UV-Vis Activated Cu2O/SnO2/WO3 Heterostructure for Photocatalytic Removal of Pesticides
Abstract
:1. Introduction
2. Experimental
2.1. Material Synthesis and Heterostructure Deposition
2.1.1. Heterostructure Powder Synthesis
2.1.2. Film Deposition
2.2. Photocatalytic Experiments
2.3. Characterization
3. Results and Discussion
3.1. Composition and Morphology
3.2. Photocatalytic Activity
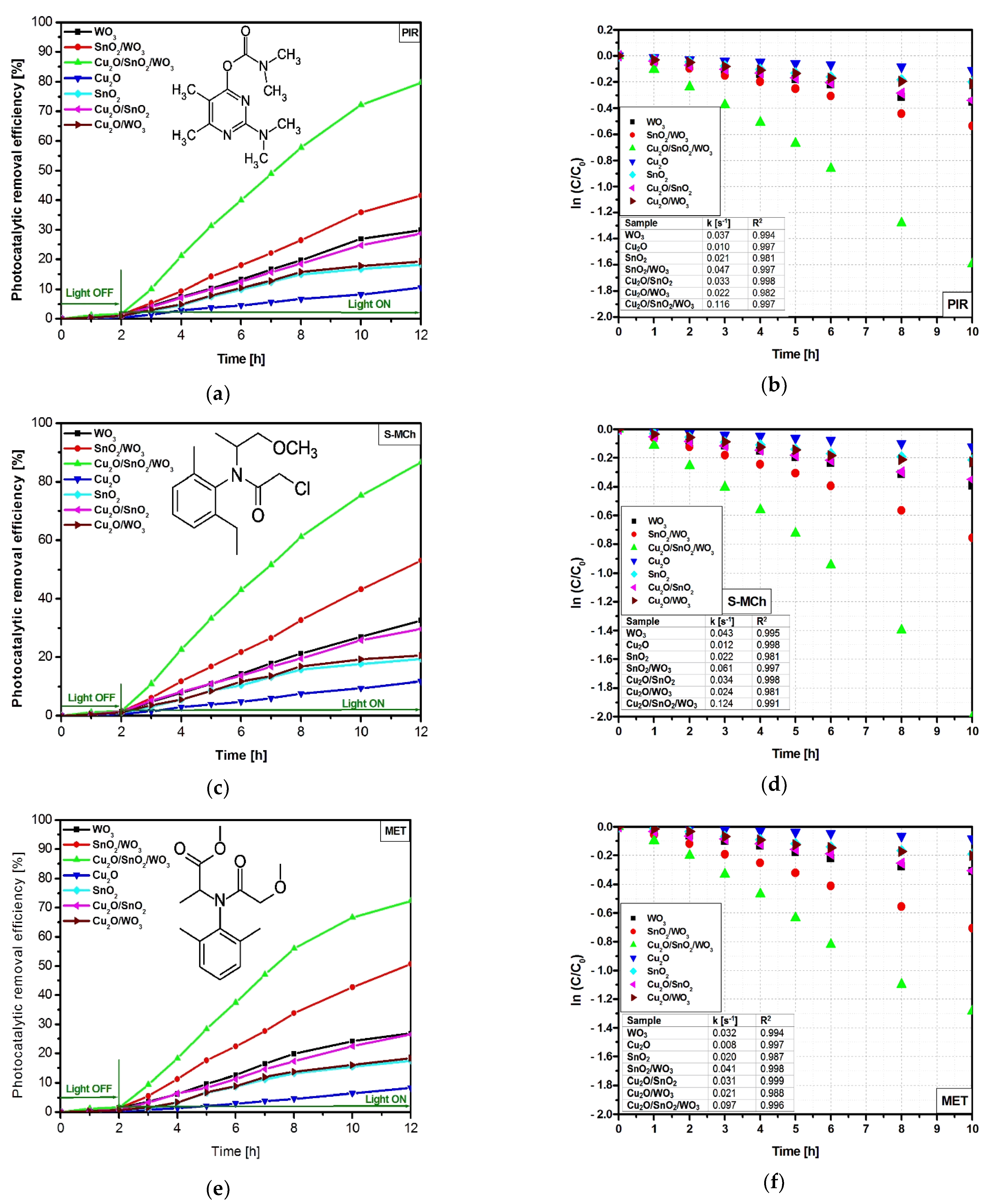
3.2.1. Degradation Efficiency and Kinetics
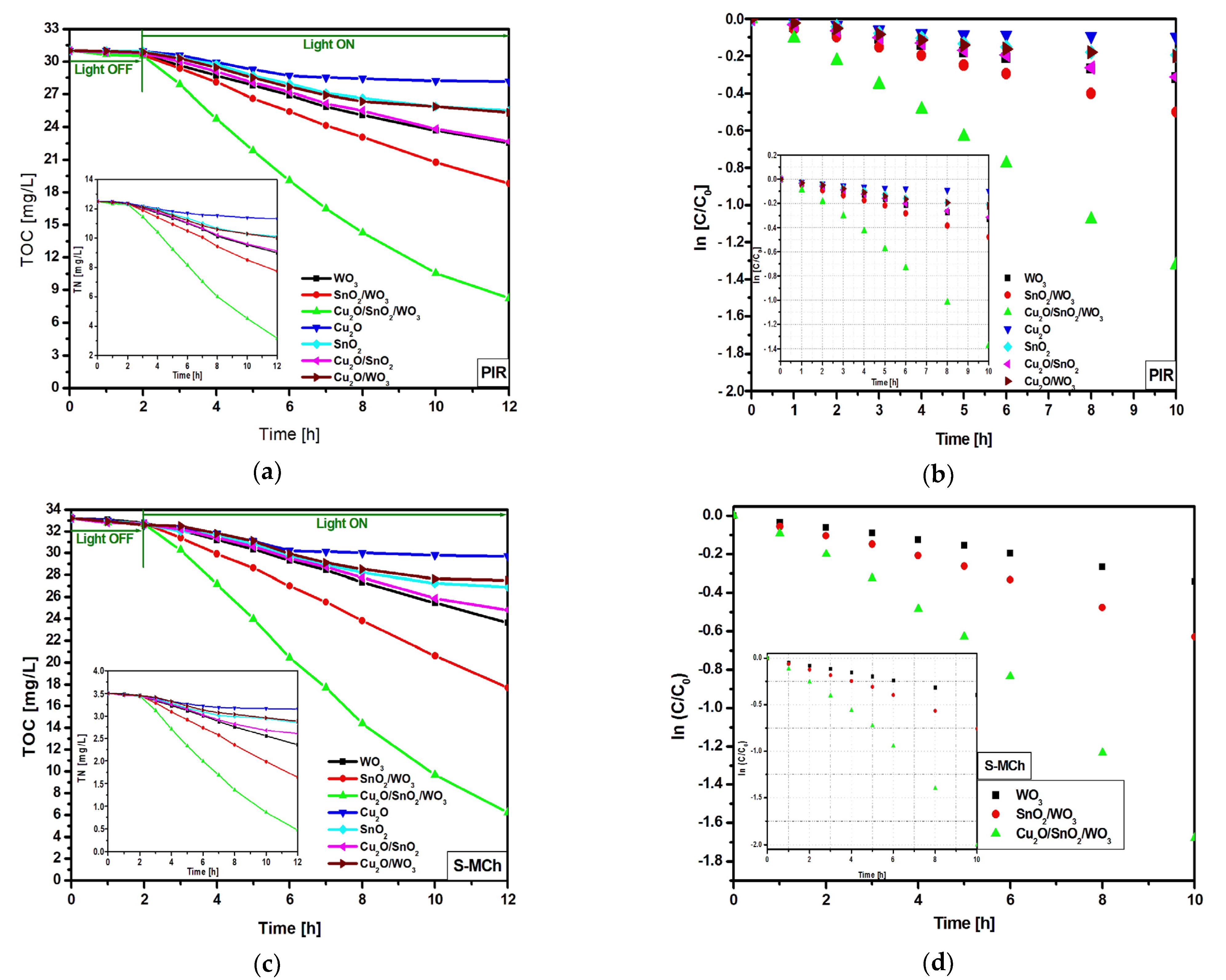
3.2.2. Reusability and Mechanism of Charge Carrier Generation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandrino, D.A.M.; Almeida, C.M.R.; Carvalho, M.F. Revisiting pesticide pollution: The case of fluorinated pesticides. Environ. Pollut. 2022, 292, 118315. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Tan, P.; Wu, Z. Advances in organophosphorus pesticides pollution: Current status and challenges in ecotoxicological, sustainable agriculture, and degradation strategies. J. Hazard. Mater. 2022, 424, 127494. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, G.; Joris, I.; Seuntjens, P. A spatial approach to identify priority areas for pesticide pollution mitigation. J. Environ. Manag. 2019, 246, 583–593. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Wu, Z.; Yu, B. Biochar for simultaneously enhancing the slow-release performance of fertilizers and minimizing the pollution of pesticides. J. Hazard. Mater. 2021, 407, 124865. [Google Scholar] [CrossRef]
- Tsaboula, A.; Menexes, G.; Papadopoulou-Mourkidou, E. Assessment and management of pesticide pollution at a river basin level part II: Optimization of pesticide monitoring networks on surface aquatic ecosystems by data analysis methods. Sci. Total Environ. 2019, 653, 1612–1622. [Google Scholar] [CrossRef]
- Dudita, M.; Bogatu, C.; Enesca, A.; Duta, A. The influence of the additives composition and concentration on the properties of SnOx thin films used in photocatalysis. Mater. Lett. 2011, 65, 2185–2189. [Google Scholar] [CrossRef]
- Kaushal, J.; Khatri, M.; Arya, S.K. A treatise on Organophosphate pesticide pollution: Current strategies and advancements in their environmental degradation and elimination. Ecotox. Environ. Saf. 2021, 207, 111483. [Google Scholar] [CrossRef]
- Gong, W.; Barrett, H.; Qu, H. Application of biochar: An approach to attenuate the pollution of the chiral pesticide fipronil and its metabolites in leachate from activated sludge. Process Saf. Environ. 2021, 149, 936–945. [Google Scholar] [CrossRef]
- Sahin, C.; Ekrem Karpuzcu, M. Mitigation of organophosphate pesticide pollution in agricultural watersheds. Mitigation of organophosphate pesticide pollution in agricultural watersheds. Sci. Total Environ. 2020, 710, 136261. [Google Scholar] [CrossRef]
- Wołejko, E.; Jabłońska-Trypuć, A.; Łozowicka, B. Soil biological activity as an indicator of soil pollution with pesticides—A review. Appl. Soil Ecol. 2020, 147, 103356. [Google Scholar] [CrossRef]
- Parada, J.; Rubilar, O.; Tortella, G.R. Combined pollution of copper nanoparticles and atrazine in soil: Effects on dissipation of the pesticide and on microbiological community profiles. J. Hazard. Mater. 2019, 361, 228–236. [Google Scholar] [CrossRef]
- Solé, M.; Bonsignore, M.; Freitas, R. Exploring alternative biomarkers of pesticide pollution in clams. Mar. Pollut. Bull. 2018, 136, 61–67. [Google Scholar] [CrossRef]
- Wan, N.F.; Ji, X.Y.; Li, B. An eco-engineering assessment index for chemical pesticide pollution management strategies to complex agro-ecosystems. Ecol. Eng. 2013, 52, 203–210. [Google Scholar] [CrossRef]
- Enesca, A.; Duta, A.; Schoonman, J. Influence of tantalum dopant ions (Ta5+) on the efficiency of the tungsten trioxide photoelectrode. Phys. Status Solidi A 2008, 205, 2038–2041. [Google Scholar] [CrossRef]
- Horne, G.P.; Zalupski, P.R.; Mincher, B.J. Radiolytic degradation of formic acid and formate in aqueous solution: Modeling the final stages of organic mineralization under advanced oxidation process conditions. Water Res. 2020, 186, 116314. [Google Scholar] [CrossRef]
- Mukherjee, A.; Mullick, A.; Moulik, S. Performance and energetic analysis of hydrodynamic cavitation and potential integration with existing advanced oxidation processes: A case study for real life greywater treatment. Ultrason. Sonochem. 2020, 66, 105116. [Google Scholar] [CrossRef]
- Chen, C.J.; Wu, C.C.; Hsieh, L.T.; Chen, K.C. Treatment of Trichloroethylene with Photocatalyst-Coated Optical Fiber. Water 2019, 11, 2391. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Zhao, Y.; Pu, M.; Zhang, Y.; Zhang, H.; Cheng, C. The Effect of Sintering Oxygen Partial Pressure on a SmBiO3 Buffer Layer for Coated Conductors via Chemical Solution Deposition. Coatings 2016, 6, 50. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Dai, W. Microwave-Hydrothermal Synthesis of SnO2-CNTs Hybrid Nanocomposites with Visible Light Photocatalytic Activity. Nanomaterials 2017, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Antoniadou, M.; Arfanis, M.K.; Ibrahim, I.; Falaras, P. Bifunctional g-C3N4/WO3 Thin Films for Photocatalytic Water Purification. Water 2019, 11, 2439. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Virtanen, S. Anodic ZnO Microsheet Coating on Zn with Sub-Surface Microtrenched Zn Layer Reduces Risk of Localized Corrosion and Improves Bioactivity of Pure Zn. Coatings 2021, 11, 486. [Google Scholar] [CrossRef]
- Liao, Y.K.; Liu, Y.T.; Hsieh, D.H.; Shen, T.L.; Hsieh, M.Y.; Tzou, A.J.; Chen, S.C.; Tsai, Y.L.; Lin, W.S.; Chan, S.W.; et al. Breakthrough to Non-Vacuum Deposition of Single-Crystal, Ultra-Thin, Homogeneous Nanoparticle Layers: A Better Alternative to Chemical Bath Deposition and Atomic Layer Deposition. Nanomaterials 2017, 7, 78. [Google Scholar] [CrossRef]
- Ramesh, K.; Gnanavel, B.; Shkir, M. Enhanced visible light photocatalytic degradation of bisphenol A (BPA) by reduced graphene oxide (RGO)–metal oxide (TiO2, ZnO and WO3) based nanocomposites. Diam. Relat. Mater. 2021, 118, 108514. [Google Scholar] [CrossRef]
- Zhang, P.; Liang, H.; Li, C. A novel Z-scheme BiOI/BiOCl nanofibers photocatalyst prepared by one-pot solvothermal with efficient visible-light-driven photocatalytic activity. Mater. Chem. Phys. 2021, 272, 125031. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, G.; Fan, W. The effect of piezo-photocatalysis on enhancing the charge carrier separation in BaTiO3/KNbO3 heterostructure photocatalyst. Appl. Surf. Sci. 2021, 562, 150164. [Google Scholar] [CrossRef]
- Baneto, M.; Enesca, A.; Mihoreanu, C.; Lare, Y.; Jondo, K.; Napo, K.; Duta, A. Effects of the growth temperature on the properties of spray deposited CuInS2 thin films for photovoltaic applications. Ceram. Int. 2015, 41, 4742–4749. [Google Scholar] [CrossRef]
- Koutavarapu, R.; Syed, K.; Shim, J. An effective CuO/Bi2WO6 heterostructured photocatalyst: Analyzing a charge-transfer mechanism for the enhanced visible-light-driven photocatalytic degradation of tetracycline and organic pollutants. Chemosphere 2022, 287, 132015. [Google Scholar] [CrossRef]
- Sridharan, M.; Maiyalagan, T. Recent progress in Tungsten disulphide based Photocatalyst for Hydrogen Production and Environmental Remediation. Chem. Eng. J. 2021, 424, 130393. [Google Scholar] [CrossRef]
- Liu, T.; Yang, K.; Jin, Z. Visible-light driven S-scheme Mn0.2Cd0.8S/CoTiO3 heterojunction for photocatalytic hydrogen evolution. Renew. Energ. 2021, 173, 389–400. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, D. Caihong Fang, CdS/CeO2 heterostructures as visible-light photocatalysts for the reduction of nitro to amine organics. J. Alloys Compd. 2021, 885, 160961. [Google Scholar] [CrossRef]
- Flihh, S.M.; Ammar, S.H. Fabrication and photocatalytic degradation activity of core/shell ZIF-67@CoWO4@CoS heterostructure photocatalysts under visible light. Environ. Nanotech. Monit. Manag. 2021, 16, 100595. [Google Scholar] [CrossRef]
- Zhai, D.; Lv, Z.; Lu, J. Pre-deposition growth of interfacial SiO2 layer by low-oxygen-partial-pressure oxidation in the Al2O3/4H-SiC MOS structure. Microelectron. Eng. 2021, 244–246, 111574. [Google Scholar] [CrossRef]
- Semenov, M.Y.; Semenov, Y.M.; Silaev, A.V.; Begunova, L.A. Assessing the Self-Purification Capacity of Surface Waters in Lake Baikal Watershed. Water 2019, 11, 1505. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-Q.; Liu, L.; Fu, X.; Tang, D.; Wang, Y.; Hu, S.; Yan, Q.-L. Transformation of Combustion Nanocatalysts inside Solid Rocket Motor under Various Pressures. Nanomaterials 2019, 9, 381. [Google Scholar] [CrossRef] [Green Version]
- Domene-López, D.; Sarabia-Riquelme, R.; García-Quesada, J.C.; Martin-Gullon, I. Custom-Made Chemically Modified Graphene Oxide to Improve the Anti-Scratch Resistance of Urethane-Acrylate Transparent Coatings. Coatings 2019, 9, 408. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Sun, D.; Shi, J.W. The synergy between electronic anchoring effect and internal electric field in CdS quantum dots decorated dandelion-like Fe-CeO2 nanoflowers for improved photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2019, 549, 179–188. [Google Scholar] [CrossRef]
- Enesca, A.; Duta, A. Tailoring WO3 thin layers using spray pyrolysis technique. Phys. Status Solidi C 2008, 5, 3499–3502. [Google Scholar] [CrossRef]
- Güneri, E.; Göde, F.; Saatçi, B. The effect of Cu2O layer on characteristic properties of n-CdS/p-Cu2O heterojunction. J. Mol. Struct. 2021, 1241, 130679. [Google Scholar] [CrossRef]
- Li, X.; Raza, S.; Liu, C. Directly electrospinning synthesized Z-scheme heterojunction TiO2@Ag@Cu2O nanofibers with enhanced photocatalytic degradation activity under solar light irradiation. J. Environ. Chem. Eng. 2021, 9, 106133. [Google Scholar] [CrossRef]
- Fentahun, D.A.; Tyagi, A.; Kar, K.K. Numerically investigating the AZO/Cu2O heterojunction solar cell using ZnO/CdS buffer layer. Optik 2021, 228, 166228. [Google Scholar] [CrossRef]
- Moghanlou, A.O.; Bezaatpour, A.; Salimi, F. Cu2O/rGO as an efficient photocatalyst for transferring of nitro group to amine group under visible light irradiation. Mater. Sci. Semicon. Proc. 2021, 130, 105838. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, Z.; Liu, Z. FeOOH as hole transfer layer to retard the photocorrosion of Cu2O for enhanced photoelctrochemical performance. Appl. Catal. B 2020, 260, 118213. [Google Scholar] [CrossRef]
- Zhu, Z.; Chu, H.; Jiang, L. Anti-microbial corrosion performance of concrete treated by Cu2O electrodeposition: Influence of different treatment parameters. Cem. Concr. Comp. 2021, 123, 104195. [Google Scholar] [CrossRef]
- Jepson, P.C.; Murray, K.; Neumeister, L. Selection of pesticides to reduce human and environmental health risks: A global guideline and minimum pesticides list. Lancet Planet. Health 2020, 4, e56–e63. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, G.; Atreya, K.; Geissen, V. Concentration and distribution of pesticide residues in soil: Non-dietary human health risk assessment. Chemosphere 2020, 253, 126594. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, W.; Gong, W. Assessing transfer of pesticide residues from chrysanthemum flowers into tea solution and associated health risks. Ecotox. Environ. Saf. 2020, 187, 109859. [Google Scholar] [CrossRef]
- Mandić-Rajčević, S.; Rubino, F.R.; Colosio, C. Establishing health-based biological exposure limits for pesticides: A proof of principle study using mancozeb. Regul. Toxicol. Pharm. 2020, 115, 104689. [Google Scholar] [CrossRef]
- Gamboa, L.C.; Diaz, K.S.; van Wendel de Joode, B. Passive monitoring techniques to evaluate environmental pesticide exposure: Results from the Infant’s Environmental Health study (ISA). Environ. Res. 2020, 184, 109243. [Google Scholar] [CrossRef]
- Mouchaal, Y.; Enesca, A.; Mihoreanu, C.; Khelil, A.; Duta, A. Tuning the opto-electrical properties of SnO2 thin films by Ag+1 and In+3 co-doping. Mater. Sci. Eng. B 2015, 199, 22–29. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Wang, H. Well-dispersed Pt nanocrystals on the heterostructured TiO2/SnO2 nanofibers and the enhanced photocatalytic properties. Appl. Surf. Sci. 2014, 319, 21–28. [Google Scholar] [CrossRef]
- Hamadanian, M.; Jabbari, V.; Mutlay, I. Preparation of novel hetero-nanostructures and high efficient visible light-active photocatalyst using incorporation of CNT as an electron-transfer channel into the support TiO2 and PbS. J. Taiwan Inst. Chem. Eng. 2013, 44, 748–757. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, X.; Zhu, R. Ag and Cu2O modified 3D flower-like ZnO nanocomposites and evaluated by photocatalysis oxidation activity regulation. Ceram. Int. 2019, 45, 23310–23319. [Google Scholar] [CrossRef]
- Tobajas, M.; Belver, C.; Rodríguez, J.J. Degradation of emerging pollutants in water under solar irradiation using novel TiO2-ZnO/clay nanoarchitectures. Chem. Eng. J. 2017, 309, 596–606. [Google Scholar] [CrossRef]
- Mohammadiyan, E.; Ghafuri, H.; Kakanejadifard, A. Synthesis and characterization of a magnetic Fe3O4@CeO2 nanocomposite decorated with Ag nanoparticle and investigation of synergistic effects of Ag on photocatalytic activity. Optik 2018, 166, 39–48. [Google Scholar] [CrossRef]
- Enesca, A.; Andronic, L.; Duta, A. The influence of surfactants on the crystalline structure, electrical and photocatalytic properties of hybrid multi-structured (SnO2, TiO2 and WO3) thin films. Appl. Surf. Sci. 2012, 258, 4339–4346. [Google Scholar] [CrossRef]
- Gao, C.; Li, J.; Shan, Z.; Huang, F.; Shen, H. Preparation and visible-light photocatalytic activity of In2S3/TiO2 composite. Mater. Chem. Phys. 2010, 122, 183–187. [Google Scholar] [CrossRef]
- Mise, T.; Nakada, T. Low temperature growth and properties of Cu–In–Te based 433 thin films for narrow bandgap solar cells. Thin Solid Films 2010, 518, 5604–5609. [Google Scholar] [CrossRef]

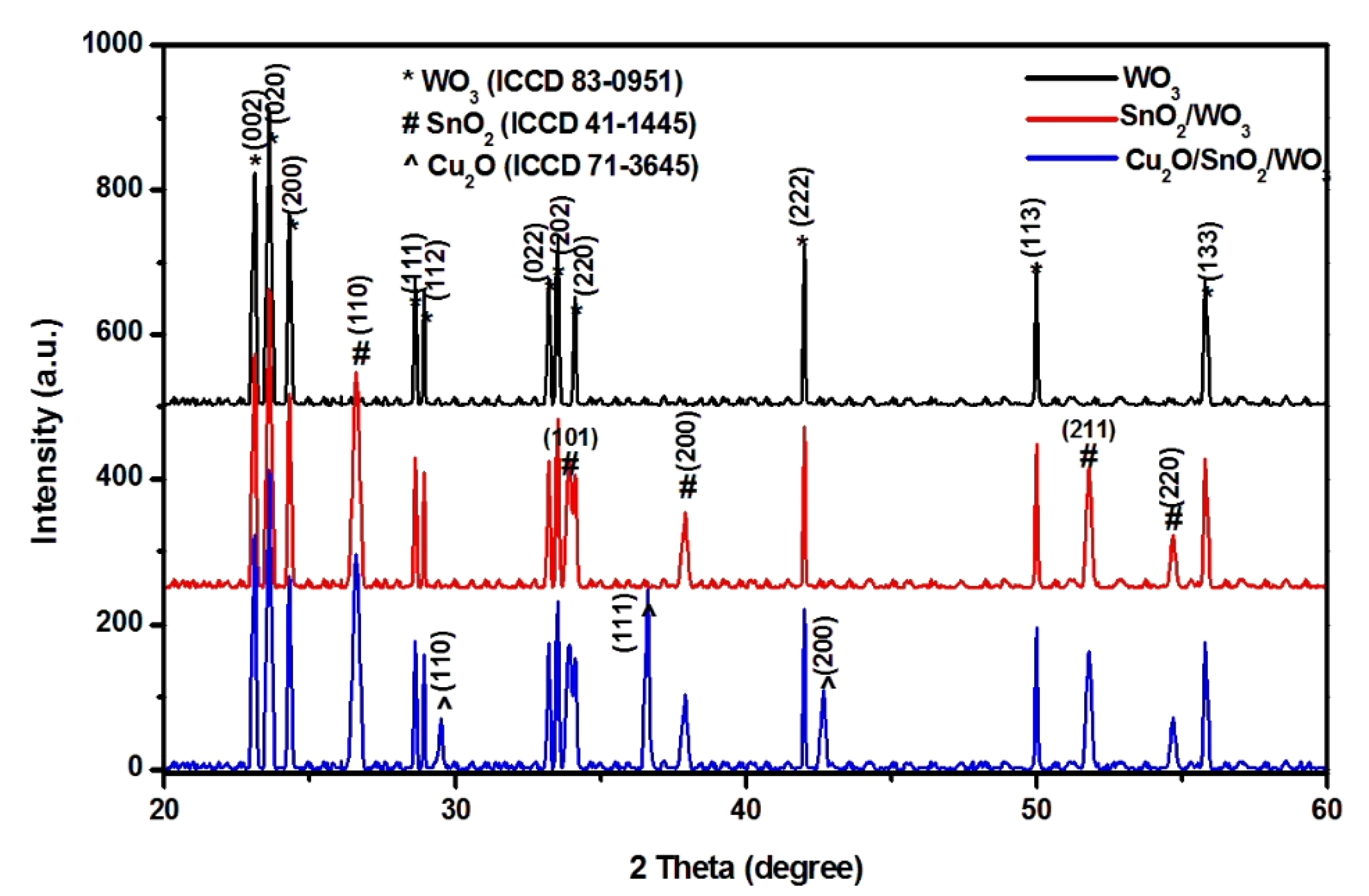
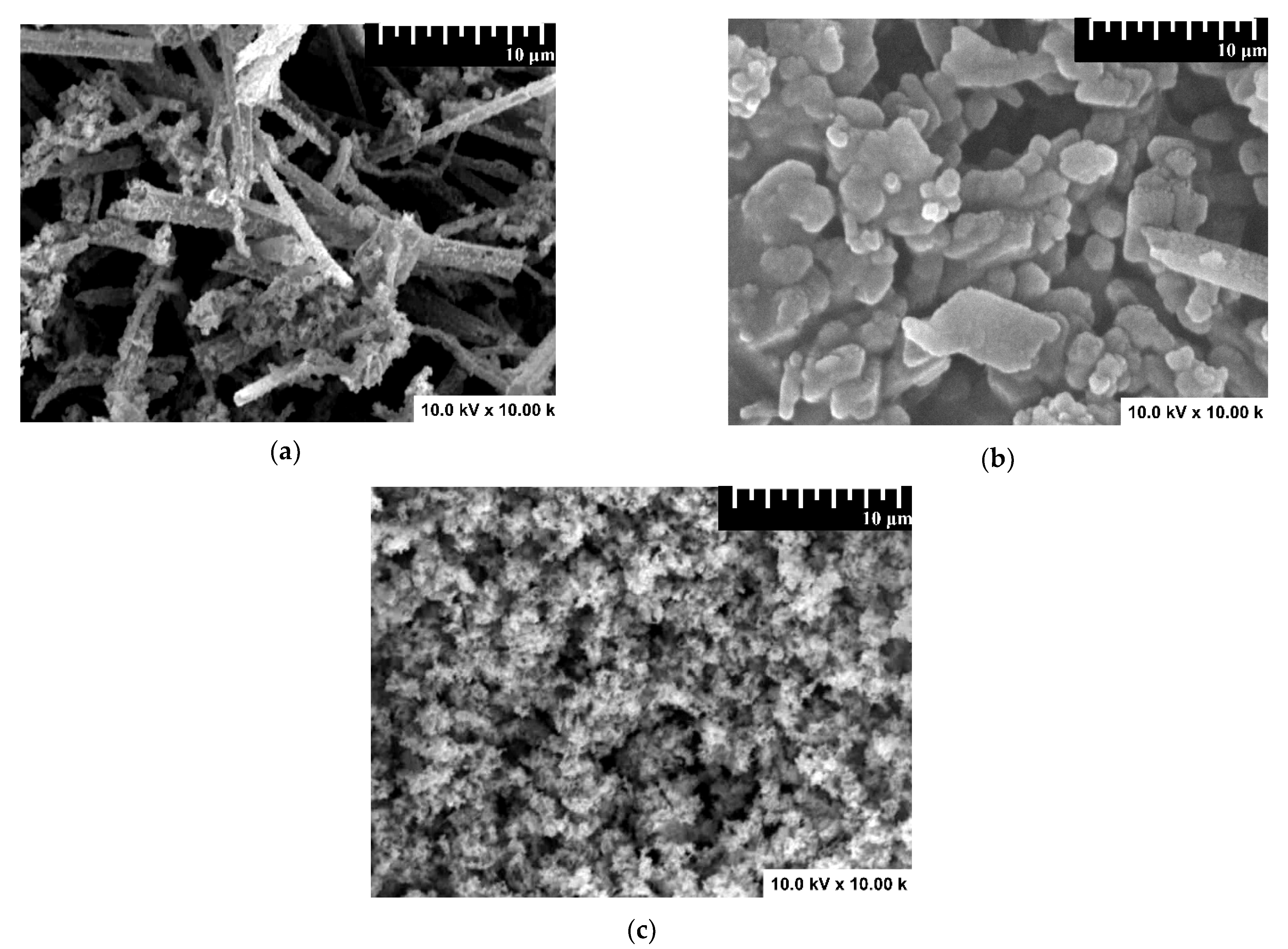

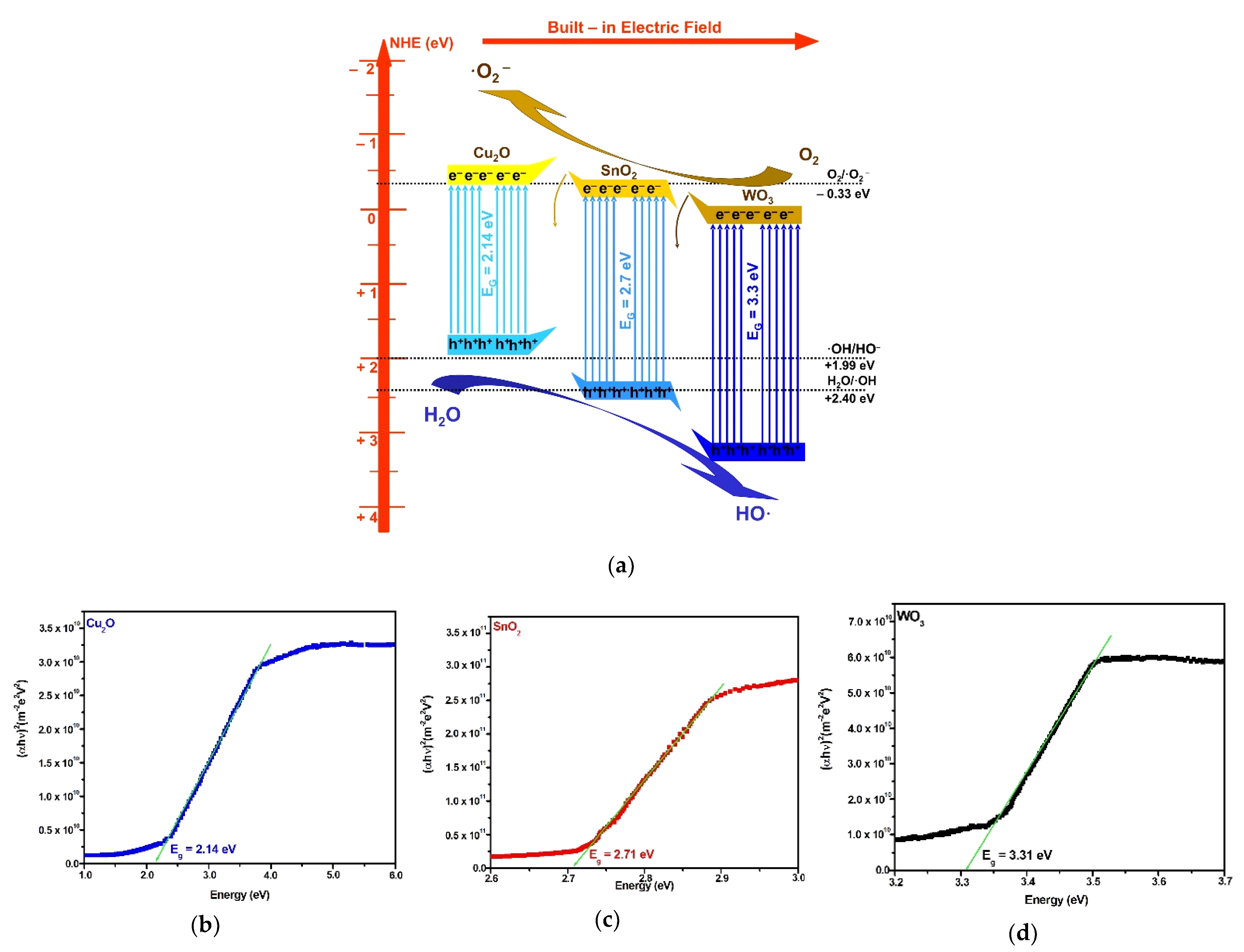
| Photocatalyst | Crystallite Size (Å) | ||
|---|---|---|---|
| WO3 | SnO2 | Cu2O | |
| WO3 | 80.5 | - | - |
| SnO2/WO3 | 81.8 | 77.1 | - |
| Cu2O/SnO2/WO3 | 81.6 | 77.3 | 42.4 |
| Samples | Elemental Composition [at.%] | ||||
|---|---|---|---|---|---|
| W | Sn | Cu | O | Oth 1 | |
| WO3 | 23.8 | - | - | 76.2 | 71.4 |
| SnO2/WO3 | 12.4 | 14.7 | - | 72.9 | 68.9 |
| Cu2O/SnO2/WO3 | 10.6 | 11.9 | 13.3 | 64.2 | 62.2 |
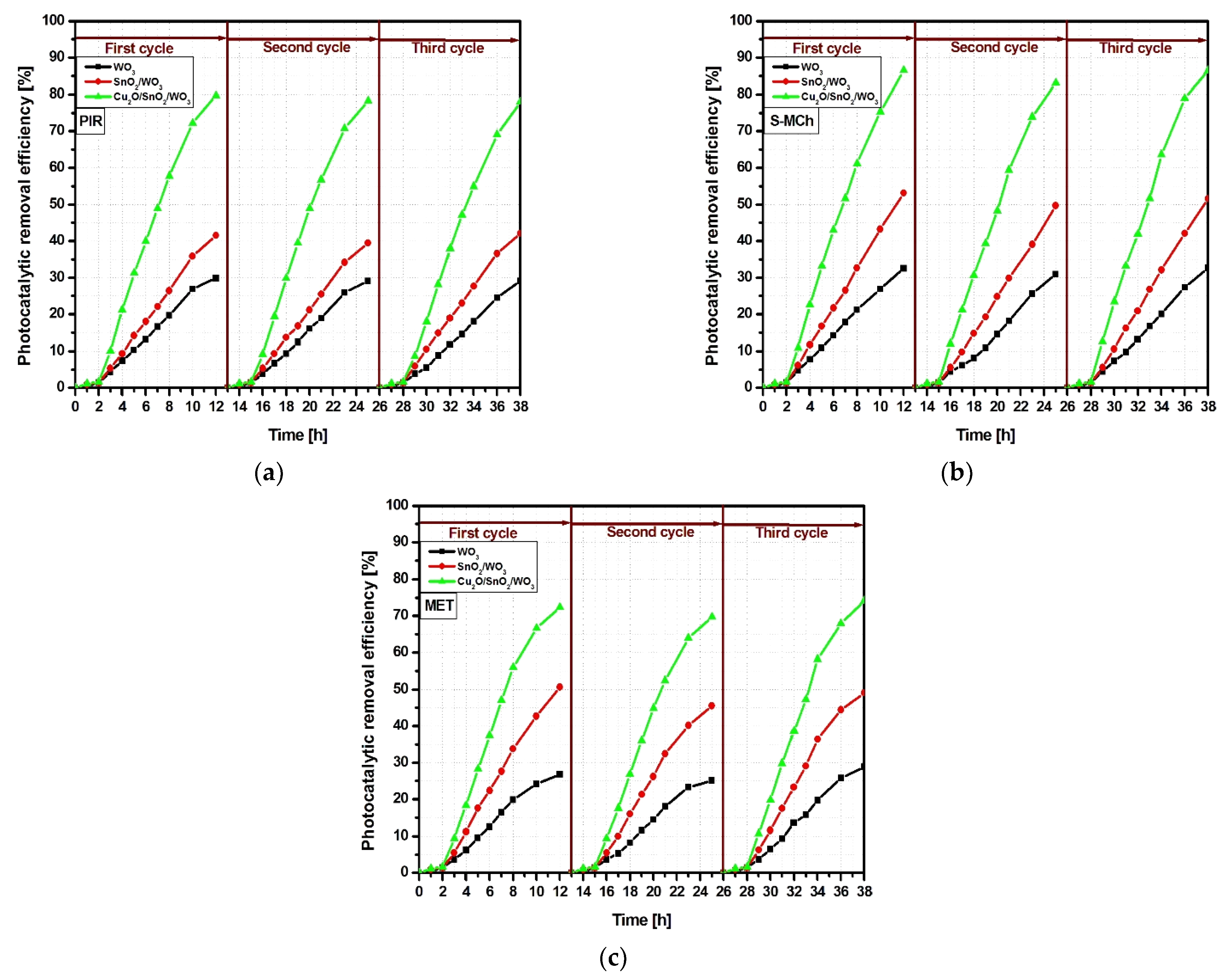
| Kinetic Parameters | PIR | S-MCh | MET | ||||
|---|---|---|---|---|---|---|---|
| TOC | TN | TOC | TN | TOC | TN | ||
| WO3 | k (s−1) | 0.031 | 0.033 | 0.033 | 0.038 | 0.030 | 0.032 |
| R2 | 0.997 | 0.998 | 0.998 | 0.999 | 0.998 | 0.994 | |
| Cu2O | k (s−1) | 0.010 | 0.009 | 0.011 | 0.009 | 0.006 | 0.005 |
| R2 | 0.911 | 0.947 | 0.919 | 0.928 | 0.920 | 0.866 | |
| SnO2 | k (s−1) | 0.020 | 0.022 | 0.022 | 0.019 | 0.019 | 0.020 |
| R2 | 0.978 | 0.989 | 0.982 | 0.965 | 0.981 | 0.969 | |
| SnO2/WO3 | k (s−1) | 0.049 | 0.047 | 0.062 | 0.074 | 0.045 | 0.043 |
| R2 | 0.999 | 0.998 | 0.995 | 0.994 | 0.998 | 0.998 | |
| Cu2O/SnO2 | k (s−1) | 0.031 | 0.032 | 0.030 | 0.030 | 0.030 | 0.031 |
| R2 | 0.998 | 0.998 | 0.998 | 0.989 | 0.999 | 0.999 | |
| Cu2O/WO3 | k (s−1) | 0.021 | 0.022 | 0.020 | 0.019 | 0.020 | 0.020 |
| R2 | 0.972 | 0.987 | 0.975 | 0.978 | 0.973 | 0.963 | |
| Cu2O/SnO2/WO3 | k (s−1) | 0.135 | 0.137 | 0.168 | 0.195 | 0.120 | 0.119 |
| R2 | 0.998 | 0.994 | 0.990 | 0.986 | 0.997 | 0.998 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enesca, A.; Andronic, L. UV-Vis Activated Cu2O/SnO2/WO3 Heterostructure for Photocatalytic Removal of Pesticides. Nanomaterials 2022, 12, 2648. https://doi.org/10.3390/nano12152648
Enesca A, Andronic L. UV-Vis Activated Cu2O/SnO2/WO3 Heterostructure for Photocatalytic Removal of Pesticides. Nanomaterials. 2022; 12(15):2648. https://doi.org/10.3390/nano12152648
Chicago/Turabian StyleEnesca, Alexandru, and Luminita Andronic. 2022. "UV-Vis Activated Cu2O/SnO2/WO3 Heterostructure for Photocatalytic Removal of Pesticides" Nanomaterials 12, no. 15: 2648. https://doi.org/10.3390/nano12152648
APA StyleEnesca, A., & Andronic, L. (2022). UV-Vis Activated Cu2O/SnO2/WO3 Heterostructure for Photocatalytic Removal of Pesticides. Nanomaterials, 12(15), 2648. https://doi.org/10.3390/nano12152648







