Electrodeless Synthesis of Low Dispersity Au Nanoparticles and Nanoclusters at an Immiscible Micro Water/Ionic Liquid Interface
Abstract
1. Introduction
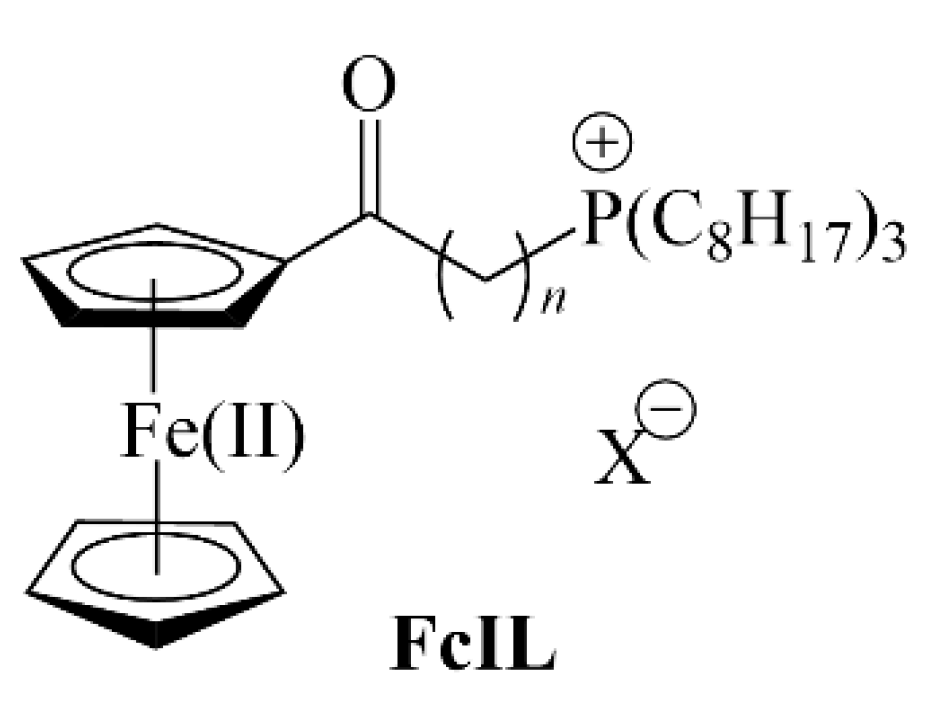
2. Materials and Methods
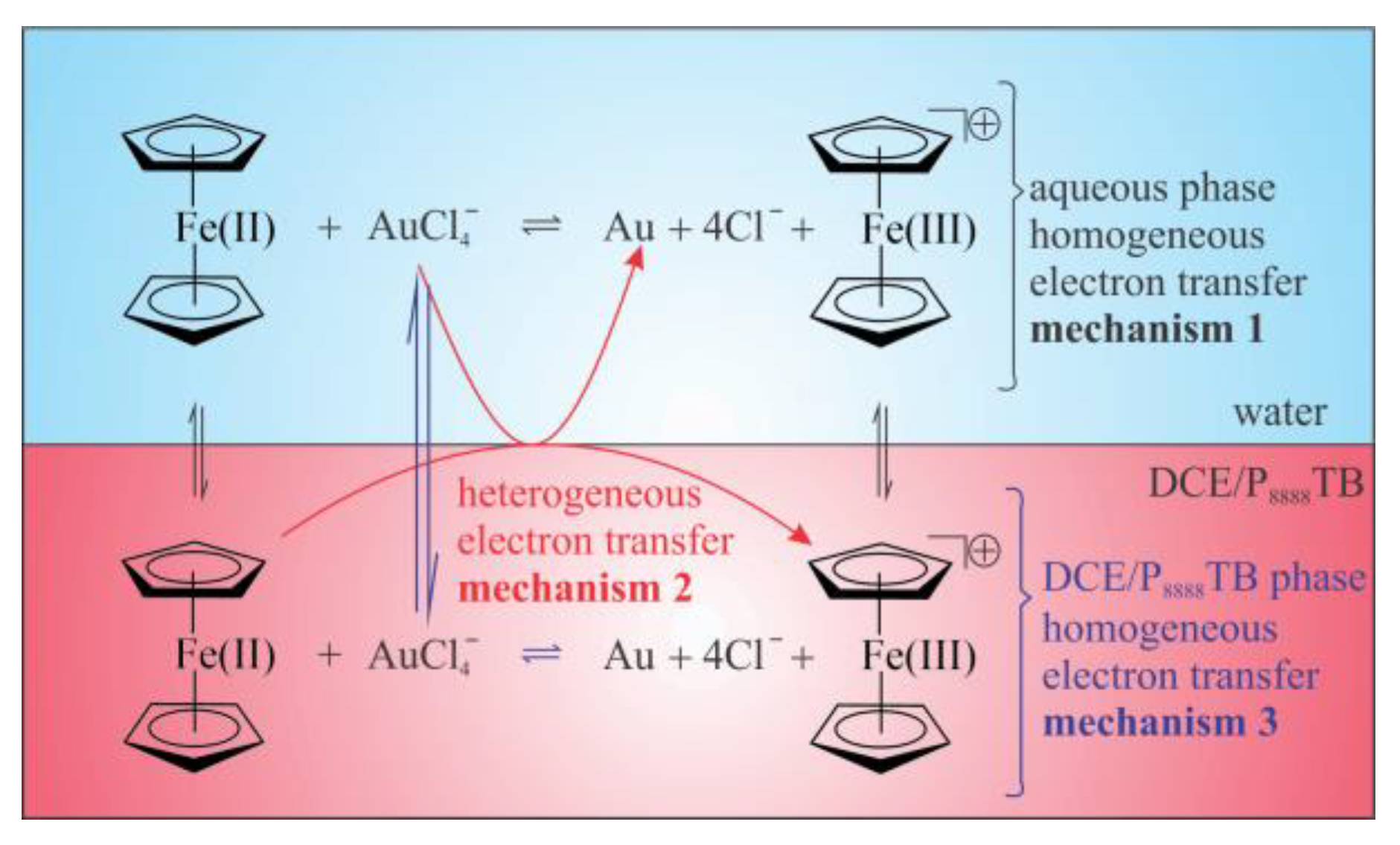
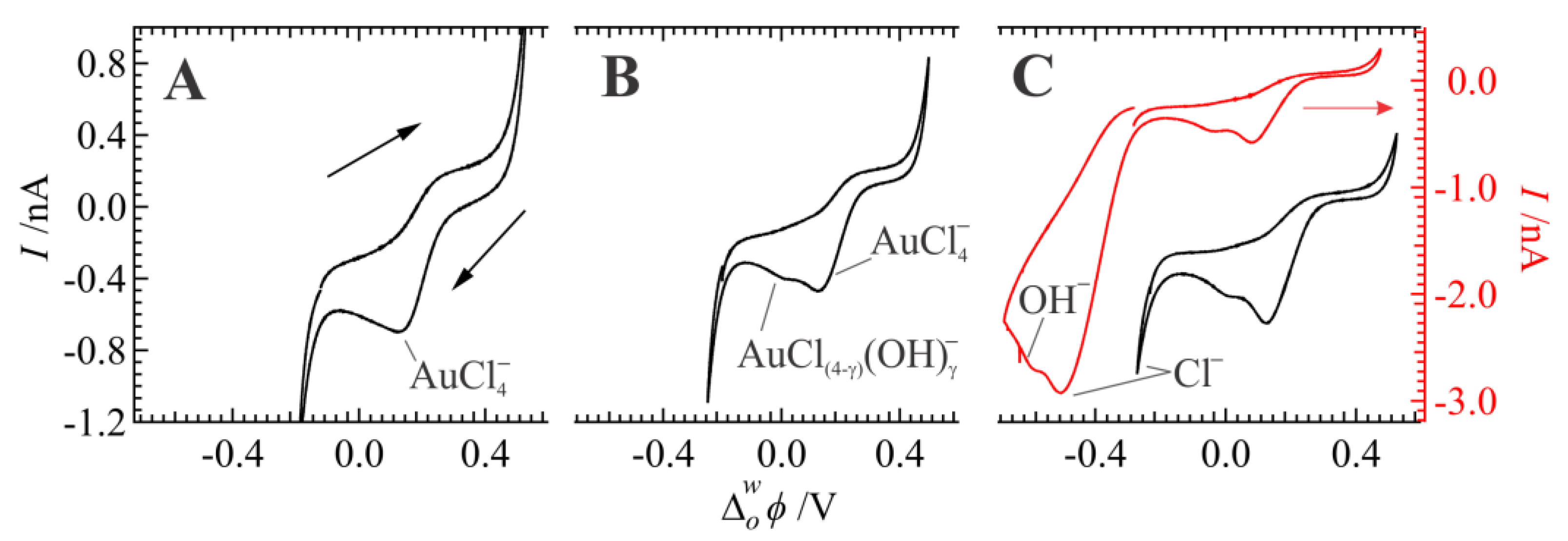
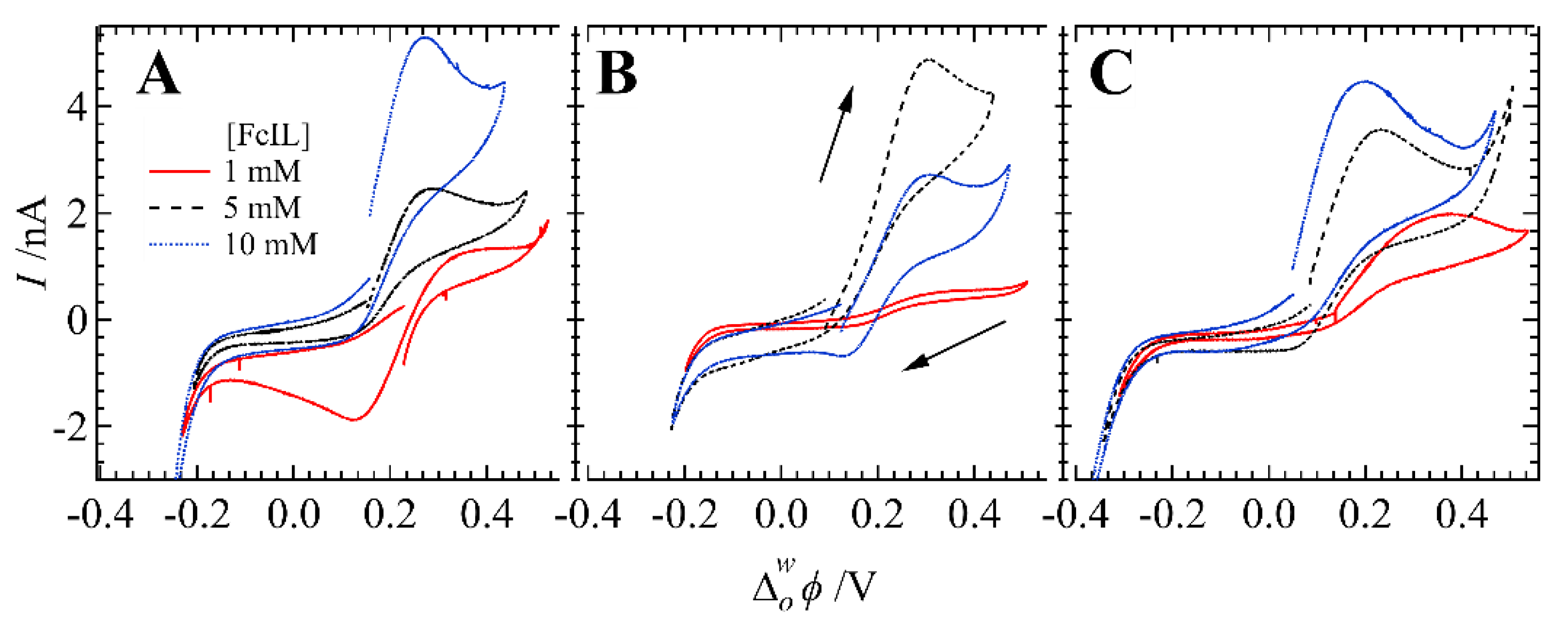
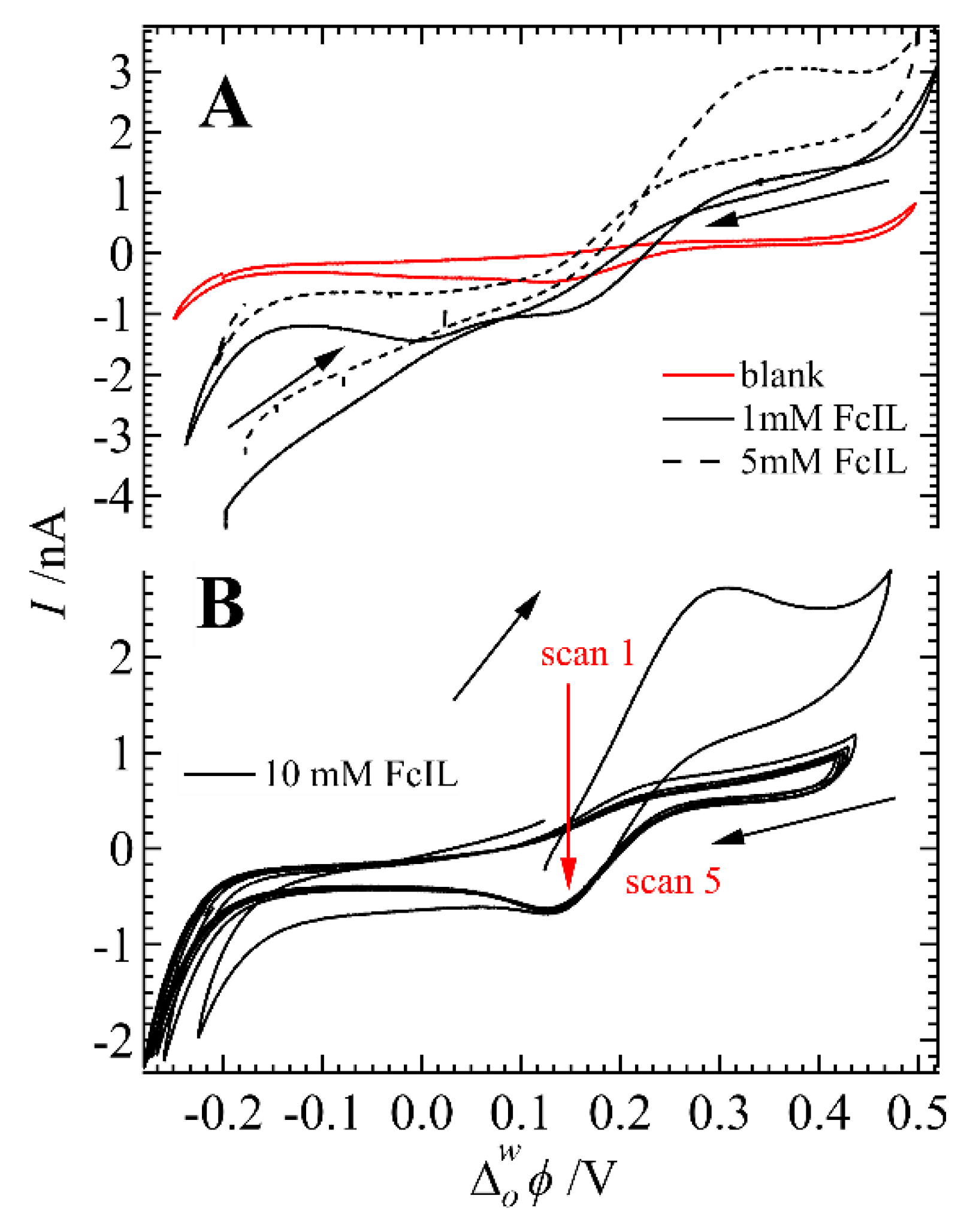
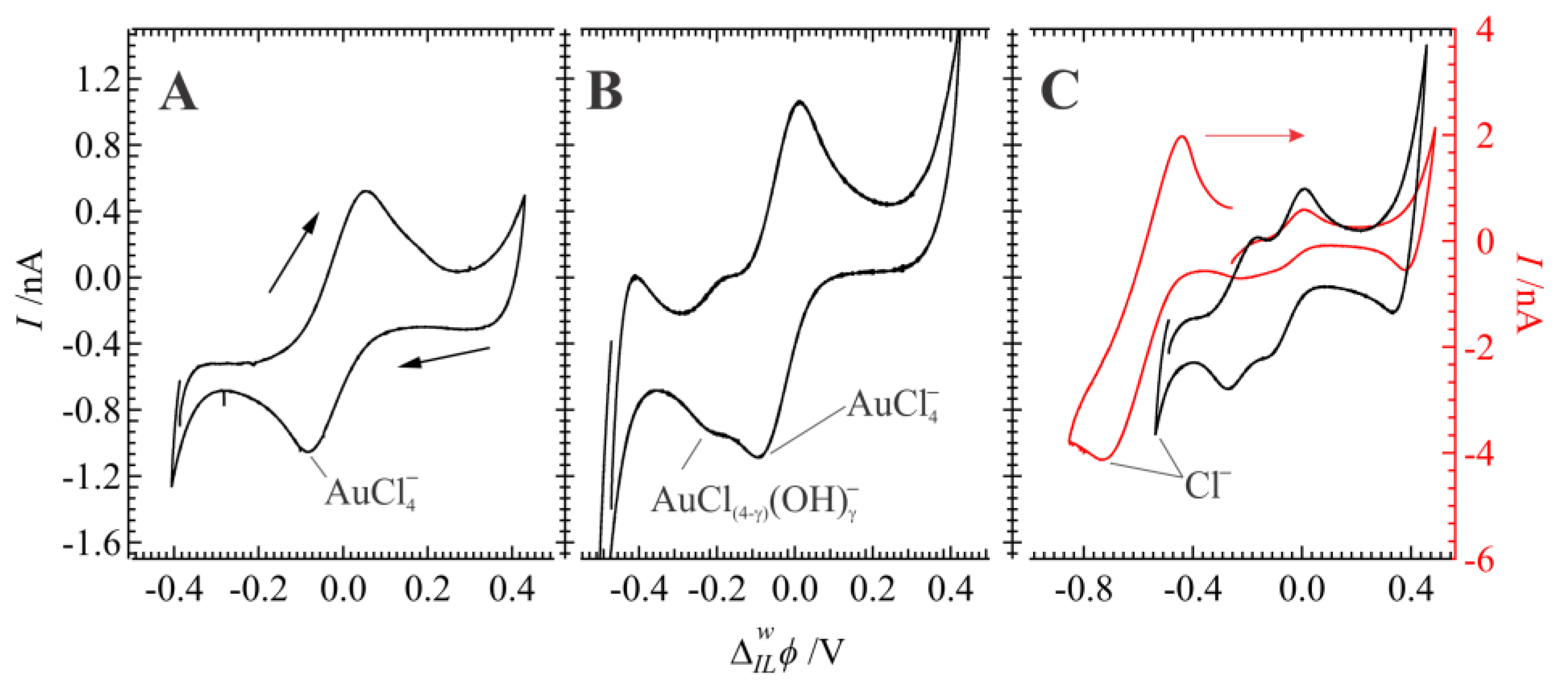
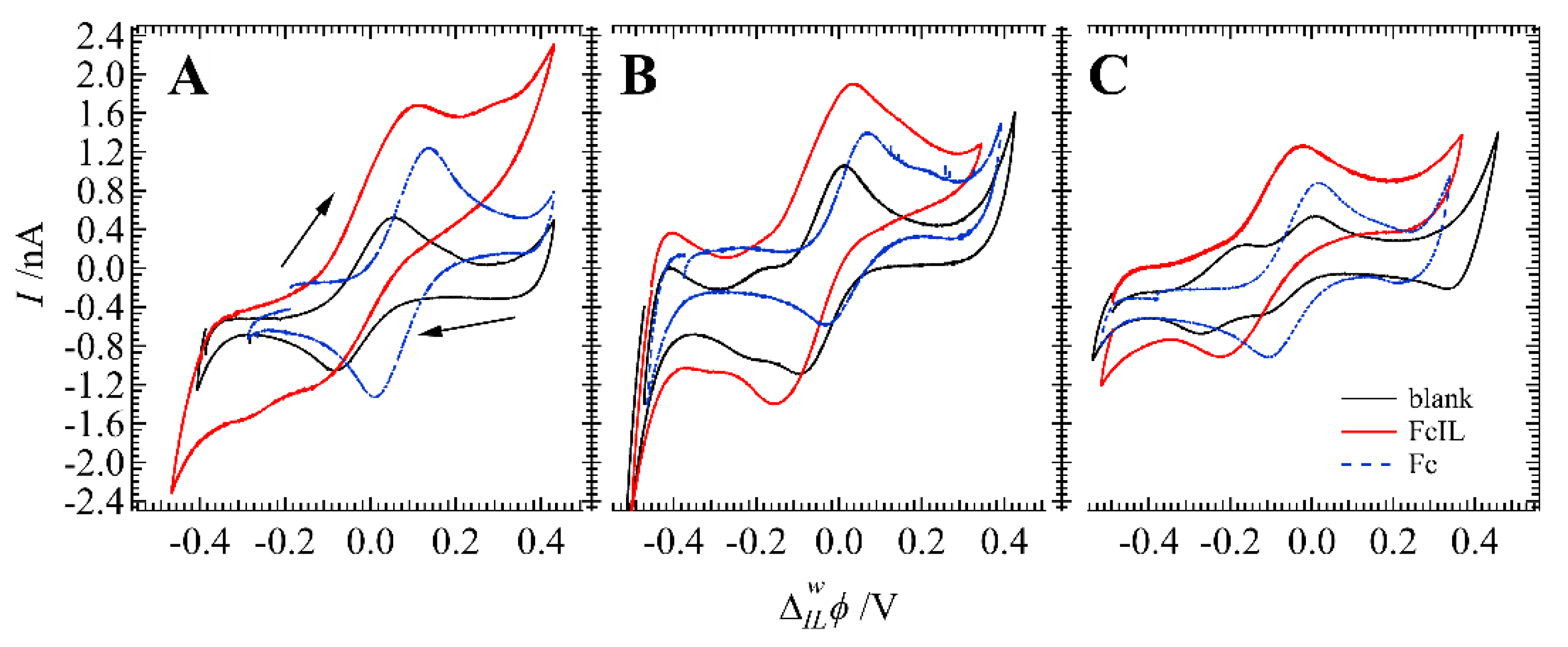
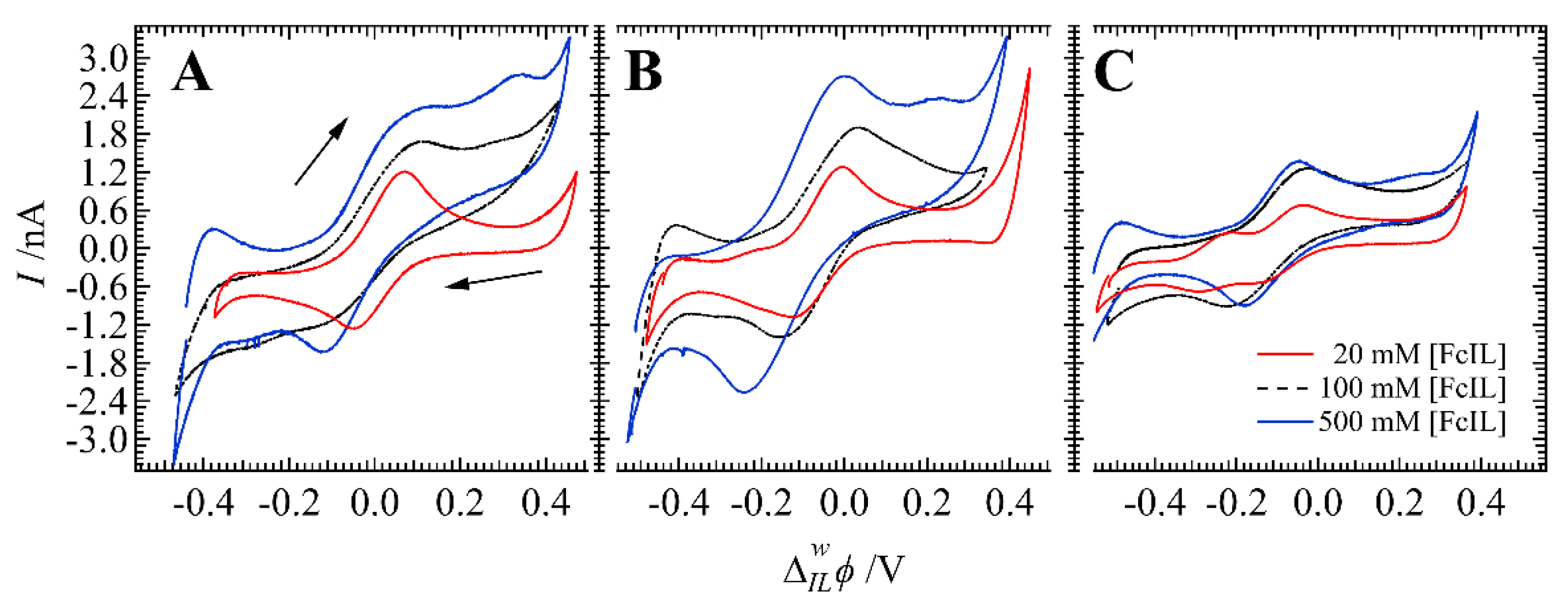
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, D.; Pal, A.; Pal, T. Electrochemical aspects of coinage metal nanoparticles for catalysis and spectroscopy. RSC Adv. 2022, 12, 12116–12135. [Google Scholar] [CrossRef]
- Scanlon, M.D.; Smirnov, E.; Stockmann, T.J.; Peljo, P. Gold Nanofilms at Liquid–Liquid Interfaces: An Emerging Platform for Redox Electrocatalysis, Nanoplasmonic Sensors, and Electrovariable Optics. Chem. Rev. 2018, 118, 3722–3751. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid-Liquid system. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Uehara, A.; Booth, S.G.; Chang, S.Y.; Schroeder, S.L.M.; Imai, T.; Hashimoto, T.; Mosselmans, J.F.W.; Dryfe, R.A.W. Electrochemical Insight into the Brust–Schiffrin Synthesis of Au Nanoparticles. J. Am. Chem. Soc. 2015, 137, 15135–15144. [Google Scholar] [CrossRef] [PubMed]
- Al Nasser, H.A.; Kim, C.; Li, Q.; Bissett, M.A.; Haigh, S.J.; Dryfe, R.A.W. The modified liquid | liquid interface: An electrochemical route for the electrode-less synthesis of MoS2 metal composite thin films. Electrochim. Acta 2022, 424, 140609. [Google Scholar] [CrossRef]
- Al Nasser, H.A.; Bissett, M.A.; Dryfe, R.A.W. The Modified Liquid-Liquid Interface: The Effect of an Interfacial Layer of MoS2 on Ion Transfer. Chem. Electro. Chem. 2021, 8, 4445–4455. [Google Scholar] [CrossRef]
- Koya, I.; Yokoyama, Y.; Sakka, T.; Nishi, N. Formation of Au Nanofiber/Fullerene Nanowhisker 1D/1D Composites via Reductive Deposition at the Interface between an Ionic Liquid and Water. Chem. Lett. 2022, 51, 643–645. [Google Scholar] [CrossRef]
- Kuroyama, Y.; Nishi, N.; Sakka, T. Electrochemical liquid-liquid interface between oil and ionic liquid for reductive deposition of metal nanostructures. J. Electroanal. Chem. 2021, 881, 114959. [Google Scholar] [CrossRef]
- Zhang, Y.; Nishi, N.; Sakka, T. Template-Free and Spontaneous Formation of Vertically Aligned Pd Nanofiber Arrays at the Liquid–Liquid Interface between Redox-Active Ionic Liquid and Water. ACS Appl. Mater. Interfaces 2019, 11, 23731–23740. [Google Scholar] [CrossRef] [PubMed]
- Nishi, N.; Yajima, I.; Amano, K.-i.; Sakka, T. Janus-Type Gold/Polythiophene Composites Formed via Redox Reaction at the Ionic Liquid|Water Interface. Langmuir 2018, 34, 2441–2447. [Google Scholar] [CrossRef]
- Lehane, R.A.; Gamero-Quijano, A.; Malijauskaite, S.; Holzinger, A.; Conroy, M.; Laffir, F.; Kumar, A.; Bangert, U.; McGourty, K.; Scanlon, M.D. Electrosynthesis of Biocompatible Free-Standing PEDOT Thin Films at a Polarized Liquid|Liquid Interface. J. Am. Chem. Soc. 2022, 144, 4853–4862. [Google Scholar] [CrossRef] [PubMed]
- Robayo-Molina, I.; Molina-Osorio, A.F.; Guinane, L.; Tofail, S.A.M.; Scanlon, M.D. Pathway Complexity in Supramolecular Porphyrin Self-Assembly at an Immiscible Liquid–Liquid Interface. J. Am. Chem. Soc. 2021, 143, 9060–9069. [Google Scholar] [CrossRef] [PubMed]
- Molina-Osorio, A.F.; Yamamoto, S.; Robayo-Molina, I.; Gamero-Quijano, A.; Nagatani, H.; Scanlon, M.D. A soft on/off switch based on the electrochemically reversible H–J interconversion of a floating porphyrin membrane. Chem. Sci. 2021, 12, 10227–10232. [Google Scholar] [CrossRef] [PubMed]
- Moshrefi, R.; Connors, E.P.; Merschrod, E.; Stockmann, T.J. Simultaneous electropolymerization/Au nanoparticle generation at an electrified liquid/liquid micro-interface. Electrochim. Acta 2022, 426, 140749. [Google Scholar] [CrossRef]
- Moshrefi, R.; Suryawanshi, A.; Stockmann, T.J. Electrochemically controlled Au nanoparticle nucleation at a micro liquid/liquid interface using ferrocene as reducing agent. Electrochem. Commun. 2021, 122, 106894. [Google Scholar] [CrossRef]
- Nieminen, E.; Murtomäki, L. Kinetics of Cu2+ Reduction and Nanoparticle Nucleation at Micro-scale 1,2-Dichlorobenzene-water Interface Studied by Cyclic Voltammetry and Square-wave Voltammetry. Electroanalysis 2021, 33, 2087–2095. [Google Scholar] [CrossRef]
- Sipa, K.; Kowalewska, K.; Leniart, A.; Walcarius, A.; Herzog, G.; Skrzypek, S.; Poltorak, L. Electrochemically assisted polyamide deposition at three-phase junction. Electrochem. Commun. 2021, 123, 106910. [Google Scholar] [CrossRef]
- Gamero-Quijano, A.; Dossot, M.; Walcarius, A.; Scanlon, M.D.; Herzog, G. Electrogeneration of a Free-Standing Cytochrome c—Silica Matrix at a Soft Electrified Interface. Langmuir 2021, 37, 4033–4041. [Google Scholar] [CrossRef]
- Cheng, Y.; Schiffrin, D.J. Electrodeposition of metallic gold clusters at the water/1,2-dichloroethane interface. J. Chem. Soc. Faraday Trans. 1996, 92, 3865–3871. [Google Scholar] [CrossRef]
- Johans, C.; Liljeroth, P.; Kontturi, K. Electrodeposition at polarisable liquid|liquid interfaces: The role of interfacial tension on nucleation kinetics. Phys Chem Chem Phys 2002, 4, 1067–1071. [Google Scholar] [CrossRef]
- Johans, C.; Kontturi, K.; Schiffrin, D.J. Nucleation at liquid∣liquid interfaces: Galvanostatic study. J. Electroanal. Chem. 2002, 526, 29–35. [Google Scholar] [CrossRef]
- Johans, C.; Clohessy, J.; Fantini, S.; Kontturi, K.; Cunnane, V.J. Electrosynthesis of polyphenylpyrrole coated silver particles at a liquid–liquid interface. Electrochem. Commun. 2002, 4, 227–230. [Google Scholar] [CrossRef]
- Johans, C.; Lahtinen, R.; Kontturi, K.; Schiffrin, D.J. Nucleation at liquid∣liquid interfaces: Electrodeposition without electrodes. J. Electroanal. Chem. 2000, 488, 99–109. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Chen, M.; Shi, J.; Yang, J.; Zhang, D. Fabrication of “clean” nano-structured metal materials on ionic liquid/water interface. Mater. Lett. 2014, 132, 153–156. [Google Scholar] [CrossRef]
- Takagi, S.; Nishi, N.; Sakka, T. Ionic Liquid-in-Water Emulsion-templated Synthesis of Gold Nanoshells at the Liquid-Liquid Interface between Water and Primary Ammonium-based Ionic Liquids. Chem. Lett. 2019, 48, 589–592. [Google Scholar] [CrossRef]
- Seitkalieva, M.M.; Samoylenko, D.E.; Lotsman, K.A.; Rodygin, K.S.; Ananikov, V.P. Metal nanoparticles in ionic liquids: Synthesis and catalytic applications. Coord. Chem. Rev. 2021, 445, 213982. [Google Scholar] [CrossRef]
- Ahmadinasab, N.; Stockmann, T.J. Single-Entity Electrochemical Detection of As-Prepared Metallic and Dielectric Nanoparticle Stochastic Impacts in a Phosphonium Ionic Liquid. Chem. Electro. Chem. 2022, 9, e202200162. [Google Scholar] [CrossRef]
- Stockmann, T.J.; Lemineur, J.-F.; Liu, H.; Cometto, C.; Robert, M.; Combellas, C.; Kanoufi, F. Single LiBH4 nanocrystal stochastic impacts at a micro water|ionic liquid interface. Electrochim. Acta 2019, 299, 222–230. [Google Scholar] [CrossRef]
- Uematsu, T.; Baba, M.; Oshima, Y.; Tsuda, T.; Torimoto, T.; Kuwabata, S. Atomic Resolution Imaging of Gold Nanoparticle Generation and Growth in Ionic Liquids. J. Am. Chem. Soc. 2014, 136, 13789–13797. [Google Scholar] [CrossRef]
- Bryant, K.; Hammond-Pereira, E.; Saunders, S.R. Ionic Liquid Aggregation Mechanism for Nanoparticle Synthesis. J. Phys. Chem. B 2021, 125, 253–263. [Google Scholar] [CrossRef]
- Nishi, N.; Kakinami, T.; Sakka, T. Dendritic nanofibers of gold formed by the electron transfer at the interface between water and a highly hydrophobic ionic liquid. Chem. Commun. 2015, 51, 13638–13641. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.E.F.; Breadner, D.; Deng, F.; Ramjee, B.; Ragogna, P.J.; Murray, R.W. Electrochemistry of Ferrocene-Functionalized Phosphonium Ionic Liquids. J. Phys. Chem. C 2011, 115, 19379–19385. [Google Scholar] [CrossRef]
- Stockmann, T.J.; Ding, Z. Tetraoctylphosphonium Tetrakis(pentafluorophenyl)borate Room Temperature Ionic Liquid toward Enhanced Physicochemical Properties for Electrochemistry. J. Phys. Chem. B 2012, 116, 12826–12834. [Google Scholar] [CrossRef]
- Stockmann, T.J.; Guterman, R.; Ragogna, P.J.; Ding, Z. Trends in Hydrophilicity/Lipophilicity of Phosphonium Ionic Liquids as Determined by Ion-Transfer Electrochemistry. Langmuir 2016, 32, 12966–12974. [Google Scholar] [CrossRef] [PubMed]
- Olaya, A.J.; Méndez, M.A.; Cortes-Salazar, F.; Girault, H.H. Voltammetric determination of extreme standard Gibbs ion transfer energy. J. Electroanal. Chem. 2010, 644, 60–66. [Google Scholar] [CrossRef]
- Stockmann, T.J.; Montgomery, A.-M.; Ding, Z. Determination of alkali metal ion transfers at liquid|liquid interfaces stabilized by a micropipette. J. Electroanal. Chem. 2012, 684, 6–12. [Google Scholar] [CrossRef]
- Zhou, M.; Gan, S.; Zhong, L.; Dong, X.; Ulstrup, J.; Han, D.; Niu, L. Improvement in the assessment of direct and facilitated ion transfers by electrochemically induced redox transformations of common molecular probes. Phys. Chem. Chem. Phys. 2012, 14, 3659–3668. [Google Scholar] [CrossRef]
- Liu, S.; Li, Q.; Shao, Y. Electrochemistry at micro- and nanoscopic liquid/liquid interfaces. Chem. Soc. Rev. 2011, 40, 2236–2253. [Google Scholar] [CrossRef]
- Ying, Y.-L.; Ding, Z.; Zhan, D.; Long, Y.-T. Advanced electroanalytical chemistry at nanoelectrodes. Chem. Sci. 2017, 8, 3338–3348. [Google Scholar] [CrossRef]
- Uehara, A.; Chang, S.-Y.; Booth, S.G.; Schroeder, S.L.M.; Mosselmans, J.F.W.; Dryfe, R.A.W. Redox and Ligand Exchange during the Reaction of Tetrachloroaurate with Hexacyanoferrate(II) at a Liquid-Liquid Interface: Voltammetry and X-ray Absorption Fine-Structure Studies. Electrochim. Acta 2016, 190, 997–1006. [Google Scholar] [CrossRef]
- Usher, A.; McPhail, D.C.; Brugger, J. A spectrophotometric study of aqueous Au(III) halide–hydroxide complexes at 25–80°C. Geochim. Cosmochim. Acta 2009, 73, 3359–3380. [Google Scholar] [CrossRef]
- Méndez, M.A.; Partovi-Nia, R.; Hatay, I.; Su, B.; Ge, P.Y.; Olaya, A.; Younan, N.; Hojeij, M.; Girault, H.H. Molecular electrocatalysis at soft interfaces. Phys. Chem. Chem. Phys. 2010, 12, 15163–15171. [Google Scholar] [CrossRef] [PubMed]
- Vanýsek, P. Electrochemical Series. In CRC Handbook of Chemistry and Physics; Haynes, W.M., Ed.; CRC Press/Taylor: Boca Raton, FL, USA, 2021; pp. 8.20–8.29. [Google Scholar]
- Stockmann, T.J.; Boyle, P.D.; Ding, Z. Preparation and crystal structure of tetraoctylphosphonium tetrakis(pentafluorophenyl)borate ionic liquid for electrochemistry at its interface with water. Catal. Today 2017, 295, 89–94. [Google Scholar] [CrossRef]
- Batchelor-McAuley, C.; Kätelhön, E.; Barnes, E.O.; Compton, R.G.; Laborda, E.; Molina, A. Recent Advances in Voltammetry. Chem. Open 2015, 4, 224–260. [Google Scholar] [CrossRef]
- Silvester, D.S.; Jamil, R.; Doblinger, S.; Zhang, Y.; Atkin, R.; Li, H. Electrical Double Layer Structure in Ionic Liquids and Its Importance for Supercapacitor, Battery, Sensing, and Lubrication Applications. J. Phys. Chem. C 2021, 125, 13707–13720. [Google Scholar] [CrossRef]
- Yasui, Y.; Kitazumi, Y.; Mizunuma, H.; Nishi, N.; Kakiuchi, T. A comparison of the ultraslow relaxation processes at the ionic liquid/water interface for three hydrophobic ionic liquids. Electrochem. Commun. 2010, 12, 1479–1482. [Google Scholar] [CrossRef]
- Rastgar, S.; Teixeira Santos, K.; Angelucci, C.A.; Wittstock, G. Catalytic Activity of Alkali Metal Cations for the Chemical Oxygen Reduction Reaction in a Biphasic Liquid System Probed by Scanning Electrochemical Microscopy. Chem. Eur. J. 2020, 26, 10882–10890. [Google Scholar] [CrossRef]
- Stockmann, T.J.; Deng, H.; Peljo, P.; Kontturi, K.; Opallo, M.; Girault, H.H. Mechanism of oxygen reduction by metallocenes near liquid|liquid interfaces. J. Electroanal. Chem. 2014, 729, 43–52. [Google Scholar] [CrossRef]
- Sharma, N.; Ajay, J.K.; Venkatasubbaiah, K.; Lourderaj, U. Mechanisms and dynamics of protonation and lithiation of ferrocene. Phys. Chem. Chem. Phys. 2015, 17, 22204–22209. [Google Scholar] [CrossRef]
- Alvarez, M.M.; Khoury, J.T.; Schaaff, T.G.; Shafigullin, M.; Vezmar, I.; Whetten, R.L. Critical sizes in the growth of Au clusters. Chem. Phys. Lett. 1997, 266, 91–98. [Google Scholar] [CrossRef]
- Zanatta, M.; Antunes, V.U.; Tormena, C.F.; Dupont, J.; dos Santos, F.P. Dealing with supramolecular structure for ionic liquids: A DOSY NMR approach. Phys. Chem. Chem. Phys. 2019, 21, 2567–2571. [Google Scholar] [CrossRef] [PubMed]
- Barmparis, G.D.; Lodziana, Z.; Lopez, N.; Remediakis, I.N. Nanoparticle shapes by using Wulff constructions and first-principles calculations. Beilstein J. Nanotechnol. 2015, 6, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Jorabchi, M.N.; Abbaspour, M.; Goharshadi, E.K.; Wohlrab, S. Ag, Au, Pt, and Au-Pt nanoclusters in [N1114][C1SO3] ionic liquid: A molecular dynamics study. J. Mol. Liq. 2022, 360, 119447. [Google Scholar] [CrossRef]



| [FcIL]/mM | pH | Ep,fwd/V | Ep,rev/V | ΔE/V | ip/nA | Q/µC | |
|---|---|---|---|---|---|---|---|
| 20 | 2 | 0.069 | −0.051 | 0.120 | 0.0086 | 1.38 | 38.7 |
| 100 | 2 | 0.108 | −0.147 | 0.255 | −0.0194 | 2.03 | 160.2 |
| 500 | 2 | 0.124 | −0.122 | 0.245 | 0.00124 | 2.21 | 282.2 |
| 20 | 5.5–6 | −0.092 | −0.125 | 0.034 | −0.109 | 1.23 | 123.2 |
| 100 | 5.5–6 | 0.031 | −0.157 | 0.188 | −0.063 | 1.52 | 96.0 |
| 500 | 5.5–6 | −0.001 | −0.243 | 0.242 | −0.122 | 2.81 | 265.2 |
| 20 | 8.5 | −0.038 | −0.160 | 0.122 | −0.099 | 0.45 | 41.8 |
| 100 | 8.5 | −0.066 | −0.266 | 0.200 | −0.127 | 0.95 | 160.3 |
| 500 | 8.5 | −0.048 | −0.182 | 0.134 | −0.115 | 1.18 | 166.4 |
| [Fc]/mM | |||||||
| 100 | 2 | 0.136 | 0.008 | 0.128 | 0.072 | 1.58 | 28.3 |
| 100 | 5.5–6 | 0.068 | −0.032 | 0.101 | 0.018 | 1.23 | 82.4 |
| 100 | 8.5 | 0.017 | −0.107 | 0.124 | −0.045 | 1.08 | 21.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moshrefi, R.; Stockmann, T.J. Electrodeless Synthesis of Low Dispersity Au Nanoparticles and Nanoclusters at an Immiscible Micro Water/Ionic Liquid Interface. Nanomaterials 2022, 12, 2748. https://doi.org/10.3390/nano12162748
Moshrefi R, Stockmann TJ. Electrodeless Synthesis of Low Dispersity Au Nanoparticles and Nanoclusters at an Immiscible Micro Water/Ionic Liquid Interface. Nanomaterials. 2022; 12(16):2748. https://doi.org/10.3390/nano12162748
Chicago/Turabian StyleMoshrefi, Reza, and Talia Jane Stockmann. 2022. "Electrodeless Synthesis of Low Dispersity Au Nanoparticles and Nanoclusters at an Immiscible Micro Water/Ionic Liquid Interface" Nanomaterials 12, no. 16: 2748. https://doi.org/10.3390/nano12162748
APA StyleMoshrefi, R., & Stockmann, T. J. (2022). Electrodeless Synthesis of Low Dispersity Au Nanoparticles and Nanoclusters at an Immiscible Micro Water/Ionic Liquid Interface. Nanomaterials, 12(16), 2748. https://doi.org/10.3390/nano12162748







