Gamma-Ray-Induced Structural Transformation of GQDs towards the Improvement of Their Optical Properties, Monitoring of Selected Toxic Compounds, and Photo-Induced Effects on Bacterial Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of Graphene Quantum Dots
2.3. Characterization of Graphene Quantum Dots
2.3.1. Atomic Force Microscopy (AFM)
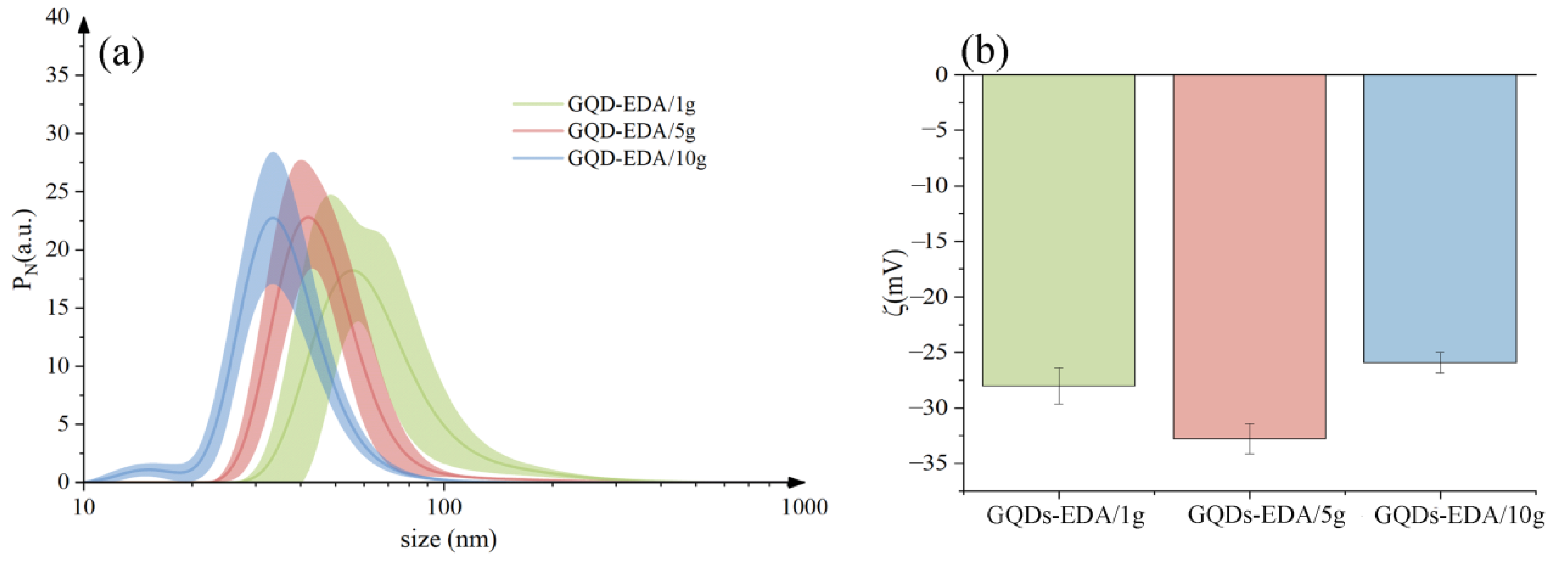
2.3.2. Dynamic Light Scattering and ξ Potential Measurement
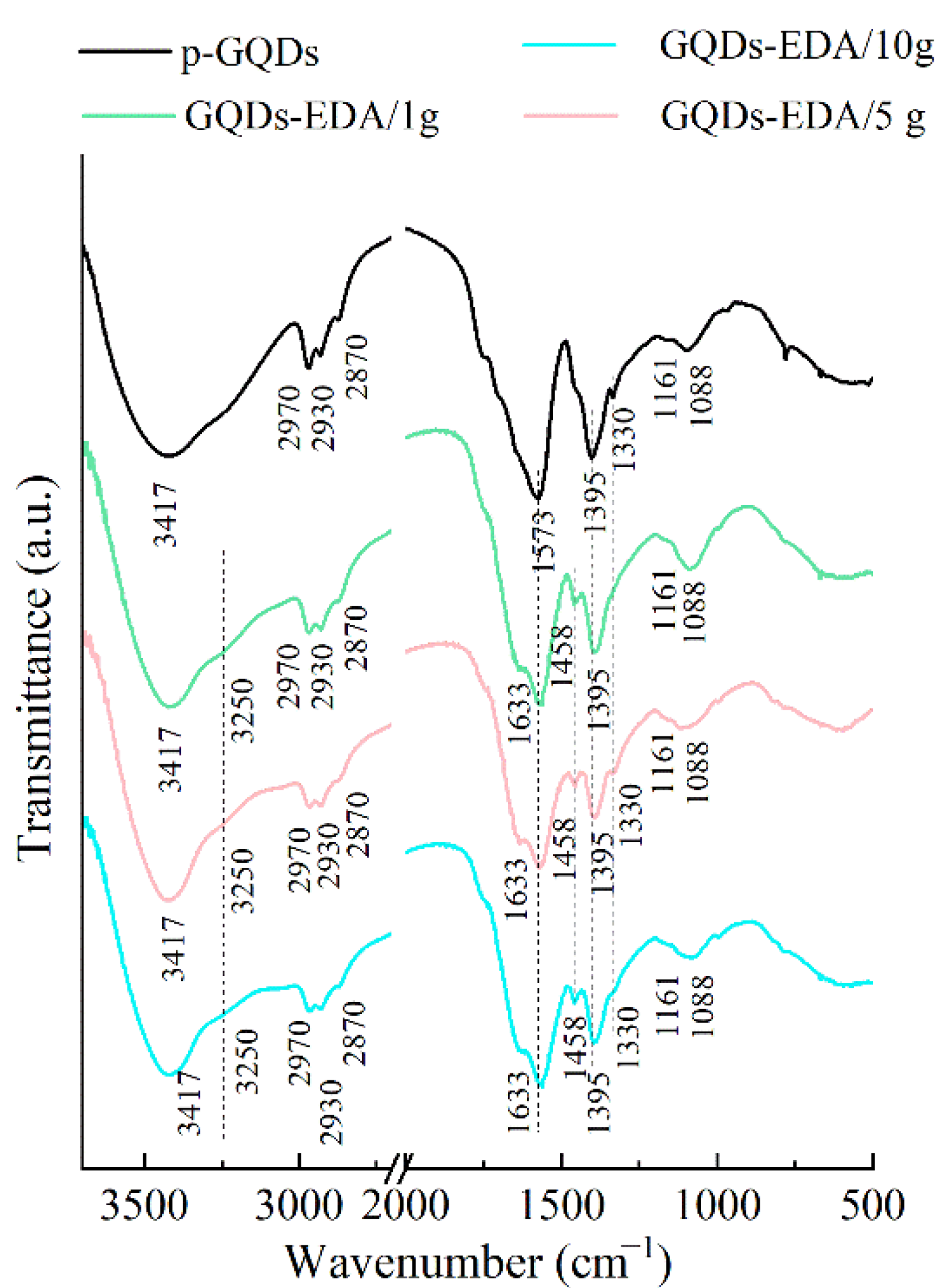
2.3.3. Infrared Spectroscopy with Fourier Transformation (FTIR)
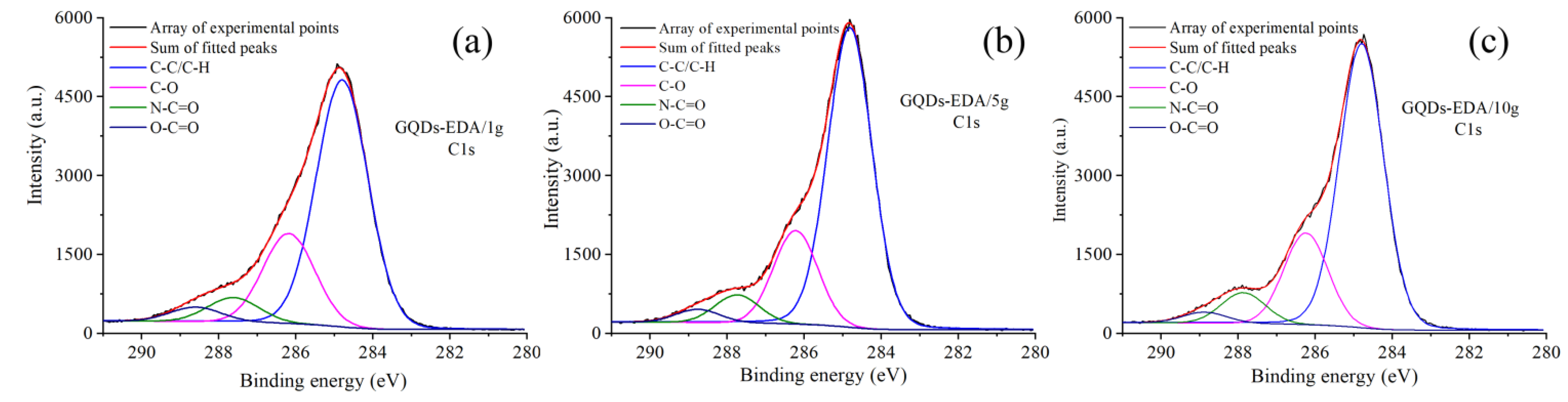
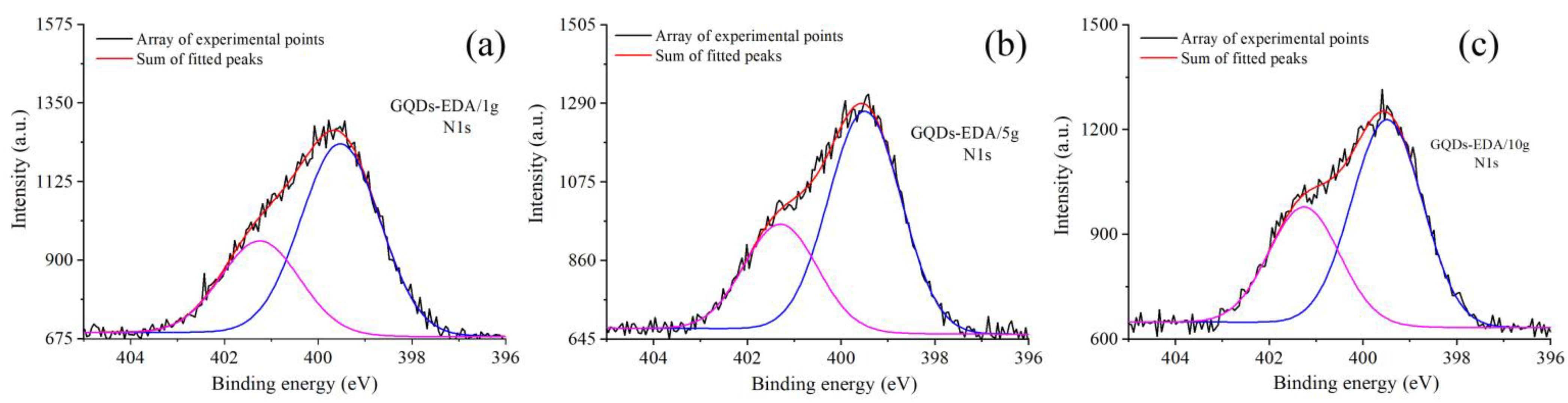
2.3.4. X-ray Photoelectron Spectroscopy (XPS)
2.3.5. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.3.6. UV-Vis Spectroscopy
2.3.7. Photoluminescence (PL) Spectroscopy
2.3.8. Photo-Induced Antibacterial Activity
2.4. Detection of Carbofuran and 3-Amino-1,2,4-triazole
2.4.1. Sample Preparation
2.4.2. Photoluminescence Measurement
3. Results and Discussion
3.1. Characterization of GQDs
3.1.1. Morphology Analysis of Gamma-Irradiated GQDs
3.1.2. Structural Characterization of GQDs
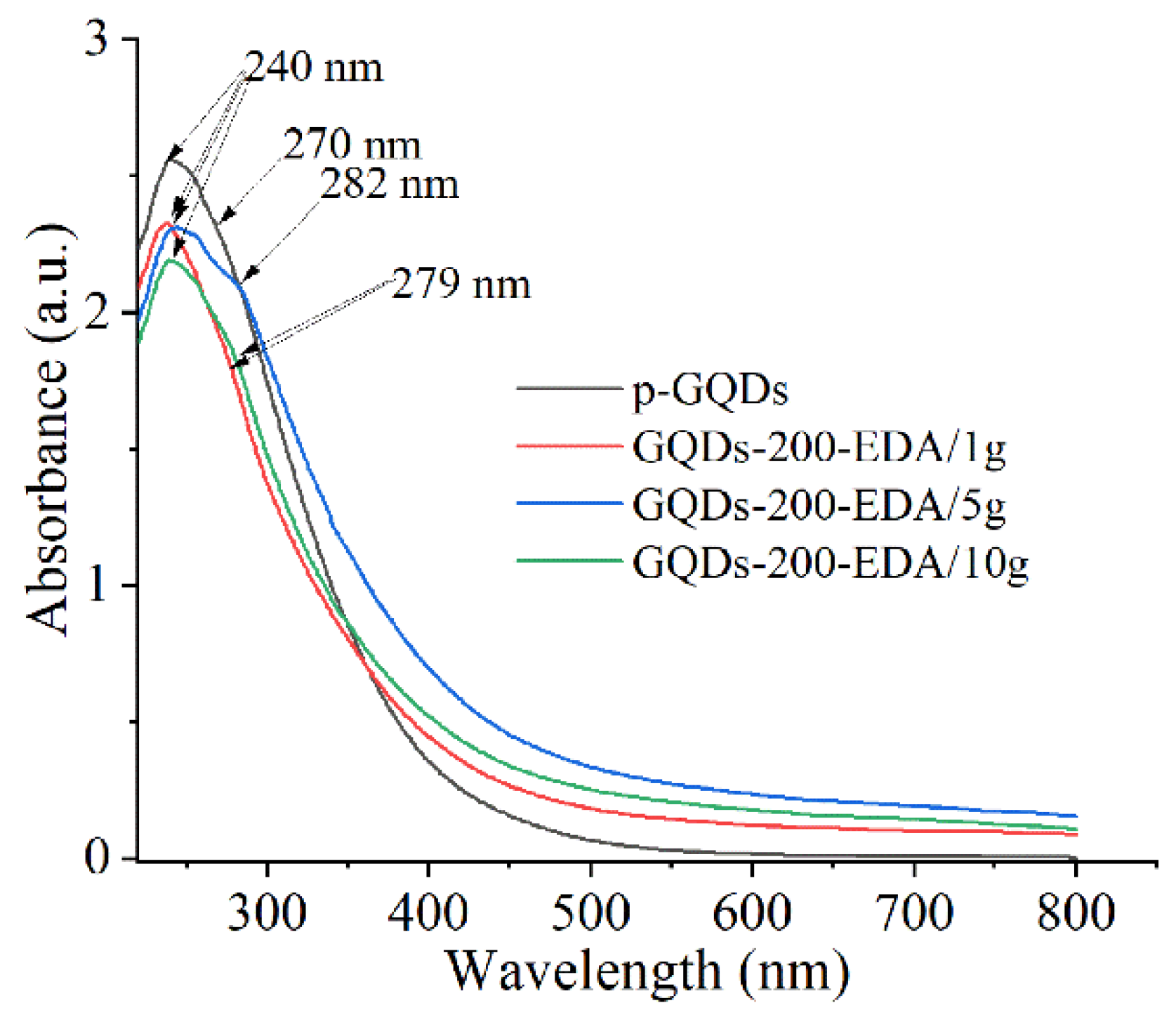
3.1.3. Optical Properties of GQDs
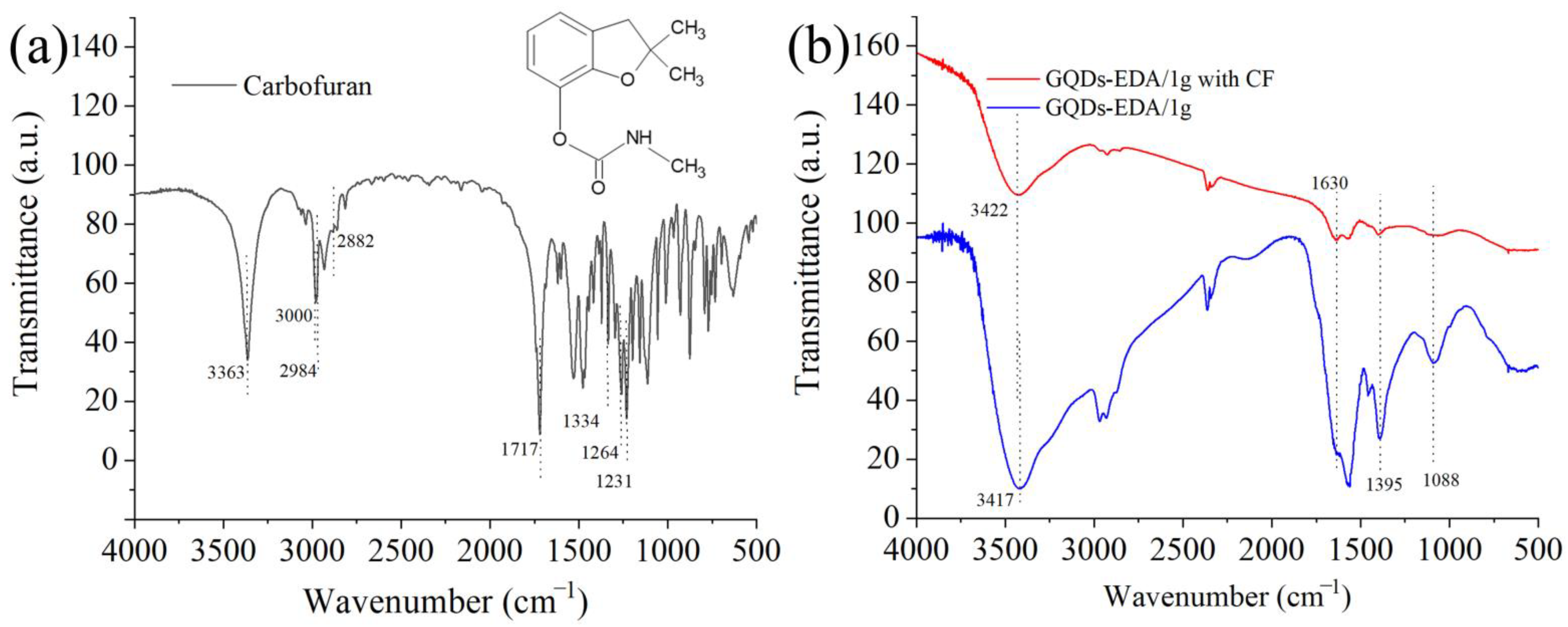
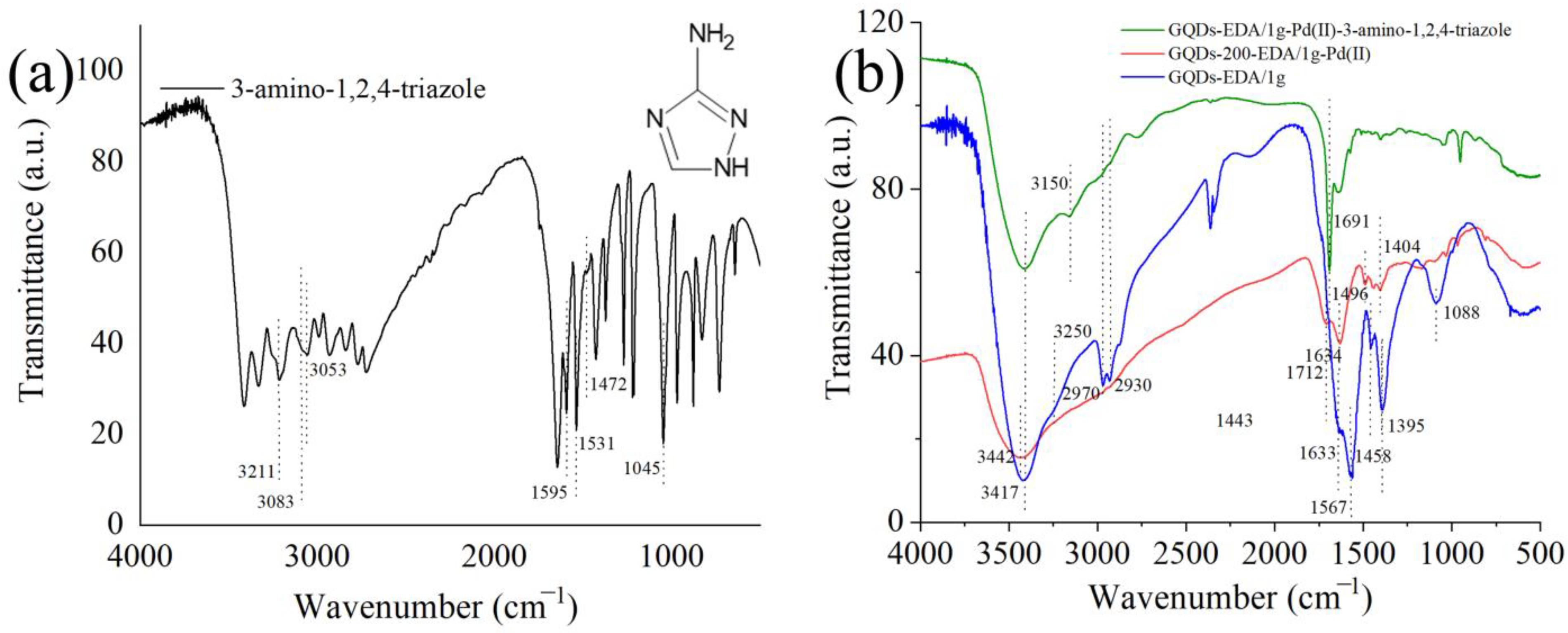
3.2. Detection of CF and 3-Amino-1,2,4-triazole
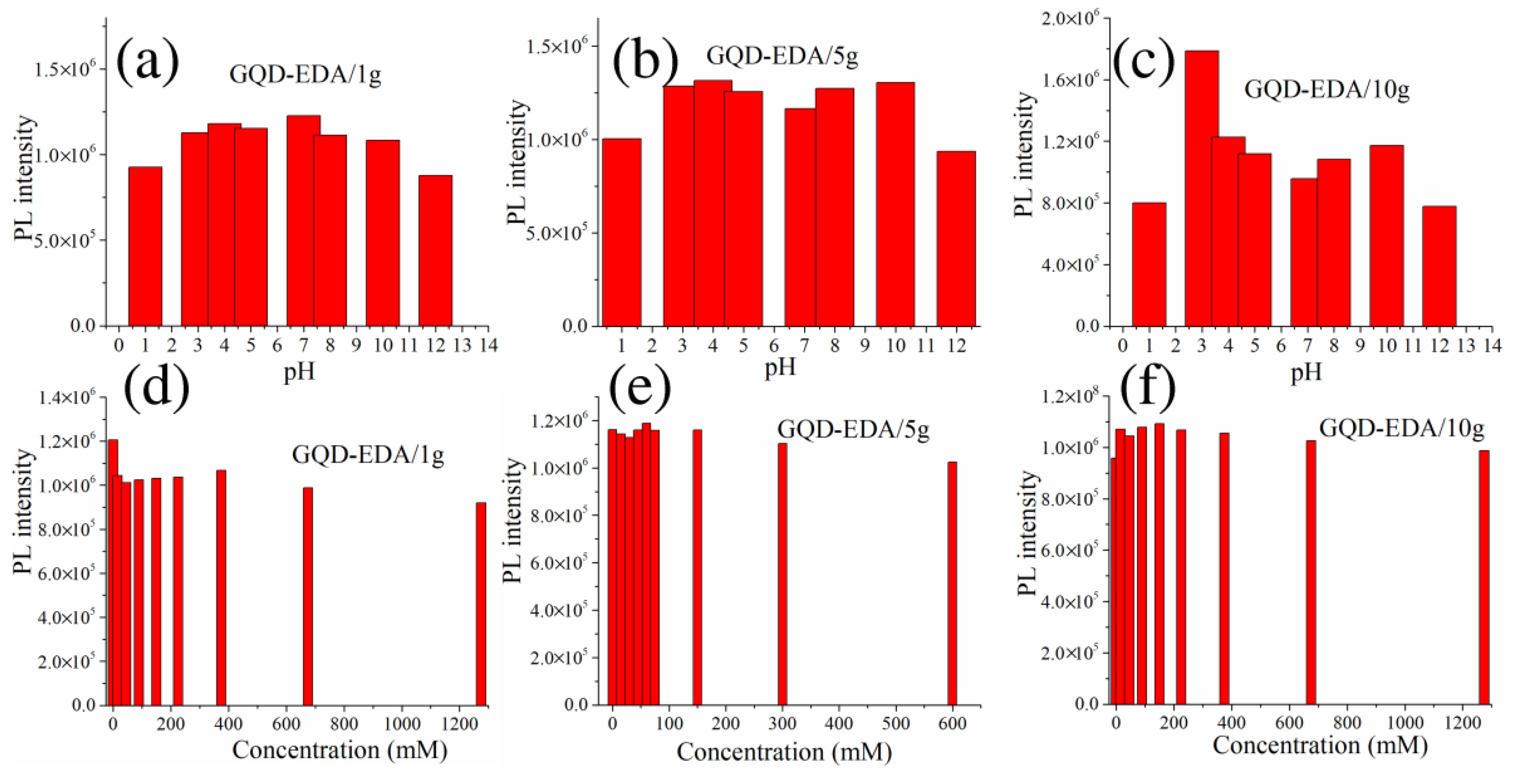
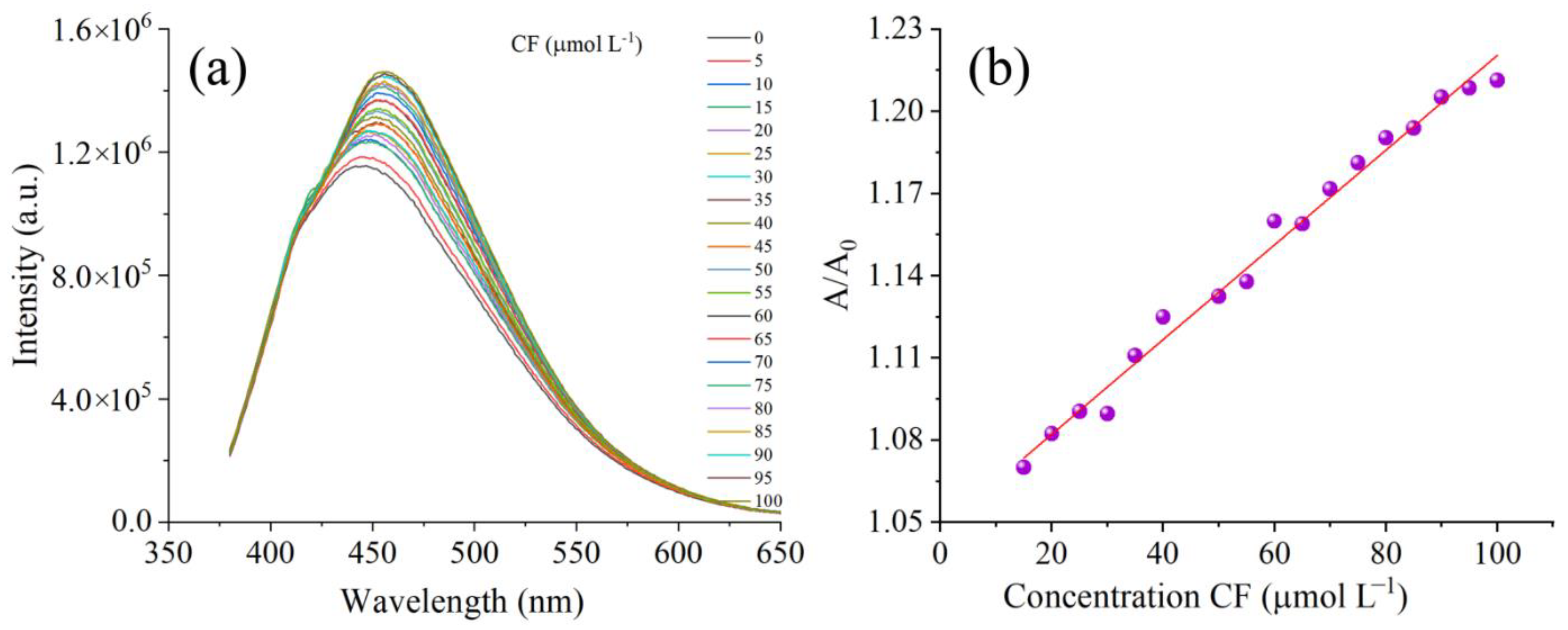
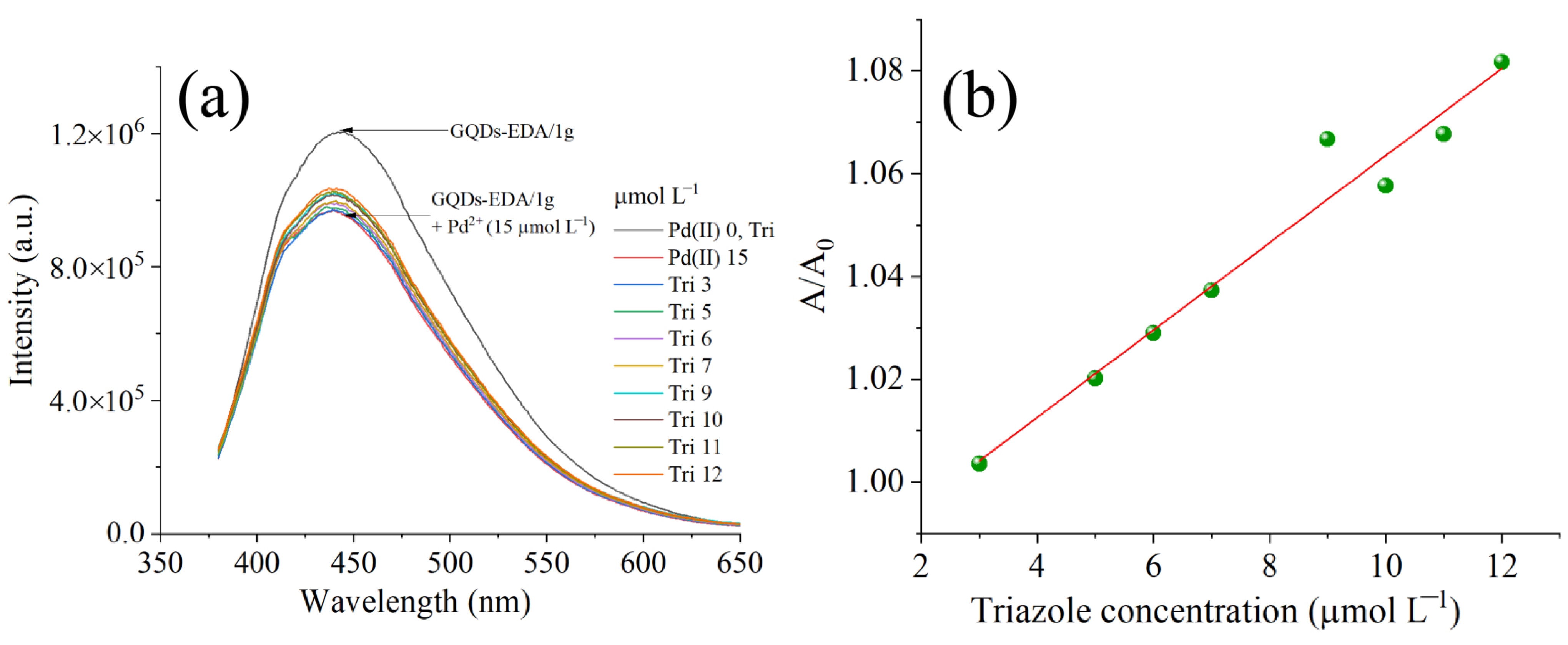
3.2.1. PL Spectroscopy
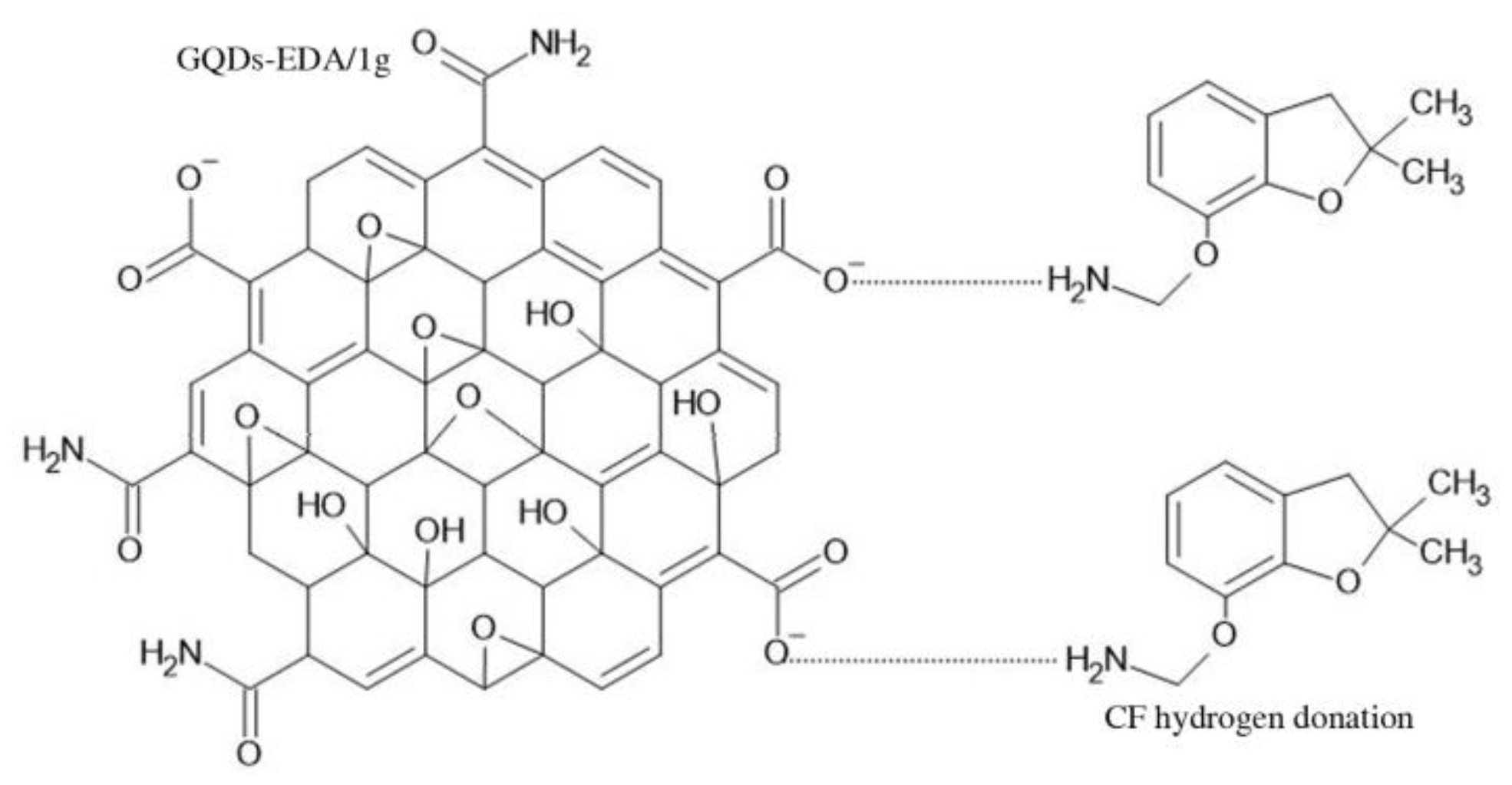
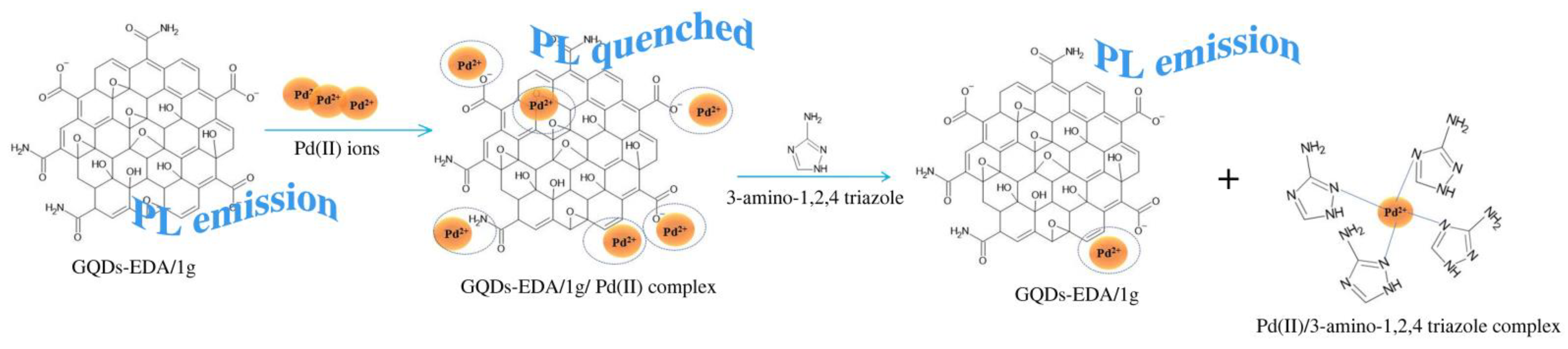
3.2.2. Detection Mechanism
3.3. Photo-Induced Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ponomarenko, L.A.; Schedin, F.; Katsnelson, M.I.; Yang, R.; Hill, E.W.; Novoselov, K.S.; Geim, A.K. Chaotic Dirac billiard in graphene quantum dots. Science 2008, 320, 356–358. [Google Scholar] [CrossRef]
- Kumar, S.; Ojha, A.K.; Ahmed, B.; Kumar, A.; Das, J.; Materny, A. Tunable (violet to green) emission by high-yield graphene quantum dots and exploiting its unique properties towards sun-light-driven photocatalysis and supercapacitor electrode materials. Mater. Today Commun. 2017, 11, 76–86. [Google Scholar] [CrossRef]
- Algarra, M.; Moreno, V.; Lázaro-Martínez, J.M.; Rodríguez-Castellón, E.; Soto, J.; Morales, J.; Benítez, A. Insights into the formation of N doped 3D-graphene quantum dots. Spectroscopic and computational approach. J. Colloid Interface Sci. 2020, 561, 678–686. [Google Scholar] [CrossRef]
- Tian, R.; Zhong, S.; Wu, J.; Jiang, W.; Shen, Y.; Jiang, W.; Wang, T. Solvothermal method to prepare graphene quantum dots by hydrogen peroxide. Opt. Mater. 2016, 60, 204–208. [Google Scholar] [CrossRef]
- Jovanović, S.; Marković, Z.M.; Budimir, M.; Špitálský, Z.; Vidoeski, B.A.; Todorović Marković, B.M. Effects of low gamma irradiation dose on the photoluminescence properties of graphene quantum dots. Opt. Quantum Electron. 2016, 48, 259. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, S.; Liu, J.; Zhong, X.; He, J.; Chen, X.; Feng, B.; Chen, Y.; Ostrikov, K.K. Cancer-Targeting Graphene Quantum Dots: Fluorescence Quantum Yields, Stability, and Cell Selectivity. Adv. Funct. Mater. 2019, 29, 1805860. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Xie, W.; Wang, Y.; Jia, X.; Nawaz, A.; Song, M.; Gong, J.R. Preparation of blue- and green-emissive nitrogen-doped graphene quantum dots from graphite and their application in bioimaging. Mater. Sci. Eng. C 2021, 119, 111642. [Google Scholar] [CrossRef]
- Jovanović, S.P.; Syrgiannis, Z.; Marković, Z.M.; Bonasera, A.; Kepić, D.P.; Budimir, M.D.; Milivojević, D.D.; Spasojević, V.D.; Dramićanin, M.D.; Pavlović, V.B.; et al. Modification of Structural and Luminescence Properties of Graphene Quantum Dots by Gamma Irradiation and Their Application in a Photodynamic Therapy. ACS Appl. Mater. Interfaces 2015, 7, 25865–25874. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of Carbon and Graphene Quantum Dots for Sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Tan, X.; Li, Y.; Li, X.; Zhou, S.; Fan, L.; Yang, S. Electrochemical synthesis of small-sized red fluorescent graphene quantum dots as a bioimaging platform. Chem. Commun. 2015, 51, 2544–2546. [Google Scholar] [CrossRef]
- Wang, S.; Cole, I.S.; Li, Q. The toxicity of graphene quantum dots. RSC Adv. 2016, 6, 89867–89878. [Google Scholar] [CrossRef]
- Tabish, T.A.; Scotton, C.J.; Ferguson, D.C.J.; Lin, L.; der Veen, A.V.; Lowry, S.; Ali, M.; Jabeen, F.; Ali, M.; Winyard, P.G.; et al. Biocompatibility and toxicity of graphene quantum dots for potential application in photodynamic therapy. Nanomedicine 2018, 13, 1923–1937. [Google Scholar] [CrossRef]
- Nair, R.V.; Thomas, R.T.; Mohamed, A.P.; Pillai, S. Fluorescent turn-off sensor based on sulphur-doped graphene quantum dots in colloidal and film forms for the ultrasensitive detection of carbamate pesticides. Microchem. J. 2020, 157, 104971. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Y.; Liu, S. Graphene quantum dots enhanced photocatalytic activity of zinc porphyrin toward the degradation of methylene blue under visible-light irradiation. J. Mater. Chem. A 2015, 3, 8552–8558. [Google Scholar] [CrossRef]
- Younis, M.R.; He, G.; Lin, J.; Huang, P. Recent Advances on Graphene Quantum Dots for Bioimaging Applications. Front. Chem. 2020, 8, 424. [Google Scholar] [CrossRef]
- Kang, G.-S.; Lee, S.; Yeo, J.-S.; Choi, E.-S.; Lee, D.C.; Na, S.-I.; Joh, H.-I. Graphene quantum dots with nitrogen and oxygen derived from simultaneous reaction of solvent as exfoliant and dopant. Chem. Eng. J. 2019, 372, 624–630. [Google Scholar] [CrossRef]
- Dorontić, S.; Jovanović, S.; Bonasera, A. Shedding Light on Graphene Quantum Dots: Key Synthetic Strategies, Characterization Tools, and Cutting-Edge Applications. Materials 2021, 14, 6153. [Google Scholar] [CrossRef]
- Ansón-Casaos, A.; Puértolas, J.A.; Pascual, F.J.; Hernández-Ferrer, J.; Castell, P.; Benito, A.M.; Maser, W.K.; Martínez, M.T. The effect of gamma-irradiation on few-layered graphene materials. Appl. Surf. Sci. 2014, 301, 264–272. [Google Scholar] [CrossRef]
- Aujara, K.M.; Chieng, B.W.; Ibrahim, N.A.; Zainuddin, N.; Thevy Ratnam, C. Gamma-Irradiation Induced Functionalization of Graphene Oxide with Organosilanes. Int. J. Mol. Sci. 2019, 20, 1910. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Li, L.; Ma, H.; Yu, M.; Li, J. γ-ray irradiation effects on graphene oxide in an ethylenediamine aqueous solution. Radiat. Phys. Chem. 2014, 94, 80–83. [Google Scholar] [CrossRef]
- He, Y.-L.; Li, J.-H.; Li, L.-F.; Chen, J.-B.; Li, J.-Y. The synergy reduction and self-assembly of graphene oxide via gamma-ray irradiation in an ethanediamine aqueous solution. Nucl. Sci. Tech. 2016, 27, 61. [Google Scholar] [CrossRef]
- Milenković, M.; Mišović, A.; Jovanović, D.; Popović Bijelić, A.; Ciasca, G.; Romanò, S.; Bonasera, A.; Mojsin, M.; Pejić, J.; Stevanović, J.; et al. Facile Synthesis of L-Cysteine Functionalized Graphene Quantum Dots as a Bioimaging and Photosensitive Agent. Nanomaterials 2021, 11, 1879. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, B.; Cao, Y.; Guo, M.; Yu, Y. Fluorescence Determination of Omethoate Based on a Dual Strategy for Improving Sensitivity. J. Agric. Food Chem. 2017, 65, 3065–3073. [Google Scholar] [CrossRef]
- Babazadeh, S.; Moghaddam, P.A.; Keshipour, S.; Mollazade, K. Colorimetric sensing of imidacloprid in cucumber fruits using a graphene quantum dot/Au (III) chemosensor. Sci. Rep. 2020, 10, 14327. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-López, J.; Llorent-Martínez, E.J.; Ortega-Barrales, P.; Ruiz-Medina, A. Graphene quantum dots-silver nanoparticles as a novel sensitive and selective luminescence probe for the detection of glyphosate in food samples. Talanta 2020, 207, 120344. [Google Scholar] [CrossRef] [PubMed]
- Lofty, H.M.; El-Aleem, A.E.-A.A.A.; Monir, H.H. Determination of insecticides malathion and lambda-cyhalothrin residues in zucchini by gas chromatography. Bull. Fac. Pharm. Cairo Univ. 2013, 51, 255–260. [Google Scholar] [CrossRef][Green Version]
- Çakır, O.; Bakhshpour, M.; Göktürk, I.; Yılmaz, F.; Baysal, Z. Sensitive and selective detection of amitrole based on molecularly imprinted nanosensor. J. Mol. Recognit. 2021, 34, e2929. [Google Scholar] [CrossRef] [PubMed]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Ramdzan, N.S.M.; Saleviter, S. Development of Graphene Quantum Dots-Based Optical Sensor for Toxic Metal Ion Detection. Sensors 2019, 19, 3850. [Google Scholar] [CrossRef]
- Long, F.; Zhu, A.; Shi, H. Recent advances in optical biosensors for environmental monitoring and early warning. Sensors 2013, 13, 13928–13948. [Google Scholar] [CrossRef] [PubMed]
- Sahub, C.; Tuntulani, T.; Nhujak, T.; Tomapatanaget, B. Effective biosensor based on graphene quantum dots via enzymatic reaction for directly photoluminescence detection of organophosphate pesticide. Sens. Actuators B Chem. 2018, 258, 88–97. [Google Scholar] [CrossRef]
- Nemati, F.; Hosseini, M.; Zare-Dorabei, R.; Ganjali, M.R. Sensitive recognition of ethion in food samples using turn-on fluorescence N and S co-doped graphene quantum dots. Anal. Methods 2018, 10, 1760–1766. [Google Scholar] [CrossRef]
- Roushani, M.; Kohzadi, S.; Haghjoo, S.; Azadbakht, A. Dual detection of Malation and Hg (II) by fluorescence switching of graphene quantum dots. Environ. Nanotechnol. Monit. Manag. 2018, 10, 308–313. [Google Scholar] [CrossRef]
- Du, F.; Sun, L.; Zen, Q.; Tan, W.; Cheng, Z.; Ruan, G.; Li, J. A highly sensitive and selective “on-off-on” fluorescent sensor based on nitrogen doped graphene quantum dots for the detection of Hg2+ and paraquat. Sens. Actuators B Chem. 2019, 288, 96–103. [Google Scholar] [CrossRef]
- Talari, F.F.; Bozorg, A.; Faridbod, F.; Vossoughi, M. A novel sensitive aptamer-based nanosensor using rGQDs and MWCNTs for rapid detection of diazinon pesticide. J. Environ. Chem. Eng. 2021, 9, 104878. [Google Scholar] [CrossRef]
- Jovanović, S.; Dorontić, S.; Jovanović, D.; Ciasca, G.; Budimir, M.; Bonasera, A.; Scopelliti, M.; Marković, O.; Todorović Marković, B. Gamma irradiation of graphene quantum dots with ethylenediamine: Antioxidant for ion sensing. Ceram. Int. 2020, 46, 23611–23622. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Raşa, M.; Kuipers, B.W.M.; Philipse, A.P. Atomic Force Microscopy and Magnetic Force Microscopy Study of Model Colloids. J. Colloid Interface Sci. 2002, 250, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Dimkić, I.; Ristivojević, P.; Janakiev, T.; Berić, T.; Trifković, J.; Milojković-Opsenica, D.; Stanković, S. Phenolic profiles and antimicrobial activity of various plant resins as potential botanical sources of Serbian propolis. Ind. Crops Prod. 2016, 94, 856–871. [Google Scholar] [CrossRef]
- Kuo, N.-J.; Chen, Y.-S.; Wu, C.-W.; Huang, C.-Y.; Chan, Y.-H.; Chen, I.W.P. One-Pot Synthesis of Hydrophilic and Hydrophobic N-Doped Graphene Quantum Dots via Exfoliating and Disintegrating Graphite Flakes. Sci. Rep. 2016, 6, 30426. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.T.; Gonzalez-Rodriguez, R.; Ryan, C.; Faerber, N.; Coffer, J.L.; Naumov, A.V. Photo-and Electroluminescence from Nitrogen-Doped and Nitrogen–Sulfur Codoped Graphene Quantum Dots. Adv. Funct. Mater. 2018, 28, 1804337. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, S.; Liu, J.; Wei, Y. Nanospace-confined preparation of uniform nitrogen-doped graphene quantum dots for highly selective fluorescence dual-function determination of Fe3+ and ascorbic acid. RSC Adv. 2018, 8, 5500–5508. [Google Scholar] [CrossRef]
- Feipeng, d.; Cao, N.-N.; Zhang, Y.-F.; Fu, P.; Wu, Y.-G.; Lin, Z.; Shi, R.; Amini, A.; C, C. PEDOT:PSS/graphene quantum dots films with enhanced thermoelectric properties via strong interfacial interaction and phase separation. Sci. Rep. 2018, 8, 6441. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Meng, X.; Ding, T.; Xu, Y.; Xu, H.; Ren, Y.; Liu, B.; Huang, J.; Yang, J.; et al. Graphenol defects induced blue emission enhancement in chemically reduced graphene quantum dots. Phys. Chem. Chem. Physic 2015, 17. [Google Scholar] [CrossRef]
- Temerov, F.; Belyaev, A.; Ankudze, B.; Pakkanen, T. Preparation and Photoluminescence Properties of Graphene Quantum Dots by Decomposition of Graphene-encapsulated Metal Nanoparticles Derived from Kraft Lignin and Transition Metal Salts. J. Lumin. 2018, 206, 403–411. [Google Scholar] [CrossRef]
- Lai, S.; Jin, Y.; Shi, L.; Zhou, R.; Zhou, Y.; An, D. Mechanisms behind excitation- and concentration-dependent multicolor photoluminescence in graphene quantum dots. Nanoscale 2020, 12, 591–601. [Google Scholar] [CrossRef]
- Xu, Q.F.; Zhou, Q.; Hua, Z.; Xue, Q.; Zhang, C.F.; Wang, X.Y.; Pan, D.Y.; Xiao, M. Single-Particle Spectroscopic Measurements of Fluorescent Graphene Quantum Dots. Acs Nano 2013, 7, 10654–10661. [Google Scholar] [CrossRef]
- Yanyan, Z.; Lin, J.; Xie, L.; Tang, H.; Wang, K.; Liu, J. One-Step Preparation of Nitrogen-Doped Graphene Quantum Dots With Anodic Electrochemiluminescence for Sensitive Detection of Hydrogen Peroxide and Glucose. Front. Chem. 2021, 9, 394. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, X.; Pang, A.; Yang, J. Facile synthesis and photoluminescence characteristics of blue-emitting nitrogen-doped graphene quantum dots. Nanotechnology 2016, 27, 165704. [Google Scholar] [CrossRef]
- Qu, D.; Zheng, M.; Zhang, L.; Zhao, H.; Xie, Z.; Jing, X.; Haddad, R.E.; Fan, H.; Sun, Z. Formation mechanism and optimization of highly luminescent N-doped graphene quantum dots. Sci. Rep. 2014, 4, 5294. [Google Scholar] [CrossRef]
- Ocak, M.; Gumrukcuoglu, N.; Ocak, Ü.; Buschmann, H.-J.; Schollmeyer, E. The Synthesis of New Triazole Ligands and Determination of Complex Stability Constants with Transition Metal Cations in Aqueous Media. J. Solut. Chem. 2008, 37, 1489–1497. [Google Scholar] [CrossRef]
- Weerasooriya, R.; Indraratne, S.P.; Nanayakkara, N.; Jayarathne, L.; Dissanayake, C.B.; Walalawela, N.; Bandara, A. Probing pyrite–carbofuran interactions with ζ potential and IR spectroscopic measurements. Colloids Surf. A Physicochem. Eng. Asp. 2012, 396, 219–223. [Google Scholar] [CrossRef]
- Vimal, V.; Patel, M.; Mohan, D. Aqueous carbofuran removal using slow pyrolyzed sugarcane bagasse biochar: Equilibrium and fixed-bed studies. RSC Adv. 2019, 9, 26338–26350. [Google Scholar] [CrossRef]
- Vithanage, M.; Mayakaduwa, S.S.; Herath, I.; Ok, Y.S.; Mohan, D. Kinetics, thermodynamics and mechanistic studies of carbofuran removal using biochars from tea waste and rice husks. Chemosphere 2016, 150, 781–789. [Google Scholar] [CrossRef]
- Mayakaduwa, S.S.; Herath, I.; Ok, Y.S.; Mohan, D.; Vithanage, M. Insights into aqueous carbofuran removal by modified and non-modified rice husk biochars. Environ. Sci. Pollut. Res. 2017, 24, 22755–22763. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Hu, Z.-J.; Ji, R. Removal of carbofuran from aqueous solution by orange peel. Desalination Water Treat. 2012, 49, 106–114. [Google Scholar] [CrossRef]
- Mohapatra, S.; Bera, M.K.; Das, R.K. Rapid “turn-on” detection of atrazine using highly luminescent N-doped carbon quantum dot. Sens. Actuators B Chem. 2018, 263, 459–468. [Google Scholar] [CrossRef]
- Das, R.K.; Mohapatra, S. Highly luminescent, heteroatom-doped carbon quantum dots for ultrasensitive sensing of glucosamine and targeted imaging of liver cancer cells. J. Mater. Chem. B 2017, 5, 2190–2197. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.-Y.; Liang, R.-P.; Li, Y.-H.; Qiu, J.-D. Boron-Doped Graphene Quantum Dots for Selective Glucose Sensing Based on the “Abnormal” Aggregation-Induced Photoluminescence Enhancement. Anal. Chem. 2014, 86, 4423–4430. [Google Scholar] [CrossRef]
- Trivedi, M.; Tallapragada, R.M.; Branton, A.; Trivedi, D.; Nayak, G.; Mishra, R.; Jana, S. Characterization of Physical, Spectral and Thermal Properties of Biofield Treated 1,2,4-Triazole. Mol. Pharm. Org. Process Res. 2015, 3, 128. [Google Scholar] [CrossRef]
- Jber, N. Journal of Global Pharma Technology Synthesis, Characterization and Evaluation the Biological activity for New Mesogenic 1, 3-oxazepine-4, 7-dione Derived from Trimesic Acid. J. Glob. Pharma Technol. 2020, 11, 388–395. [Google Scholar]
- He, P.; Yu, Q.; Shao, L.; Lü, F. Removal of Copper (II) by Biochar Mediated by Dissolved Organic Matter. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bisauriya, R.; Antonaroli, S.; Ardini, M.; Angelucci, F.; Ricci, A.; Pizzoferrato, R. Tuning the Sensing Properties of N and S Co-Doped Carbon Dots for Colorimetric Detection of Copper and Cobalt in Water. Sensors 2022, 22, 2487. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Li, H.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Cadmium removal from aqueous solution by biochar obtained by co-pyrolysis of sewage sludge with tea waste. Res. Chem. Intermed. 2018, 44, 135–154. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Chao, H.P. Effect of pyrolysis temperatures and times on the adsorption of cadmium onto orange peel derived biochar. Waste Manag. Res. J. Sustain. Circ. Econ. 2016, 34, 129–138. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Z.X.; Li, R.S.; Zhang, H.Z.; Huang, C.Z.; Wang, J. The aggregation induced emission quenching of graphene quantum dots for visualizing the dynamic invasions of cobalt(ii) into living cells. J. Mater. Chem. B 2017, 5, 6394–6399. [Google Scholar] [CrossRef]
- Liu, L.; Fan, S. Removal of cadmium in aqueous solution using wheat straw biochar: Effect of minerals and mechanism. Environ. Sci. Pollut. Res. 2018, 25, 8688–8700. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Z.; Xue, Z.; Huang, C.; Shan, Y.; Liu, C.; Qin, X.; Yang, W.; Chen, X.; Wang, T. Understanding the Selective Detection of Fe3+ Based on Graphene Quantum Dots as Fluorescent Probes: The Ksp of a Metal Hydroxide-Assisted Mechanism. Anal. Chem. 2017, 89, 12054–12058. [Google Scholar] [CrossRef]
- Marković, Z.M.; Jovanović, S.P.; Mašković, P.Z.; Danko, M.; Mičušík, M.; Pavlovic, V.; Milivojevic, D.; Kleinova, A.; Špitalský, Z.; Todorovic Markovic, B. Photo-induced antibacterial activity of four graphene based nanomaterials on a wide range of bacteria. RSC Adv. 2018, 8, 31337–31347. [Google Scholar] [CrossRef]
- Jovanović, S.P.; Syrgiannis, Z.; Budimir, M.D.; Milivojević, D.D.; Jovanovic, D.J.; Pavlović, V.B.; Papan, J.M.; Bartenwerfer, M.; Mojsin, M.M.; Stevanović, M.J. Graphene quantum dots as singlet oxygen producer or radical quencher—The matter of functionalization with urea/thiourea. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110539. [Google Scholar] [CrossRef] [PubMed]
- Marković, Z.M.; Jovanović, S.P.; Mašković, P.Z.; Mojsin, M.M.; Stevanović, M.J.; Danko, M.; Mičušík, M.; Jovanović, D.J.; Kleinová, A.; Špitalský, Z. Graphene oxide size and structure pro-oxidant and antioxidant activity and photoinduced cytotoxicity relation on three cancer cell lines. J. Photochem. Photobiol. B Biol. 2019, 200, 111647. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.S.; Yeh, T.S.; Chang, C.Y.; Liu, J.C.; Chen, C.H.; So, E.C.; Wu, P.C. Amino-functionalized nitrogen-doped graphene quantum dots for efficient enhancement of two-photon-excitation photodynamic therapy: Functionalized nitrogen as a bactericidal and contrast agent. Int. J. Nanomed. 2020, 15, 6961–6973. [Google Scholar] [CrossRef]
- Kuo, W.S.; Shao, Y.T.; Huang, K.S.; Chou, T.M.; Yang, C.H. Antimicrobial Amino-Functionalized Nitrogen-Doped Graphene Quantum Dots for Eliminating Multidrug-Resistant Species in Dual-Modality Photodynamic Therapy and Bioimaging under Two-Photon Excitation. ACS Appl. Mater. Interfaces 2018, 10, 14438–14446. [Google Scholar] [CrossRef]
- Sen, P.; Nyokong, T. Promising photodynamic antimicrobial activity of polyimine substituted zinc phthalocyanine and its polycationic derivative when conjugated to nitrogen, sulfur, co-doped graphene quantum dots against Staphylococcus aureus. Photodiagn. Photodyn. Ther. 2021, 34, 102300. [Google Scholar] [CrossRef]
- Kuo, W.S.; Lin, Y.S.; Wu, P.C.; Chang, C.Y.; Wang, J.Y.; Chen, P.C.; Hsieh, M.H.; Kao, H.F.; Lin, S.H.; Chang, C.C. Two-Photon–Near Infrared-II Antimicrobial Graphene-Nanoagent for Ultraviolet–Near Infrared Imaging and Photoinactivation. Int. J. Mol. Sci. 2022, 23, 3230. [Google Scholar] [CrossRef]





| Sample | %C | %O | %N |
|---|---|---|---|
| p-GQDs [35] | 63.00 | 37.00 | 0.00 |
| GQDs- EDA/1 g | 76.25 | 17.36 | 6.39 |
| GQDs- EDA/5 g | 77.18 | 16.10 | 6.71 |
| GQDs- EDA/10 g | 76.60 | 16.35 | 7.05 |
| Sample | Peak Position (nm) | Intensity/cps |
|---|---|---|
| p-GQDs | 430Ex 300 437Ex 320 454Ex 340 464Ex 360 472Ex 380 492Ex 400 | 1,068,712 1,504,852 1,595,944 1,522,472 1,217,330 833,934 |
| GQDs-EDA/1 g | 441Ex 300 436Ex 320 439Ex 340 447Ex 360 457Ex 380 465Ex 400 | 1,095,082 1,682,314 2,197,876 2,599,566 2,590,304 2,584,144 |
| GQDs-EDA/5 g | 438Ex 300 439Ex 320 442Ex 340 446Ex 360 458Ex 380 465Ex 400 | 1,239,596 1,931,034 2,576,008 3,029,036 3,071,398 2,936,478 |
| GQDs-EDA/10 g | 439Ex 300 436Ex 320 439Ex 340 444Ex 360 458Ex 380 466Ex 400 | 941,924 1,455,166 1,916,766 2,265,520 2,272,670 2,092,036 |
| Sample | QY360 (%) |
|---|---|
| p-GQDs [35] | 1.45 |
| GQDs-EDA/1 g | 7.24 |
| GQDs-EDA/5 g | 6.77 |
| GQDs-EDA/10 g | 5.82 |
| Rhodamine B | 31 |
| Bacterial Strain | GQD-EDA/1 g | GQD-EDA/10 g | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| E. coli ATCC 35218 | >0.8 | - | >0.8 | - |
| S. aureus ATCC 29213 | >0.8 | - | >0.8 | - |
| OD Values | |||
|---|---|---|---|
| E. coli | S. aureus | ||
| 0.85 | 0.77 | 0.63 | 0.49 |
| 0.78 | 0.70 | 0.51 | 0.50 |
| 0.67 | 0.61 | 0.50 | 0.43 |
| 0.69 | 0.63 | 0.90 | 0.42 |
| 0.66 | 0.62 | 0.47 | 0.50 |
| 0.9 | 0.89 | 0.50 | 0.47 |
| 0.91 | 0.72 | 0.52 | 0.56 |
| 0.05 | 0.05 | 0.05 | 0.05 |
| Material | Bacterial Strain | Illumination Wavelength (WL) | Effects |
|---|---|---|---|
| nitrogen-doped GQDs and amino-functionalized N-GQDs (amino-N-GQDs) [71] | Bacillus subtilis | WL 720–820 nm, power of 2.8 mW, 394.24 nJ pixel−1, exposure time 3.171 s | amino-N-GQDs6 μg mL−1 100% elimination |
| amino-N-GQDs [72] | Methicillin-resistant Staphyococcus aureus (MRSA) | power: 239.36 nJ pixel−1, exposure time 12 s, WL 800 nm | 0.25 µg mL−1 Incubation 3 h at 37 °C in the dark |
| octa-imine substituted Zinc phthalocyanines with nitrogen, sulfur co-doped graphene quantum dots (N,S-GQDs) [73] | S. aureus | a diode laser WL 670 nm, 20 MHz repetition rate, 44 ps pulse width | 80 min irradiation 100% elimination |
| amino-N-GQDs [74] | E. coli | WL 960 nm, laser source, 222.7 nJ pixel−1, total illumination: ~1.11 s | 0.75 µg mL−1 elimination > 99% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorontic, S.; Bonasera, A.; Scopelliti, M.; Markovic, O.; Bajuk Bogdanović, D.; Ciasca, G.; Romanò, S.; Dimkić, I.; Budimir, M.; Marinković, D.; et al. Gamma-Ray-Induced Structural Transformation of GQDs towards the Improvement of Their Optical Properties, Monitoring of Selected Toxic Compounds, and Photo-Induced Effects on Bacterial Strains. Nanomaterials 2022, 12, 2714. https://doi.org/10.3390/nano12152714
Dorontic S, Bonasera A, Scopelliti M, Markovic O, Bajuk Bogdanović D, Ciasca G, Romanò S, Dimkić I, Budimir M, Marinković D, et al. Gamma-Ray-Induced Structural Transformation of GQDs towards the Improvement of Their Optical Properties, Monitoring of Selected Toxic Compounds, and Photo-Induced Effects on Bacterial Strains. Nanomaterials. 2022; 12(15):2714. https://doi.org/10.3390/nano12152714
Chicago/Turabian StyleDorontic, Sladjana, Aurelio Bonasera, Michelangelo Scopelliti, Olivera Markovic, Danica Bajuk Bogdanović, Gabriele Ciasca, Sabrina Romanò, Ivica Dimkić, Milica Budimir, Dragana Marinković, and et al. 2022. "Gamma-Ray-Induced Structural Transformation of GQDs towards the Improvement of Their Optical Properties, Monitoring of Selected Toxic Compounds, and Photo-Induced Effects on Bacterial Strains" Nanomaterials 12, no. 15: 2714. https://doi.org/10.3390/nano12152714
APA StyleDorontic, S., Bonasera, A., Scopelliti, M., Markovic, O., Bajuk Bogdanović, D., Ciasca, G., Romanò, S., Dimkić, I., Budimir, M., Marinković, D., & Jovanovic, S. (2022). Gamma-Ray-Induced Structural Transformation of GQDs towards the Improvement of Their Optical Properties, Monitoring of Selected Toxic Compounds, and Photo-Induced Effects on Bacterial Strains. Nanomaterials, 12(15), 2714. https://doi.org/10.3390/nano12152714














