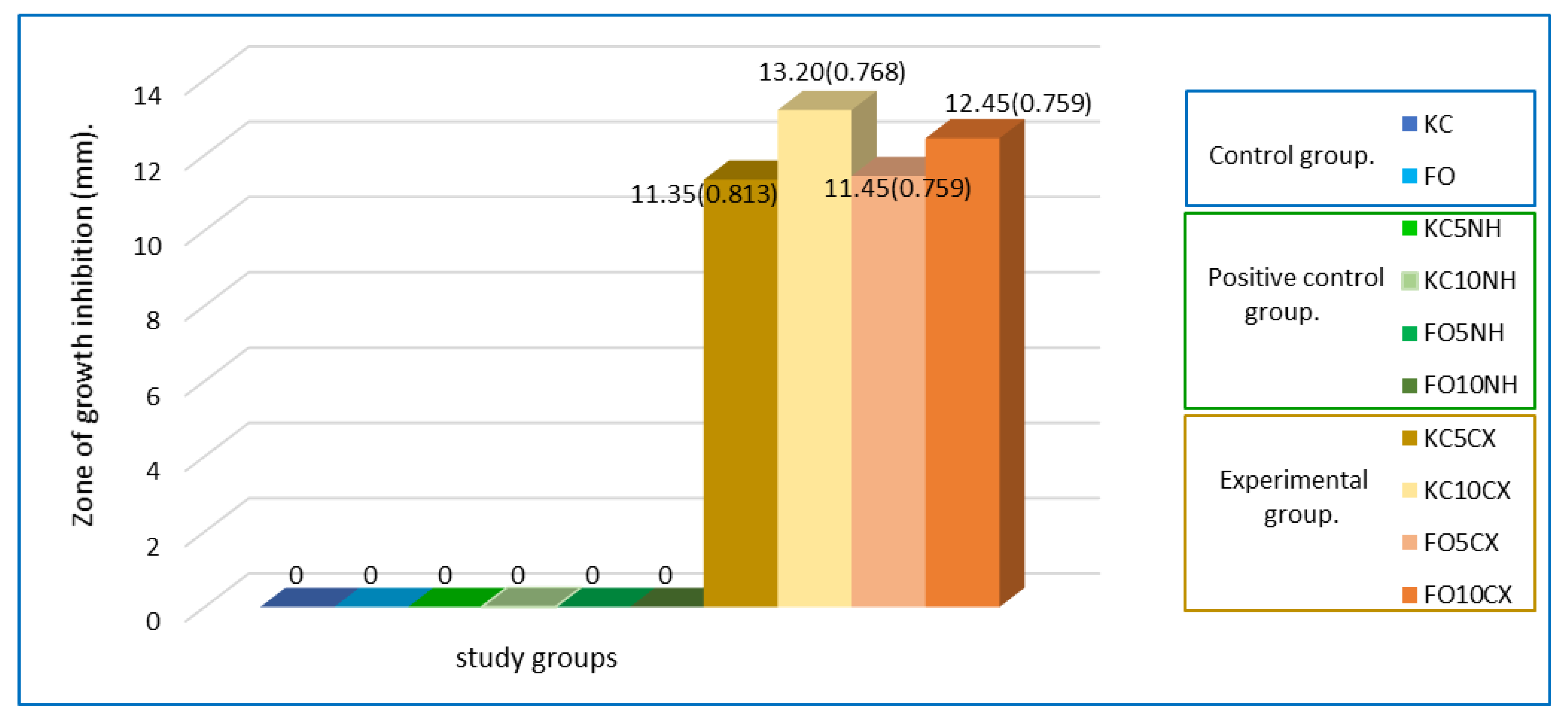
| Groups | Mean (SD) |
|---|---|
| Fuji ortho 5% of NH + CX. (FO5CX) | 11.45 (0.759) |
| Fuji Ortho 10% of NH + CX. (FO10CX) | 12.45 (0.759) |
| Ketac Cem 5% of NH + CX. (KC5CX) | 11.35 (0.813) |
| Ketac Cem 10% of NH + CX. (KC10CX) | 13.20 (0.768) |
| Total samples | 80 |
| Contrast Statistics | 41.735 |
| Degrees of freedom | 3 |
| p value Kruskal Wallis Test | 0.001 * |
| Groups | Contrast Statistics | Contrast Statistics Deviation | p Value |
|---|---|---|---|
| KC5CX-FO5CX | −1.725 | −0.244 | 1.000 |
| KC5CX-FO10CX | −24.025 | −3.400 | 0.004 * |
| KC5CX-KC10CX | −38.760 | −5.485 | 0.001 * |
| FO5CX-FO10CX | −22.300 | −3.156 | 0.010 * |
| FO5CX-KC10CX | 37.025 | 5.240 | 0.001 * |
| FO10CX-KC10CX | 14.725 | 2.084 | 0.223 |
| Groups | VMHN Mean (SD) | ST Mean (SD) | CS Mean (SD) |
|---|---|---|---|
| KC (control group) | 80.03 (4.56) | 7.56 (0.024) | 84.16 (0.92) |
| KC5CX | 77.87 (3.63) | 7.56 (0.017) | 88.78 (1.12) |
| KC10CX | 77.66 (2.99) | 7.56 (0.019) | 93.96 (1.66) |
| Total degrees of freedom | 74 | 59 | 59 |
| Sum of squares | 1119.245 | 0.025 | 1056.608 |
| Fisher’s statistic | 2.992 | 0.697 | 293.83 |
| ANOVA test FO groups | 0.056 | 0.502 | 0.001 * |
| FO (control group) | 68.83 (5.26) | 9.57 (0.011) | 125.42 (1.79) |
| FO5CX | 67.96 (5.85) | 9.57 (0.014) | 128.26 (2.26) |
| FO10CX | 67.66 (6.50) | 9.56 (0.018) | 133.17 (2.13) |
| Total degrees of freedom | 74 | 59 | 59 |
| Sum of squares | 2520.976 | 0.014 | 860.412 |
| Fisher’s statistic | 0.267 | 1.043 | 71.39 |
| ANOVA test FO groups | 0.766 | 0.359 | 0.001 * |
| Groups | Mean Difference | 95% Confidence Intervals | p Value |
|---|---|---|---|
| Control-KC5CX | −4.62800 | IL: −5.6022, SL: −3.6538 | 0.001 * |
| Control-KC10CX | −9.80900 | IL: −10.7832, SL: −8.8348 | 0.001 * |
| KC5CX-KC10CX | −5.18100 | IL: −6.1552, SL: −4.2068 | 0.001 * |
| Control-FO5CX | −2.84500 | IL: −4.4242, SL: −1.2658 | 0.001 * |
| Control-FO10CX | −7.75100 | IL: −9.3302, SL: −6.1718 | 0.001 * |
| FO5CX-FO10CX | −4.90600 | IL: −6.4852, SL: −3.3268 | 0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmerón-Valdés, E.N.; Cruz-Mondragón, A.C.; Toral-Rizo, V.H.; Jiménez-Rojas, L.V.; Correa-Prado, R.; Lara-Carrillo, E.; Morales-Valenzuela, A.A.; Scougall-Vilchis, R.J.; López-Flores, A.I.; Hoz-Rodriguez, L.; et al. Mechanical Properties and Antibacterial Effect on Mono-Strain of Streptococcus mutans of Orthodontic Cements Reinforced with Chlorhexidine-Modified Nanotubes. Nanomaterials 2022, 12, 2891. https://doi.org/10.3390/nano12172891
Salmerón-Valdés EN, Cruz-Mondragón AC, Toral-Rizo VH, Jiménez-Rojas LV, Correa-Prado R, Lara-Carrillo E, Morales-Valenzuela AA, Scougall-Vilchis RJ, López-Flores AI, Hoz-Rodriguez L, et al. Mechanical Properties and Antibacterial Effect on Mono-Strain of Streptococcus mutans of Orthodontic Cements Reinforced with Chlorhexidine-Modified Nanotubes. Nanomaterials. 2022; 12(17):2891. https://doi.org/10.3390/nano12172891
Chicago/Turabian StyleSalmerón-Valdés, Elias Nahum, Ana Cecilia Cruz-Mondragón, Víctor Hugo Toral-Rizo, Leticia Verónica Jiménez-Rojas, Rodrigo Correa-Prado, Edith Lara-Carrillo, Adriana Alejandra Morales-Valenzuela, Rogelio José Scougall-Vilchis, Alejandra Itzel López-Flores, Lia Hoz-Rodriguez, and et al. 2022. "Mechanical Properties and Antibacterial Effect on Mono-Strain of Streptococcus mutans of Orthodontic Cements Reinforced with Chlorhexidine-Modified Nanotubes" Nanomaterials 12, no. 17: 2891. https://doi.org/10.3390/nano12172891
APA StyleSalmerón-Valdés, E. N., Cruz-Mondragón, A. C., Toral-Rizo, V. H., Jiménez-Rojas, L. V., Correa-Prado, R., Lara-Carrillo, E., Morales-Valenzuela, A. A., Scougall-Vilchis, R. J., López-Flores, A. I., Hoz-Rodriguez, L., & Velásquez-Enríquez, U. (2022). Mechanical Properties and Antibacterial Effect on Mono-Strain of Streptococcus mutans of Orthodontic Cements Reinforced with Chlorhexidine-Modified Nanotubes. Nanomaterials, 12(17), 2891. https://doi.org/10.3390/nano12172891







