Effectiveness of Silver Nanoparticles Deposited in Facemask Material for Neutralising Viruses
Abstract
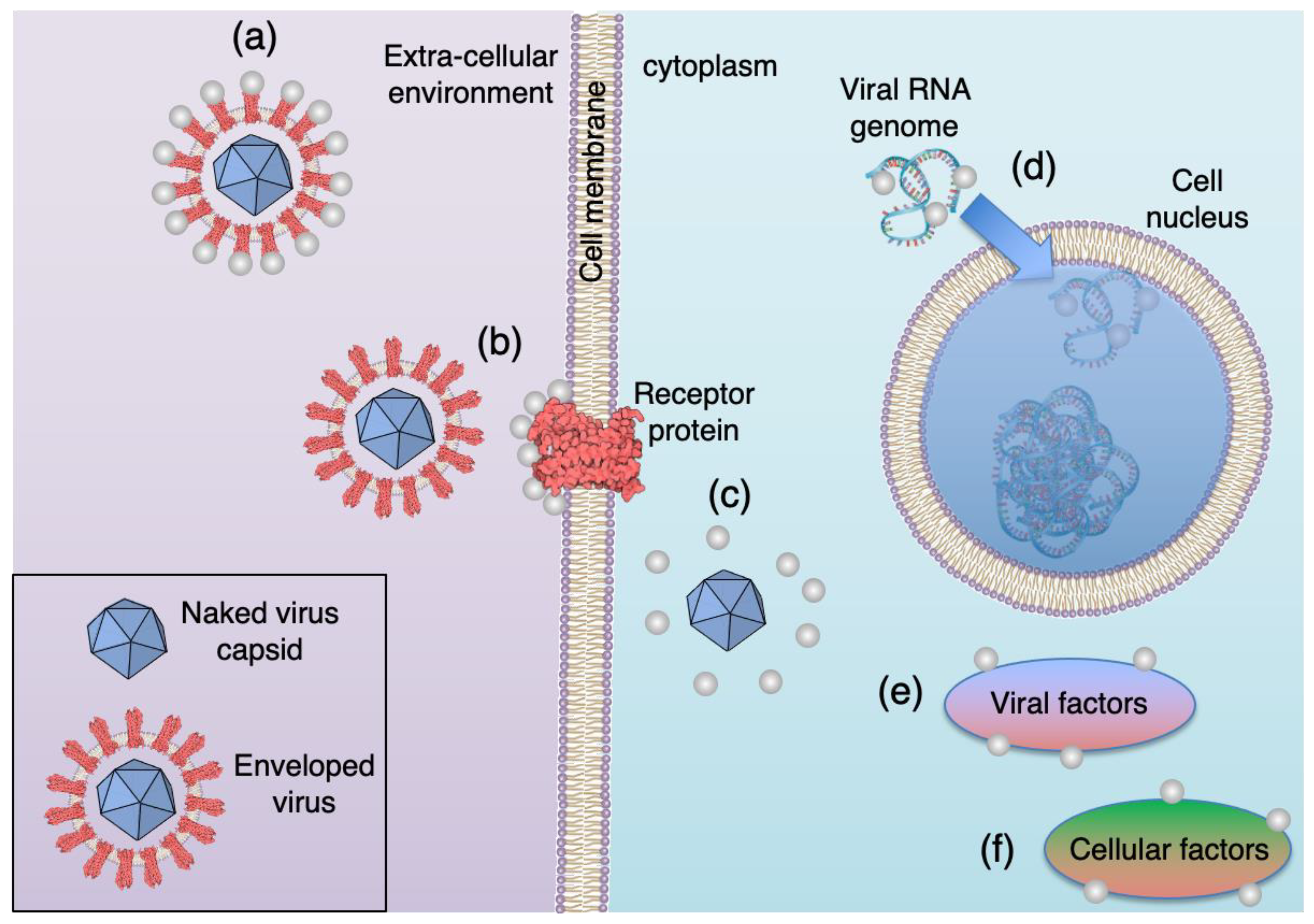
:1. Introduction
2. Materials and Methods
2.1. Ag Nanoparticle Synthesis and Cloth Coating
2.2. Nanoparticle Characterisation
2.3. Determining the Antiviral Performance of Coated Cloth
3. Results
3.1. Characterisation of Gas Phase Nanoparticles
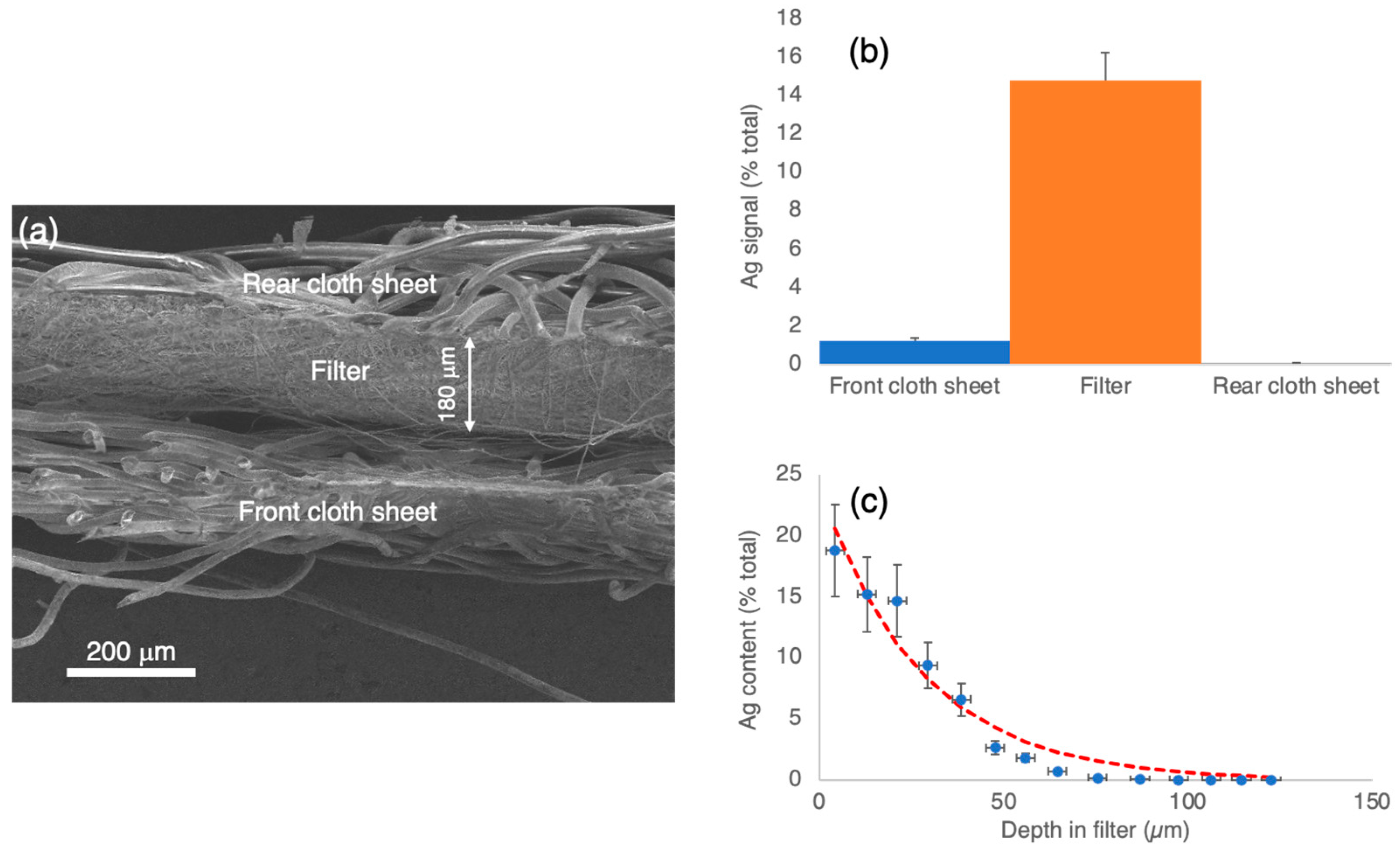
3.2. Characterisation of Nanoparticles Deposited in Cloth
3.3. Antiviral Performance of Coated Cloth vs. Nanoparticle Deposition Time
3.4. Antiviral Performance of Coated Cloth vs. Source Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Castellano, J.J.; Shafii, S.M.; Ko, F.; Donate, G.; Wright, T.E.; Mannari, R.J.; Payne, W.G.; Smith, D.J.; Robson, M.C. Comparative Evaluation of Silver-Containing Antimicrobial Dressings and Drugs. Int. Wound J. 2007, 4, 114–122. [Google Scholar] [CrossRef]
- Martínez-Castañón, G.A.; Niño-Martínez, N.; Martínez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis and Antibacterial Activity of Silver Nanoparticles with Different Sizes. J. Nanopart. Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Mijakovic, I. Green Synthesis and Antibacterial Applications of Gold and Silver Nanoparticles from Ligustrum Vulgare Berries. Sci. Rep. 2022, 12, 7902. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia Coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiu, Z.; Zhang, Q.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of Silver Nanoparticles with HIV-1. J. Nanobiotechnol. 2005, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerry, R.G.; Malik, S.; Redda, Y.T.; Sahoo, S.; Patra, J.K.; Majhi, S. Nano-Based Approach to Combat Emerging Viral (NIPAH Virus) Infection. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 196–220. [Google Scholar] [CrossRef]
- Rius-Rocabert, S.; Arranz-Herrero, J.; Fernández-Valdés, A.; Marciello, M.; Moreno, S.; Llinares-Pinel, F.; Presa, J.; Hernandez-Alcoceba, R.; López-Píriz, R.; Torrecillas, R.; et al. Broad Virus Inactivation Using Inorganic Micro/Nano-Particulate Materials. Mater. Today Bio 2022, 13, 100191. [Google Scholar] [CrossRef] [PubMed]
- Baram-Pinto, D.; Shukla, S.; Perkas, N.; Gedanken, A.; Sarid, R. Inhibition of Herpes Simplex Virus Type 1 Infection by Silver Nanoparticles Capped with Mercaptoethane Sulfonate. Bioconjug. Chem. 2009, 20, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Shionoiri, N.; Sato, T.; Fujimori, Y.; Nakayama, T.; Nemoto, M.; Matsunaga, T.; Tanaka, T. Investigation of the Antiviral Properties of Copper Iodide Nanoparticles against Feline Calicivirus. J. Biosci. Bioeng. 2012, 113, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Nangmenyi, G.; Li, X.; Mehrabi, S.; Mintz, E.; Economy, J. Silver-Modified Iron Oxide Nanoparticle Impregnated Fiberglass for Disinfection of Bacteria and Viruses in Water. Mater. Lett. 2011, 65, 1191–1193. [Google Scholar] [CrossRef]
- Mazurkova, N.A.; Spitsyna, Y.E.; Shikina, N.V.; Ismagilov, Z.R.; Zagrebel’nyi, S.N.; Ryabchikova, E.I. Interaction of Titanium Dioxide Nanoparticles with Influenza Virus. Nanotechnol. Russ. 2010, 5, 417–420. [Google Scholar] [CrossRef]
- Patial, S.; Kumar, A.; Raizada, P.; Van Le, Q.; Nguyen, V.-H.; Selvasembian, R.; Singh, P.; Thakur, S.; Hussain, C.M. Potential of Graphene Based Photocatalyst for Antiviral Activity with Emphasis on COVID-19: A Review. J. Environ. Chem. Eng. 2022, 10, 107527. [Google Scholar] [CrossRef]
- Rai, M.; Deshmukh, S.D.; Ingle, A.P.; Gupta, I.R.; Galdiero, M.; Galdiero, S. Metal nanoparticles: The protective nanoshield against virus infection. Crit. Rev. Microbiol. 2014, 42, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Binns, C. Magic Beacons and Magic Bullets: The Medical Applications of Functional Nanoparticles. In Introduction to Nanoscience and Nanotechnology, 2nd ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2022; p. 281. ISBN 978-1-119-17225-3. [Google Scholar]
- Wang, J.; Li, J.; Guo, G.; Wang, Q.; Tang, J.; Zhao, Y.; Qin, H.; Wahafu, T.; Shen, H.; Liu, X.; et al. Silver-Nanoparticles-Modified Biomaterial Surface Resistant to Staphylococcus: New Insight into the Antimicrobial Action of Silver. Sci. Rep. 2016, 6, 32699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Wang, Y.; Zu, Y.; Fu, Y.; Li, N.; Guo, N.; Liu, R.; Zhang, Y. Synthesis of Polyethylenimine (PEI) Functionalized Silver Nanoparticles by a Hydrothermal Method and Their Antibacterial Activity Study. Mater. Sci. Eng. C 2014, 42, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Sun, R.W.-Y.; Chen, R.; Hui, C.-K.; Ho, C.-M.; Luk, J.M.; Lau, G.K.K.; Che, C.-M. Silver Nanoparticles Inhibit Hepatitis B Virus Replication. Antivir. Ther. 2008, 13, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Maślana, K.; Kędzierski, T.; Żywicka, A.; Zielińska, B.; Mijowska, E. Design of Self-Cleaning and Self-Disinfecting Paper-Shaped Photocatalysts Based on Wood and Eucalyptus Derived Cellulose Fibers Modified with GCN/Ag Nanoparticles. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100656. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeong, J.-K.; Han, J.W.; Zhang, X.-F.; Park, J.H.; Kim, J.-H. Multidimensional Effects of Biologically Synthesized Silver Nanoparticles in Helicobacter Pylori, Helicobacter Felis, and Human Lung (L132) and Lung Carcinoma A549 Cells. Nanoscale Res. Lett. 2015, 10, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sheekh, M.M.; Shabaan, M.T.; Hassan, L.; Morsi, H.H. Antiviral Activity of Algae Biosynthesized Silver and Gold Nanoparticles against Herps Simplex (HSV-1) Virus in Vitro Using Cell-Line Culture Technique. Int. J. Environ. Health Res. 2022, 32, 616–627. [Google Scholar] [CrossRef]
- Li, K.; Jia, X.; Tang, A.; Zhu, X.; Meng, H.; Wang, Y. Preparation of Spherical and Triangular Silver Nanoparticles by a Convenient Method. Integr. Ferroelectr. 2012, 136, 9–14. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, W.; Xue, J.; Liu, Y.; Liu, Y.; Yan, P.; Liu, J.; Tang, J. Recent Advances in Synthetic Methods and Applications of Silver Nanostructures. Nanoscale Res. Lett. 2018, 13, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.; Raghavendra, G.M.; Kim, D.; Seo, J. One-Step Synthesis of Starch-Silver Nanoparticle Solution and Its Application to Antibacterial Paper Coating. Int. J. Biol. Macromol. 2018, 107, 2285–2290. [Google Scholar] [CrossRef]
- Hiragond, C.B.; Kshirsagar, A.S.; Dhapte, V.V.; Khanna, T.; Joshi, P.; More, P.V. Enhanced Anti-Microbial Response of Commercial Face Mask Using Colloidal Silver Nanoparticles. Vacuum 2018, 156, 475–482. [Google Scholar] [CrossRef]
- Gonzalez, A.; Aboubakr, H.A.; Brockgreitens, J.; Hao, W.; Wang, Y.; Goyal, S.M.; Abbas, A. Durable Nanocomposite Face Masks with High Particulate Filtration and Rapid Inactivation of Coronaviruses. Sci. Rep. 2021, 11, 24318. [Google Scholar] [CrossRef]
- Hamouda, T.; Kafafy, H.; Mashaly, H.M.; Aly, N.M. Breathability Performance of Antiviral Cloth Masks Treated with Silver Nanoparticles for Protection against COVID-19. J. Ind. Text. 2021, 51, 1494–1523. [Google Scholar] [CrossRef]
- Lee, H.Y.; Park, H.K.; Lee, Y.M.; Kim, K.; Park, S.B. A Practical Procedure for Producing Silver Nanocoated Fabric and Its Antibacterial Evaluation for Biomedical Applications. Chem. Commun. 2007, 28, 2959–2961. [Google Scholar] [CrossRef] [PubMed]
- Nageh, H.; Emam, M.H.; Ali, F.; Abdel Fattah, N.F.; Taha, M.; Amin, R.; Kamoun, E.A.; Loutfy, S.A.; Kasry, A. Zinc Oxide Nanoparticle-Loaded Electrospun Polyvinylidene Fluoride Nanofibers as a Potential Face Protector against Respiratory Viral Infections. ACS Omega 2022, 7, 14887–14896. [Google Scholar] [CrossRef] [PubMed]
- Castro-Mayorga, J.L.; Randazzo, W.; Fabra, M.J.; Lagaron, J.M.; Aznar, R.; Sánchez, G. Antiviral Properties of Silver Nanoparticles against Norovirus Surrogates and Their Efficacy in Coated Polyhydroxyalkanoates Systems. LWT—Food Sci. Technol. 2017, 79, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Karagoz, S.; Kiremitler, N.B.; Sarp, G.; Pekdemir, S.; Salem, S.; Goksu, A.G.; Onses, M.S.; Sozdutmaz, I.; Sahmetlioglu, E.; Ozkara, E.S.; et al. Antibacterial, Antiviral, and Self-Cleaning Mats with Sensing Capabilities Based on Electrospun Nanofibers Decorated with ZnO Nanorods and Ag Nanoparticles for Protective Clothing Applications. ACS Appl. Mater. Interfaces 2021, 13, 5678–5690. [Google Scholar] [CrossRef] [PubMed]
- Valerini, D.; Tammaro, L.; Vitali, R.; Guillot, G.; Rinaldi, A. Sputter-Deposited Ag Nanoparticles on Electrospun PCL Scaffolds: Morphology, Wettability and Antibacterial Activity. Coatings 2021, 11, 345. [Google Scholar] [CrossRef]
- Balagna, C.; Perero, S.; Percivalle, E.; Nepita, E.V.; Ferraris, M. Virucidal Effect against Coronavirus SARS-CoV-2 of a Silver Nanocluster/Silica Composite Sputtered Coating. Open Ceram. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Meister, T.L.; Fortmann, J.; Breisch, M.; Sengstock, C.; Steinmann, E.; Köller, M.; Pfaender, S.; Ludwig, A. Nanoscale Copper and Silver Thin Film Systems Display Differences in Antiviral and Antibacterial Properties. Sci. Rep. 2022, 12, 7193. [Google Scholar] [CrossRef] [PubMed]
- Bello-Lopez, J.M.; Silva-Bermudez, P.; Prado, G.; Martínez, A.; Ibáñez-Cervantes, G.; Cureño-Díaz, M.A.; Rocha-Zavaleta, L.; Manzo-Merino, J.; Almaguer-Flores, A.; Ramos-Vilchis, C.; et al. Biocide Effect against SARS-CoV-2 and ESKAPE Pathogens of a Noncytotoxic Silver–Copper Nanofilm. Biomed. Mater. 2021, 17, 15002. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Zhu, Z.; You, P.; Lin, J.; Cheung, C.F.; Lu, V.L.; Yan, F.; Chan, C.-Y.; Li, G. Plasmonic and Superhydrophobic Self-Decontaminating N95 Respirators. ACS Nano 2020, 14, 8846–8854. [Google Scholar] [CrossRef]
- Feng, J.; Hontañón, E.; Blanes, M.; Meyer, J.; Guo, X.; Santos, L.; Paltrinieri, L.; Ramlawi, N.; de Smet, L.C.P.M.; Nirschl, H.; et al. Scalable and Environmentally Benign Process for Smart Textile Nanofinishing. ACS Appl. Mater. Interfaces 2016, 8, 14756–14765. [Google Scholar] [CrossRef]
- Pfeiffer, T.V.; Feng, J.; Schmidt-Ott, A. New Developments in Spark Production of Nanoparticles. Adv. Powder Technol. 2014, 25, 56–70. [Google Scholar] [CrossRef]
- Binns, C. The Nanotechnology Toolkit. In Introduction to Nanoscience and Nanotechnology, 2nd ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2022; p. 131. ISBN 978-1-119-17225-3. [Google Scholar]
- Final Report of BUONAPART-E Project. Available online: https://cordis.europa.eu/project/id/280765/reporting (accessed on 15 February 2022).
- Bassi, M.R.; Sempere, R.N.; Meyn, P.; Polacek, C.; Arias, A. Extinction of Zika Virus and Usutu Virus by Lethal Mutagenesis Reveals Different Patterns of Sensitivity to Three Mutagenic Drugs. Antimicrob. Agents Chemother. 2018, 62, 9. [Google Scholar] [CrossRef] [Green Version]
- Chaudhry, Y.; Skinner, M.A.; Goodfellow, I.G. Recovery of Genetically Defined Murine Norovirus in Tissue Culture by Using a Fowlpox Virus Expressing T7 RNA Polymerase. J. Gen. Virol. 2007, 88, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, B.D. Measuring HCV Infectivity Produced in Cell Culture and In Vivo BT. In Hepatitis C: Methods and Protocols; Tang, H., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 329–336. ISBN 978-1-59745-394-3. [Google Scholar]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty per Cent Endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Preger, C.; Bulbucan, C.; Meuller, B.O.; Ludvigsson, L.; Kostanyan, A.; Muntwiler, M.; Deppert, K.; Westerström, R.; Messing, M.E. Controlled Oxidation and Self-Passivation of Bimetallic Magnetic FeCr and FeMn Aerosol Nanoparticles. J. Phys. Chem. C 2019, 123, 16083–16090. [Google Scholar] [CrossRef] [Green Version]
- Snellman, M.; Eom, N.; Ek, M.; Messing, M.E.; Deppert, K. Continuous Gas-Phase Synthesis of Core–Shell Nanoparticles via Surface Segregation. Nanoscale Adv. 2021, 3, 3041–3052. [Google Scholar] [CrossRef]
- Saiz, J.-C.; Blazquez, A.-B. Usutu Virus: Current Knowledge and Future Perspectives. Virus Adapt. Treat. 2017, 9, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Guo, J.; Song, S.; Yu, G.; Liu, H.; Yang, X.; Lu, Z. Preparation of Ag Nanoparticles by Spark Ablation in Gas as Catalysts for Electrocatalytic Hydrogen Production. RSC Adv. 2020, 10, 38583–38587. [Google Scholar] [CrossRef]
- Blackman, J.A. Chapter 2 Shell Models of Isolated Clusters. In Metallic Nanoparticles; Blackman, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 5, pp. 17–47. ISBN 1570-002X. [Google Scholar]
- Mello, V.M.; Eller, C.M.; Salvio, A.L.; Nascimento, F.F.; Figueiredo, C.M.; Silva, E.S.R.F.; Sousa, P.S.F.; Costa, P.F.; Paiva, A.A.P.; Mares-Guias, M.A.M.M.; et al. Effectiveness of face masks in blocking the transmission of SARS-CoV-2: A preliminary evaluation of masks used by SARS-CoV-2-infected individuals. PLoS ONE 2022, 17, e0264389. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Martín, R.; Rodrigo, I.; Ballesta, C.; Arias, A.; Mas, A.; Santos Burgos, B.; Normile, P.S.; De Toro, J.A.; Binns, C. Effectiveness of Silver Nanoparticles Deposited in Facemask Material for Neutralising Viruses. Nanomaterials 2022, 12, 2662. https://doi.org/10.3390/nano12152662
López-Martín R, Rodrigo I, Ballesta C, Arias A, Mas A, Santos Burgos B, Normile PS, De Toro JA, Binns C. Effectiveness of Silver Nanoparticles Deposited in Facemask Material for Neutralising Viruses. Nanomaterials. 2022; 12(15):2662. https://doi.org/10.3390/nano12152662
Chicago/Turabian StyleLópez-Martín, Raúl, Imanol Rodrigo, Carlos Ballesta, Armando Arias, Antonio Mas, Benito Santos Burgos, Peter S. Normile, Jose A. De Toro, and Chris Binns. 2022. "Effectiveness of Silver Nanoparticles Deposited in Facemask Material for Neutralising Viruses" Nanomaterials 12, no. 15: 2662. https://doi.org/10.3390/nano12152662
APA StyleLópez-Martín, R., Rodrigo, I., Ballesta, C., Arias, A., Mas, A., Santos Burgos, B., Normile, P. S., De Toro, J. A., & Binns, C. (2022). Effectiveness of Silver Nanoparticles Deposited in Facemask Material for Neutralising Viruses. Nanomaterials, 12(15), 2662. https://doi.org/10.3390/nano12152662






