Polystyrene-Modulated Polypyrrole to Achieve Controllable Electromagnetic-Wave Absorption with Enhanced Environmental Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of PPy/PS Composites
2.3. Characterization
3. Results and Discussion
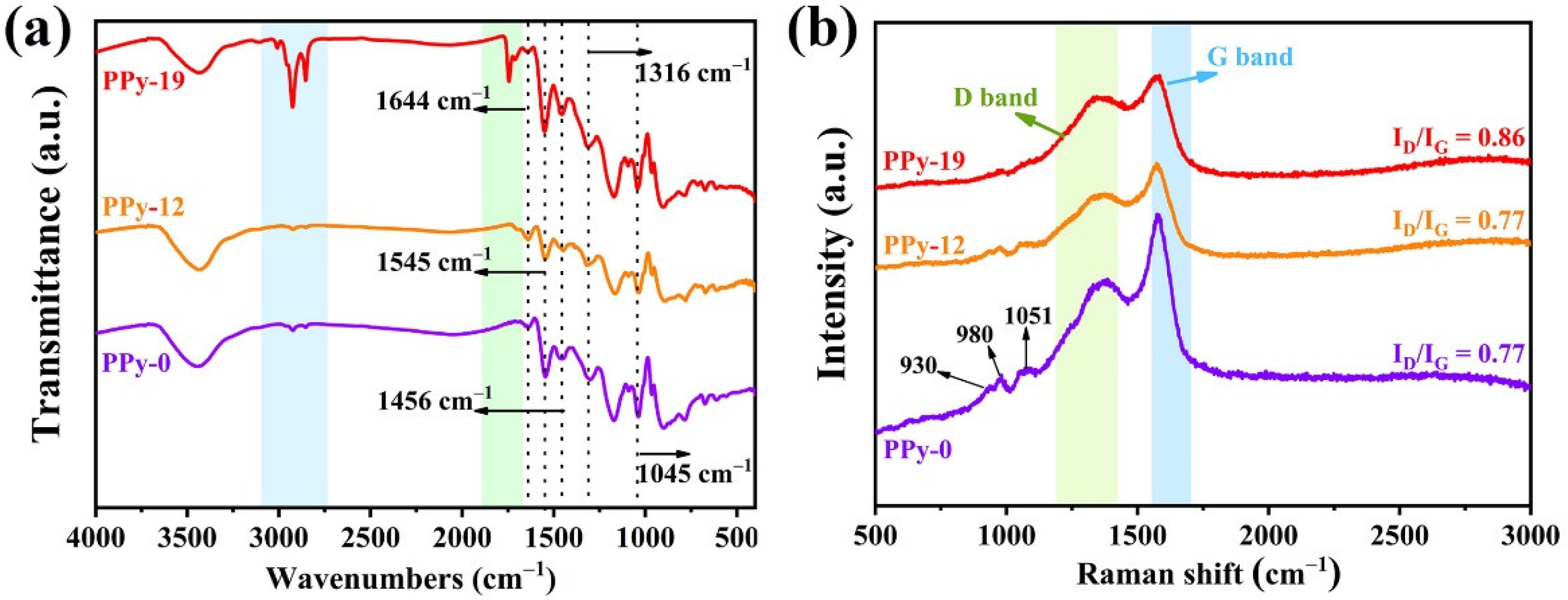
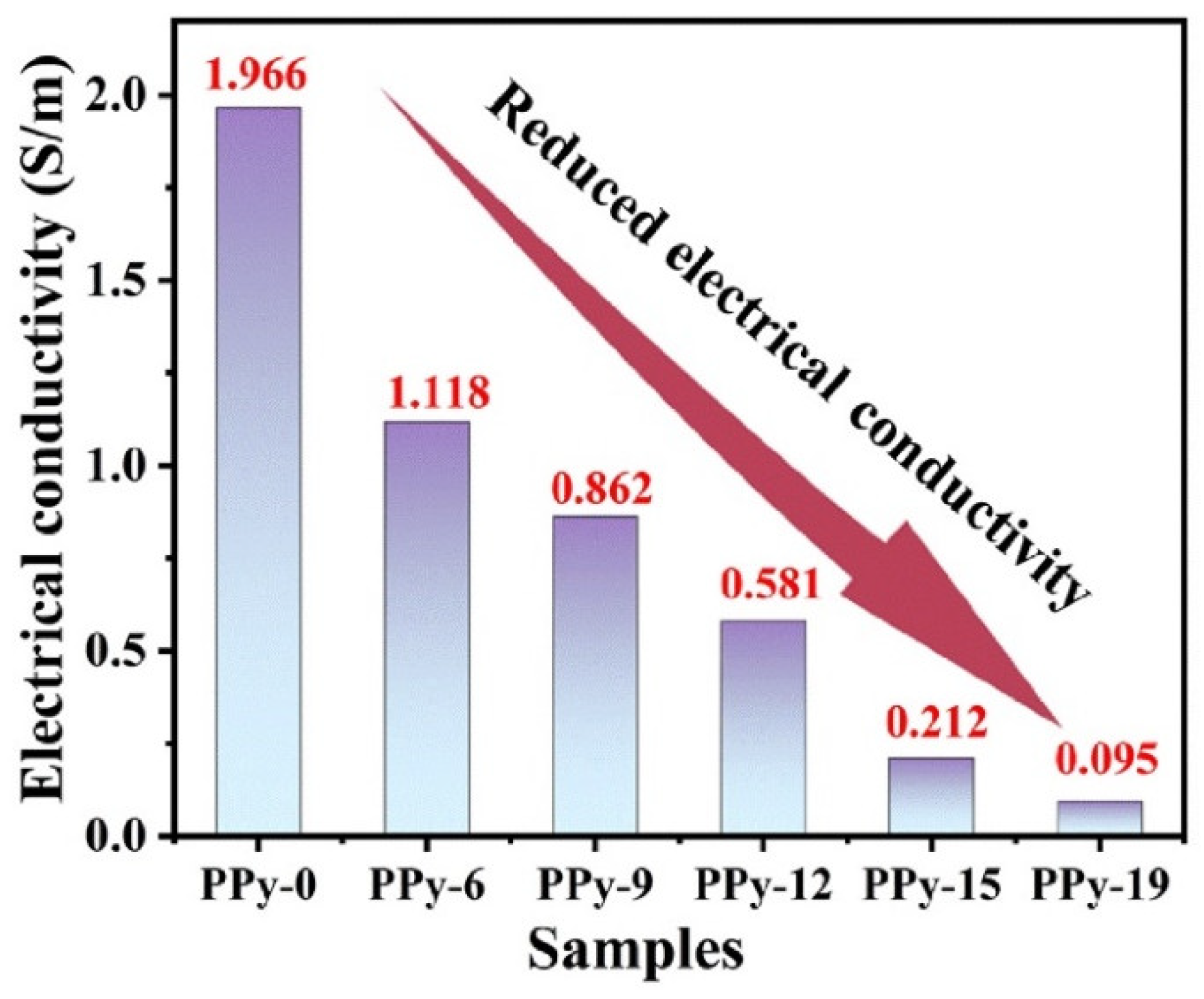
3.1. Structural, Morphology and Component Analysis
3.2. Morphological Analysis
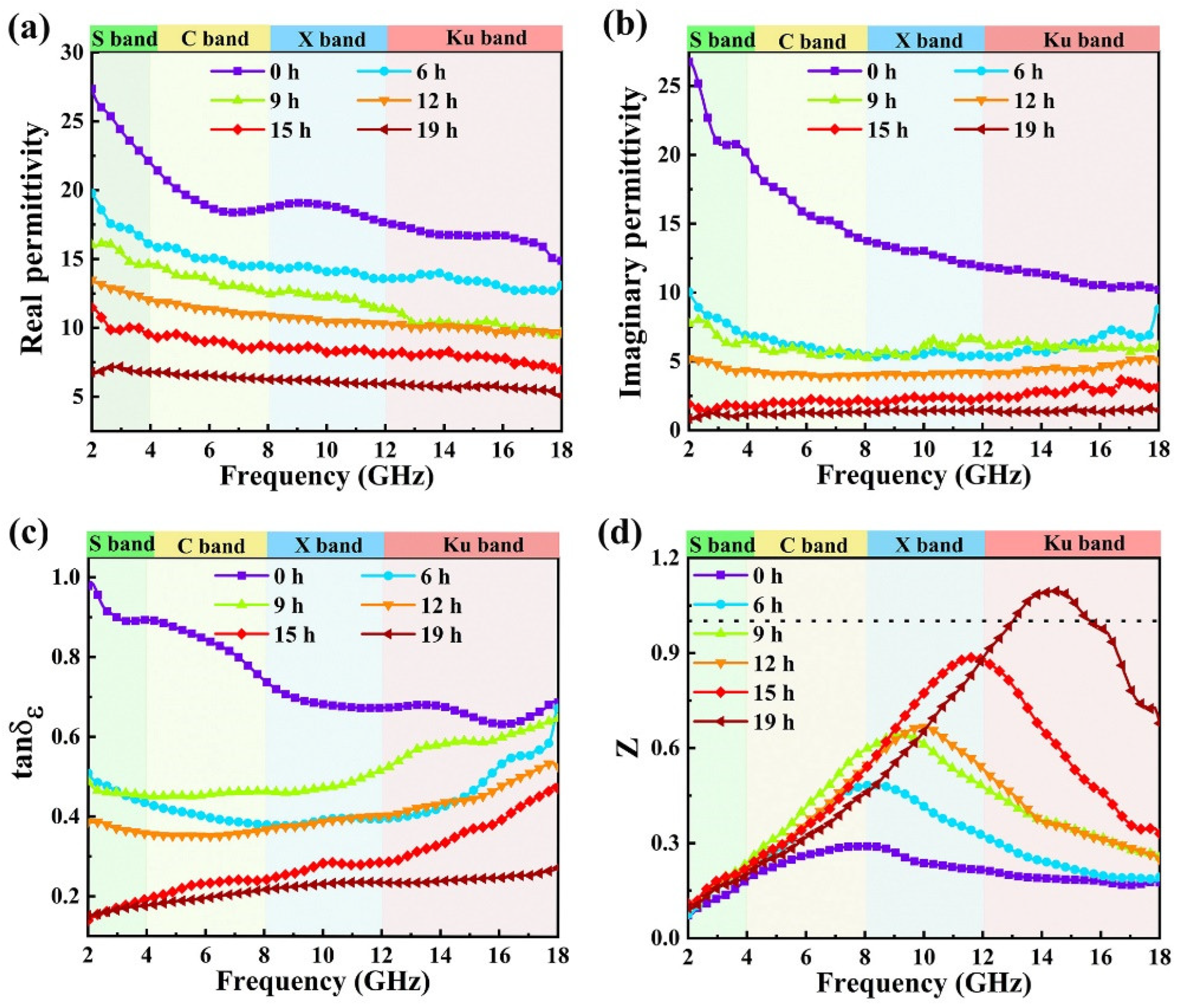
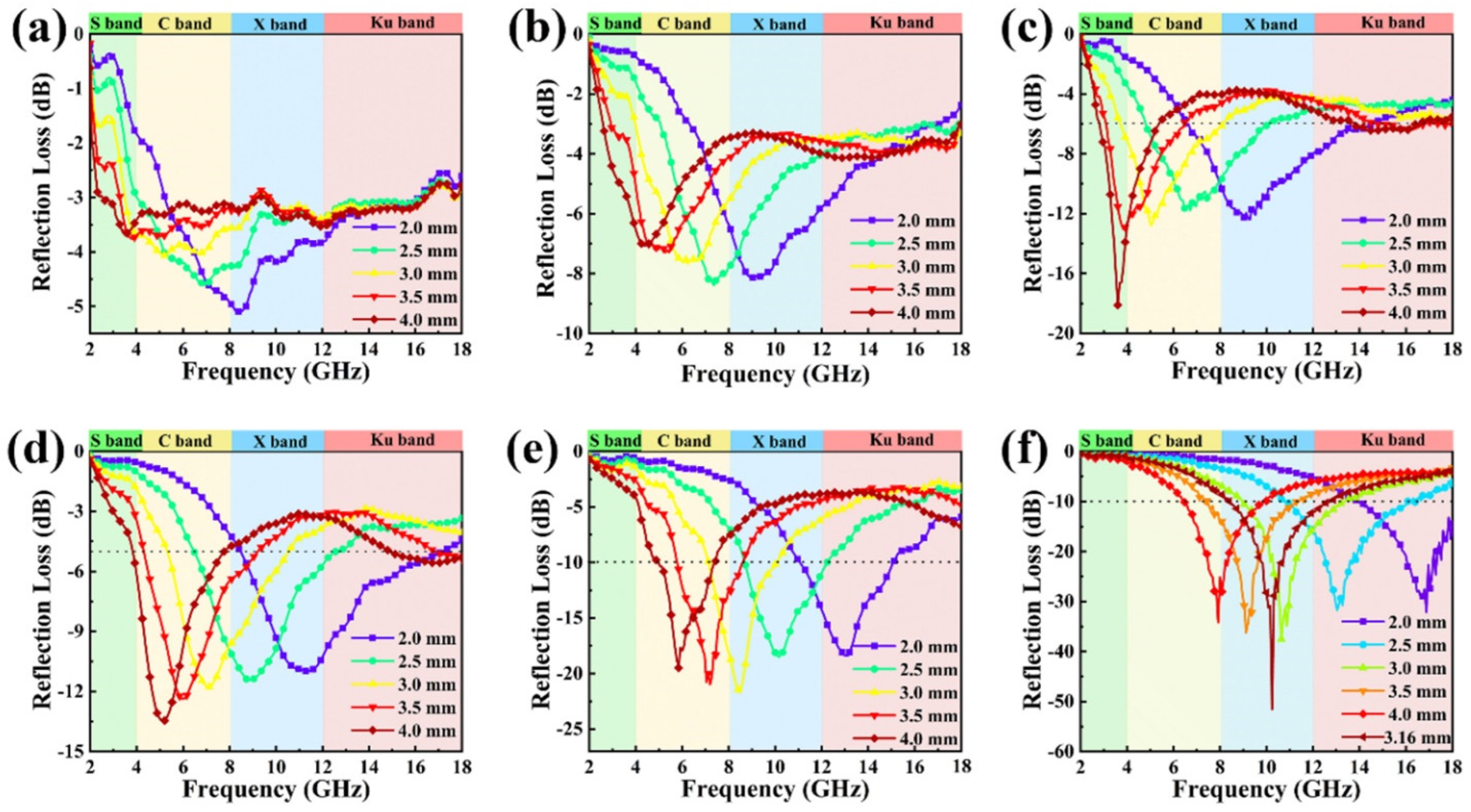
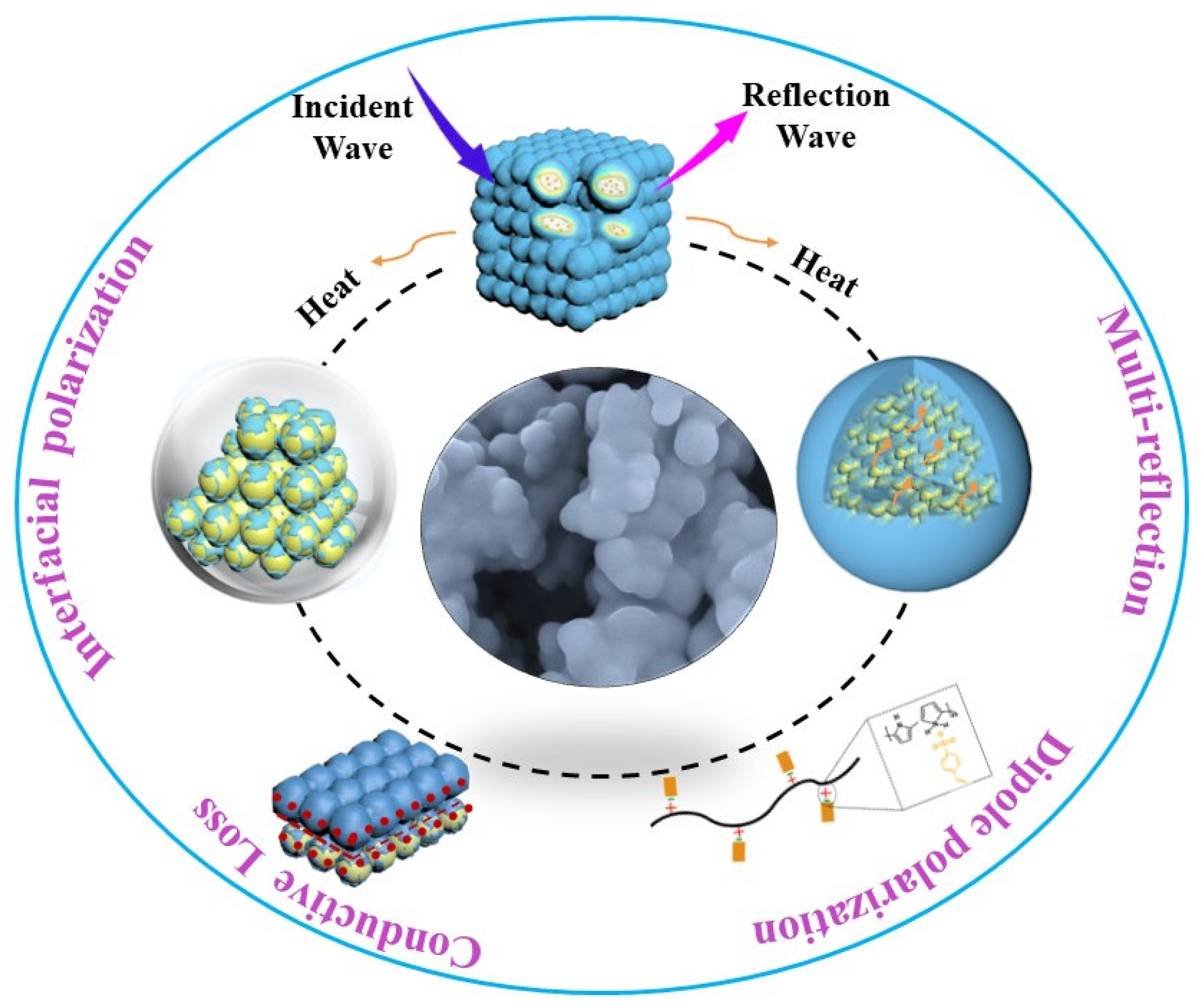
3.3. Microwave-Absorption Property
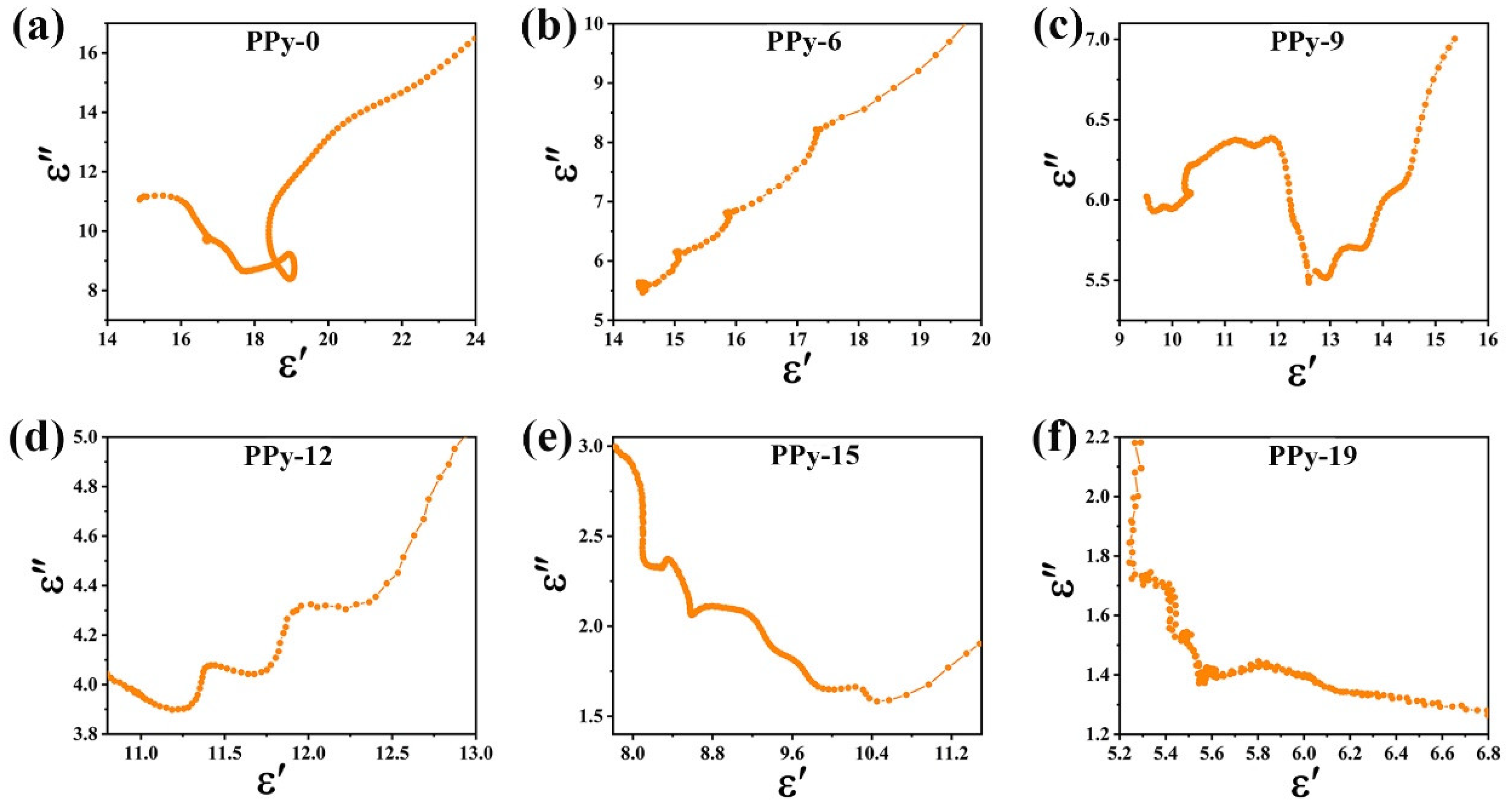
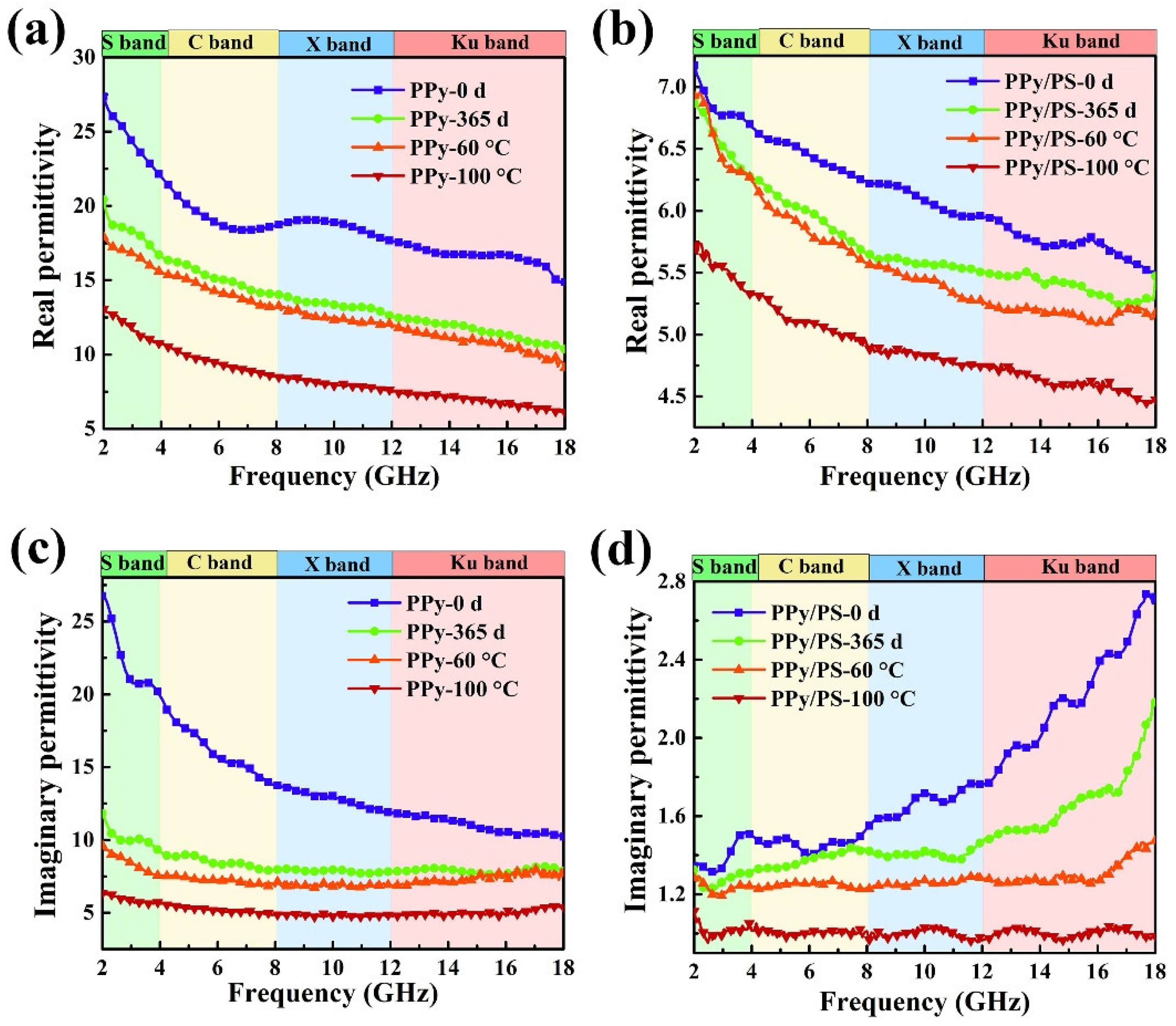
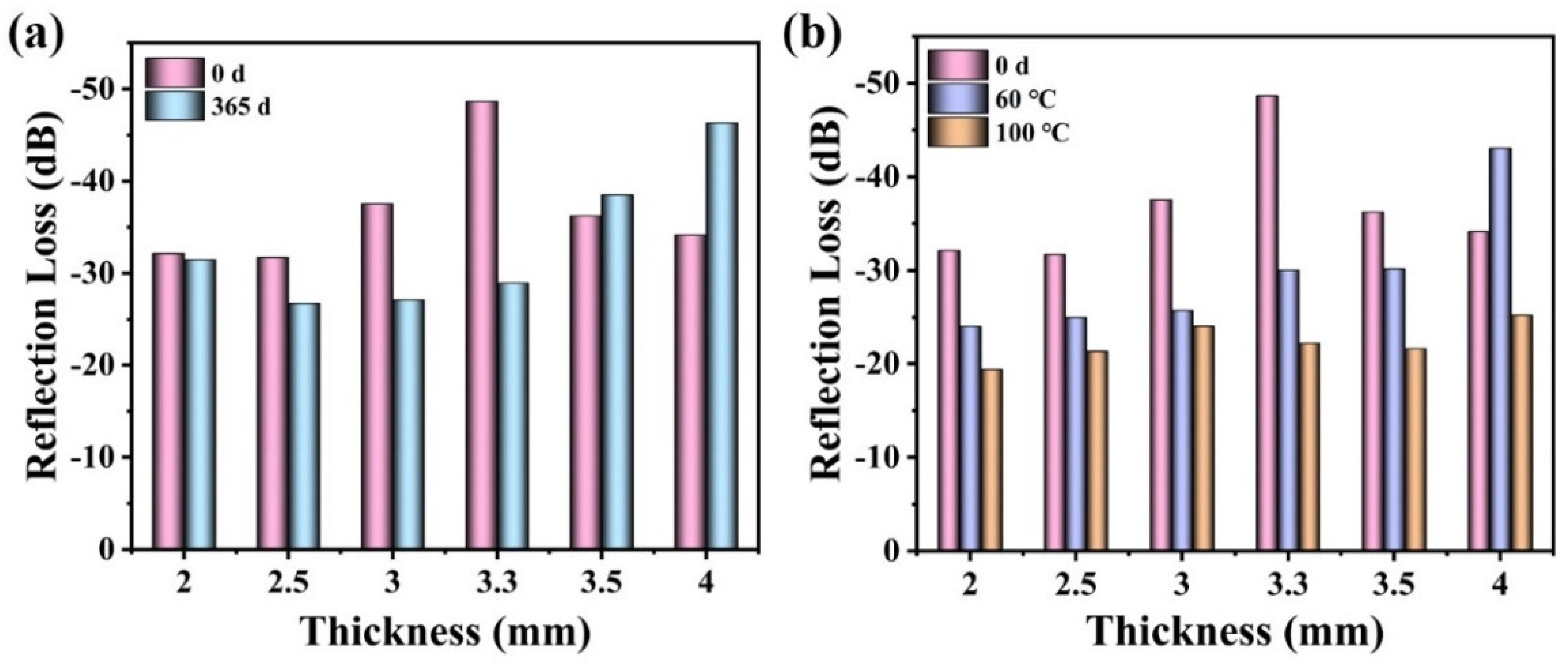
3.4. Environmental Stability of Microwave Absorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Yu, G.; Zhang, Y.; Zhang, M.; Shao, G. Design of Cellular Structure of Graphene Aerogels for Electromagnetic Wave Absorption. Chem. Eng. J. 2021, 426, 131894. [Google Scholar] [CrossRef]
- Wu, Z.; Pei, K.; Xing, L.; Yu, X.; You, W.; Che, R. Enhanced Microwave Absorption Performance from Magnetic Coupling of Magnetic Nanoparticles Suspended within Hierarchically Tubular Composite. Adv. Funct. Mater. 2019, 29, 1901448. [Google Scholar] [CrossRef]
- Guan, H.; Wang, Q.; Wu, X.; Pang, J.; Jiang, Z.; Chen, G.; Dong, C.; Wang, L.; Gong, C. Biomass Derived Porous Carbon (BPC) and Their Composites as Lightweight and Efficient Microwave Absorption Materials. Compos. Part B Eng. 2021, 207, 108562. [Google Scholar] [CrossRef]
- Zhu, T.; Chang, S.; Song, Y.-F.; Lahoubi, M.; Wang, W. PVP-Encapsulated CoFe2O4/RGO Composites with Controllable Electromagnetic Wave Absorption Performance. Chem. Eng. J. 2019, 373, 755–766. [Google Scholar] [CrossRef]
- Wang, H.; Ren, H.; Jing, C.; Li, J.; Zhou, Q.; Meng, F. Two Birds with One Stone: Graphene Oxide@Sulfonated Polyaniline Nanocomposites towards High-Performance Electromagnetic Wave Absorption and Corrosion Protection. Compos. Sci. Technol. 2021, 204, 108630. [Google Scholar] [CrossRef]
- Lyu, L.; Liu, J.; Liu, H.; Liu, C.; Lu, Y.; Sun, K.; Fan, R.; Wang, N.; Lu, N.; Guo, Z.; et al. An Overview of Electrically Conductive Polymer Nanocomposites toward Electromagnetic Interference Shielding. Eng. Sci. 2018, 2, 26–42. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, X.; Huang, J.; Chen, S.; Zhang, Y.; Dong, C.; Chen, G.; Wang, L.; Guan, H. Enhanced Microwave Absorption of Biomass Carbon/Nickel/Polypyrrole (C/Ni/PPy) Ternary Composites through the Synergistic Effects. J. Alloys Compd. 2022, 890, 161887. [Google Scholar] [CrossRef]
- Wang, C.; Gao, H.; Liang, D.; Liu, S.; Zhang, H.; Guan, H.; Wu, Y.; Zhang, Y. Effective Fabrication of Flexible Nickel Chains/Acrylate Composite Pressure-Sensitive Adhesives with Layered Structure for Tunable Electromagnetic Interference Shielding. Adv. Compos. Hybrid Mater. 2022. [Google Scholar] [CrossRef]
- Guo, X.; Facchetti, A. The Journey of Conducting Polymers from Discovery to Application. Nat. Mater. 2020, 19, 922–928. [Google Scholar] [CrossRef]
- Lan, D.; Gao, Z.; Zhao, Z.; Kou, K.; Wu, H. Application Progress of Conductive Conjugated Polymers in Electromagnetic Wave Absorbing Composites. Compos. Commun. 2021, 26, 100767. [Google Scholar] [CrossRef]
- Lee, K.; Cho, S.; Heum Park, S.; Heeger, A.J.; Lee, C.-W.; Lee, S.-H. Metallic Transport in Polyaniline. Nature 2006, 441, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, T.; Yang, Z. Flexible Polyethylene Terephthalate/Polyaniline Composite Paper with Bending Durability and Effective Electromagnetic Shielding Performance. Chem. Eng. J. 2020, 389, 124433. [Google Scholar] [CrossRef]
- Mahun, A.; Abbrent, S.; Bober, P.; Brus, J.; Kobera, L. Effect of Structural Features of Polypyrrole (PPy) on Electrical Conductivity Reflected on 13C SsNMR Parameters. Synth. Met. 2020, 259, 116250. [Google Scholar] [CrossRef]
- Liu, M.; Yang, X.; Shao, W.; Wu, T.; Ji, R.; Fan, B.; Tong, G. Superior Microwave Absorbing Properties of O, S, N Codoped Carbon Planar Helixes via Carbonization of Polypyrrole Spiral Nanowires. Carbon 2021, 174, 625–637. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; Liu, X.; Zhao, X.X.; Li, T.; Zhao, Y.; Liu, P. Polypyrrole-Based Composite Materials for Electromagnetic Wave Absorption. Polym. Rev. 2021, 61, 646–687. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, J.; Liang, H.; Chen, G. Facile Synthesis of Adjustable High-Entropy Alloy/Polypyrrole Electromagnetic Wave Absorber. J. Mater. Sci. Mater. Electron. 2021, 32, 26074–26085. [Google Scholar] [CrossRef]
- Ren, H.; Li, T.; Wang, H.; Guo, Z.; Chen, T.; Meng, F. Two Birds with One Stone: Superhelical Chiral Polypyrrole towards High-Performance Electromagnetic Wave Absorption and Corrosion Protection. Chem. Eng. J. 2022, 427, 131582. [Google Scholar] [CrossRef]
- Ding, L.; Zhao, X.; Huang, Y.; Yan, J.; Li, T.; Liu, P. Ultra-Broadband and Covalently Linked Core–Shell CoFe2O4@PPy Nanoparticles with Reduced Graphene Oxide for Microwave Absorption. J. Colloid Interface Sci. 2021, 595, 168–177. [Google Scholar] [CrossRef]
- Su, Q.; Wang, B.; Mu, C.; Zhai, K.; Nie, A.; Xiang, J.; Wen, F. Polypyrrole Coated 3D Flower MoS2 Composites with Tunable Impedance for Excellent Microwave Absorption Performance. J. Alloys Compd. 2021, 888, 161487. [Google Scholar] [CrossRef]
- Tansley, T.L.; Maddison, D.S. Conductivity Degradation in Oxygen-Aged Polypyrrole. J. Appl. Phys. 1991, 69, 7711–7713. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, J.; Tang, Y.; Kong, J.; Liu, T.; Gu, J. Polymer Matrix Wave-Transparent Composites: A Review. J. Mater. Sci. Technol. 2021, 75, 225–251. [Google Scholar] [CrossRef]
- Li, W.; Qiu, T.; Wang, L.; Ren, S.; Zhang, J.; He, L.; Li, X. Preparation and Electromagnetic Properties of Core/Shell Polystyrene@Polypyrrole@Nickel Composite Microspheres. ACS Appl. Mater. Interfaces 2013, 5, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.G.; Li, Y.X.; Li, C.H.; Wang, J.Y.; Wang, Z.H.; Zhang, Y.H.; Qi, F.; Zhang, X.F. High Performance Microwave Absorption through Multi-Scale Metacomposite by Intergrating Ni@C Nanocapsules with Millimetric Polystyrene Sphere. J. Phys. D Appl. Phys. 2018, 51, 365303. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, H.; Jin, C.; Yang, B.; Xu, C.; Pei, K.; Zhang, H.; Yang, Z.; Che, R. Dimensional Design and Core–Shell Engineering of Nanomaterials for Electromagnetic Wave Absorption. Adv. Mater. 2022, 34, 2107538. [Google Scholar] [CrossRef]
- Yan, D.; Ren, P.; Pang, H.; Fu, Q.; Yang, M.; Li, Z. Efficient Electromagnetic Interference Shielding of Lightweight Graphene/Polystyrene Composite. J. Mater. Chem. 2012, 22, 18772. [Google Scholar] [CrossRef]
- Shakir, M.F.; Tariq, A.; Rehan, Z.A.; Nawab, Y.; Abdul Rashid, I.; Afzal, A.; Hamid, U.; Raza, F.; Zubair, K.; Rizwan, M.S.; et al. Effect of Nickel-Spinal-Ferrites on EMI Shielding Properties of Polystyrene/Polyaniline Blend. SN Appl. Sci. 2020, 2, 706. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Wang, D.; Li, Y.; Zheng, W.; Zhang, Q.; Kong, J. The Effect of Microspheres Surface Morphology on the Enhanced Microwave Absorbing Properties of MWCNTs. J. Polym. Res. 2021, 28, 91. [Google Scholar] [CrossRef]
- Wu, J.; Chen, T.; Luo, X.; Han, D.; Wang, Z.; Wu, J. TG/FTIR Analysis on Co-Pyrolysis Behavior of PE, PVC and PS. Waste Manag. 2014, 34, 676–682. [Google Scholar] [CrossRef]
- Liang, M.; Zhao, M.; Wang, H.; Shen, J.; Song, X. Enhanced Cycling Stability of Hierarchical NiCo2S4@Ni(OH)2@PPy Core–Shell Nanotube Arrays for Aqueous Asymmetric Supercapacitors. J. Mater. Chem. A 2018, 6, 2482–2493. [Google Scholar] [CrossRef]
- Omastová, M.; Trchová, M.; Kovářová, J.; Stejskal, J. Synthesis and Structural Study of Polypyrroles Prepared in the Presence of Surfactants. Synth. Met. 2003, 138, 447–455. [Google Scholar] [CrossRef]
- Liu, P.; Qiu, J.; Wu, X. Synthesis of Polyaniline Nanorods/Polystyrene Composite via Facile in Situ Radical Bulk Polymerization. J. Taiwan Inst. Chem. Eng. 2013, 44, 686–690. [Google Scholar] [CrossRef]
- Lourenco, E.; Felisberti, M.I. PS/EPDM Blends Prepared by In Situ Polymerization of Styrene. J. Appl. Polym. Sci. 2008, 110, 1804–1813. [Google Scholar] [CrossRef]
- Su, N.; Li, H.B.; Yuan, S.J.; Yi, S.P.; Yin, E.Q. Synthesis and Characterization of Polypyrrole Doped with Anionic Spherical Polyelectrolyte Brushes. Express Polym. Lett. 2012, 6, 697–705. [Google Scholar] [CrossRef]
- Wu, F.; Xie, A.; Sun, M.; Wang, Y.; Wang, M. Reduced Graphene Oxide (RGO) Modified Spongelike Polypyrrole (PPy) Aerogel for Excellent Electromagnetic Absorption. J. Mater. Chem. A 2015, 3, 14358–14369. [Google Scholar] [CrossRef]
- Zhou, X.; Wen, J.; Wang, Z.; Ma, X.; Wu, H. Broadband High-Performance Microwave Absorption of the Single-Layer Ti3C2Tx MXene. J. Mater. Sci. Technol. 2022, 115, 148–155. [Google Scholar] [CrossRef]
- Zhang, K.; Li, X.; Li, Y.; Lv, J.; Qin, R.; Wang, X.; Liu, X.; Liu, Y. Enhanced Microwave Absorption Property of Ferroferric Oxide: The Role of Magnetoelectric Resonance. Chem. Eng. J. 2022, 433, 134455. [Google Scholar] [CrossRef]
- Palsaniya, S.; Mukherji, S. Enhanced Dielectric and Electrostatic Energy Density of Electronic Conductive Organic-Metal Oxide Frameworks at Ultra-High Frequency. Carbon 2022, 196, 749–762. [Google Scholar] [CrossRef]
- Zhang, P.; Yao, Y.; Zhou, W.; Liu, Y.; Cao, X.; Zhang, Z. NiMnO3 Anchored on Reduced Graphene Oxide Nanosheets: A New High-Performance Microwave Absorbing Material. Nanomaterials 2022, 12, 1089. [Google Scholar] [CrossRef]
- Mei, Q.; Xiao, H.; Ding, G.; Liu, H.; Zhao, C.; Wang, R.; Huang, Z. Ultralight Open-Cell Graphene Aerogels with Multiple, Gradient Microstructures for Efficient Microwave Absorption. Nanomaterials 2022, 12, 1896. [Google Scholar] [CrossRef]
- Shi, Y.; Li, D.; Si, H.; Jiang, Z.; Li, M.; Gong, C. TiN/BN Composite with Excellent Thermal Stability for Efficiency Microwave Absorption in Wide Temperature Spectrum. J. Mater. Sci. Technol. 2022, 130, 249–255. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, Y.; Liu, P.; Ding, X.; Zong, M.; Wang, M. Synthesis of Magnetical Nanoparticles Decorated with Reduced Graphene Oxide as an Efficient Broad Band EM Wave Absorber. J. Alloys Compd. 2017, 692, 639–646. [Google Scholar] [CrossRef]
- Gao, H.; Wang, C.; Yang, Z.; Zhang, Y. 3D porous nickel metal foam/polyaniline heterostructure with excellent electromagnetic interference shielding capability and superior absorption based on pre-constructed macroscopic conductive framework. Compos. Sci. Technol. 2021, 213, 108896. [Google Scholar] [CrossRef]
- Pan, F.; Liu, Z.; Deng, B.; Dong, Y.; Zhu, X.; Huang, C.; Lu, W. Lotus Leaf-Derived Gradient Hierarchical Porous C/MoS2 Morphology Genetic Composites with Wideband and Tunable Electromagnetic Absorption Performance. Nano-Micro Lett. 2021, 13, 43. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, H.; Huang, J.; Li, N.; Yang, H.; Wang, Y.; Zhang, Y.; Dong, C.; Chen, G.; Guan, H. Polystyrene-Modulated Polypyrrole to Achieve Controllable Electromagnetic-Wave Absorption with Enhanced Environmental Stability. Nanomaterials 2022, 12, 2698. https://doi.org/10.3390/nano12152698
Gu H, Huang J, Li N, Yang H, Wang Y, Zhang Y, Dong C, Chen G, Guan H. Polystyrene-Modulated Polypyrrole to Achieve Controllable Electromagnetic-Wave Absorption with Enhanced Environmental Stability. Nanomaterials. 2022; 12(15):2698. https://doi.org/10.3390/nano12152698
Chicago/Turabian StyleGu, Huiling, Ji Huang, Na Li, Hua Yang, Yin Wang, Yang Zhang, Chengjun Dong, Gang Chen, and Hongtao Guan. 2022. "Polystyrene-Modulated Polypyrrole to Achieve Controllable Electromagnetic-Wave Absorption with Enhanced Environmental Stability" Nanomaterials 12, no. 15: 2698. https://doi.org/10.3390/nano12152698
APA StyleGu, H., Huang, J., Li, N., Yang, H., Wang, Y., Zhang, Y., Dong, C., Chen, G., & Guan, H. (2022). Polystyrene-Modulated Polypyrrole to Achieve Controllable Electromagnetic-Wave Absorption with Enhanced Environmental Stability. Nanomaterials, 12(15), 2698. https://doi.org/10.3390/nano12152698







