Effect of Ionic Liquids in the Elaboration of Nanofibers of Cellulose Bagasse from Agave tequilana Weber var. azul by Electrospinning Technique
Abstract
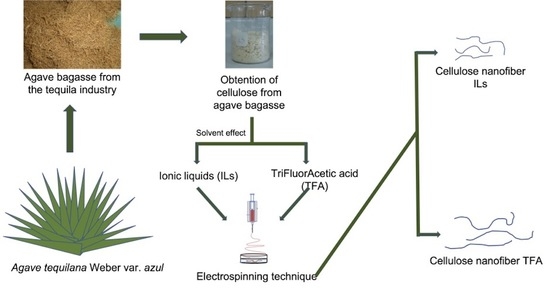
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Extraction of Cellulose
2.3. Preparation of Polymer Solution Using Ionic Liquids (ILs) Solvent
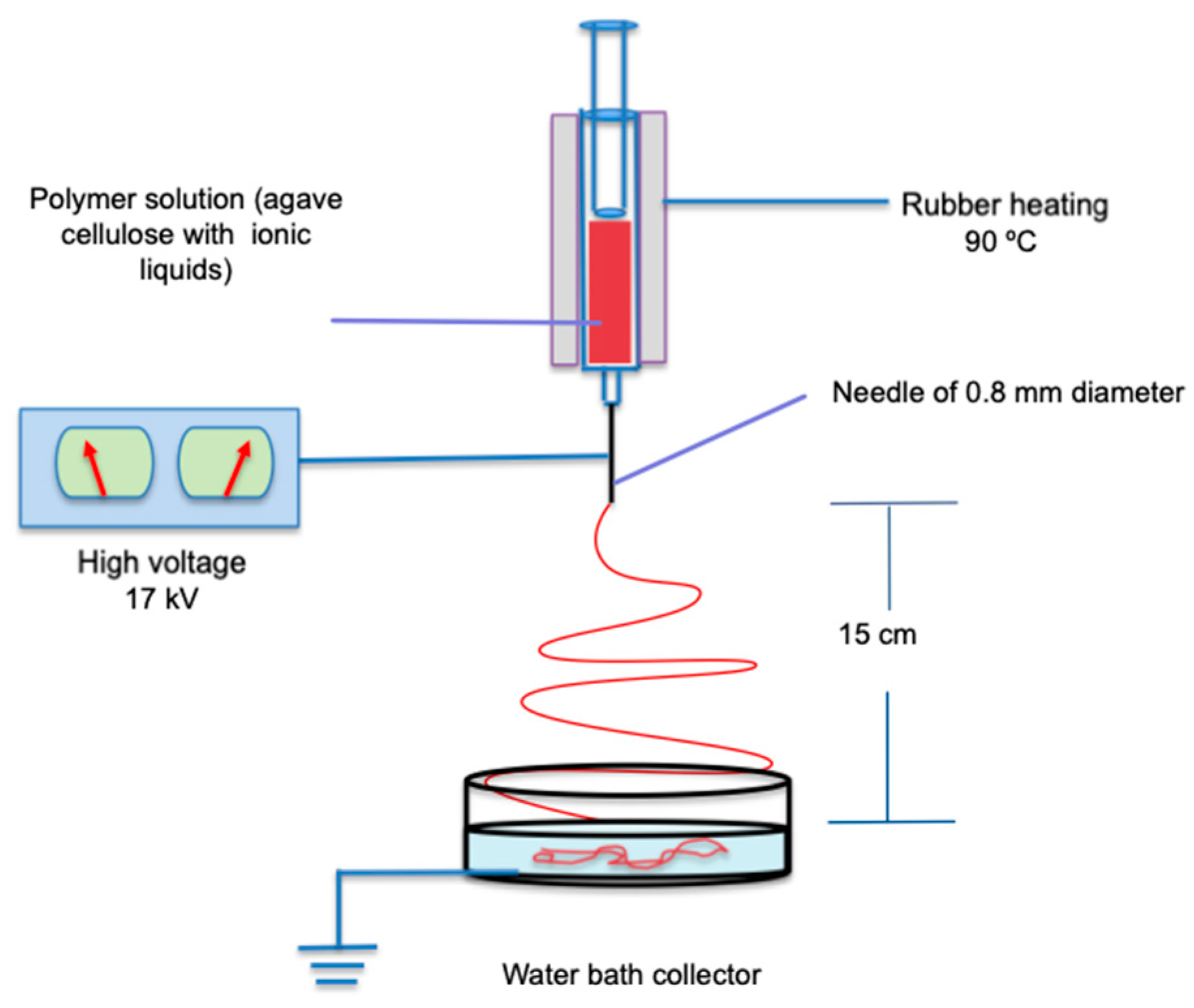
2.4. Nanofiber Elaboration by Electrospinning Process
2.5. Characterization of Cellulose Nanofibers Elaborated with IL
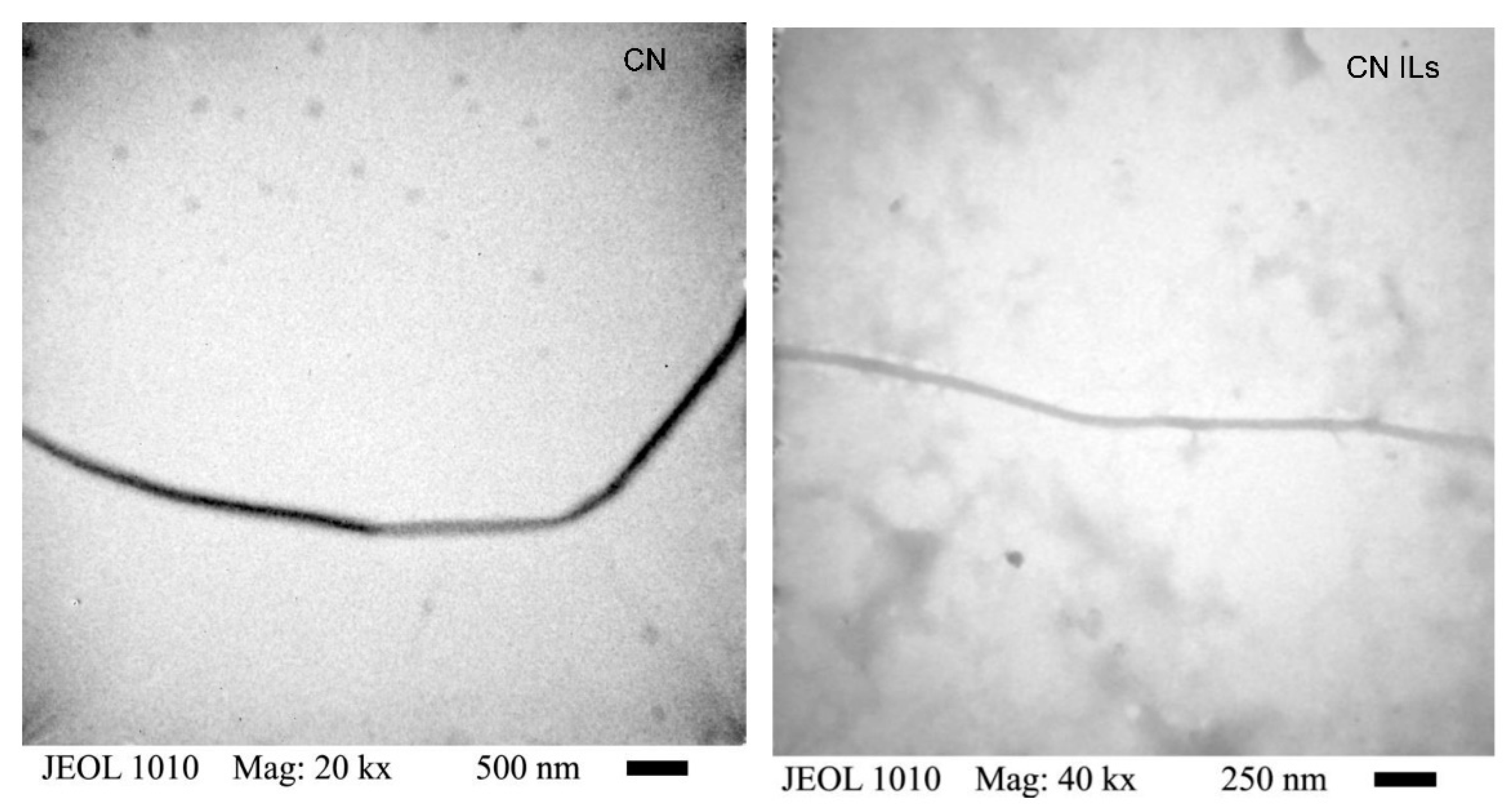
2.5.1. Transmission Electron Microscopy (TEM)
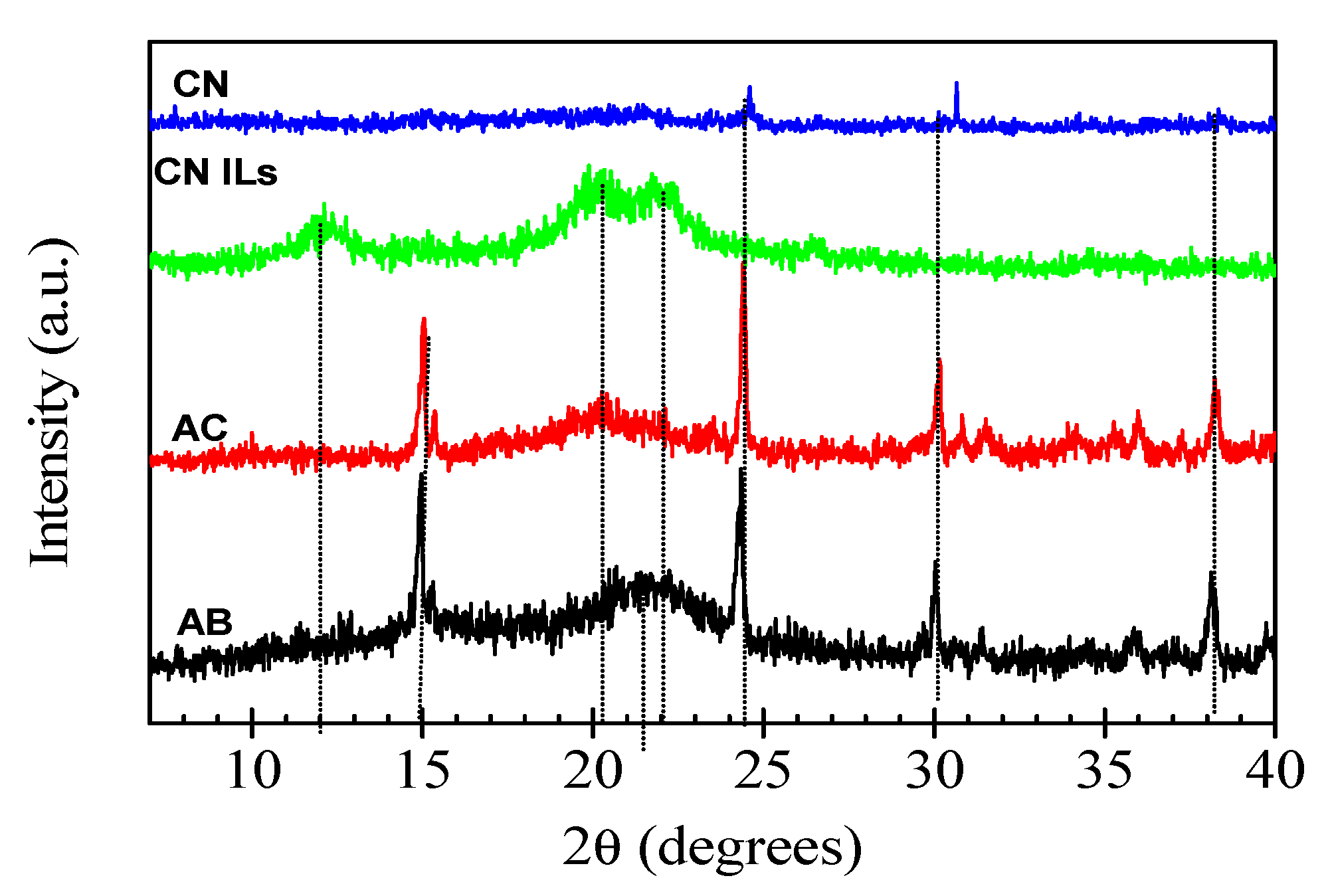
2.5.2. X-ray Diffraction (XRD)
2.5.3. Fourier Transform Infrared with Attenuated Total Reflectance (FTIR-ATR)
2.5.4. Thermogravimetric Analysis (TGA)
3. Results and Discussion
3.1. Elaboration Conditions of CN ILs
3.2. Transmission Electron Microscopy (TEM)
3.3. X-ray Diffraction (XRD)
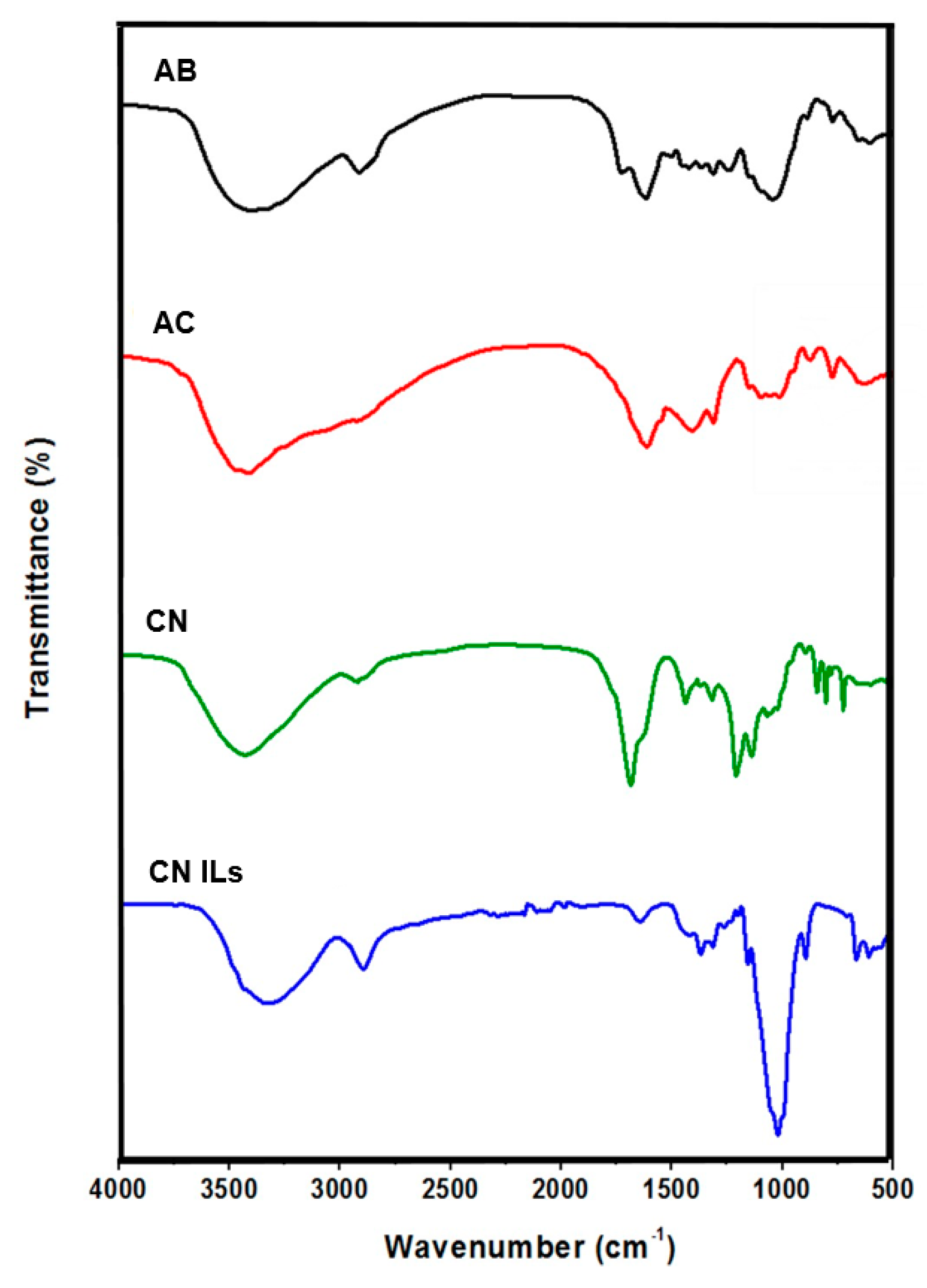
3.4. FTIR-ATR Spectroscopy
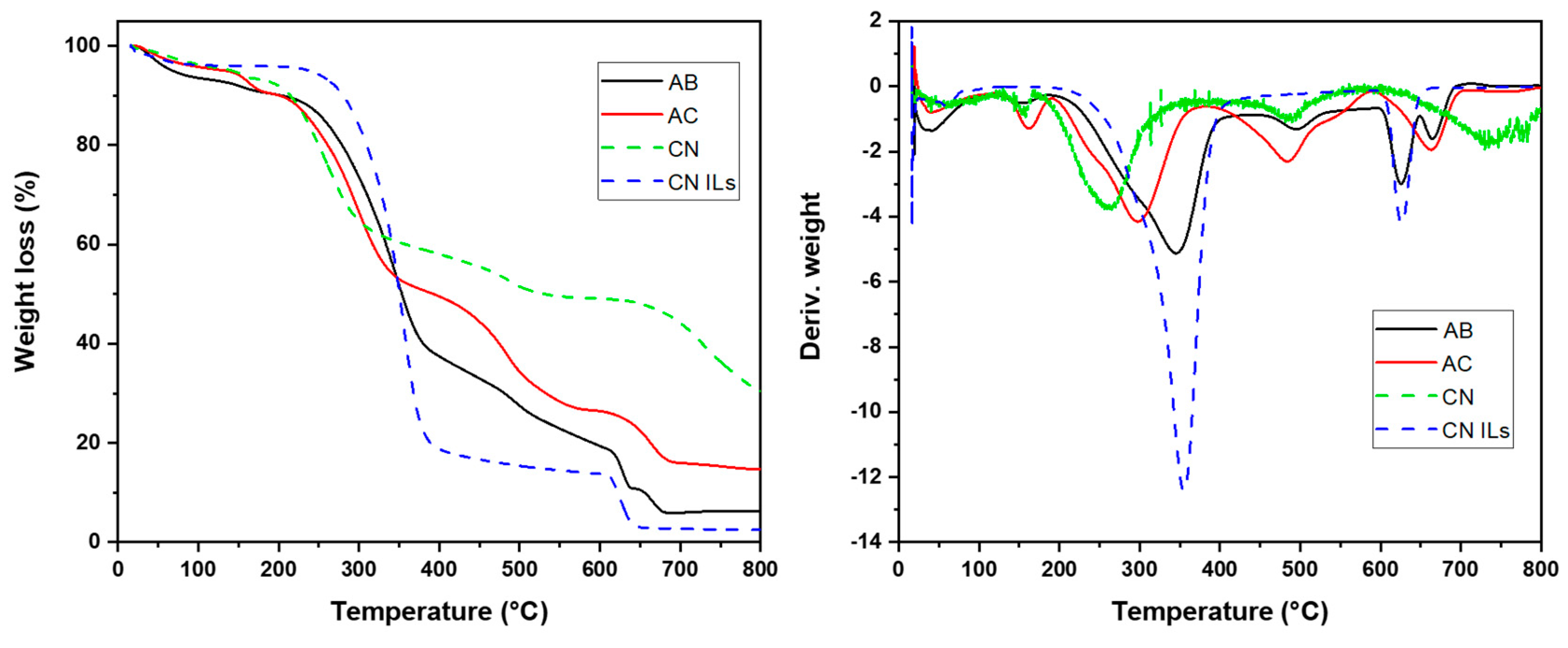
3.5. Thermogravimetric Analysis (TGA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Íñiguez, G.; Acosta, N.; Martínez, L.; Parra, J.; González, O. Utilización de subproductos de la industria tequilera. Parte 7. Compostaje de bagazo de agave y vinazas tequileras. Rev. Int. Contam. Ambient. 2005, 21, 37–50. [Google Scholar]
- López-López, A.; Davila-Vazquez, G.; León-Becerril, E.; Villegas-García, E.; Gallardo-Valdez, J. Tequila vinasses: Generation and full scale treatment processes. Rev. Environ. Sci. Bio-Technol. 2010, 9, 109–116. [Google Scholar] [CrossRef]
- CRT (Consejo Regulador del Tequila), Información Estadística. Consumo de Agave Para Tequila y Tequila 100% de Agave. 2022. Available online: https://www.crt.org.mx/EstadisticasCRTweb/ (accessed on 25 January 2022).
- Cedeño, C.M. Tequila production. Crit. Rev. Biotechnol. 1995, 15, 1–11. [Google Scholar] [CrossRef]
- Ramírez-Cortina, C.R.; Alonso-Gutiérrez, M.S.; Rigal, L. Tratamiento alcalino de los residuos agroindustriales de la producción del tequila, para su uso como complemento de alimento de rumiantes. Rev. AIDIS De Ing. Y Cienc. Ambient. Investig. Desarro. Y Práctica 2012, 5, 69–77. [Google Scholar] [CrossRef]
- Shatkin, J.A.; Wegner, T.H.; Bilek, E.M.; Cowie, J. Market projections of cellulose nanomaterial-enabled products—Part 1: Applications. TAPPI J. 2014, 13, 9–16. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent developments on nanocellulose reinforced polymer nanocomposites: A review. Polymer 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Holland, C.; Perzon, A.; Cassland, P.R.; Jensen, J.P.; Langebeck, B.; Sørensen, O.B.; Whale, E.; Hepworth, D.; Plaice-Inglis, R.; Moestrup, Ø.; et al. Nanofibers produced from agro-industrial plant waste using entirely enzymatic pretreatments. Biomacromolecules 2018, 20, 443–453. [Google Scholar] [CrossRef]
- Härdelin, L.; Thunberg, J.; Perzon, E.; Westman, G.; Walkenström, P.; Gatenholm, P. Electrospinning of cellulose nanofibers from ionic liquids: The effect of different cosolvents. J. Appl. Polym. Sci. 2012, 125, 1901–1909. [Google Scholar] [CrossRef]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef]
- Xu, L.; Lu, X.; Cheng, X. Preparation of modified cotton cellulose in ionic liquid and its adsorption of Cu(II) and Ni(II) from aqueous solutions. RSC Adv. 2015, 5, 79022–79030. [Google Scholar] [CrossRef]
- Coseri, S. Cellulose: To depolymerize or not to? Biotechnol. Adv. 2017, 35, 251–266. [Google Scholar] [CrossRef]
- Zhu, K.; Qiu, C.; Lu, A.; Luo, L.; Guo, J.; Cong, H.; Chen, F.; Liu, X.; Zhang, X.; Wang, H.; et al. Mechanically Strong Multifilament Fibers Spun from Cellulose Solution via Inducing Formation of Nanofibers. ACS Sustain. Chem. Eng. 2018, 6, 5314–5321. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Y.; Chen, Q.; Yu, Z.; Wang, C.; Jin, S.; Ding, Y.; Wu, G. Dissolution of cellulose with ionic liquids and its application: A mini-review. Green Chem. 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Olsson, C.; Hedlund, A.; Idström, A.; Westman, G. Effect of methylimidazole on cellulose/ionic liquid solutions and regenerated material therefrom. J. Mater. Sci. 2014, 49, 3423–3433. [Google Scholar] [CrossRef]
- Wu, K.C.W. Synthesis of Multi-functionalized Mesoporous Silica Nanoparticles for Cellulosic Biomass Conversion. In Heterogeneous Catalysis for Today’s Challenges: Synthesis, Characterization and Applications; Royal Society of Chemistry: London, UK, 2015; pp. 1–27. [Google Scholar] [CrossRef]
- Niroomand, F.; Khosravani, A.; Younesi, H. Fabrication and properties of cellulose-nanochitosan biocomposite film using ionic liquid. Cellulose. 2016, 23, 1311–1324. [Google Scholar] [CrossRef]
- He, Z.; Alexandridis, P. Ionic liquid and nanoparticle hybrid systems: Emerging applications. Adv. Colloid Interface Sci. 2017, 244, 54–70. [Google Scholar] [CrossRef]
- Colburn, A.; Wanninayake, N.; Kim, D.Y.; Bhattacharyya, D. Cellulose-graphene quantum dot composite membranes using ionic liquid. J. Membr. Sci. 2018, 556, 293–302. [Google Scholar] [CrossRef]
- Hermenegildo, B.; Meira, R.M.; Díez, A.G.; Correia, D.M.; Ribeiro, S.; Serra, J.P.; Ribeiro, C.; Pérez-Álvarez, L.; Vilas-Vilela, J.L.; Lanceros-Méndez, S. Ionic liquid modified electroactive polymer-based microenvironments for tissue engineering. Polymer 2022, 246, 124731. [Google Scholar] [CrossRef]
- Qasim, U.; Rafiq, S.; Jamil, F.; Ahmed, A.; Ali, T.; Kers, J.; Khurram, M.S.; Hussain, M.; Inayat, A.; Park, Y.K. Processing of lignocellulose in ionic liquids: A cleaner and sustainable approach. J. Clean. Prod. 2021, 323, 129189. [Google Scholar] [CrossRef]
- Lu, J.; Yan, F.; Texter, J. Advanced applications of ionic liquids in polymer science. Prog. Polym. Sci. 2009, 34, 431–448. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Patil, R.; Rutman, D.; Kucknoor, A.S.; Wang, A.; Guo, Z. Ionic liquid assisted electrospinning of quantum dots/elastomer composite nanofibers. Polymer 2011, 52, 1954–1962. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Yu, J.; Zhang, X.; He, J.; Zhang, J. Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: State of the art and future trends. Mater. Chem. Front. 2017, 1, 1273–1290. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, B.; Dou, H.; Zhang, L.; Tantai, X.; Sun, Y.; Zhang, H. Highly efficient and reversible capture of low partial pressure SO2 by functional deep eutectic solvents. Energy Fuels. 2018, 32, 10737–10744. [Google Scholar] [CrossRef]
- da Silva, B.A.; de Sousa Cunha, R.; Valerio, A.; Junior, A.D.N.; Hotza, D.; González, S.Y.G. Electrospinning of cellulose using ionic liquids: An overview on processing and applications. Eur. Polym. J. 2021, 147, 110283. [Google Scholar] [CrossRef]
- Krugly, E.; Pauliukaityte, I.; Ciuzas, D.; Bulota, M.; Peciulyte, L.; Martuzevicius, D. Cellulose electrospinning from ionic liquids: The effects of ionic liquid removal on the fiber morphology. Carbohydr. Polym. 2022, 285, 119260. [Google Scholar] [CrossRef]
- Hermanutz, F.; Gähr, F.; Uerdingen, E.; Meister, F.; Kosan, B. New developments in dissolving and processing of cellulose in ionic liquids. In Macromolecular Symposia; Wiley VCH: Weinheim, Germany, 2008; Volume 262, pp. 23–27. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Del-Toro-Sánchez, C.L.; Cinco-Moroyoqui, F.J.; Ruiz-Cruz, S.; Juárez, J.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; López-Ahumada, G.A.; Rodríguez-Félix, F. Gallic Acid-Loaded Zein Nanoparticles by Electrospraying Process. J. Food Sci. 2019, 84, 818–831. [Google Scholar] [CrossRef]
- Yu, D.G.; Zheng, X.L.; Yang, Y.; Li, X.Y.; Williams, G.R.; Zhao, M. Immediate release of helicid from nanoparticles produced by modified coaxial electrospraying. Appl. Surf. Sci. 2019, 473, 148–155. [Google Scholar] [CrossRef]
- Freire, M.G.; Teles, A.R.R.; Ferreira, R.A.; Carlos, L.D.; Lopes-da-Silva, J.A.; Coutinho, J.A. Electrospun nanosized cellulose fibers using ionic liquids at room temperature. Green Chem. 2011, 13, 3173–3180. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, M.; Liu, Y.; Liu, H.; Qu, J.; Li, J. Ionic Liquid Assisted Electrospun Cellulose Acetate Fibers for Aqueous Removal of Triclosan. Langmuir 2015, 31, 1820–1827. [Google Scholar] [CrossRef]
- Hsin-Fu, Y.; Sheng-Yuan, K.; Hsin-Che, L.; Yi-Feng, L.; Feng, H.; Hao-Wei, P.; Vittal, R.; Jiang-Jen, L.; Kuo-Chuan, H. Electrospun nanofibers composed of poly(vinylidene fluoride-cohexafluoropropylene) and poly(oxyethylene)-imideimidazolium tetrafluoroborate as electrolytes for solid-state electrochromic devices. Sol. Energy Mater. Sol. Cells. 2018, 177, 32–43. [Google Scholar] [CrossRef]
- Bozkaya, O.; Arat, E.; Gök, Z.G.; Yiğitoğlu, M.; Vargel, I. Production and characterization of hybrid nanofiber wound dressing containing Centella asiatica coated silver nanoparticles by mutual electrospinning method. Eur. Polym. J. 2022, 166, 111023. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Kim, I.G.; Lee, J.H.; Unnithan, A.R.; Park, C.H.; Kim, C.S. A comprehensive electric field analysis of cylinder-type multi-nozzle electrospinning system for mass production of nanofibers. J. Ind. Eng. Chem. 2015, 31, 251–256. [Google Scholar] [CrossRef]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Yang, T.; Yao, Y.; Lin, Y.; Wang, B.; Xiang, R.; Wu, Y.; Wu, D. Electrospinning of polyacrylonitrile fibers from ionic liquid solution. Appl. Phys. A 2010, 98, 517–523. [Google Scholar] [CrossRef]
- Hu, S.; Qin, Z.; Cheng, M.; Chen, Y.; Liu, J.; Zhang, Y. Improved properties and drug delivery behaviors of electrospun cellulose acetate nanofibrous membranes by introducing carboxylated cellulose nanocrystals. Cellulose 2018, 25, 1883–1898. [Google Scholar] [CrossRef]
- Torres-Salcido, C.E.; Rodríguez-León, E.; Castillo-Ortega, M.M.; Ramírez-Bon, R.; Moreno-Corral, R.; Burruel-Ibarra, S.E.; Corella-Madueño, M.A.G.; Armenta-Villegas, L. Poly (Lactic Acid) Electrospun fibers loaded with Rumex hymenosepalus extract: Preparation and characterization. Biotecnia. 2020, 22, 108–115. [Google Scholar] [CrossRef]
- Robles-García, M.Á.; Del-Toro-Sánchez, C.L.; Márquez-Ríos, E.; Barrera-Rodríguez, A.; Aguilar, J.; Aguilar, J.A.; Reynoso-Marín, F.J.; Ceja, I.; Dórame-Miranda, R.; Rodríguez-Félix, F. Nanofibers of cellulose bagasse from Agave tequilana Weber var. azul by electrospinning: Preparation and characterization. Carbohydr. Polym. 2018, 192, 69–74. [Google Scholar] [CrossRef]
- Quan, S.L.; Kang, S.G.; Chin, I.J. Characterization of cellulose fibers electrospun using ionic liquid. Cellulose 2010, 17, 223–230. [Google Scholar] [CrossRef]
- Wang, W.; Nie, Y.; Liu, Y.; Bai, L.; Gao, J.; Zhang, S. Preparation of cellulose/multi-walled carbon nanotube composite membranes with enhanced conductive property regulated by ionic liquids. Fibers Polym. 2017, 18, 1780–1789. [Google Scholar] [CrossRef]
- Ninomiya, K.; Abe, M.; Tsukegi, T.; Kuroda, K.; Tsuge, Y.; Ogino, C.; Taki, K.; Taima, T.; Saito, J.; Kimizu, M.; et al. Lignocellulose nanofibers prepared by ionic liquid pretreatment and subsequent mechanical nanofibrillation of bagasse powder: Application to esterified bagasse/polypropylene composites. Carbohydr. Polym. 2018, 182, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Ullah, A.; Lee, J.; Jeong, Y.; Hashmi, M.; Zhu, C.; Joo, K., II; Cha, H.J.; Kim, I.S. Reusability comparison of melt-blown vs. nanofiber face mask filters for use in the coronavirus pandemic. ACS Appl. Nano Mater. 2020, 3, 7231–7241. [Google Scholar] [CrossRef]
- Niu, X.; Huan, S.; Li, H.; Pan, H.; Rojas, O.J. Transparent films by ionic liquid welding of cellulose nanofibers and polylactide: Enhanced biodegradability in marine environments. J. Hazard. Mater. 2021, 402, 124073. [Google Scholar] [CrossRef] [PubMed]
- Khanyile, N. Advances in Nanostructured Polyamide-Based Chemical Sensors. J. Nanomater. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Wu, C.; McClements, D.J.; Li, L.; He, M.; Li, Y.; Teng, F. Fabrication of composite hydrogels by assembly of okara cellulose nanofibers and gum Arabic in ionic liquids: Structure and properties. J. Mol. Liq. 2022, 349, 118132. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, J.; He, A.; Li, J.; Zhang, H.; Han, C.C. Electrospinning of native cellulose from nonvolatile solvent system. Polymer 2008, 49, 2911–2917. [Google Scholar] [CrossRef]
- Kamide, K. Cellulose in aqueous sodium hydroxide. In Cellulose and Cellulose Derivatives: Molecular Characterization and Uts Applications, 1st ed.; Elsevier BV: Amsterdam, The Netherlands, 2005; pp. 448–449. [Google Scholar] [CrossRef]
- Moscoso-Sánchez, F.J.; Ríos-Díaz, O.; Flores, J.; Martínez, L.; Fernández, V.V.A.; Barrera, A.; Canché-Escamilla, G. Effect of the cellulose of Agave tequilana Weber onto the mechanical properties of foamed and unfoamed polypropylene composites. Polym. Bull. 2013, 70, 837–847. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Deganello, S. The structure of whewellite, CaC2O4. H2O at 328 K. Acta Crystallogr. Sect. B-Struct. Sci. 1981, 37, 826–829. [Google Scholar] [CrossRef]
- Monje, P.V.; Baran, E.J. Characterization of calcium oxalate biominerals in some (non-cactaceae) succulent plant species. Z. Für Nat. C. 2010, 65, 429–432. [Google Scholar] [CrossRef]
- Perez-Pimienta, J.A.; Lopez-Ortega, M.G.; Chavez-Carvayar, J.A.; Varanasi, P.; Stavila, V.; Cheng, G.; Singh, S.; Simmons, B.A. Characterization of agave bagasse as a function of ionic liquid pretreatment. Biomass Bioenergy 2015, 75, 180–188. [Google Scholar] [CrossRef]
- Langan, P.; Nishiyama, Y.; Chanzy, H. X-ray structure of mercerized cellulose II at 1 Å resolution. Biomacromolecules 2001, 2, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on cellulose nanocrystals produced from cellulose sources with various polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.N.; Schliesser, J.; Mittal, A.; Decker, S.R.; Santos, A.F.L.; Freitas, V.L.; Urbas, A.; Lang, B.E.; Heiss, C.; da Silva, R.M.D.; et al. A thermodynamic investigation of the cellulose allomorphs: Cellulose (am), cellulose Iβ (cr), cellulose II (cr), and cellulose III (cr). J. Chem. Thermodyn. 2015, 81, 184–226. [Google Scholar] [CrossRef]
- Phinichka, N.; Kaenthong, S. Regenerated cellulose from high alpha cellulose pulp of steam-exploded sugarcane bagasse. J. Mater. Res. Technol. 2018, 7, 55–65. [Google Scholar] [CrossRef]
- Poletto, M.; Pistor, V.; Zattera, A.J. Structural characteristics and thermal properties of native cellulose. Cellul.—Fundam. Asp. 2013, 2, 45–68. [Google Scholar] [CrossRef]
- Abdul-Rahman, N.H.; Chieng, B.W.; Ibrahim, N.A.; Abdul Rahman, N. Extraction and Characterization of Cellulose Nanocrystals from Tea Leaf Waste Fibers. Polymers 2017, 9, 588. [Google Scholar] [CrossRef]
- Mozhzhukhina, N.; Méndez De Leo, L.P.; Calvo, E.J. Infrared spectroscopy studies on stability of dimethyl sulfoxide for application in a Li–air battery. J. Phys. Chem. C. 2013, 117, 18375–18380. [Google Scholar] [CrossRef]
- Hasegawa, M.; Isogai, A.; Onabe, F.; Usuda, M. Dissolving States of Cellulose and Chitosan in Trifluoroacetic Acid. J. Appl. Polym. Sci. 1992, 45, 1857–1863. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Z.-L. Research progress on dissolution and functional modification of cellulose in ionic liquids. J. Mol. Liq. 2008, 142, 1–5. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Feng, Y.; Lan, J.; Ma, P.; Dong, X.; Qu, J.; He, H. Chemical structure and thermal properties of lignin modified with polyethylene glycol during steam explosion. Wood Sci. Technol. 2017, 51, 135–150. [Google Scholar] [CrossRef]
- Yu-Chuan, L.; Cho, J.; Tompsett, G.A.; Westmoreland, P.R.; Huber, G.W. Kinetics and Mechanism of Cellulose Pyrolysis. J. Phys. Chem. C. 2009, 113, 20097–20107. [Google Scholar] [CrossRef]
- Mukarakate, C.; Mittal, A.; Ciesielski, P.N.; Budhi, S.; Thompson, L.; Iisa, K.; Nimlos, M.R.; Donohoe, B.S. Influence of crystal allomorph and crystallinity on the products and behavior of cellulose during fast pyrolysis. ACS Sustain. Chem. Eng. 2016, 4, 4662–4674. [Google Scholar] [CrossRef]
- Leal, G.F.; Ramos, L.A.; Barrett, D.H.; Curvelo, A.A.S.; Rodella, C.B. A thermogravimetric analysis (TGA) method to determine the catalytic conversion of cellulose from carbon-supported hydrogenolysis process. Thermochim. Acta. 2015, 616, 9–13. [Google Scholar] [CrossRef]
- Young-Min, K.; Tae, U.H.; ByeongAh, H.; Yunhee, L.; Atsushi, W.; Norio, T.; Seung-Soo, K.; Young-Kwon, P.; Seungdo, K. New approach for the kinetic analysis of cellulose using EGA-MS. Polym. Test. 2017, 60, 12–17. [Google Scholar] [CrossRef]
- Bernard, F.L.; Duczinski, R.B.; Rojas, M.F.; Fialho, M.C.C.; Carreno, L.A.; Chaban, V.V.; Vecchia, F.D.; Einloft, S. Cellulose based poly (ionic liquids): Tuning cation-anion interaction to improve carbon dioxide sorption. Fuel 2018, 211, 76–86. [Google Scholar] [CrossRef]
- Wang, Z.; McDonald, A.G.; Westerhof, R.J.; Kersten, S.R.; Cuba-Torres, C.M.; Ha, S.; Pecha, B.; Garcia-Perez, M. Effect of cellulose crystallinity on the formation of a liquid intermediate and on product distribution during pyrolysis. J. Anal. Appl. Pyrolysis 2013, 100, 56–66. [Google Scholar] [CrossRef]
- Kestur, G.S.; Flores-Sahagun, T.H.S.; Dos Santos, L.P.; Dos Santos, J.; Mazzaro, I.; Mikowski, A. Characterization of blue agave bagasse fibers of Mexico. Compos. Part A-Appl. Sci. Manuf. 2013, 45, 153–161. [Google Scholar] [CrossRef]
| Cellulose (g) | BMIMCl (g) | Polymer Concentration (wt%) | DMSO (g) | Dissolution Temperature (°C) | Dissolution Time (min) | Result |
|---|---|---|---|---|---|---|
| 0.015 | 1.5 | 1 | 0.001 | 90 | 35 | Dropped |
| 0.030 | 1.5 | 2 | 0.001 | 90 | 35 | No jet formed |
| 0.040 | 1.5 | 2.5 | 0.001 | 90 | 35 | No jet formed |
| 0.063 | 1.5 | 4 | 0.005 | 90 | 45 | Jet formed |
| 0.075 | 1.5 | 5 | 0.005 | 90 | 45 | Not jet formed |
| 0.12 | 1.5 | 8 | 0.005 | 90 | 45 | Not jet formed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez-Ríos, E.; Robles-García, M.Á.; Rodríguez-Félix, F.; Aguilar-López, J.A.; Reynoso-Marín, F.J.; Tapia-Hernández, J.A.; Cinco-Moroyoqui, F.J.; Ceja-Andrade, I.; González-Vega, R.I.; Barrera-Rodríguez, A.; et al. Effect of Ionic Liquids in the Elaboration of Nanofibers of Cellulose Bagasse from Agave tequilana Weber var. azul by Electrospinning Technique. Nanomaterials 2022, 12, 2819. https://doi.org/10.3390/nano12162819
Márquez-Ríos E, Robles-García MÁ, Rodríguez-Félix F, Aguilar-López JA, Reynoso-Marín FJ, Tapia-Hernández JA, Cinco-Moroyoqui FJ, Ceja-Andrade I, González-Vega RI, Barrera-Rodríguez A, et al. Effect of Ionic Liquids in the Elaboration of Nanofibers of Cellulose Bagasse from Agave tequilana Weber var. azul by Electrospinning Technique. Nanomaterials. 2022; 12(16):2819. https://doi.org/10.3390/nano12162819
Chicago/Turabian StyleMárquez-Ríos, Enrique, Miguel Ángel Robles-García, Francisco Rodríguez-Félix, José Antonio Aguilar-López, Francisco Javier Reynoso-Marín, José Agustín Tapia-Hernández, Francisco Javier Cinco-Moroyoqui, Israel Ceja-Andrade, Ricardo Iván González-Vega, Arturo Barrera-Rodríguez, and et al. 2022. "Effect of Ionic Liquids in the Elaboration of Nanofibers of Cellulose Bagasse from Agave tequilana Weber var. azul by Electrospinning Technique" Nanomaterials 12, no. 16: 2819. https://doi.org/10.3390/nano12162819
APA StyleMárquez-Ríos, E., Robles-García, M. Á., Rodríguez-Félix, F., Aguilar-López, J. A., Reynoso-Marín, F. J., Tapia-Hernández, J. A., Cinco-Moroyoqui, F. J., Ceja-Andrade, I., González-Vega, R. I., Barrera-Rodríguez, A., Aguilar-Martínez, J., Omar-Rueda-Puente, E., & Del-Toro-Sánchez, C. L. (2022). Effect of Ionic Liquids in the Elaboration of Nanofibers of Cellulose Bagasse from Agave tequilana Weber var. azul by Electrospinning Technique. Nanomaterials, 12(16), 2819. https://doi.org/10.3390/nano12162819