Water-Induced Nanometer-Thin Crystalline Indium-Praseodymium Oxide Channel Layers for Thin-Film Transistors
Abstract
:1. Introduction
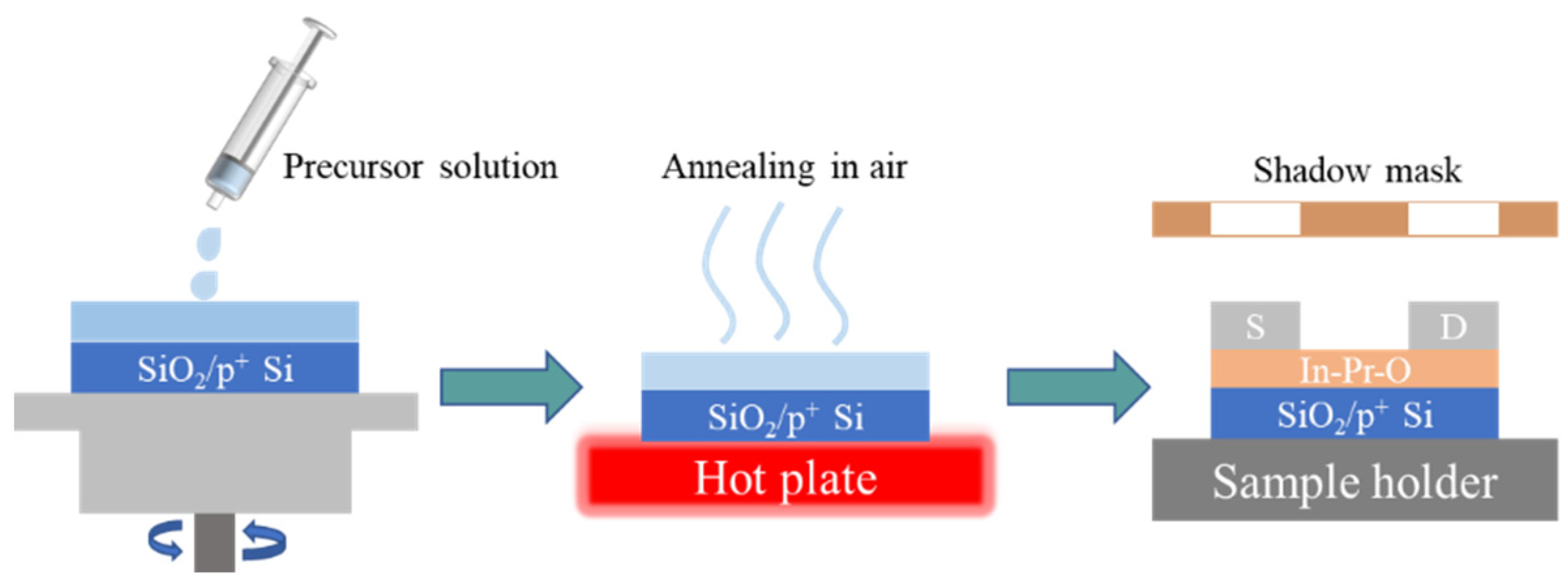
2. Experimental Section
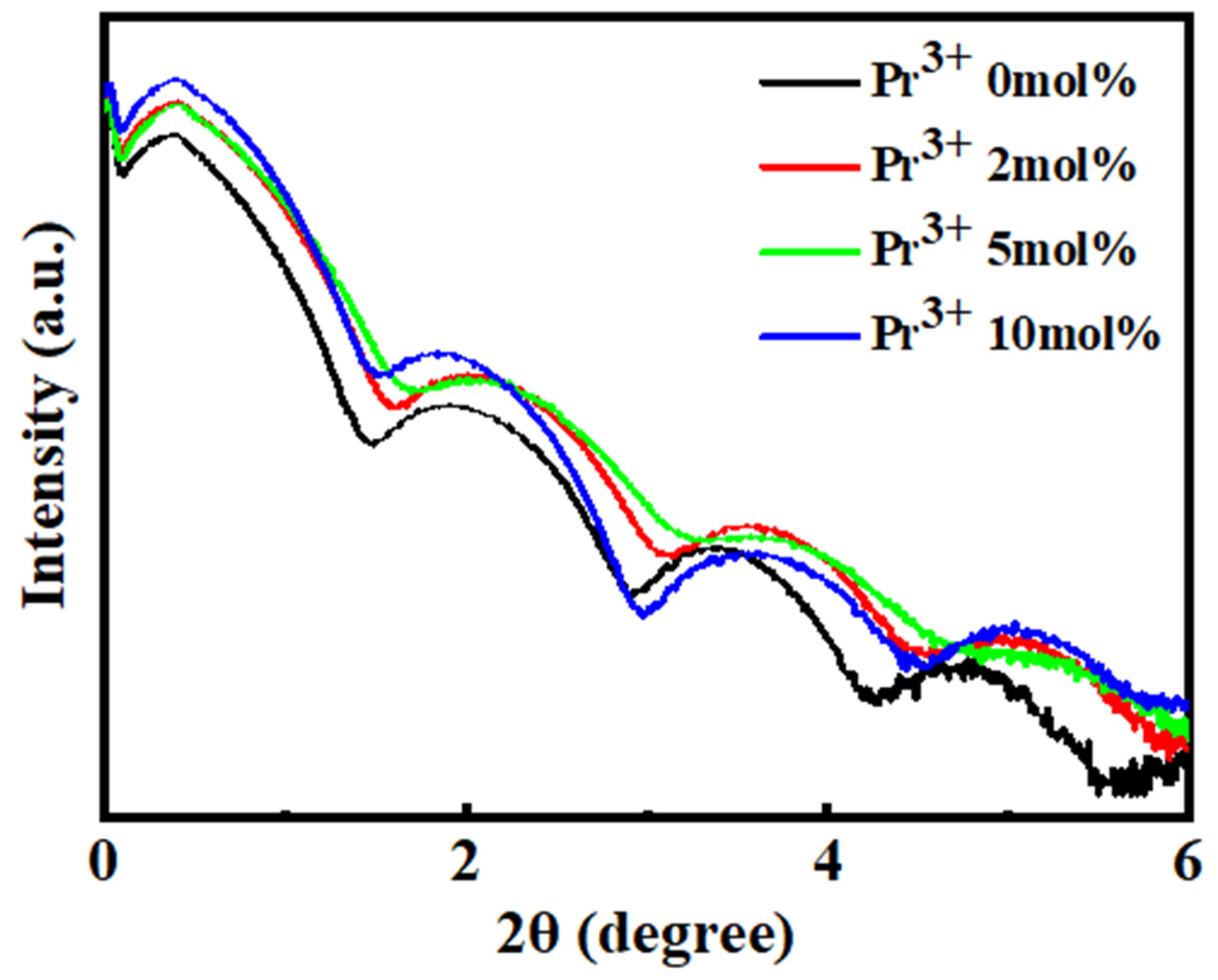
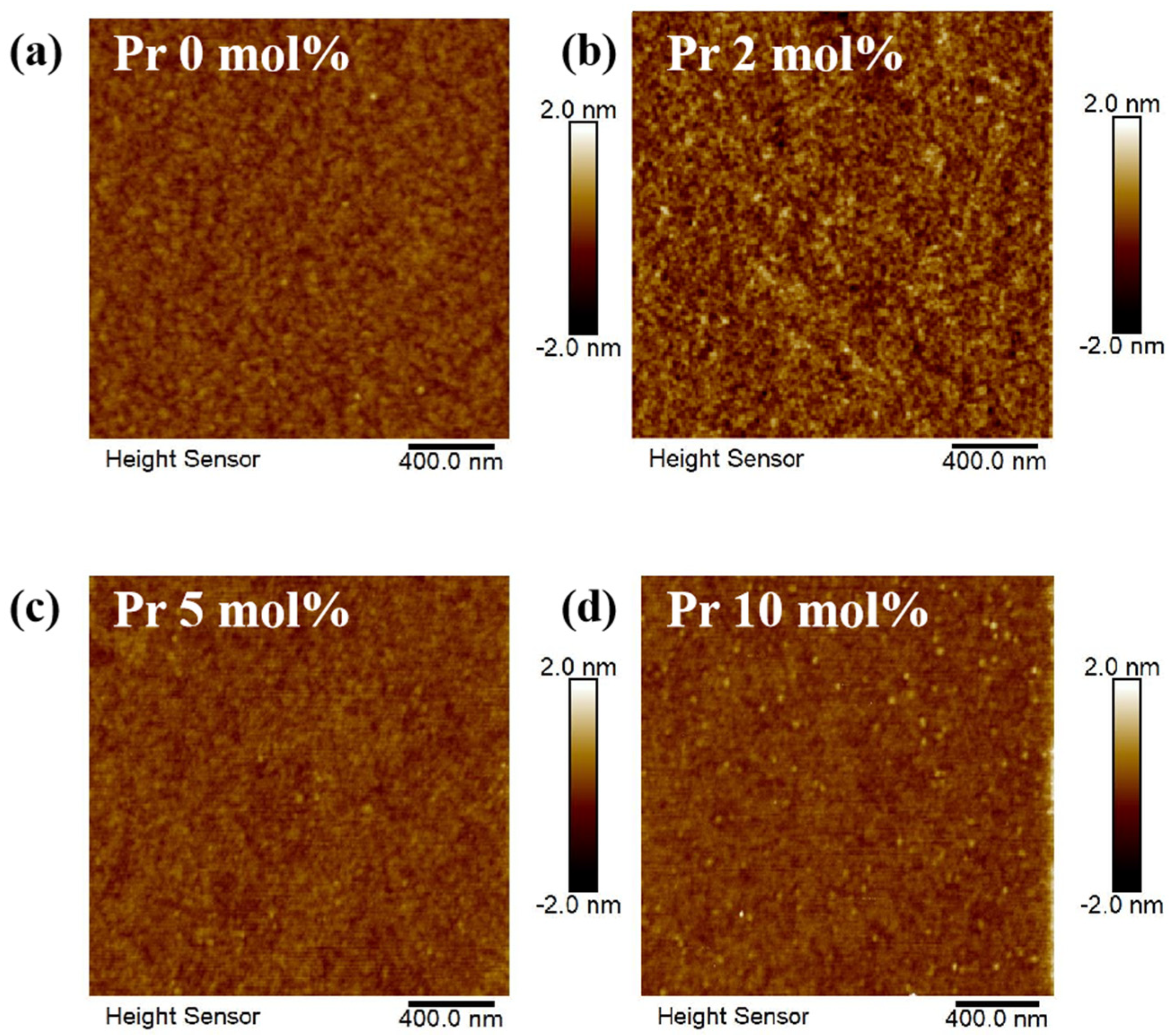
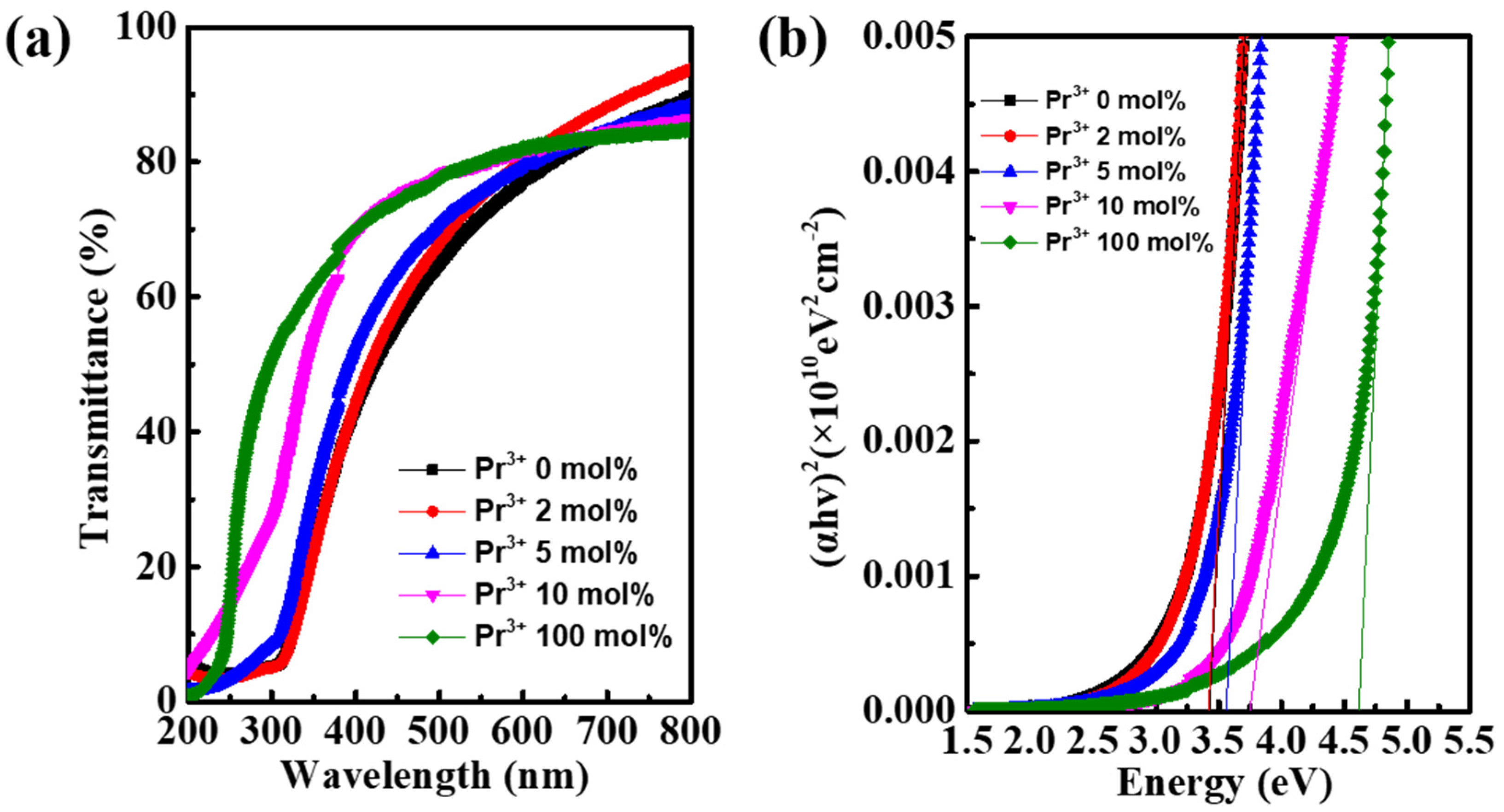
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fortunato, E.; Barquinha, P.; Martins, R. Oxide Semiconductor Thin-Film Transistors: A Review of Recent Advances. Adv. Mater. 2012, 24, 2945–2986. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, H.; Xu, J.-B.; Wang, L. Recent Advances of Solution-Processed Metal Oxide Thin-Film Transistors. ACS Appl. Mater. Interfaces 2018, 10, 25878–25901. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Lan, L. Solution-processed metal-oxide thin-film transistors: A review of recent developments. Nanotechnology 2019, 30, 312001. [Google Scholar] [CrossRef]
- Zhu, L.; He, G.; Li, W.; Yang, B.; Fortunato, E.; Martins, R. Nontoxic, Eco-friendly Fully Water-Induced Ternary Zr-Gd-O Dielectric for High-Performance Transistors and Unipolar Inverters. Adv. Electron. Mater. 2018, 4, 1800100. [Google Scholar] [CrossRef]
- Yu, X.; Marks, T.J.; Facchetti, A. Metal oxides for optoelectronic applications. Nat. Mater. 2016, 15, 383–396. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Xie, F.; Chen, J.; Cao, H.; Xu, J.-B. Facile and Environmentally Friendly Solution-Processed Aluminum Oxide Dielectric for Low-Temperature, High-Performance Oxide Thin-Film Transistors. ACS Appl. Mater. Interfaces 2015, 7, 5803–5810. [Google Scholar] [CrossRef]
- Thomas, S.R.; Pattanasattayavong, P.; Anthopoulos, T.D. Solution-processable metal oxide semiconductors for thin-film transistor applications. Chem. Soc. Rev. 2013, 42, 6910–6923. [Google Scholar] [CrossRef]
- Kamiya, T.; Hosono, H. Material characteristics and applications of transparent amorphous oxide semiconductors. NPG Asia Mater. 2010, 2, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Kim, C.H.; Lee, W.J.; Sung, S.; Yoon, M.-H. Sol-gel metal oxide dielectrics for all-solution-processed electronics. Mater. Sci. Eng. R Rep. 2017, 114, 1–22. [Google Scholar] [CrossRef]
- Li, S.; Tian, M.; Gao, Q.; Wang, M.; Li, T.; Hu, Q.; Li, X.; Wu, Y. Nanometre-thin indium tin oxide for advanced high-performance electronics. Nat. Mater. 2019, 18, 1091–1097. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Huang, W.; Chen, Y.; Wang, G.; Zeng, L.; Zhu, W.; Bedzyk, M.J.; Zhang, W.; Medvedeva, J.E.; et al. Synergistic Boron Doping of Semiconductor and Dielectric Layers for High-Performance Metal Oxide Transistors: Interplay of Experiment and Theory. J. Am. Chem. Soc. 2018, 140, 12501–12510. [Google Scholar] [CrossRef] [PubMed]
- Kirmani, A.R.; Chen, H.; Stafford, C.M.; Bittle, E.G.; Richter, L.J. Coating Thickness Controls Crystallinity and Enables Homoepitaxial Growth of Ultra-Thin-Channel Blade-Coated In2O3 Transistors. Adv. Electron. Mater. 2020, 6, 2000354. [Google Scholar] [CrossRef]
- Liu, G.; Liu, A.; Zhu, H.; Shin, B.; Fortunato, E.; Martins, R.; Wang, Y.; Shan, F. Low-Temperature, Nontoxic Water-Induced Metal-Oxide Thin Films and Their Application in Thin-Film Transistors. Adv. Funct. Mater. 2015, 25, 2564–2572. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Seo, J.-S.; Yun, J.M.; Park, H.; Yang, S.; Park, S.-H.K.; Bae, B.-S. An ‘aqueous route’ for the fabrication of low-temperature-processable oxide flexible transparent thin-film transistors on plastic substrates. NPG Asia Mater. 2013, 5, e45. [Google Scholar] [CrossRef] [Green Version]
- Parthiban, S.; Kwon, J.-Y. Role of dopants as a carrier suppressor and strong oxygen binder in amorphous indium-oxide-based field effect transistor. J. Mater. Res. 2014, 29, 1585–1596. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhu, D.; Xu, W.; Han, S.; Fang, M.; Liu, W.; Cao, P.; Lu, Y. High-mobility nanometer-thick crystalline In–Sm–O thin-film transistors via aqueous solution processing. J. Mater. Chem. C 2020, 8, 310–318. [Google Scholar] [CrossRef]
- Lin, Z.; Lan, L.; Sun, S.; Li, Y.; Song, W.; Gao, P.; Song, E.; Zhang, P.; Li, M.; Wang, L.; et al. Solution-processed high-mobility neodymium-substituted indium oxide thin-film transistors formed by facile patterning based on aqueous precursors. Appl. Phys. Lett. 2017, 110, 133502. [Google Scholar] [CrossRef]
- Li, Y.; Xu, W.; Liu, W.; Han, S.; Cao, P.; Fang, M.; Zhu, D.; Lu, Y. High-Performance Thin-Film Transistors with Aqueous Solution-Processed NiInO Channel Layer. ACS Appl. Electron. Mater. 2019, 1, 1842–1851. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, T.; Lee, J.; Avis, C.; Jang, J. Solution-processed gadolinium doped indium-oxide thin-film transistors with oxide passivation. Appl. Phys. Lett. 2017, 110, 122102. [Google Scholar] [CrossRef]
- Kim, J.-S.; Na, C.; Kwak, C.-H.; Li, H.-Y.; Yoon, J.; Kim, J.-H.; Jeong, S.-Y.; Lee, J.-H. Humidity-Independent Gas Sensors Using Pr-Doped In2O3 Macroporous Spheres: Role of Cyclic Pr3+/Pr4+ Redox Reactions in Suppression of Water-Poisoning Effect. ACS Appl. Mater. Interfaces 2019, 11, 25322–25329. [Google Scholar] [CrossRef]
- Park, H.; Nam, Y.; Jin, J.; Bae, B.-S. Improvement of bias stability of oxyanion-incorporated aqueous sol–gel processed indium zinc oxide TFTs. J. Mater. Chem. C 2014, 2, 5998–6003. [Google Scholar] [CrossRef]
- Jeong, J.K.; Yang, H.W.; Jeong, J.H.; Mo, Y.-G.; Kim, H.D. Origin of threshold voltage instability in indium-gallium-zinc oxide thin film transistors. Appl. Phys. Lett. 2008, 93, 123508. [Google Scholar] [CrossRef]
- Liu, P.-T.; Chou, Y.-T.; Teng, L.-F. Environment-dependent metastability of passivation-free indium zinc oxide thin film transistor after gate bias stress. Appl. Phys. Lett. 2009, 95, 233504. [Google Scholar] [CrossRef] [Green Version]
- Si, M.; Lin, Z.; Chen, Z.; Sun, X.; Wang, H.; Ye, P.D. Scaled indium oxide transistors fabricated using atomic layer deposition. Nat. Electron. 2022, 5, 164–170. [Google Scholar] [CrossRef]
- Datta, R.S.; Syed, N.; Zavabeti, A.; Jannat, A.; Mohiuddin, M.; Rokunuzzaman, M.; Zhang, B.Y.; Rahman, A.; Atkin, P.; Messalea, K.A.; et al. Flexible two-dimensional indium tin oxide fabricated using a liquid metal printing technique. Nat. Electron. 2020, 3, 51–58. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Bae, B.S. Effect of Aluminum and Gallium Doping on the Performance of Solution-Processed Indium Oxide Thin-Film Transistors. J. Disp. Technol. 2013, 9, 704–709. [Google Scholar] [CrossRef]
- Hennek, J.W.; Jeremy, S.; Aiming, Y.; Myung-Gil, K.; Wei, Z.; Dravid, V.P.; Antonio, F.; Marks, T.J. Oxygen “getter” effects on microstructure and carrier transport in low temperature combustion-processed a-InXZnO (X = Ga, Sc, Y, La) transistors. J. Am. Chem. Soc. 2013, 135, 10729–10741. [Google Scholar] [CrossRef]
- Yu, X.; Smith, J.; Zhou, N.; Zeng, L.; Guo, P.; Xia, Y.; Alvarez, A.; Aghion, S.; Lin, H.; Yu, J.; et al. Spray-combustion synthesis: Efficient solution route to high-performance oxide transistors. Proc. Natl. Acad. Sci. USA 2015, 112, 3217–3222. [Google Scholar] [CrossRef] [Green Version]
- Zhong, D.Y.; Li, J.; Zhao, C.Y.; Huang, C.X.; Zhang, J.H.; Li, X.F.; Jiang, X.Y.; Zhang, Z.L. Enhanced Electrical Performance and Negative Bias Illumination Stability of Solution-Processed InZnO Thin-Film Transistor by Boron Addition. IEEE Trans. Electron Devices 2018, 65, 520–525. [Google Scholar] [CrossRef]
- Kim, T.; Jang, B.; Bae, J.H.; Park, H.; Cho, C.S.; Kwon, H.J.; Jang, J. Improvement in the Performance of Sol–Gel Processed In2O3 Thin-Film Transistor Depending on Sb Dopant Concentration. IEEE Electron Device Lett. 2017, 38, 1027. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Li, J.; Zhong, D.Y.; Huang, C.X.; Zhang, J.H.; Li, X.F.; Jiang, X.Y.; Zhang, Z.L. Mg Doping to Simultaneously Improve the Electrical Performance and Stability of MgInO Thin-Film Transistors. IEEE Trans. Electron Devices 2017, 64, 2216. [Google Scholar] [CrossRef]
- Song, A.; Javaid, K.; Yu, L.; Wu, W.; Yu, J.; Liang, L.; Zhang, H.; Lan, L.; Chang, T.C.; Cao, H. Design, Properties, and TFT Application of Solution-Processed In-Ga-Cd-O Thin Films. Phys. Status Solidi (RRL)—Rapid Res. Lett. 2018, 12, 1800034. [Google Scholar] [CrossRef]
- Jaehnike, F.; Pham, D.V.; Bock, C.; Kunze, U. Role of gallium and yttrium dopants on the stability and performance of solution processed indium oxide thin-film transistors. J. Mater. Chem. C 2019, 7, 7627–7635. [Google Scholar] [CrossRef]
- Cheng, J.; Li, X.Y.; Guo, J.; Xu, H.F.; Chen, Y.H.; He, Y.F.; Xue, J.S.; Zhang, T.; Yu, Z.N. The role of the sequence of plasma treatment and high temperature annealing on solution-processed a-IMZO thin film transistor. J. Alloys Compd. 2019, 793, 369–374. [Google Scholar] [CrossRef]
- Hoang, H.; Hori, T.; Yasuda, T.-o.; Kizu, T.; Tsukagoshi, K.; Nabatame, T.; Trinh, B.; Fujiwara, A. Erratum: Si-doping effect on solution-processed In-O thin-film transistors (2019 Mater. Res. Express 6 026410). Mater. Res. Express 2019, 6, 039601. [Google Scholar] [CrossRef]
- Hong, L.; Xu, W.; Liu, W.; Han, S.; Cao, P.; Fang, M.; Zhu, D.; Lu, Y. High performance indium dysprosium oxide thin-film transistors grown from aqueous solution. Appl. Surf. Sci. 2020, 504, 144499. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, D.; Han, S.; Lu, Y.; Fang, M.; Liu, W.; Cao, P.; Xu, W. Aqueous solution deposition of amorphous gallium tin oxide for thin-film transistors applications. Ceram. Int. 2020, 46, 19557–19563. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, D.-W.; Lee, W.-Y.; Lee, S.-H.; Bae, J.-H.; Kang, I.-M.; Jang, J. Improved Negative Bias Stress Stability of Sol–Gel-Processed Li-Doped SnO2 Thin-Film Transistors. Electronics 2021, 10, 1629. [Google Scholar] [CrossRef]
- Kim, H.; Maeng, S.; Lee, S.; Kim, J. Improved Performance and Operational Stability of Solution-Processed InGaSnO (IGTO) Thin Film Transistors by the Formation of Sn–O Complexes. ACS Appl. Electron. Mater. 2021, 3, 1199–1210. [Google Scholar] [CrossRef]

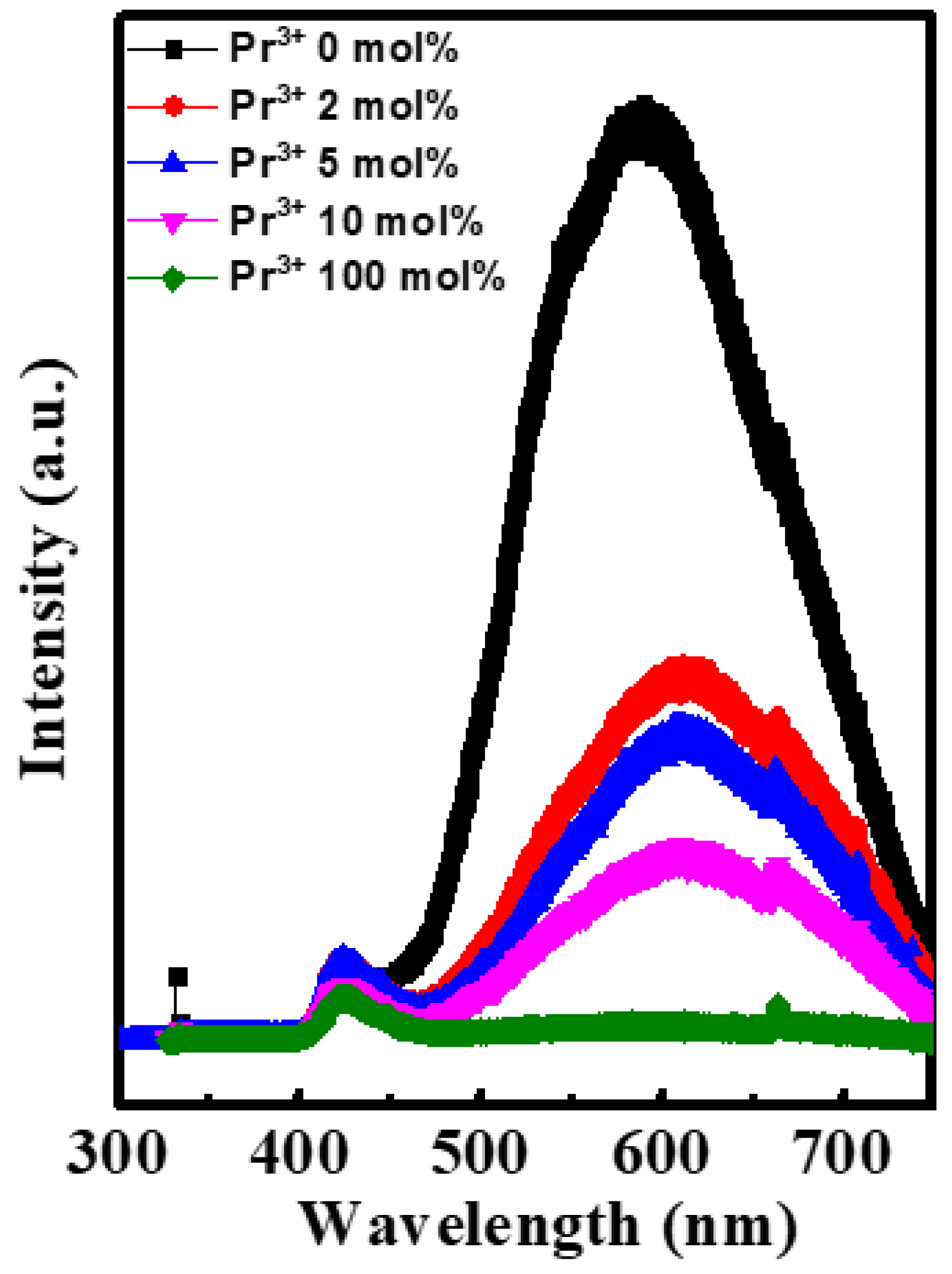


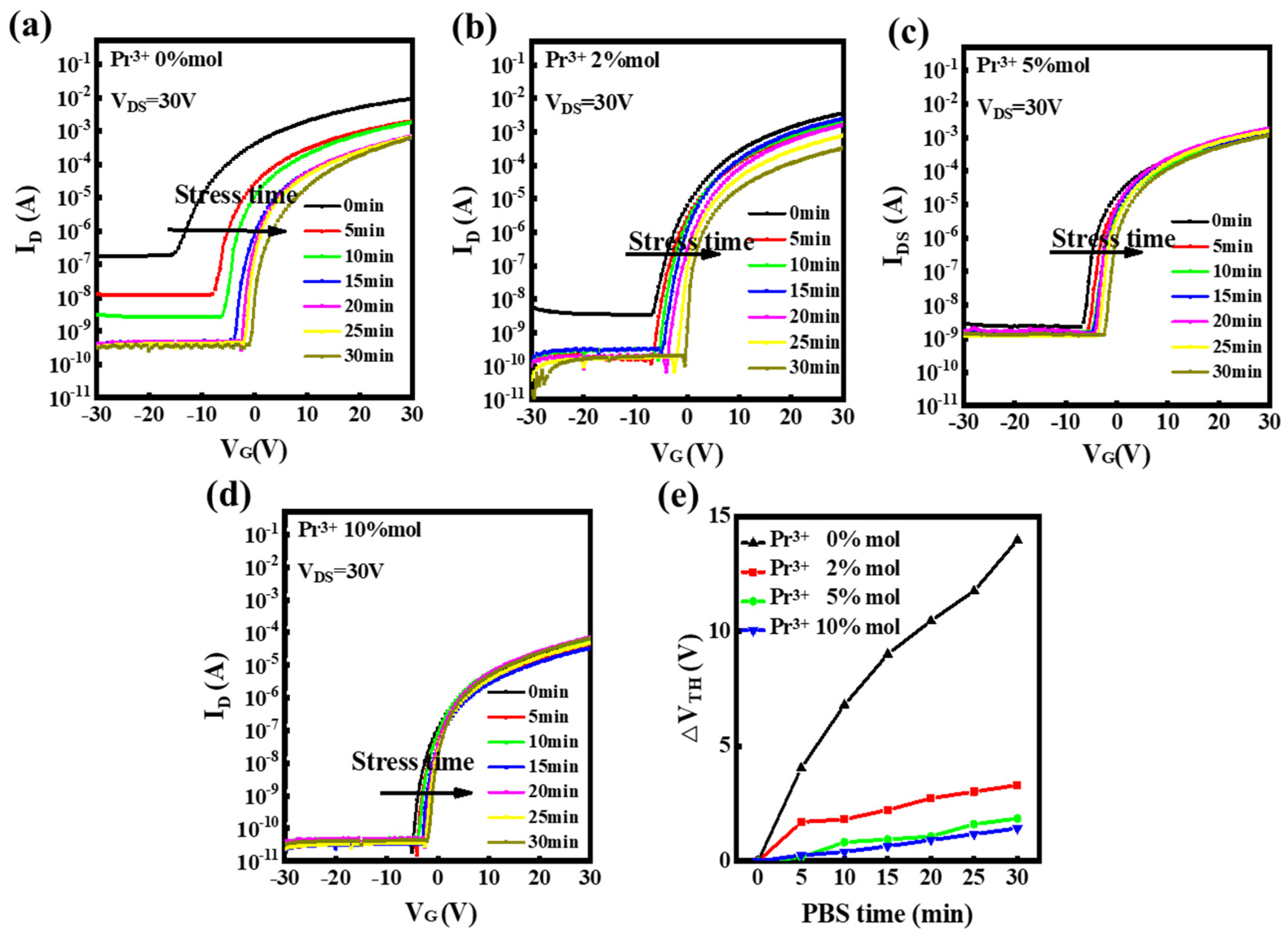

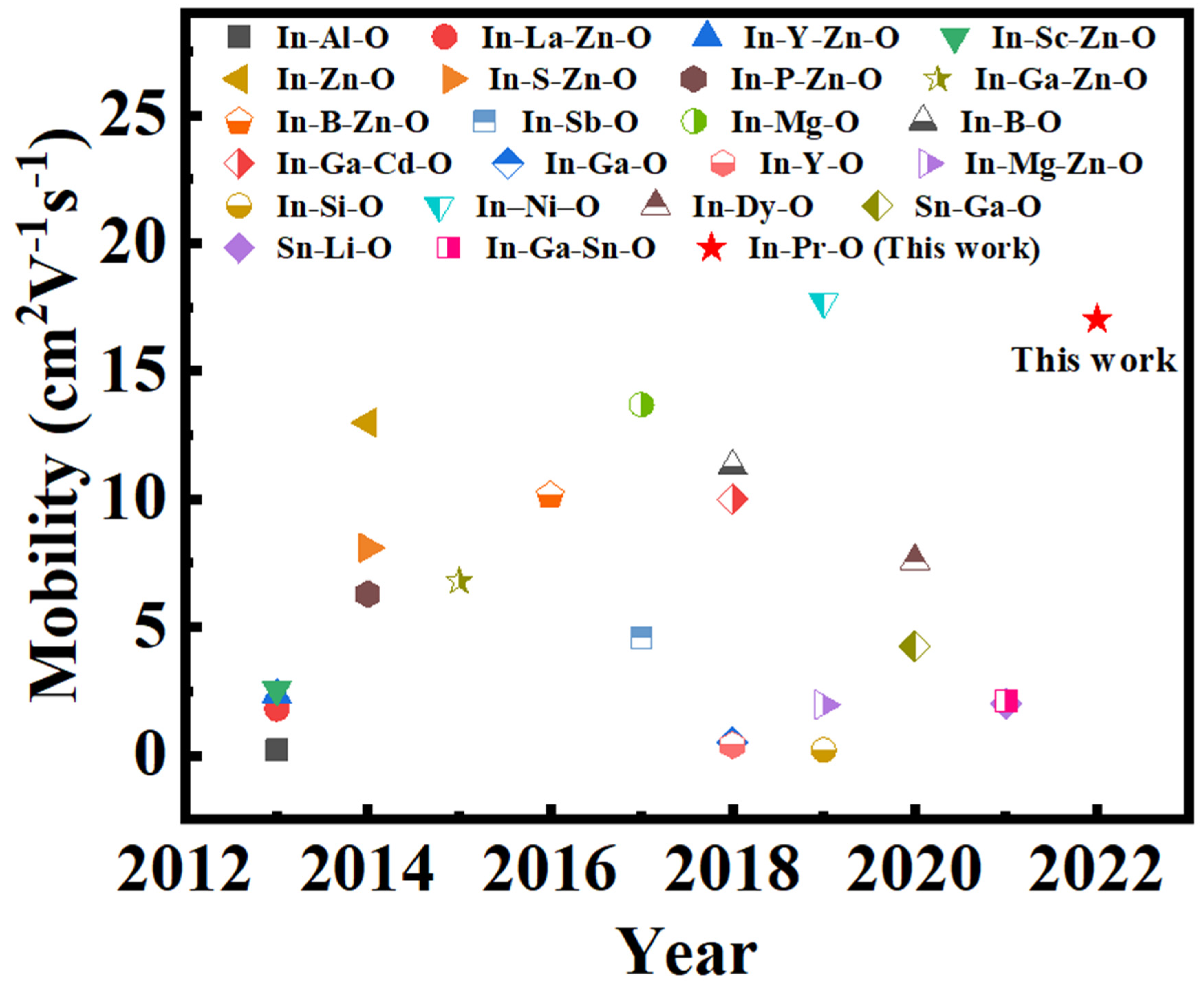
| Pr Ratio (%) | μ (cm2V−1s−1) | Ion/Ioff | S (V/dec) | VT (V) | Hysteresis (V) | VT Shift under PBS for 30 min (V) | VT Shift under NBS for 30 min (V) |
|---|---|---|---|---|---|---|---|
| 0 | 28.15 ± 1.27 | 5.30 × 104 | 2.50 ± 0.13 | −6.88 ± 0.27 | 1.32 | 13.96 | −6.14 |
| 2 | 21.87 ± 1.45 | 7.05 × 105 | 1.46 ± 0.09 | 2.37 ± 0.41 | 0.96 | 3.31 | −5.09 |
| 5 | 17.03 ± 1.19 | 2.15×106 | 1.32 ± 0.06 | 4.86 ± 0.32 | 0.44 | 1.86 | −2.09 |
| 10 | 0.76 ± 0.14 | 1.26 × 107 | 0.51 ± 0.11 | 5.77 ± 0.48 | 0.15 | 1.43 | −1.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Xu, C.; Zhang, Z.; Huang, W.; Lin, Q.; Zhuo, S.; Xu, F.; Liu, X.; Zhu, D.; Zhao, C. Water-Induced Nanometer-Thin Crystalline Indium-Praseodymium Oxide Channel Layers for Thin-Film Transistors. Nanomaterials 2022, 12, 2880. https://doi.org/10.3390/nano12162880
Xu W, Xu C, Zhang Z, Huang W, Lin Q, Zhuo S, Xu F, Liu X, Zhu D, Zhao C. Water-Induced Nanometer-Thin Crystalline Indium-Praseodymium Oxide Channel Layers for Thin-Film Transistors. Nanomaterials. 2022; 12(16):2880. https://doi.org/10.3390/nano12162880
Chicago/Turabian StyleXu, Wangying, Chuyu Xu, Zhibo Zhang, Weicheng Huang, Qiubao Lin, Shuangmu Zhuo, Fang Xu, Xinke Liu, Deliang Zhu, and Chun Zhao. 2022. "Water-Induced Nanometer-Thin Crystalline Indium-Praseodymium Oxide Channel Layers for Thin-Film Transistors" Nanomaterials 12, no. 16: 2880. https://doi.org/10.3390/nano12162880
APA StyleXu, W., Xu, C., Zhang, Z., Huang, W., Lin, Q., Zhuo, S., Xu, F., Liu, X., Zhu, D., & Zhao, C. (2022). Water-Induced Nanometer-Thin Crystalline Indium-Praseodymium Oxide Channel Layers for Thin-Film Transistors. Nanomaterials, 12(16), 2880. https://doi.org/10.3390/nano12162880








