Photocatalytic Hydrogen Production from Aqueous Solutions of Glucose and Xylose over Layered Perovskite-like Oxides HCa2Nb3O10, H2La2Ti3O10 and Their Inorganic-Organic Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Initial Protonated Oxides
2.2. Synthesis of Inorganic−Organic Derivatives
2.3. Investigation of Photocatalytic Activity
2.3.1. Testing the Activity of Bare Samples (No Cocatalyst)
2.3.2. Testing the Activity of Pt-Loaded Samples
2.4. Instrumentation
2.4.1. XRD
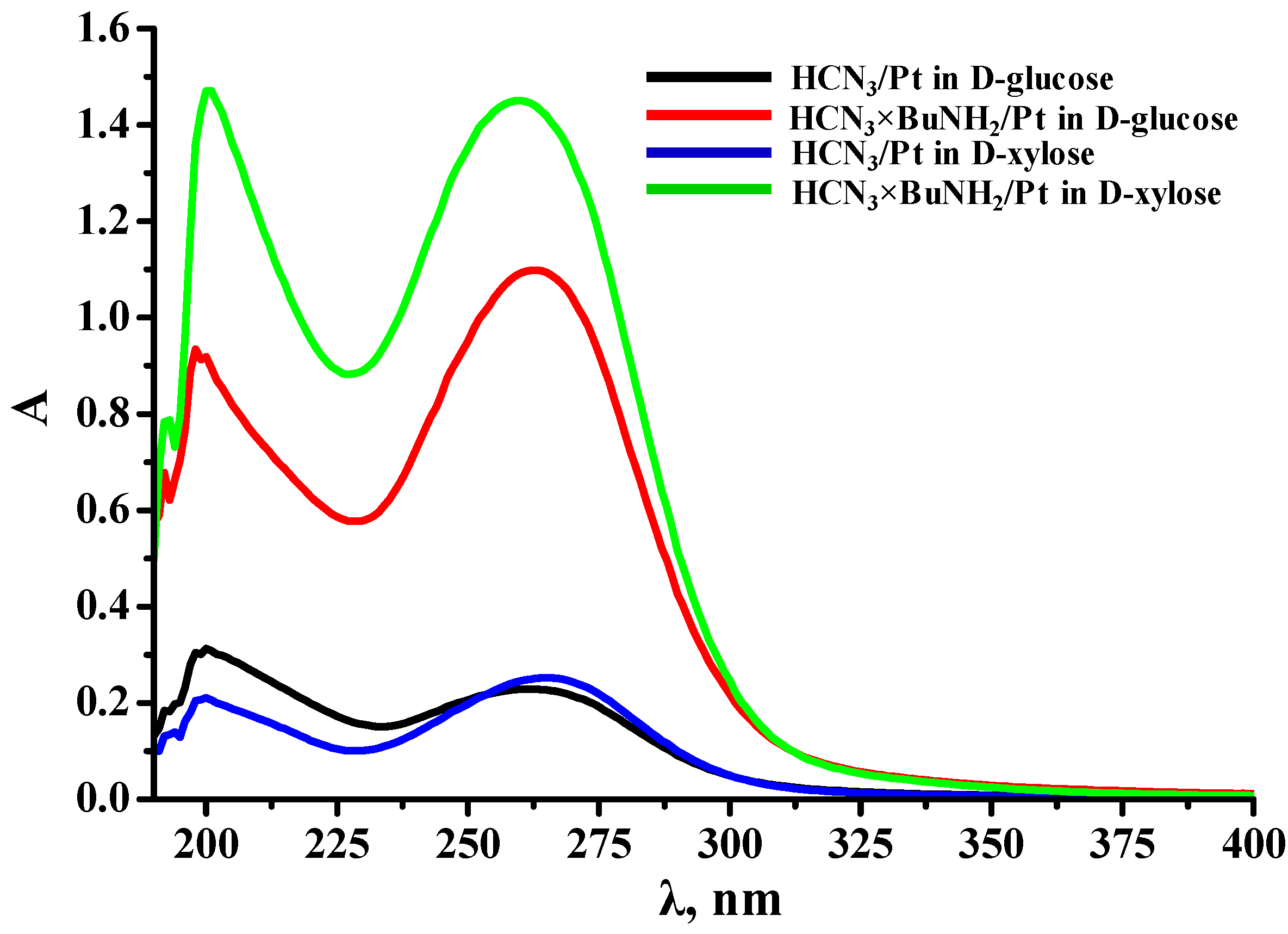
2.4.2. Spectrophotometry
2.4.3. pH Measurement
2.4.4. Other Methods of Analysis
3. Results and Discussion
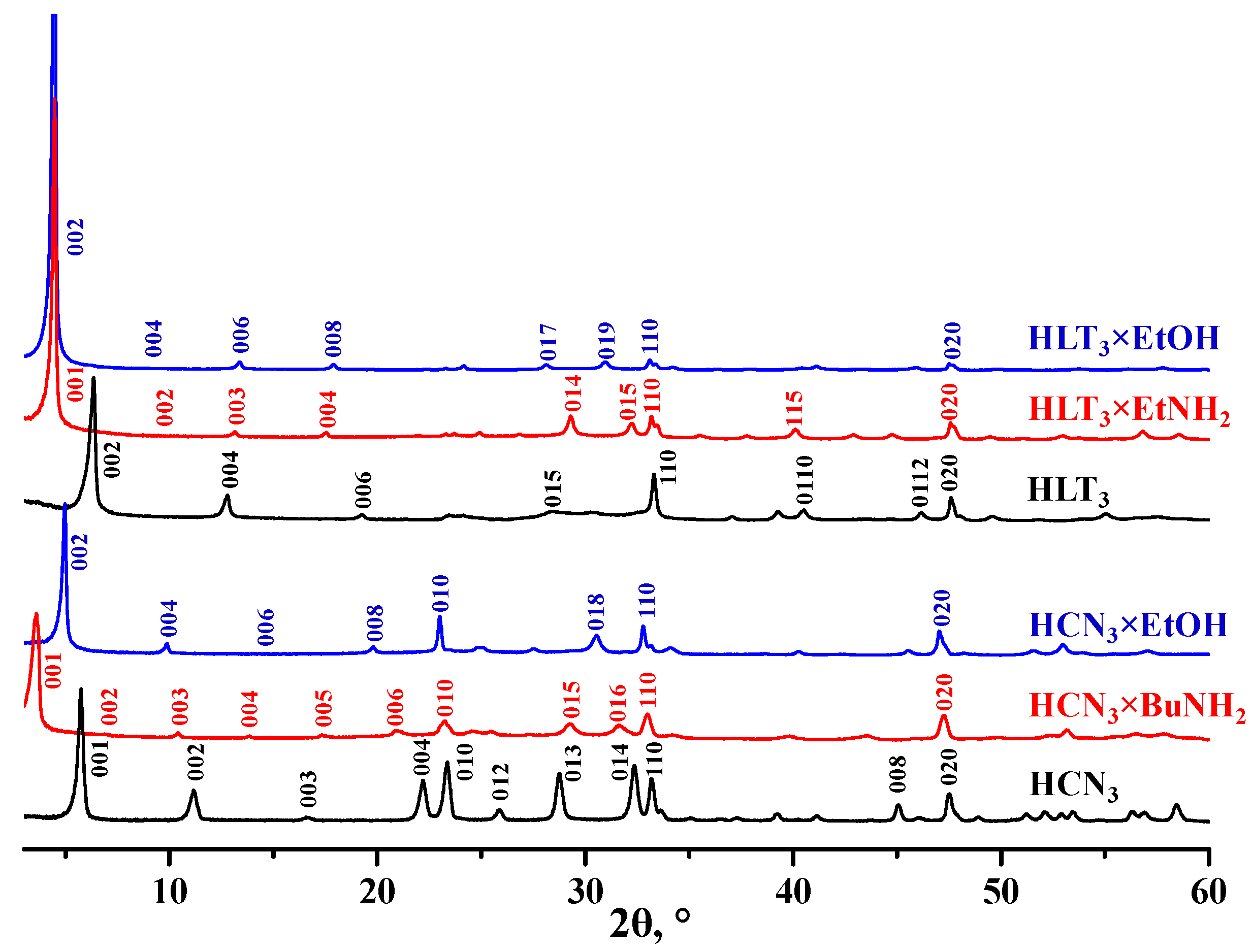
3.1. Characterization of the Protonated Oxides and Their Inorganic−Organic Derivatives
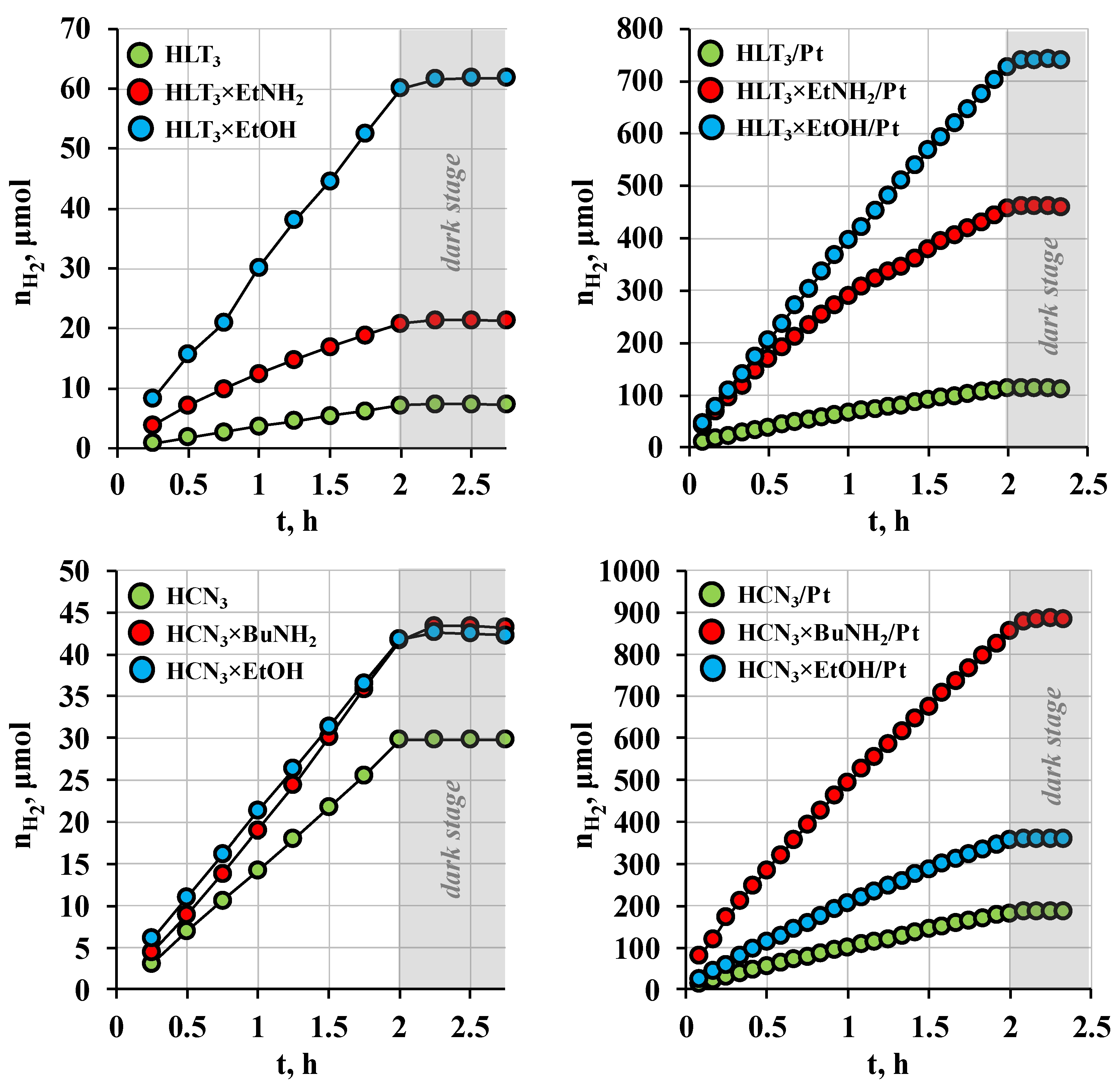
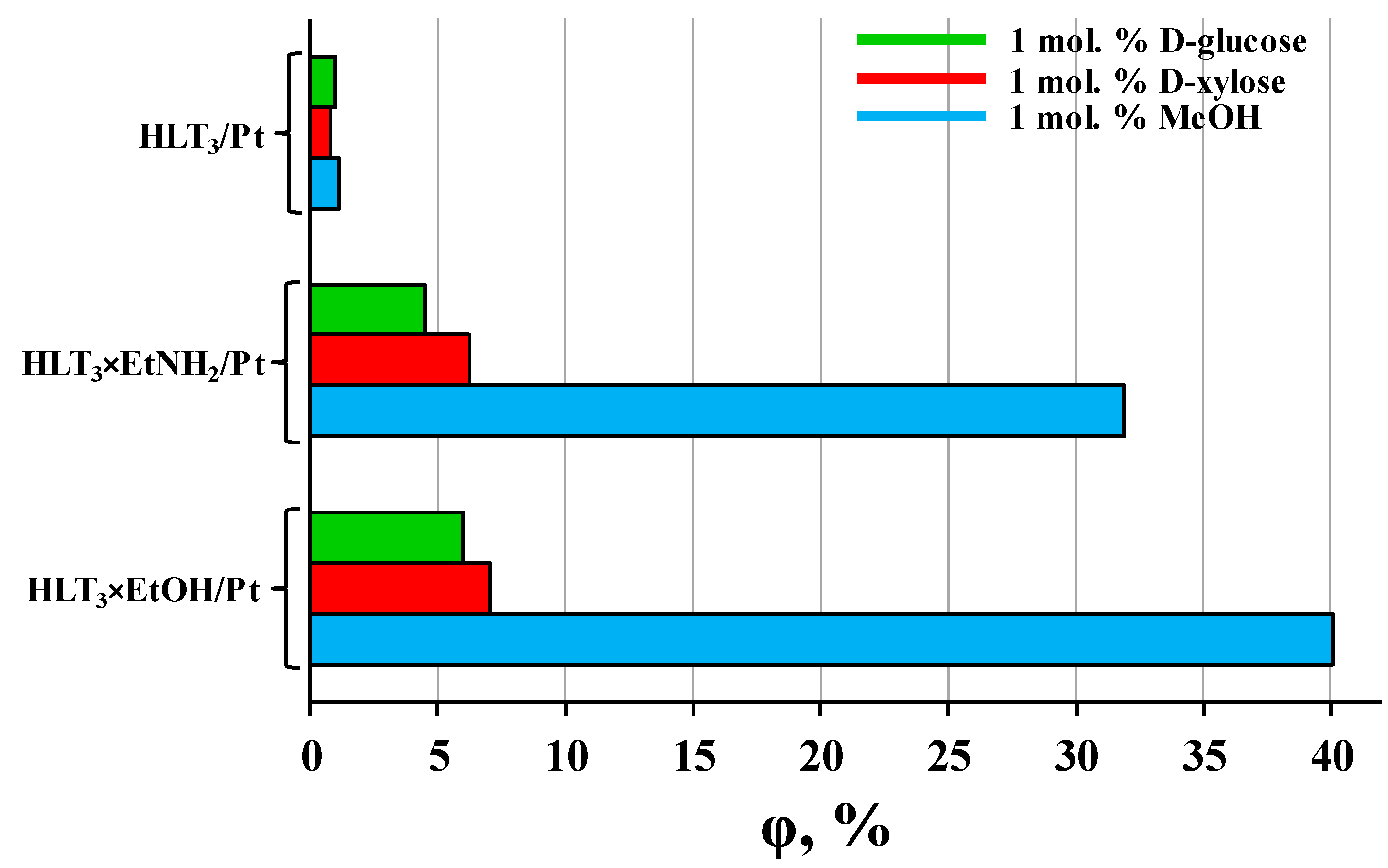
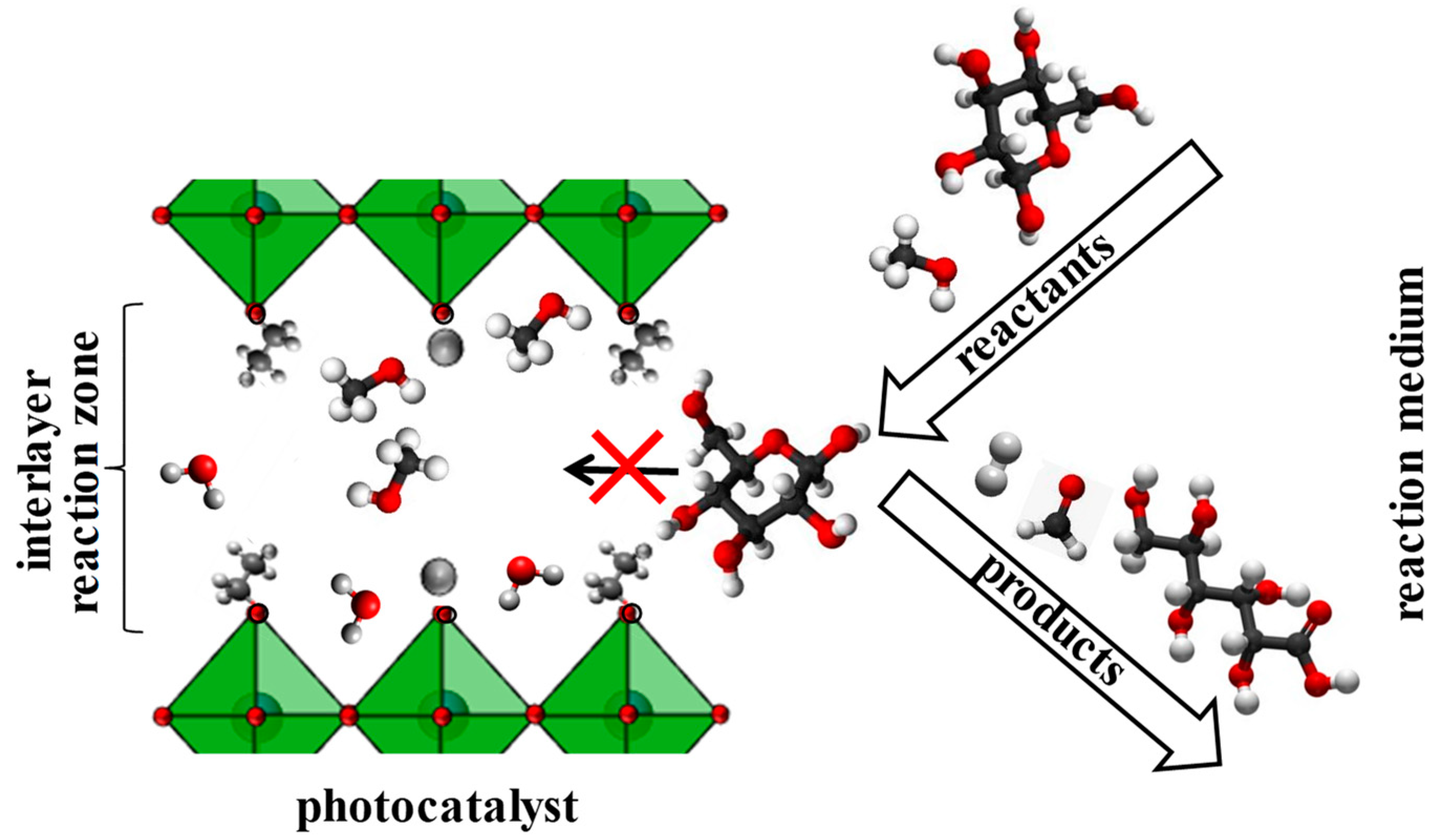
3.2. Photocatalytic Activity with Respect to Carbohydrates Reforming
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, K.A.; Yoo, S.; Shuler, E.W.; Sherman, B.D.; Lee, S.; Leem, G. Photocatalytic hydrogen evolution from biomass conversion. Nano Converg. 2021, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Omri, M.; Sauvage, F.; Golonu, S.; Wadouachi, A.; Pourceau, G. Photocatalyzed transformation of free carbohydrates. Catalysts 2018, 8, 672. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, X.; Li, Z.; Meng, X. Photocatalytic reforming for hydrogen evolution: A review. Catalysts 2020, 10, 335. [Google Scholar] [CrossRef]
- Kennedy, J.; Bahruji, H.; Bowker, M.; Davies, P.R.; Bouleghlimat, E.; Issarapanacheewin, S. Hydrogen generation by photocatalytic reforming of potential biofuels: Polyols, cyclic alcohols, and saccharides. J. Photochem. Photobiol. A Chem. 2018, 356, 451–456. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Zvereva, I.A. Photocatalytic activity of layered perovskite-like oxides in practically valuable chemical reactions. Russ. Chem. Rev. 2016, 85, 248–279. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Li, B.; Sun, H.; Wang, S. Engineered Graphitic Carbon Nitride-Based Photocatalysts for Visible-Light-Driven Water Splitting: A Review. Energy Fuels 2021, 35, 6504–6526. [Google Scholar] [CrossRef]
- Bellardita, M.; García-López, E.I.; Marcì, G.; Palmisano, L. Photocatalytic formation of H2 and value-added chemicals in aqueous glucose (Pt)-TiO2 suspension. Int. J. Hydrog. Energy 2016, 41, 5934–5947. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.; Peng, S.; Lu, G.; Li, S. Effect of epimerization of D-glucose on photocatalytic hydrogen generation over Pt/TiO2. Catal. Commun. 2012, 18, 21–25. [Google Scholar] [CrossRef]
- Bahadori, E.; Ramis, G.; Zanardo, D.; Menegazzo, F.; Signoretto, M.; Gazzoli, D.; Pietrogiacomi, D.; Michele, A.D. Photoreforming of Glucose over CuO/TiO2. Catalysts 2020, 10, 477. [Google Scholar] [CrossRef]
- Bellardita, M.; García-López, E.I.; Marcì, G.; Nasillo, G.; Palmisano, L. Photocatalytic Solar Light H2 Production by Aqueous Glucose Reforming. Eur. J. Inorg. Chem. 2018, 2018, 4522–4532. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Murcia, J.J.; Rizzo, L.; Ventre, G.; Pepe, G.; Campiglia, P.; Hidalgo, M.C.; Navío, J.A.; Sannino, D. Photocatalytic hydrogen production from degradation of glucose over fluorinated and platinized TiO2 catalysts. J. Catal. 2016, 339, 47–56. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Sannino, D.; Rizzo, L.; Ciambelli, P. Production of hydrogen from glucose by LaFeO3 based photocatalytic process during water treatment. Int. J. Hydrog. Energy 2016, 41, 959–966. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Sannino, D.; Rizzo, L.; Galluzzi, A.; Polichetti, M.; Pepe, G.; Campiglia, P. Hydrogen production from glucose degradation in water and wastewater treated by Ru-LaFeO3/Fe2O3 magnetic particles photocatalysis and heterogeneous photo-Fenton. Int. J. Hydrog. Energy 2018, 43, 2184–2196. [Google Scholar] [CrossRef]
- Zhao, H.; Li, C.F.; Yong, X.; Kumar, P.; Palma, B.; Hu, Z.Y.; van Tendeloo, G.; Siahrostami, S.; Larter, S.; Zheng, D.; et al. Coproduction of hydrogen and lactic acid from glucose photocatalysis on band-engineered Zn1-xCdxS homojunction. iScience 2021, 24, 102109. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Ming, J.; Liu, M.; Fang, P. Hydrogen generation by photocatalytic reforming of glucose with heterostructured CdS/MoS2 composites under visible light irradiation. Int. J. Hydrog. Energy 2017, 42, 16968–16978. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, X.; Liu, J.; Fu, X.; Yang, Y.; Han, H.; Fan, Y.; Zhang, S.; Meng, S.; Chen, S. Construction of NiPx/MoS2/NiS/CdS composite to promote photocatalytic H2 production from glucose solution. J. Am. Ceram. Soc. 2021, 104, 5307–5316. [Google Scholar] [CrossRef]
- Madriz, L.; Tatá, J.; Carvajal, D.; Núñez, O.; Scharifker, B.R.; Mostany, J.; Borrás, C.; Cabrerizo, F.M.; Vargas, R. Photocatalysis and photoelectrochemical glucose oxidation on Bi2WO6: Conditions for the concomitant H2 production. Renew. Energy 2020, 152, 974–983. [Google Scholar] [CrossRef]
- Speltini, A.; Scalabrini, A.; Maraschi, F.; Sturini, M.; Pisanu, A.; Malavasi, L.; Profumo, A. Improved photocatalytic H2 production assisted by aqueous glucose biomass by oxidized g-C3N4. Int. J. Hydrog. Energy 2018, 43, 14925–14933. [Google Scholar] [CrossRef]
- Bai, X.; Hou, Q.; Qian, H.; Nie, Y.; Xia, T.; Lai, R.; Yu, G.; Laiq Ur Rehman, M.; Xie, H.; Ju, M. Selective oxidation of glucose to gluconic acid and glucaric acid with chlorin e6 modified carbon nitride as metal-free photocatalyst. Appl. Catal. B Environ. 2022, 303, 120895. [Google Scholar] [CrossRef]
- Speltini, A.; Romani, L.; Dondi, D.; Malavasi, L.; Profumo, A. Carbon nitride-perovskite composites: Evaluation and optimization of photocatalytic hydrogen evolution in saccharides aqueous solution. Catalysts 2020, 10, 1259. [Google Scholar] [CrossRef]
- Kawai, T.; Sakata, T. Conversion of carbohydrate into hydrogen fuel by a photocatalytic process. Nature 1980, 286, 474–476. [Google Scholar] [CrossRef]
- Kurenkova, A.Y.; Markovskaya, D.V.; Gerasimov, E.Y.; Prosvirin, I.P.; Cherepanova, S.V.; Kozlova, E.A. New insights into the mechanism of photocatalytic hydrogen evolution from aqueous solutions of saccharides over CdS-based photocatalysts under visible light. Int. J. Hydrog. Energy 2020, 45, 30165–30177. [Google Scholar] [CrossRef]
- Kondarides, D.I.; Patsoura, A.; Verykios, X.E. Anaerobic photocatalytic oxidation of carbohydrates in aqueous Pt/TiO2 Suspensions with simultaneous production of hydrogen. J. Adv. Oxid. Technol. 2010, 13, 116–123. [Google Scholar] [CrossRef]
- Beasley, C.; Gnanamani, M.K.; Qian, D.; Hopps, S.D. Photocatalytic Reforming of Sucrose and Dextrose for Hydrogen Production on Pd/TiO2. ChemistrySelect 2021, 6, 6512–6524. [Google Scholar] [CrossRef]
- Caravaca, A.; Jones, W.; Hardacre, C.; Bowker, M. H2 production by the photocatalytic reforming of cellulose and raw biomass using Ni, Pd, Pt and Au on titania. Proc. R. Soc. Math. Phys. Eng. Sci. 2016, 472, 20160054. [Google Scholar] [CrossRef]
- Chang, C.; Skillen, N.; Nagarajan, S.; Ralphs, K.; Irvine, J.T.S.; Lawton, L.; Robertson, P.K.J. Using cellulose polymorphs for enhanced hydrogen production from photocatalytic reforming. Sustain. Energy Fuels 2019, 3, 1971–1975. [Google Scholar] [CrossRef]
- Kuehnel, M.F.; Reisner, E. Solar Hydrogen Generation from Lignocellulose. Angew. Chem. Int. Ed. 2018, 57, 3290–3296. [Google Scholar] [CrossRef]
- Ramis, G.; Bahadori, E.; Rossetti, I. Design of efficient photocatalytic processes for the production of hydrogen from biomass derived substrates. Int. J. Hydrog. Energy 2020, 46, 12105–12116. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, G.; Xu, X. One-pot photoreforming of cellulosic biomass waste to hydrogen by merging photocatalysis with acid hydrolysis. Appl. Catal. A Gen. 2018, 563, 73–79. [Google Scholar] [CrossRef]
- Schaak, R.E.; Mallouk, T.E. Perovskites by Design: A Toolbox of Solid-State Reactions. Chem. Mater. 2002, 14, 1455–1471. [Google Scholar] [CrossRef]
- Uppuluri, R.; Sen Gupta, A.; Rosas, A.S.; Mallouk, T.E. Soft chemistry of ion-exchangeable layered metal oxides. Chem. Soc. Rev. 2018, 47, 2401–2430. [Google Scholar] [CrossRef] [PubMed]
- Tani, S.; Komori, Y.; Hayashi, S.; Sugahara, Y. Local environments and dynamics of hydrogen atoms in protonated forms of ion-exchangeable layered perovskites estimated by solid-state 1H NMR. J. Solid State Chem. 2006, 179, 3357–3364. [Google Scholar] [CrossRef]
- Silyukov, O.; Chislov, M.; Burovikhina, A.; Utkina, T.; Zvereva, I. Thermogravimetry study of ion exchange and hydration in layered oxide materials. J. Therm. Anal. Calorim. 2012, 110, 187–192. [Google Scholar] [CrossRef]
- Silyukov, O.I.; Kurnosenko, S.A.; Zvereva, I.A. Intercalation of Methylamine into the Protonated Forms of Layered Perovskite-Like Oxides HLnTiO4 (Ln = La and Nd). Glas. Phys. Chem. 2018, 44, 428–432. [Google Scholar] [CrossRef]
- Kurnosenko, S.A.; Silyukov, O.I.; Mazur, A.S.; Zvereva, I.A. Synthesis and thermal stability of new inorganic-organic perovskite-like hybrids based on layered titanates HLnTiO4 (Ln = La, Nd). Ceram. Int. 2020, 46, 5058–5068. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Lushpinskaya, I.P.; Kurnosenko, S.A.; Silyukov, O.I.; Zvereva, I.A. Identification of Intercalates and Grafted Organic Derivatives of H2La2Ti3O10 by Multinuclear NMR. Russ. J. Gen. Chem. 2020, 90, 760–761. [Google Scholar] [CrossRef]
- Kurnosenko, S.A.; Silyukov, O.I.; Minich, I.A.; Zvereva, I.A. Exfoliation of Methylamine and n-Butylamine Derivatives of Layered Perovskite-Like Oxides HLnTiO4 and H2Ln2Ti3O10 (Ln = La, Nd) into Nanolayers. Glas. Phys. Chem. 2021, 47, 372–381. [Google Scholar] [CrossRef]
- Silyukov, O.I.; Minich, I.A.; Zvereva, I.A. Synthesis of Protonated Derivatives of Layered Perovskite-Like Bismuth Titanates. Glas. Phys. Chem. 2018, 44, 115–119. [Google Scholar] [CrossRef]
- Minich, I.A.; Silyukov, O.I.; Gak, V.V.; Borisov, E.V.; Zvereva, I.A. Synthesis of Organic–Inorganic Hybrids Based on Perovskite-like Bismuth Titanate H2K0.5Bi2.5Ti4O13·H2O and n-Alkylamines. ACS Omega 2020, 8, 8158. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Silyukov, O.I.; Lushpinskaia, I.P.; Kurnosenko, S.A.; Mazur, A.S.; Shenderovich, I.G.; Zvereva, I.A. NMR Study of Intercalates and Grafted Organic Derivatives of H2La2Ti3O10. Molecules 2020, 25, 5229. [Google Scholar] [CrossRef]
- Silyukov, O.I.; Kurnosenko, S.A.; Minich, I.A.; Rodionov, I.A.; Zvereva, I.A. Protonated Forms of Layered Perovskite-Like Titanate NaNdTiO4: Neutron and X-ray Diffraction Structural Analysis. Solids 2021, 2, 265–277. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Silyukov, O.I.; Andronova, E.A.; Nefedov, D.Y.; Antonenko, A.O.; Missyul, A.; Kurnosenko, S.A.; Zvereva, I.A. 1H NMR Study of the HCa2Nb3O10 Photocatalyst with Different Hydration Levels. Molecules 2021, 26, 5943. [Google Scholar] [CrossRef] [PubMed]
- Rodionov, I.A.; Silyukov, O.I.; Utkina, T.D.; Chislov, M.V.; Sokolova, Y.P.; Zvereva, I.A. Photocatalytic properties and hydration of perovskite-type layered titanates A2Ln2Ti3O10 (A = Li, Na, K; Ln = La, Nd). Russ. J. Gen. Chem. 2012, 82, 1191–1196. [Google Scholar] [CrossRef]
- Takata, T.; Furumi, Y.; Shinohara, K.; Tanaka, A.; Hara, M.; Kondo, J.N.; Domen, K. Photocatalytic Decomposition of Water on Spontaneously Hydrated Layered Perovskites. Chem. Mater. 1997, 9, 1063–1064. [Google Scholar] [CrossRef]
- Zou, Z.; Ye, J.; Arakawa, H. Substitution effects of In3+ by Fe3+ on photocatalytic and structural properties of Bi2InNbO7 photocatalysts. J. Mol. Catal. 2001, 168, 289–297. [Google Scholar] [CrossRef]
- Reddy, V.; Hwang, D.; Lee, J. Effect of Zr substitution for Ti in KLaTiO4 for photocatalytic water splitting. Catal. Lett. 2003, 90, 39–44. [Google Scholar] [CrossRef]
- Kumar, V.; Govind; Uma, S. Investigation of cation (Sn2+) and anion (N3-) substitution in favor of visible light photocatalytic activity in the layered perovskite K2La2Ti3O10. J. Hazard. Mater. 2011, 189, 502–508. [Google Scholar] [CrossRef]
- Zhou, Y.; Wen, T.; Guo, Y.; Yang, B.; Wang, Y. Controllable doping of nitrogen and tetravalent niobium affords yellow and black calcium niobate nanosheets for enhanced photocatalytic hydrogen evolution. RSC Adv. 2016, 6, 64930–64936. [Google Scholar] [CrossRef]
- Kawashima, K.; Hojamberdiev, M.; Chen, S.; Yubuta, K.; Wagata, H.; Domen, K.; Teshima, K. Understanding the effect of partial N3−-to-O2− substitution and H+-to-K+ exchange on photocatalytic water reduction activity of Ruddlesden–Popper layered perovskite KLaTiO4. Mol. Catal. 2017, 432, 250–258. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Wei, Y.; Li, Y.; Lin, J.; Wu, J. Fabrication and photocatalytic property of Pt-intercalated layered perovskite niobates H1−xLaNb2−xMoxO7 (x = 0–0.15). J. Hazard. Mater. 2009, 166, 103–108. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Wei, Y.; Huang, M.; Wu, J. Photocatalytic property of partially substituted Pt-intercalated layered perovskite, ASr2TaxNb3−xO10 (A = K, H; x = 0, 1, 1.5, 2 and 3). Sol. Energy Mater. Sol. Cells 2011, 95, 1019–1027. [Google Scholar] [CrossRef]
- Oshima, T.; Wang, Y.; Lu, D.; Yokoi, T.; Maeda, K. Photocatalytic overall water splitting on Pt nanocluster-intercalated, restacked KCa2Nb3O10 nanosheets: The promotional effect of co-existing ions. Nanoscale Adv. 2019, 1, 189–194. [Google Scholar] [CrossRef]
- Cui, W.; Qi, Y.; Liu, L.; Rana, D.; Hu, J.; Liang, Y. Synthesis of PbS–K2La2Ti3O10 composite and its photocatalytic activity for hydrogen production. Prog. Nat. Sci. Mater. Int. 2012, 22, 120–125. [Google Scholar] [CrossRef][Green Version]
- Cui, W.; Liu, L.; Ma, S.; Liang, Y.; Zhang, Z. CdS-sensitized K2La2Ti3O10 composite: A new photocatalyst for hydrogen evolution under visible light irradiation. Catal. Today 2013, 207, 44–49. [Google Scholar] [CrossRef]
- Cui, W.; Guo, D.; Liu, L.; Hu, J.; Rana, D.; Liang, Y. Preparation of ZnIn2S4/K2La2Ti3O10 composites and their photocatalytic H2 evolution from aqueous Na2S/Na2SO3 under visible light irradiation. Catal. Commun. 2014, 48, 55–59. [Google Scholar] [CrossRef]
- Saito, K.; Kozeni, M.; Sohmiya, M.; Komaguchi, K.; Ogawa, M.; Sugahara, Y.; Ide, Y. Unprecedentedly enhanced solar photocatalytic activity of a layered titanate simply integrated with TiO2 nanoparticles. Phys. Chem. Chem. Phys. 2016, 18, 30920–30925. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Y.; Lv, C.; Zhang, C.; Jin, X.; Meng, Q.; Chen, G. Construction of 2D-composite HCa2Nb3O10 /CaNb2O6 heterostructured photocatalysts with enhanced hydrogen production performance. New J. Chem. 2018, 42, 681–687. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Zhang, L.; Wong, K.H.; Chen, Z.; Yu, J.C.; Zhao, J.; Hu, C.; Chan, C.Y.; Wong, P.K. AgBr-Ag-Bi2WO6 nanojunction system: A novel and efficient photocatalyst with double visible-light active components. Appl. Catal. A Gen. 2009, 363, 221–229. [Google Scholar] [CrossRef]
- Kim, H.G.; Jeong, E.D.; Borse, P.H.; Jeon, S.; Yong, K.; Lee, J.S.; Li, W.; Oh, S.H. Photocatalytic Ohmic layered nanocomposite for efficient utilization of visible light photons. Appl. Phys. Lett. 2006, 89, 2012–2015. [Google Scholar] [CrossRef]
- Kim, H.G.; Borse, P.H.; Choi, W.; Lee, J.S. Photocatalytic Nanodiodes for Visible-Light Photocatalysis. Angew. Chem. 2005, 117, 4661–4665. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Xiong, Z.; Tang, H.; Jiang, C. Fabrication of flower-like direct Z-scheme β-Bi2O3/g-C3N4 photocatalyst with enhanced visible light photoactivity for Rhodamine B degradation. Appl. Surf. Sci. 2018, 436, 162–171. [Google Scholar] [CrossRef]
- Youngblood, W.J.; Anna Lee, S.H.; Maeda, K.; Mallouk, T.E. Visible light water splitting using dye-sensitized oxide semiconductors. Acc. Chem. Res. 2009, 42, 1966–1973. [Google Scholar] [CrossRef]
- Maeda, K.; Mallouk, T.E. Two-Dimensional Metal Oxide Nanosheets as Building Blocks for Artificial Photosynthetic Assemblies. Bull. Chem. Soc. Jpn. 2018, 92, 38–54. [Google Scholar] [CrossRef]
- Sanchez, P.G.-R. and C. Functional Hybrid Materials; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; ISBN 3527303596. [Google Scholar]
- Kickelbick, G. Hybrid Materials: Synthesis, Characterization, and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; ISBN 9783527312993. [Google Scholar]
- Mir, S.H.; Nagahara, L.A.; Thundat, T.; Mokarian-Tabari, P.; Furukawa, H.; Khosla, A. Review—Organic-Inorganic Hybrid Functional Materials: An Integrated Platform for Applied Technologies. J. Electrochem. Soc. 2018, 165, B3137–B3156. [Google Scholar] [CrossRef]
- Constantino, V.R.L.; Barbosa, C.A.S.; Bizeto, M.A.; Dias, P.M. Intercalation compounds involving inorganic layered structures. An. Acad. Bras. Cienc. 2000, 72, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.J.; Johnson, J.W.; Lewandowski, J. Intercalation of the layered solid acid HCa2Nb3O10 by organic amines. Mater. Res. Bull. 1987, 22, 45–51. [Google Scholar] [CrossRef]
- Minich, I.A.; Silyukov, O.I.; Kurnosenko, S.A.; Gak, V.V.; Kalganov, V.D.; Kolonitskiy, P.D.; Zvereva, I.A. Physical–Chemical Exfoliation of n-Alkylamine Derivatives of Layered Perovskite-like Oxide H2K0.5Bi2.5Ti4O13 into Nanosheets. Nanomaterials 2021, 11, 2708. [Google Scholar] [CrossRef]
- Tahara, S.; Sugahara, Y. Interlayer Surface Modification of the Protonated Triple-Layered Perovskite HCa2Nb3O10·xH2O with n-Alcohols. Langmuir 2003, 19, 9473–9478. [Google Scholar] [CrossRef]
- Tahara, S.; Ichikawa, T.; Kajiwara, G.; Sugahara, Y. Reactivity of the Ruddlesden−Popper Phase H2La2Ti3O10 with Organic Compounds: Intercalation and Grafting Reactions. Chem. Mater. 2007, 19, 2352–2358. [Google Scholar] [CrossRef]
- Kurnosenko, S.A.; Voytovich, V.V.; Silyukov, O.I.; Minich, I.A.; Malygina, E.N.; Zvereva, I.A. Inorganic-organic derivatives of layered perovskite-like titanates HLnTiO4 (Ln = La, Nd) with n-amines and n-alcohols: Synthesis, thermal, vacuum and hydrolytic stability. Ceram. Int. 2021, 48, 7240–7252. [Google Scholar] [CrossRef]
- Wang, C.; Tang, K.; Wang, D.; Liu, Z.; Wang, L.; Zhu, Y.; Qian, Y. A new carbon intercalated compound of Dion–Jacobson phase HLaNb2O7. J. Mater. Chem. 2012, 22, 11086. [Google Scholar] [CrossRef]
- Aznar, A.J.; Sanz, J.; Ruiz-Hitzky, E. Mechanism of the grafting of organosilanes on mineral surfaces. IV. Phenylderivatives of sepiolite and poly(organosiloxanes). Colloid Polym. Sci. 1992, 270, 165–176. [Google Scholar] [CrossRef]
- Shimada, A.; Yoneyama, Y.; Tahara, S.; Mutin, P.H.; Sugahara, Y. Interlayer surface modification of the protonated ion-exchangeable layered perovskite HLaNb2O7xH2O with organophosphonic acids. Chem. Mater. 2009, 21, 4155–4162. [Google Scholar] [CrossRef]
- Machida, M.; Mitsuyama, T.; Ikeue, K.; Matsushima, S.; Arai, M. Photocatalytic property and electronic structure of triple-layered perovskite tantalates, MCa2Ta3O10 (M = Cs, Na, H, and C6H13NH3). J. Phys. Chem. B 2005, 109, 7801–7806. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Maksimova, E.A.; Pozhidaev, A.Y.; Kurnosenko, S.A.; Silyukov, O.I.; Zvereva, I.A. Layered Titanate H2Nd2Ti3O10 Intercalated With n-Butylamine: A New Highly Efficient Hybrid Photocatalyst for Hydrogen Production From Aqueous Solutions of Alcohols. Front. Chem. 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Voytovich, V.V.; Kurnosenko, S.A.; Silyukov, O.I.; Rodionov, I.A.; Minich, I.A.; Zvereva, I.A. Study of n-alkylamine Intercalated Layered Perovskite-Like Niobates HCa2Nb3O10 as Photocatalysts for Hydrogen Production From an Aqueous Solution of Methanol. Front. Chem. 2020, 8, 300. [Google Scholar] [CrossRef]
- Voytovich, V.V.; Kurnosenko, S.A.; Silyukov, O.I.; Rodionov, I.A.; Bugrov, A.N.; Minich, I.A.; Malygina, E.N.; Zvereva, I.A. Synthesis of n-Alkoxy Derivatives of Layered Perovskite-Like Niobate HCa2Nb3O10 and Study of Their Photocatalytic Activity for Hydrogen Production from an Aqueous Solution of Methanol. Catalysts 2021, 11, 897. [Google Scholar] [CrossRef]
- Kurnosenko, S.A.; Voytovich, V.V.; Silyukov, O.I.; Rodionov, I.A.; Kirichenko, S.O.; Minich, I.A.; Malygina, E.N.; Khramova, A.D.; Zvereva, I.A. Photocatalytic Activity of n-Alkylamine and n-Alkoxy Derivatives of Layered Perovskite-like Titanates H2Ln2Ti3O10 (Ln = La, Nd) in the Reaction of Hydrogen Production from an Aqueous Solution of Methanol. Catalysts 2021, 11, 1279. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Al-Azri, Z.H.N.; Chen, W.T.; Chan, A.; Jovic, V.; Ina, T.; Idriss, H.; Waterhouse, G.I.N. The roles of metal co-catalysts and reaction media in photocatalytic hydrogen production: Performance evaluation of M/TiO2 photocatalysts (M = Pd, Pt, Au) in different alcohol-water mixtures. J. Catal. 2015, 329, 355–367. [Google Scholar] [CrossRef]
- Zhao, G.; Busser, G.W.; Froese, C.; Hu, B.; Bonke, S.A.; Schnegg, A.; Ai, Y.; Wei, D.; Wang, X.; Peng, B.; et al. Anaerobic Alcohol Conversion to Carbonyl Compounds over Nanoscaled Rh-Doped SrTiO3 under Visible Light. J. Phys. Chem. Lett. 2019, 10, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Hu, Y.; Li, B.; Zou, Y.; Li, S.; Wilma Busser, G.; Wang, X.; Zhao, G.; Muhler, M. State-of-the-art progress in the selective photo-oxidation of alcohols. J. Energy Chem. 2021, 62, 338–350. [Google Scholar] [CrossRef]
- Li, B.; Hong, J.; Ai, Y.; Hu, Y.; Shen, Z.; Li, S.; Zou, Y.; Zhang, S.; Wang, X.; Zhao, G.; et al. Visible-near-infrared-light-driven selective oxidation of alcohols over nanostructured Cu doped SrTiO3 in water under mild condition. J. Catal. 2021, 399, 142–149. [Google Scholar] [CrossRef]
- Wads, I. Photodegradation of Carbohydrates. Part IV. Direct Photolysis of D-Glucose in Aqueous Solution. Acta Chem. Scand. 1962, 16, 487–494. [Google Scholar]
| Sample | Precursor | Organic Content in the Reaction Mixture (vol. %) | Temperature (°C) | Duration (d) |
|---|---|---|---|---|
| HCN3×BuNH2 | HCN3 | 90 | 25 | 1 |
| HCN3×EtOH | HCN3 | 96 | 100 | 7 |
| HLT3×EtNH2 | HLT3×MeNH2 | 70 | 25 | 1 |
| HLT3×EtOH | HLT3×BuNH2 | 96 | 180 | 7 |
| Sample | a (Å) | c (Å) | d (Å) | x | y | Eg (eV) | λmax (nm) | S (m2/g) |
|---|---|---|---|---|---|---|---|---|
| HCN3 | 3.82 | 16.0 | 16.0 | − | 1.50 | 3.50 | 354 | 7.6 |
| HCN3×BuNH2 | 3.86 | 25.4 | 25.4 | 1.00 | 0.45 | 3.62 | 343 | 5.0 |
| HCN3×EtOH | 3.86 | 35.8 | 17.9 | 0.90 | 0.45 | 3.50 | 354 | 3.9 |
| HLT3 | 3.79 | 27.2 | 13.6 | − | 0.15 | 3.44 | 360 | 3.2 |
| HLT3×EtNH2 | 3.82 | 20.2 | 20.2 | 0.70 | 0.35 | 3.39 | 366 | − * |
| HLT3×EtOH | 3.83 | 39.5 | 19.8 | 0.85 | 0.40 | 3.41 | 364 | − * |
| Sample | ω (μmol/h) | ϕ (%) | kPt | |
|---|---|---|---|---|
| D-glucose | HCN3 | 14 | 0.2 | − |
| HCN3/Pt | 95 | 1.6 | 7 | |
| HCN3/Pt(MeOH) | 140 | 2.4 | 10 | |
| HCN3×BuNH2 | 21 | 0.4 | − | |
| HCN3×BuNH2/Pt | 450 | 7.5 | 21 | |
| HCN3×BuNH2/Pt(MeOH) | 410 | 6.8 | 20 | |
| HCN3×EtOH | 20 | 0.3 | − | |
| HCN3×EtOH/Pt | 200 | 3.3 | 10 | |
| HCN3×EtOH/Pt(MeOH) | 170 | 2.8 | 9 | |
| HLT3 | 4 | 0.1 | − | |
| HLT3/Pt | 61 | 1.0 | 15 | |
| HLT3/Pt(MeOH) | 110 | 1.8 | 28 | |
| HLT3×EtNH2 | 10 | 0.2 | − | |
| HLT3×EtNH2/Pt | 270 | 4.5 | 27 | |
| HLT3×EtNH2/Pt(MeOH) | 240 | 4.0 | 24 | |
| HLT3×EtOH | 30 | 0.5 | − | |
| HLT3×EtOH/Pt | 360 | 6.0 | 12 | |
| HLT3×EtOH/Pt(MeOH) | 120 | 2.0 | 4 | |
| D-xylose | HCN3/Pt | 88 | 1.5 | − |
| HCN3×BuNH2/Pt | 530 | 8.8 | − | |
| HCN3×EtOH/Pt | 140 | 2.3 | − | |
| HLT3/Pt | 48 | 0.80 | − | |
| HLT3×EtNH2/Pt | 380 | 6.3 | − | |
| HLT3×EtOH/Pt | 420 | 7.0 | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurnosenko, S.A.; Voytovich, V.V.; Silyukov, O.I.; Rodionov, I.A.; Zvereva, I.A. Photocatalytic Hydrogen Production from Aqueous Solutions of Glucose and Xylose over Layered Perovskite-like Oxides HCa2Nb3O10, H2La2Ti3O10 and Their Inorganic-Organic Derivatives. Nanomaterials 2022, 12, 2717. https://doi.org/10.3390/nano12152717
Kurnosenko SA, Voytovich VV, Silyukov OI, Rodionov IA, Zvereva IA. Photocatalytic Hydrogen Production from Aqueous Solutions of Glucose and Xylose over Layered Perovskite-like Oxides HCa2Nb3O10, H2La2Ti3O10 and Their Inorganic-Organic Derivatives. Nanomaterials. 2022; 12(15):2717. https://doi.org/10.3390/nano12152717
Chicago/Turabian StyleKurnosenko, Sergei A., Vladimir V. Voytovich, Oleg I. Silyukov, Ivan A. Rodionov, and Irina A. Zvereva. 2022. "Photocatalytic Hydrogen Production from Aqueous Solutions of Glucose and Xylose over Layered Perovskite-like Oxides HCa2Nb3O10, H2La2Ti3O10 and Their Inorganic-Organic Derivatives" Nanomaterials 12, no. 15: 2717. https://doi.org/10.3390/nano12152717
APA StyleKurnosenko, S. A., Voytovich, V. V., Silyukov, O. I., Rodionov, I. A., & Zvereva, I. A. (2022). Photocatalytic Hydrogen Production from Aqueous Solutions of Glucose and Xylose over Layered Perovskite-like Oxides HCa2Nb3O10, H2La2Ti3O10 and Their Inorganic-Organic Derivatives. Nanomaterials, 12(15), 2717. https://doi.org/10.3390/nano12152717







