Transparent Heat Shielding Properties of Core-Shell Structured Nanocrystalline CsxWO3@TiO2
Abstract
:1. Introduction
2. Materials and methods
2.1. Preparation of CWO Nanoparticles
2.2. Preparation of CWO@TiO2 Nanoparticles
2.3. Fabrication Process for CWO@TiO2 Coated Glass
2.4. Discrete Dipole Approximation Method
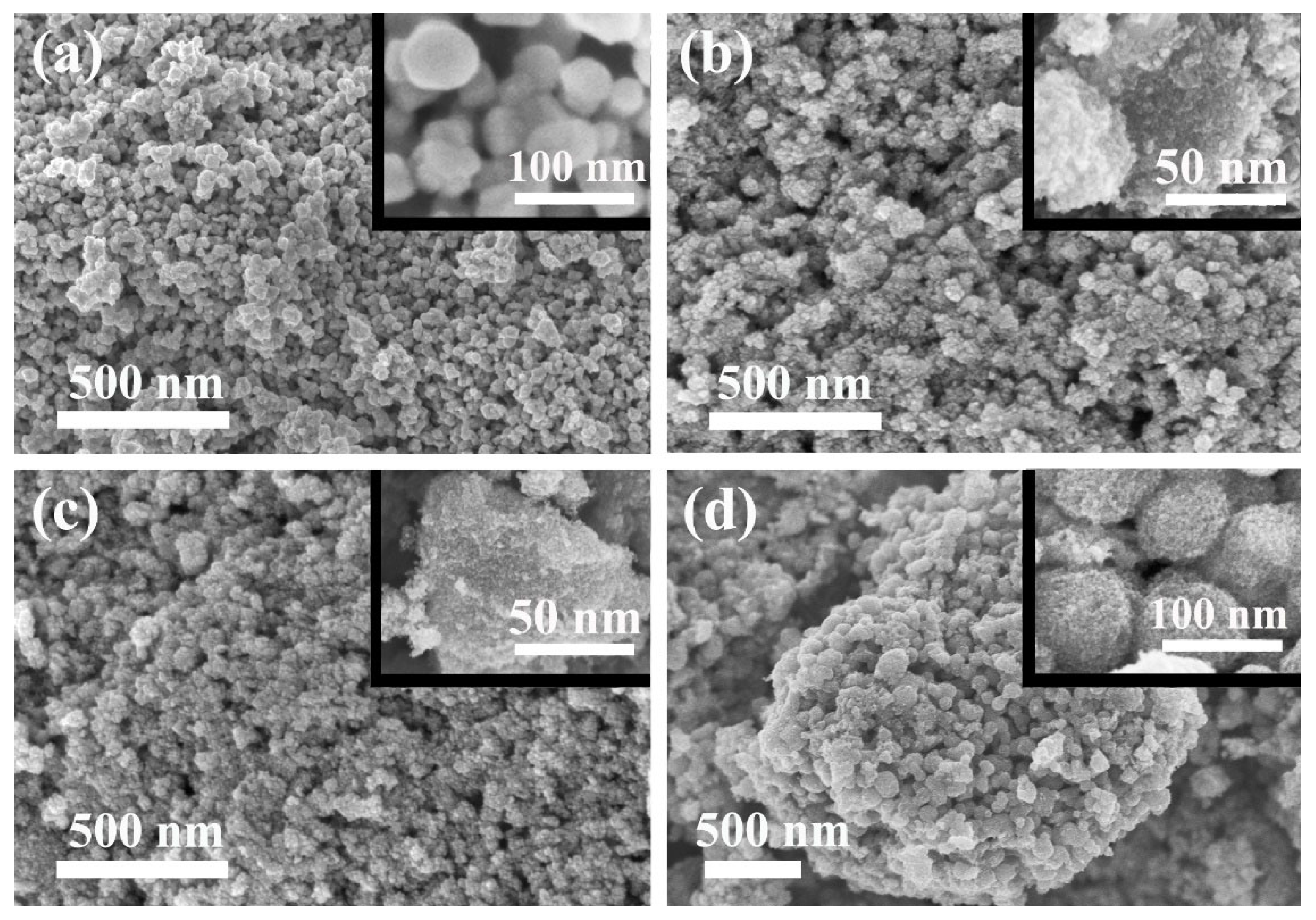
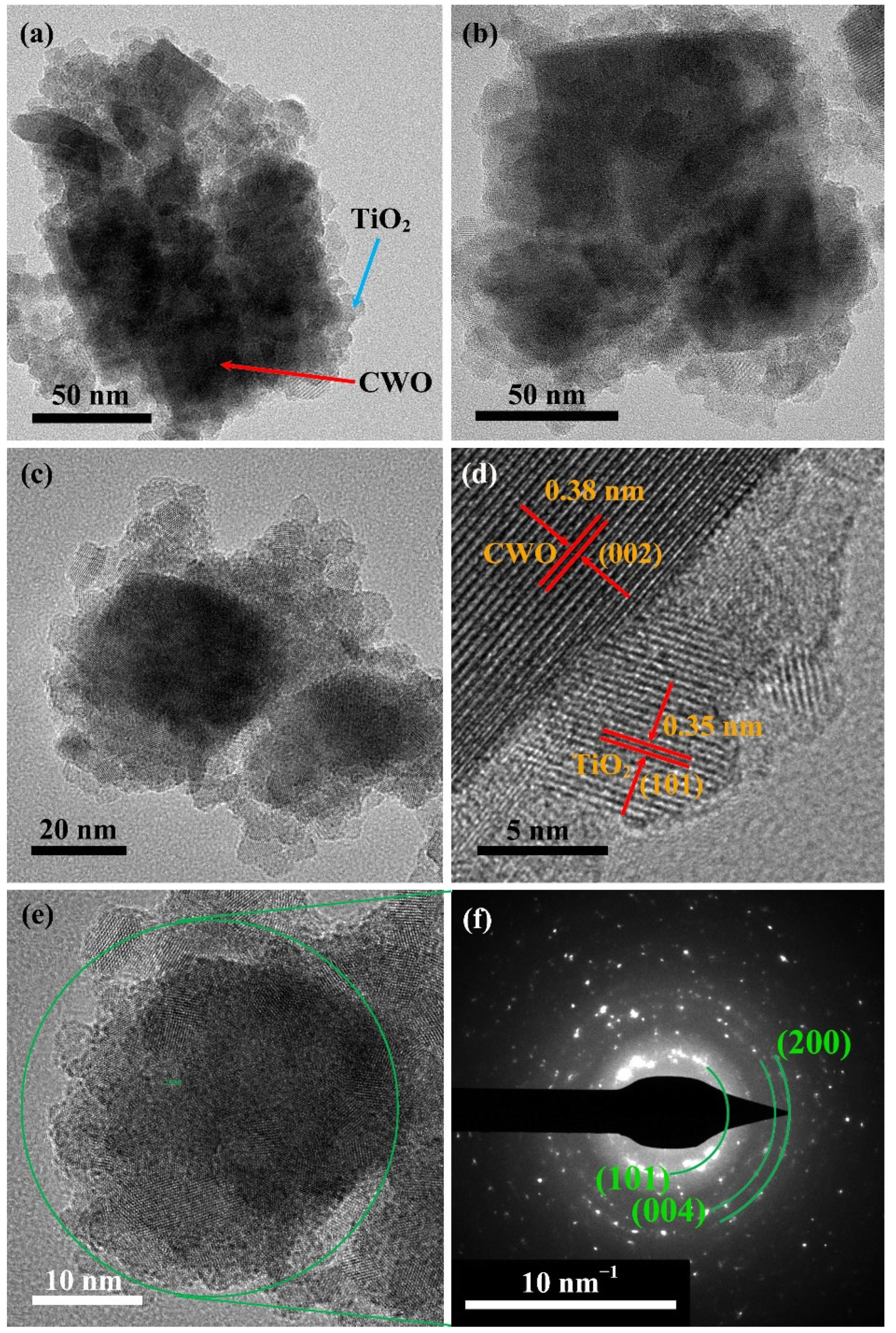
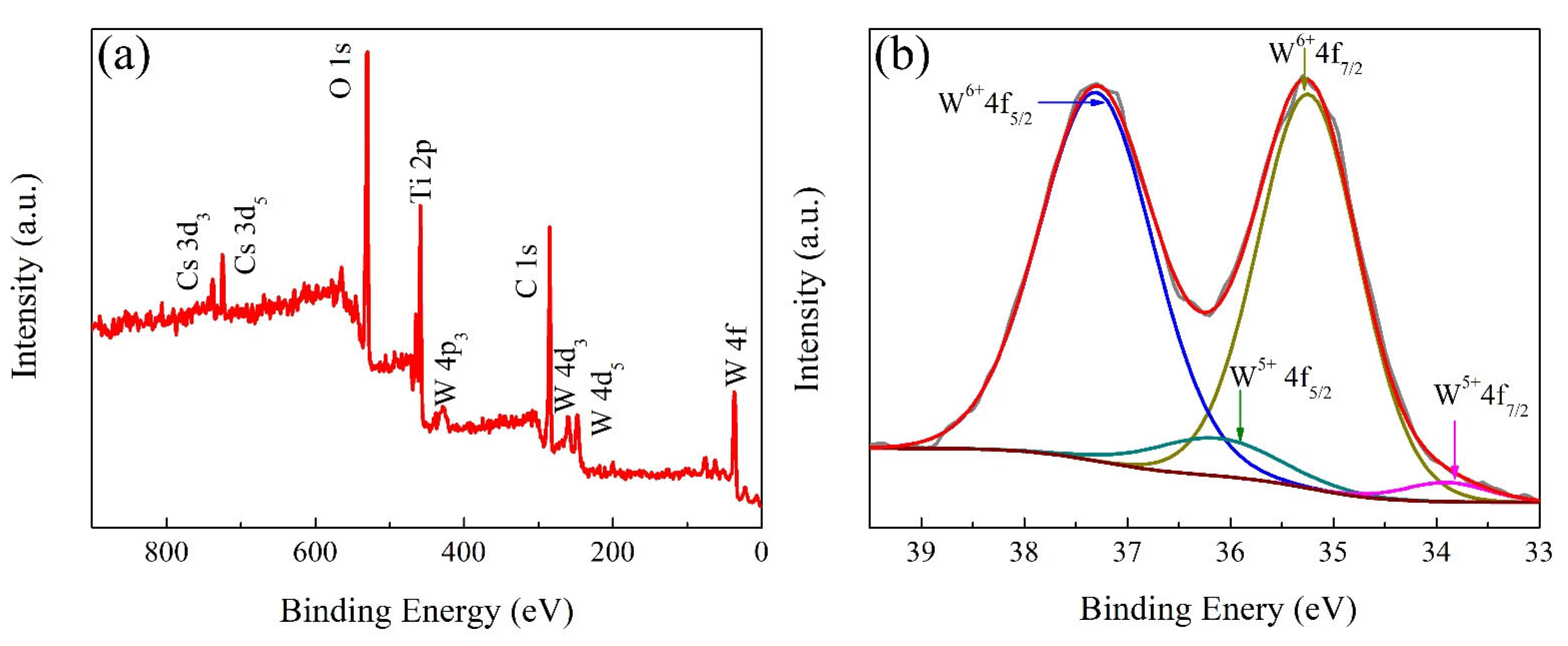
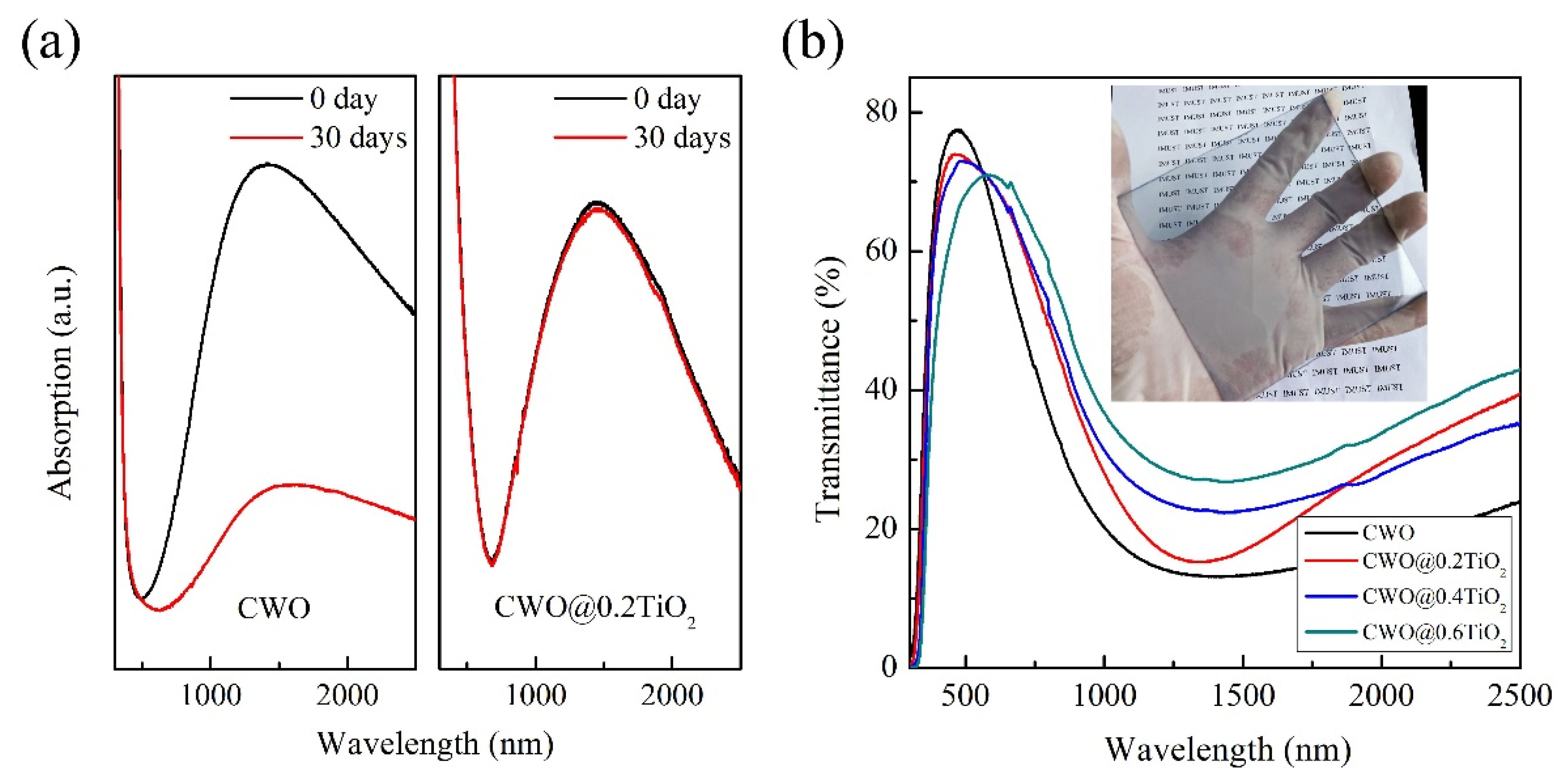
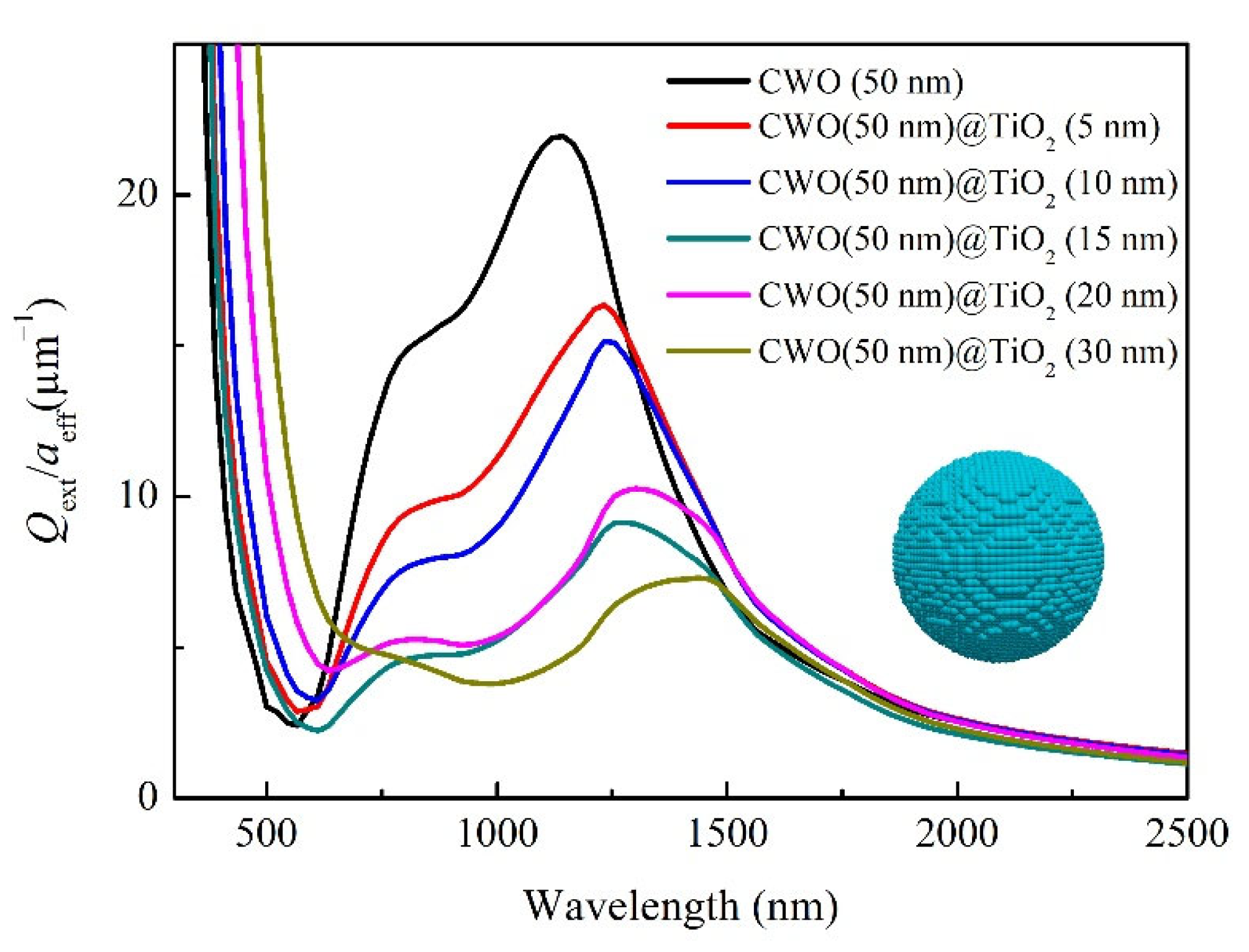
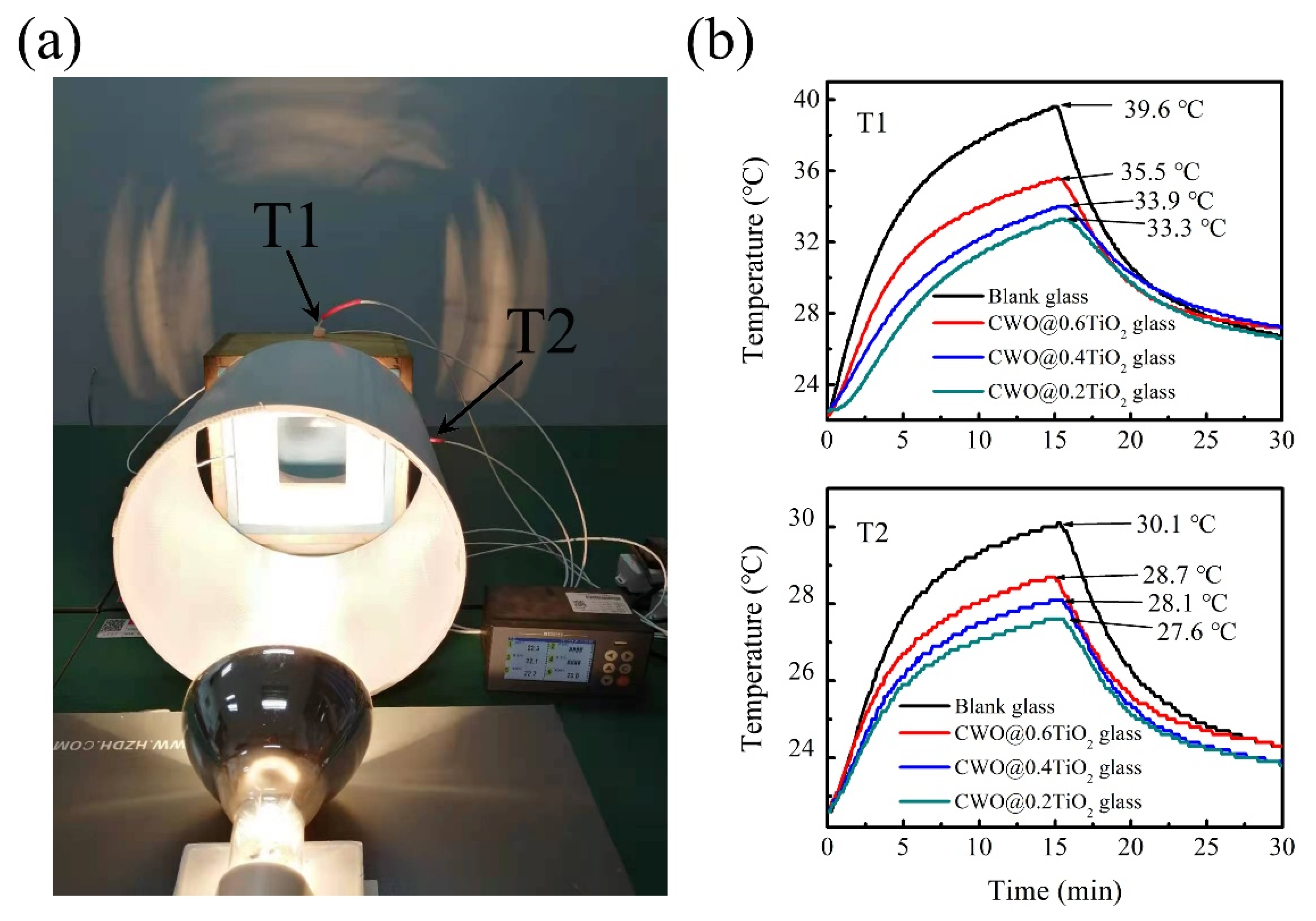
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karlsson, J.; Roos, A. Annual energy window performance vs. glazing thermal emittance—The relevance of very low emittance values. Thin. Solid. Film. 2001, 92, 345–348. [Google Scholar] [CrossRef]
- Kim, C.; Park, J.W.; Kim, J.; Hong, S.J.; Lee, M.J. A highly efficient indium tin oxide nanoparticles (ITO-NPs) transparent heater based on solution-process optimized with oxygen vacancy control. J. Alloys Compd. 2017, 726, 712–719. [Google Scholar] [CrossRef]
- Chao, L.; Bao, L.; Wei, W.; Tegus, O. A review of recent advances in synthesis, characterization and NIR shielding property of nanocrystalline rare-earth hexaborides and tungsten bronzes. Sol. Energy 2019, 190, 10–27. [Google Scholar] [CrossRef]
- Schelm, S.; Smith, G.B. Dilute LaB6 Nanoparticles in Polymer as Optimized Clear Solar Control Glazing. Appl. Phys. Lett. 2003, 82, 4346. [Google Scholar] [CrossRef]
- Takeda, H.; Kuno, H.; Adachi, K. Solar Control Dispersions and Coatings with Rare-Earth Hexaboride Nanoparticles. J. Am. Ceram. Soc. 2018, 91, 2897. [Google Scholar] [CrossRef]
- Long, L.; Ye, H.; Gao, Y.; Zou, R. Performance demonstration and evaluation of the synergetic application of vanadium dioxide glazing and phase change material in passive buildings. Appl. Energy 2014, 136, 89–97. [Google Scholar] [CrossRef]
- Shen, N.; Dong, B.; Cao, C.; Chen, Z.; Liu, J.; Luo, H.; Guo, Y. Lowered phase transition temperature and excellent solar heat shielding properties of well-crystallized VO2 by W doping. Phys. Chem. Chem. Phys. 2016, 18, 28010–28017. [Google Scholar] [CrossRef] [PubMed]
- Iken, O.; Fertahi, S.; Dlimi, M.; Agounoun, R.; Kadiri, I.; Sbai, K. Thermal and energy performance investigation of a smart double skin facade integrating vanadium dioxide through CFD simulations. Energy Convers. Manag. 2019, 195, 650–671. [Google Scholar] [CrossRef]
- Liang, X.; Guo, C.; Chen, M.; Guo, S.; Zhang, L.; Li, F.; Guo, S.; Yang, H. A roll-to-roll process for multi-responsive soft-matter composite films containing CsxWO3 nanorods for energy-efficient smart window applications. Nanoscale Horiz. 2017, 2, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; Liu, J.; Shi, F.; Fan, C.; Chen, B.; Zhang, H.; Yu, L.; Liu, S.H. Greatly improved heat-shielding performance of KxWO3 by trace Pt doping for energy-saving window glass applications. Sol. Energy. Mater. Sol. Cells 2018, 174, 342–350. [Google Scholar] [CrossRef]
- Liu, J.X.; Ando, Y.; Dong, X.L.; Shi, F.; Yin, S.; Adachi, K.; Chonan, T.; Tanaka, A.; Sato, T. Microstructure and electrical–optical properties of cesium tungsten oxides synthesized by solvothermal reaction followed by ammonia annealing. J. Solid. State. Chem. 2010, 183, 2456–2460. [Google Scholar] [CrossRef]
- Adachi, K.; Ota, Y.; Tanaka, H.; Okada, M.; Oshimura, N.; Tofuku, A. Chromatic instabilities in cesium-doped tungsten bronze nanoparticles. J. Appl. Phys. 2013, 114, 194304. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, X.; Zhou, Y.; Li, R.; Yao, H.L.; Cao, X.; Jin, P. Core-shell structured CsxWO3@ZnO with excellent stability and high performance on near-infrared shielding. Ceram. Int. 2018, 44, 2738–2744. [Google Scholar] [CrossRef]
- Zeng, X.; Zhou, Y.; Jin, S.; Luo, H.; Yao, H.; Huang, X.; Jin, P. The preparation of a high performance nearinfrared shielding CsxWO3/SiO2 composite resin coating and research on its optical stability under ultraviolet illumination. J. Mater. Chem. C 2015, 3, 8050–8060. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Chao, L. Theoretical analysis on optical properties of Cs0.33WO3 nanoparticles with different sizes, shapes and structures. Materials. Lett. 2020, 272, 127847. [Google Scholar] [CrossRef]
- Draine, B.T. The discrete-dipole approximation and its application to interstellar graphite grains. Astrophysical. J. 1988, 333, 848–872. [Google Scholar] [CrossRef]
- Draine, B.T.; Flatau, P.J. Discrete-dipole approximation for scattering calculations. J. Opt. Soc. Am. 1994, 11, 1491–1499. [Google Scholar] [CrossRef]
- Purcell, E.M.; Pennypacker, C.R. Scattering and absorption of light by nonspherical dielectric grains. Astrophysical. J. 1973, 186, 705–714. [Google Scholar] [CrossRef]
- Yurkin, M.A.; Hoekstra, A.G. The discrete-dipole-approximation code ADDA: Capabilities and known limitations. J. Quant. Spectrosc. Radiat. Transf. 2011, 112, 2234–2247. [Google Scholar] [CrossRef]
- Draine, B.T.; Flatau, P.J. User Guide for the Discrete Dipole Approximation Code DDSCAT 7.3. arXiv 2013. [Google Scholar] [CrossRef]
- Sato, Y.; Terauchi, M.; Adachi, K. High energy-resolution electron energy-loss spectroscopy study on the near-infrared scattering mechanism of Cs0.33WO3 crystals and nanoparticles. J. Appl. Phys. 2012, 112, 074308. [Google Scholar] [CrossRef]
- Li, G.; Guo, C.; Yan, M.; Liu, S. CsxWO3, nanorods: Realization of full-spectrum-responsive photocatalytic activities from UV, visible to near-infrared region. Appl. Catal. B Environ. 2016, 183, 142–148. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, L.; Sun, C.; Li, J.; Sun, M.; Liu, J.; Ma, Y. Transparent Heat Shielding Properties of Core-Shell Structured Nanocrystalline CsxWO3@TiO2. Nanomaterials 2022, 12, 2806. https://doi.org/10.3390/nano12162806
Chao L, Sun C, Li J, Sun M, Liu J, Ma Y. Transparent Heat Shielding Properties of Core-Shell Structured Nanocrystalline CsxWO3@TiO2. Nanomaterials. 2022; 12(16):2806. https://doi.org/10.3390/nano12162806
Chicago/Turabian StyleChao, Luomeng, Changwei Sun, Jiaxin Li, Miao Sun, Jia Liu, and Yonghong Ma. 2022. "Transparent Heat Shielding Properties of Core-Shell Structured Nanocrystalline CsxWO3@TiO2" Nanomaterials 12, no. 16: 2806. https://doi.org/10.3390/nano12162806
APA StyleChao, L., Sun, C., Li, J., Sun, M., Liu, J., & Ma, Y. (2022). Transparent Heat Shielding Properties of Core-Shell Structured Nanocrystalline CsxWO3@TiO2. Nanomaterials, 12(16), 2806. https://doi.org/10.3390/nano12162806






