Fe3O4-PAA–(HP-γ-CDs) Biocompatible Ferrimagnetic Nanoparticles for Increasing the Efficacy in Superparamagnetic Hyperthermia
Abstract
:1. Introduction
- (a)
- The type, size, and the surface of the magnetic particles: in this regard, nanoparticles ranging in size between 10 and 100 nm are considered suitable for in vivo administration [3]. Additionally, these nanoparticles may use the enhanced permeability and retention (EPR) effect developed by several types of tumors [6,7], a phenomenon that plays a key role in the clinical outcome [8]. In addition, when administrated at the tumor site, magnetic nanoparticles could provide insightful data on the EPR effect of tumor tissue because, as demonstrated by Chen et al. [8], the higher the contrast of the tumor tissue after the injection of magnetic nanoparticles (used as contrast agents), the better the EPR effect is represented at the tumor site. Knowing this aspect, it may lead to a better understanding of magnetic nanoparticles’ mechanism of action within the tumor tissue. On the other hand, particles with a diameter above 200 nm are prone to phagocytosis while particles with a diameter less than 10 nm are removed by the renal system, which are key factors when selecting the nanoparticle size for biomedical applications [3,9]. Moreover, in order to avoid a cytotoxic effect related to the aggregation phenomenon, metallic nanoparticles should possess a zeta potential close to −30 mV [1]. Additionally, the EPR effect is facilitated by highly negatively charged particles [10];
- (b)
- The heat dissipation features of the magnetic nanoparticles (MNPs): this aspect is of major significance for magnetically induced hyperthermia, as the newly developed MNPs should be able to induce hyperthermic conditions (around 43 °C) in the presence of an alternating magnetic field when applied under nontoxic parameters (magnetic field frequency between 100 and 300 kHz) [3]. Moreover, the superparamagnetic characteristics of MNPs offer superior advantages for biomedical applications, as according to Langevin’s theory related to paramagnetism, superparamagnetic NPs elicit high saturation magnetization when an external magnetic field is applied while no remanent magnetization is developed by MNPs when the magnetic field is removed [3,4,11]. This feature may be of real interest when administrated in vivo, as magnetic dipolar interaction may be avoided, and thus thrombus-related events are minimal [3].
2. Materials and Methods
2.1. Molecular Docking Analysis
2.2. Computational Study Method on the Specific Loss Power in Superparamagnetic Hyperthermia
2.3. Synthesis Method and Characterization Techniques of Fe3O4-PAA Magnetic Nanoparticles and Fe3O4-PAA–(HP-γCDs) Nanobioconjugates
2.3.1. Materials and Synthesis Method
Fe3O4-PAA Nanoparticles Synthesis
Synthesis of Fe3O4-PAA–(HP-γ-CDs) Nanobioconjugates
2.3.2. X-ray Diffraction Technique
2.3.3. Fourier Transformed Infrared Spectroscopy
2.3.4. Transmission Electron Microscopy
2.3.5. Dynamic Light Scattering and Zeta Potential Measurements
2.3.6. Magnetic Characterization of Fe3O4-PAA Nanoparticles for Superparamagnetic Hyperthermia
2.4. Materials and Methods for In Vitro Experimental Study
2.4.1. Cell Line and Cell Culture Conditions
2.4.2. MTT Assay under Standard Conditions
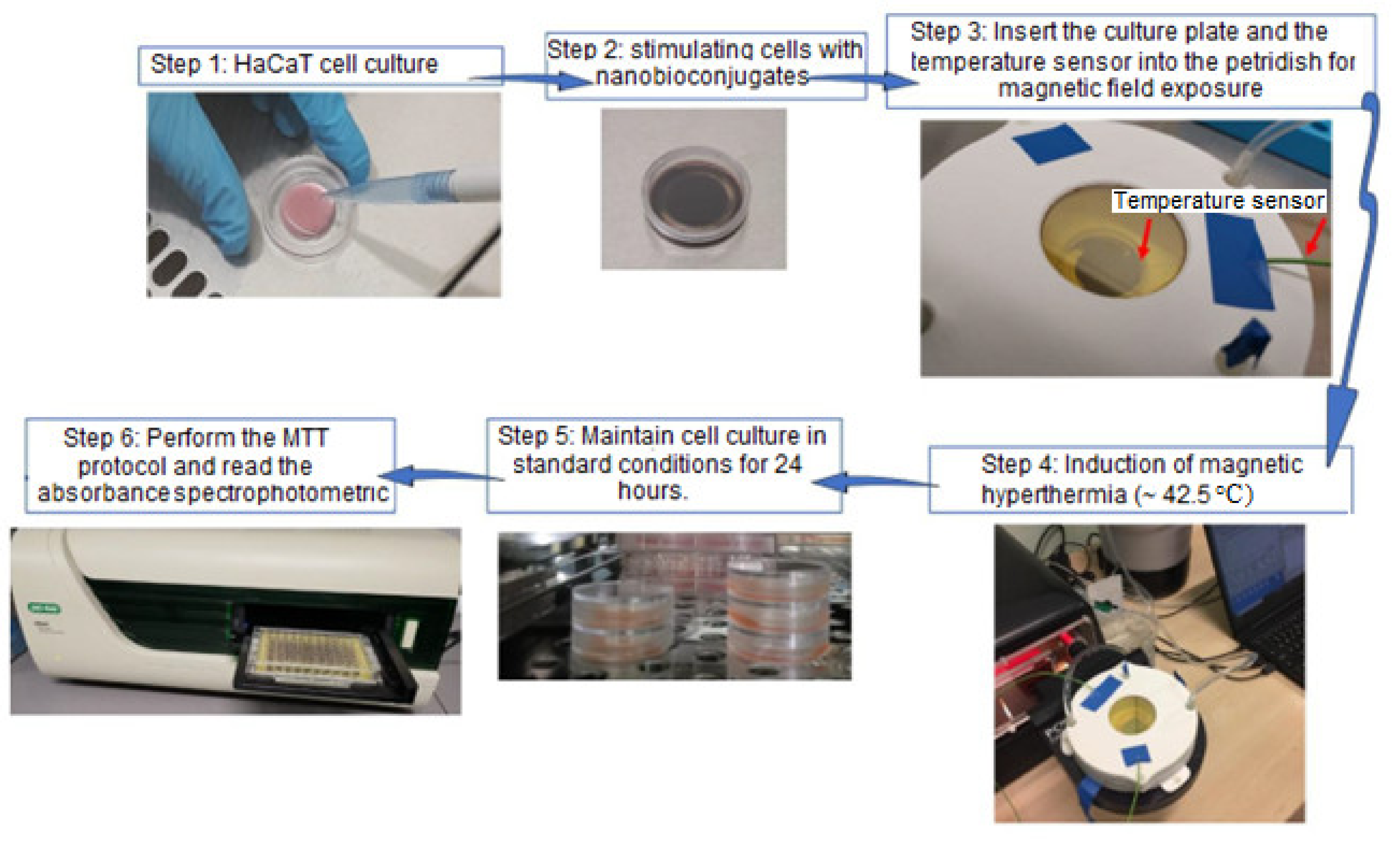
2.4.3. In Vitro Magnetically Induced Hyperthermia (MHT) Protocol
2.5. Experimental Magnetic Hyperthermia
3. Results and Discussion
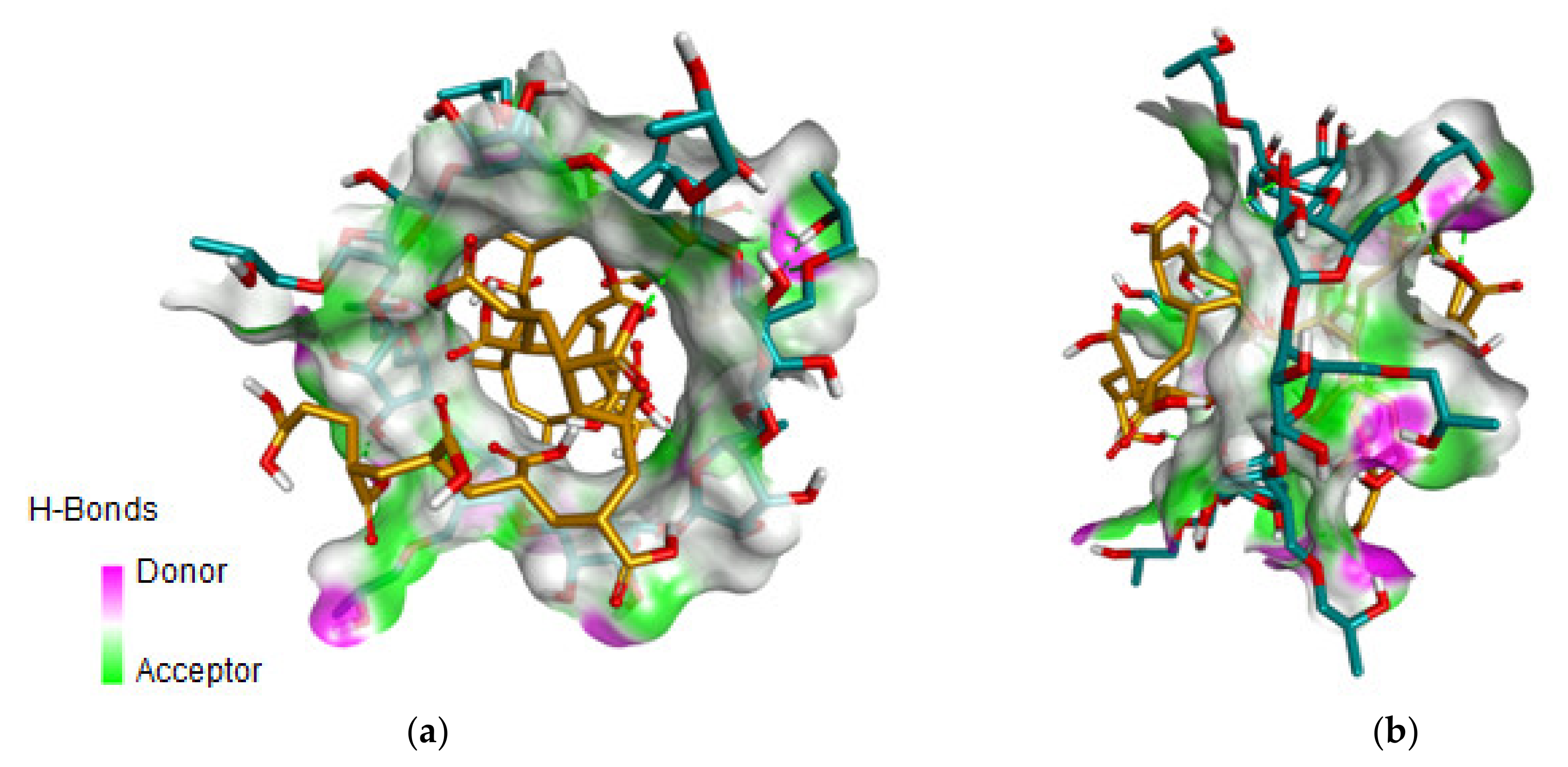
3.1. Molecular Docking
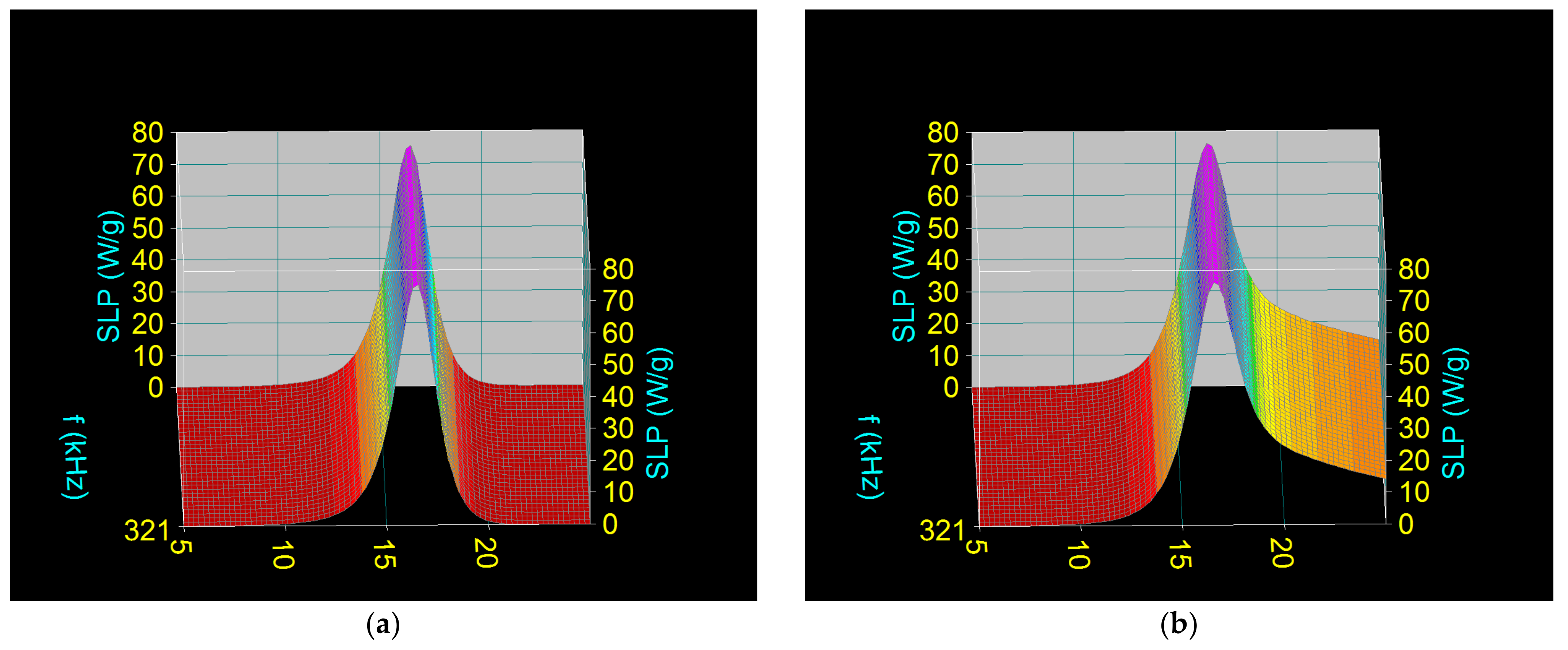
3.2. Computational Assessment of Specific Loss Power in Superparamagnetic Hyperthermia with Fe3O4-PAA Nanoparticles
3.3. Obtaining Superparamagnetic Fe3O4-PAA–(HP-γ-CDs) Nanobioconjugates
3.3.1. Characterization of Fe3O4-PAA Ferrimagnetic Nanoparticles
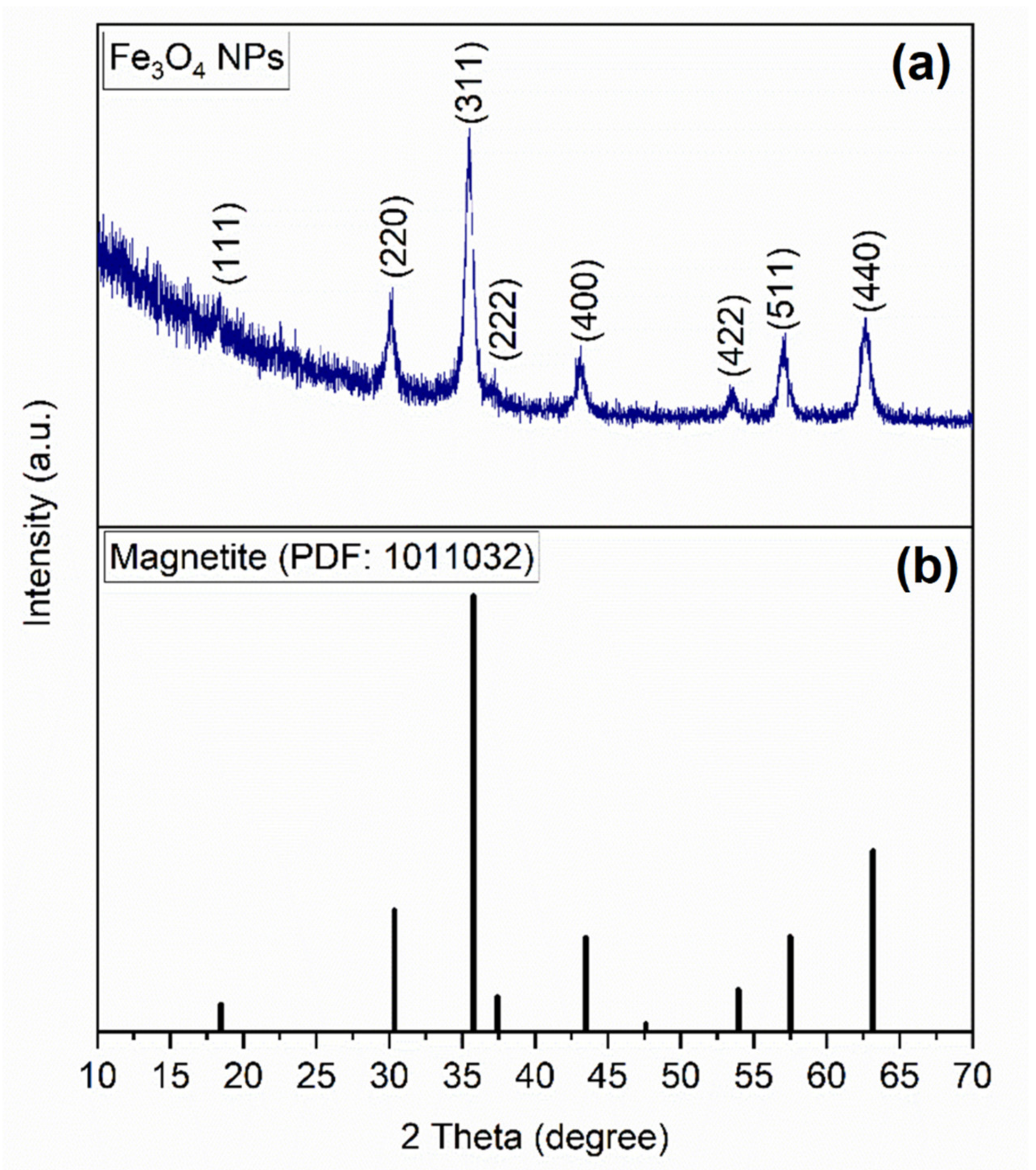
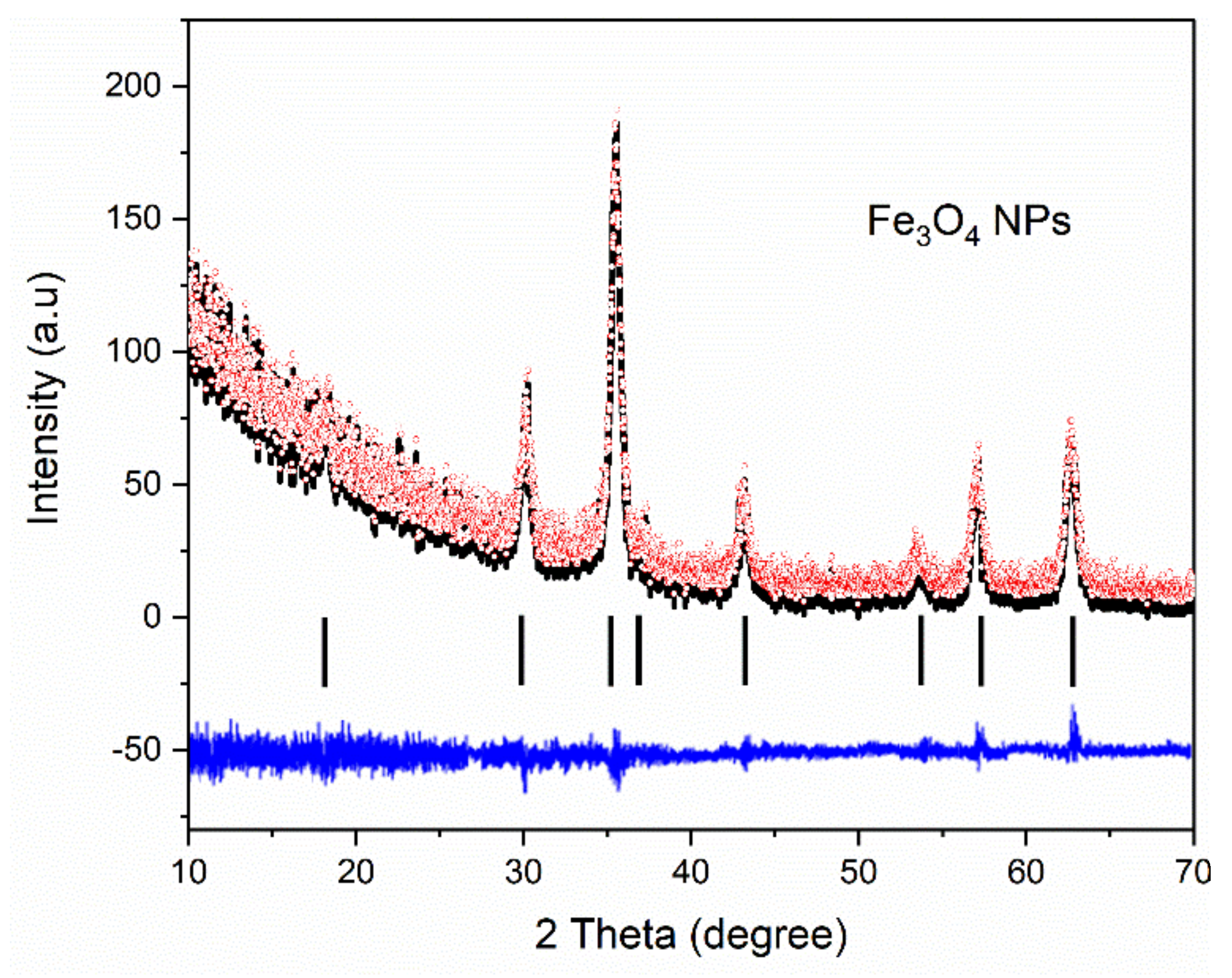
X-ray Diffraction of Fe3O4-PAA Nanoparticles
Fourier Transformed Infrared Spectroscopy of Fe3O4-PAA Nanoparticles
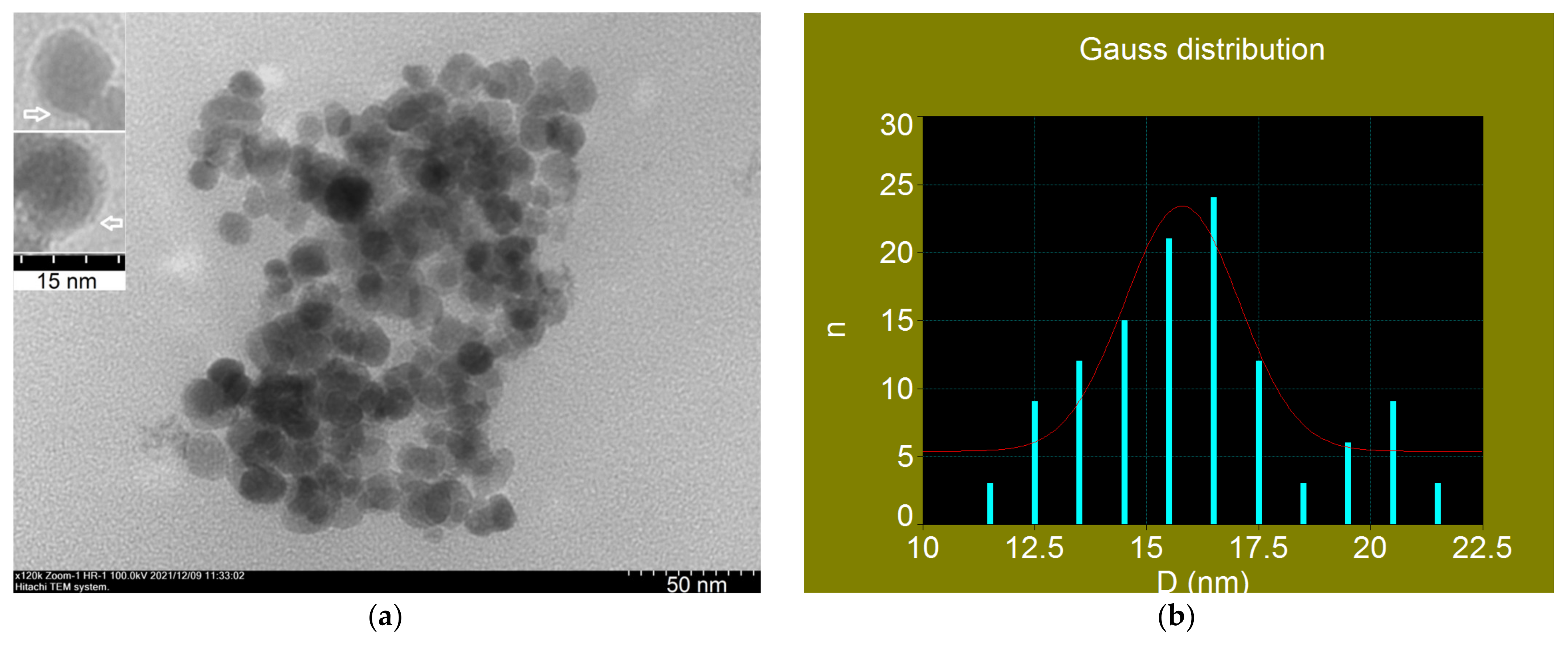
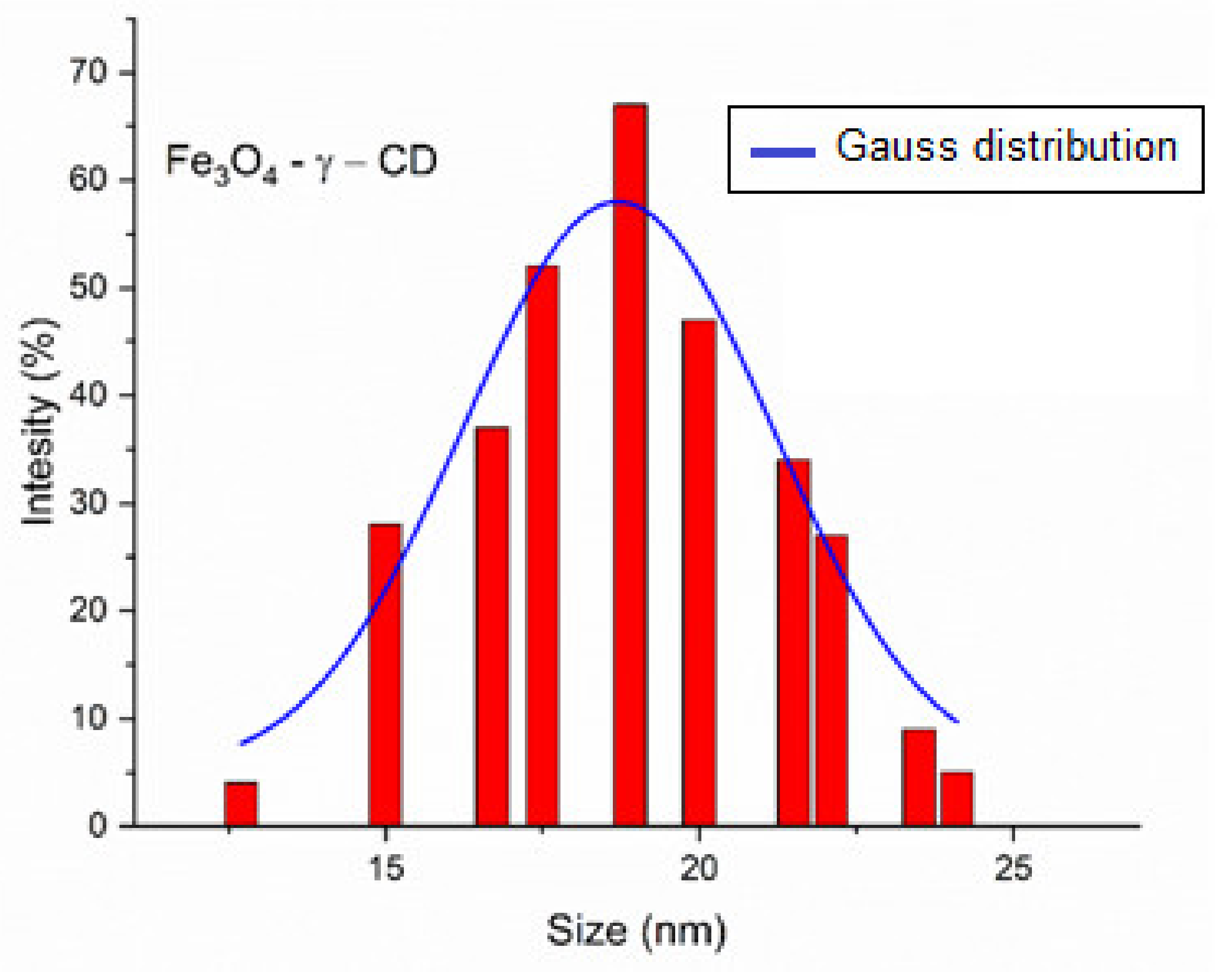
Transmission Electron Microscopy of Fe3O4-PAA Nanoparticles
Dynamic Light Scattering and Zeta Potential of Fe3O4-PAA Nanoparticles
Magnetic Behavior of Fe3O4-PAA Nanoparticles
3.3.2. Characterization of Fe3O4-PAA–(HP-γ-CDs) Nanobioconjugates
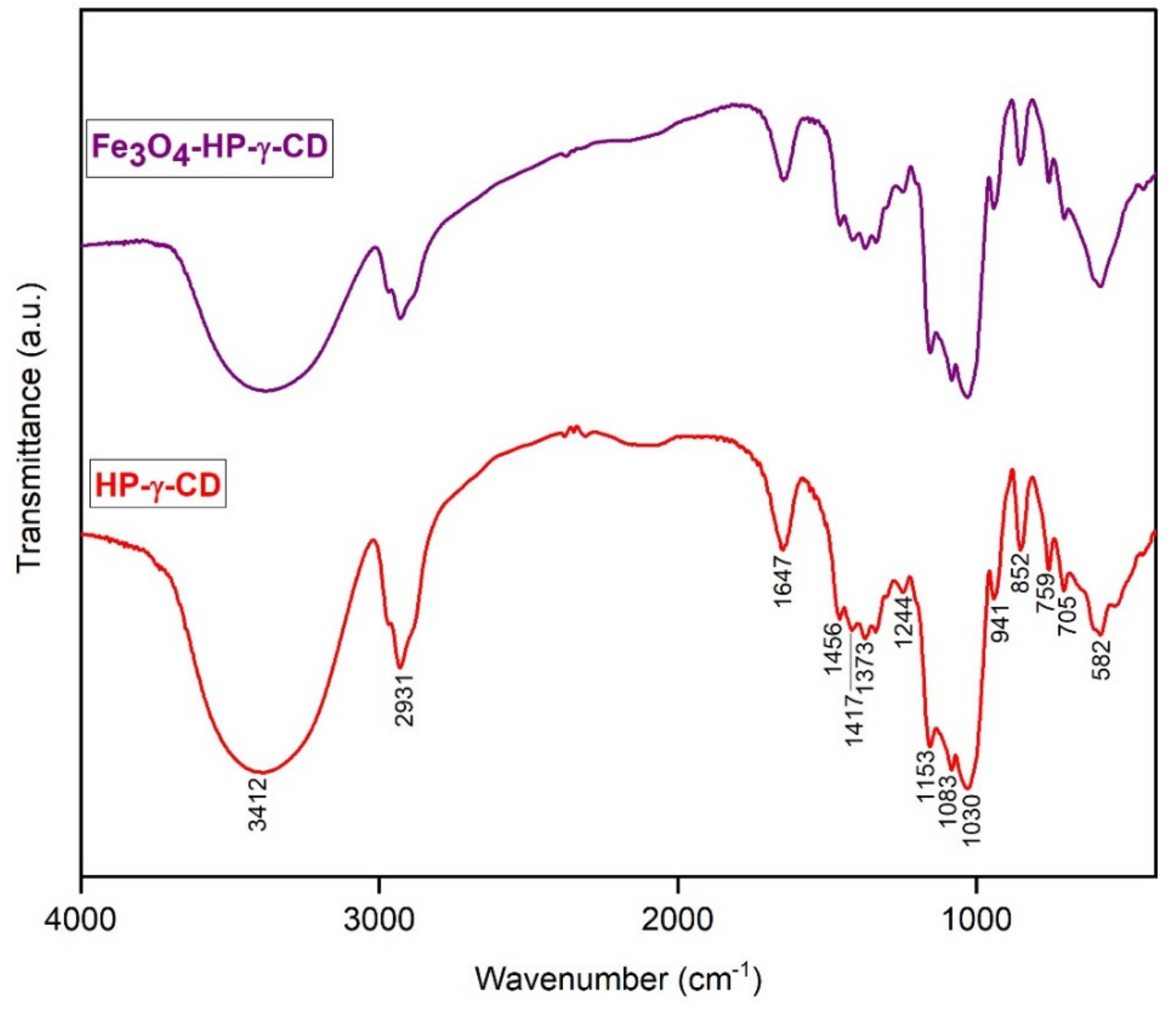
Fourier Transformed Infrared of Fe3O4-PAA–(HP-γ-CDs)
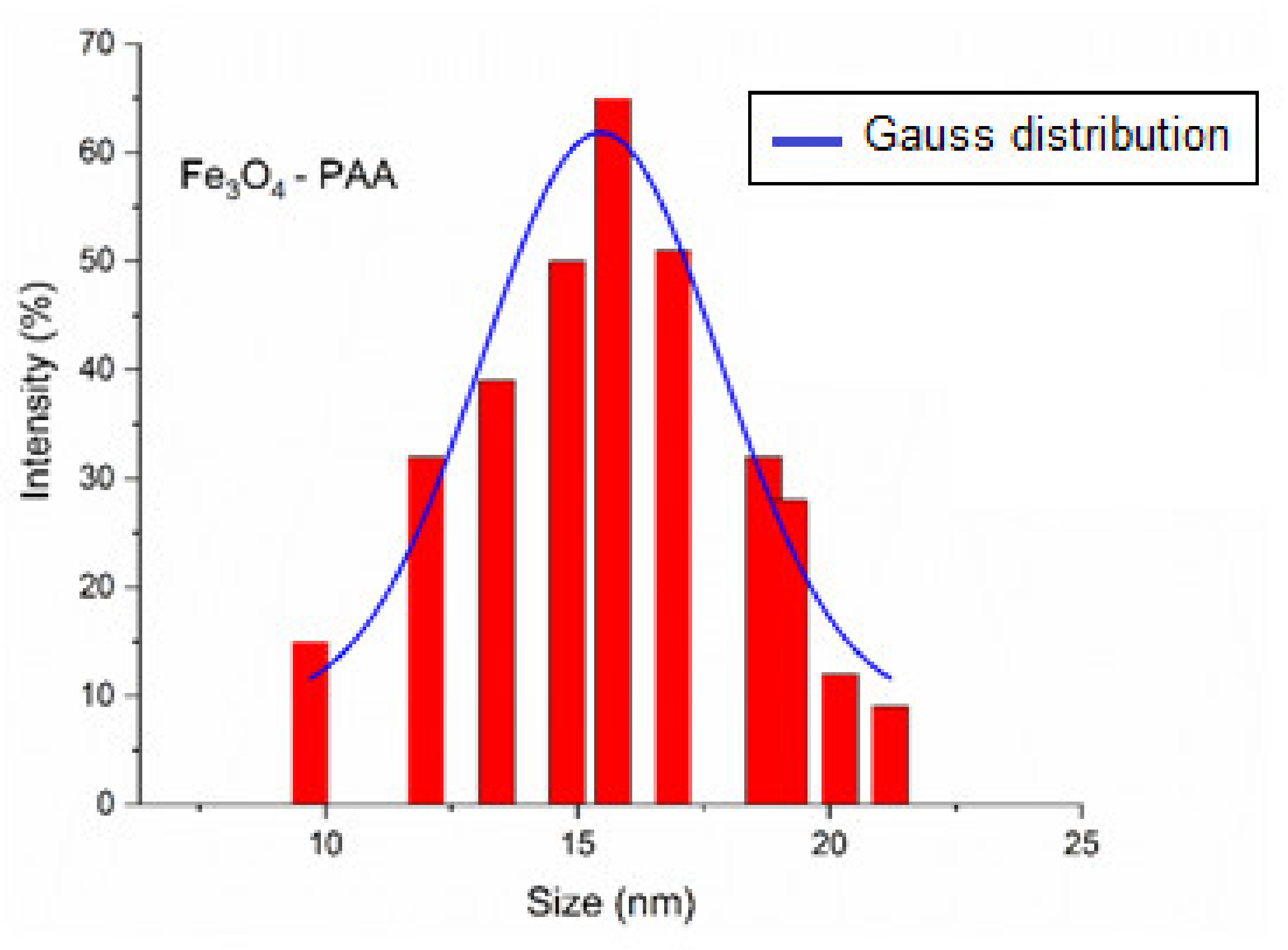
Dynamic Light Scattering and Zeta Potential Measurements of Fe3O4-PAA-(HP-γ-CDs)
3.4. Cell Viability Assessment under Standard Conditions for the Prepared Fe3O4-PAA Nanoparticles and Fe3O4-PAA–(HP-γ-CDs) Nanobioconjugates
3.5. In Vitro Superparamagnetic Hyperthermia Using Biocompatible Fe3O4-PAA–(HP-γ-CDs) Nanobioconjugates
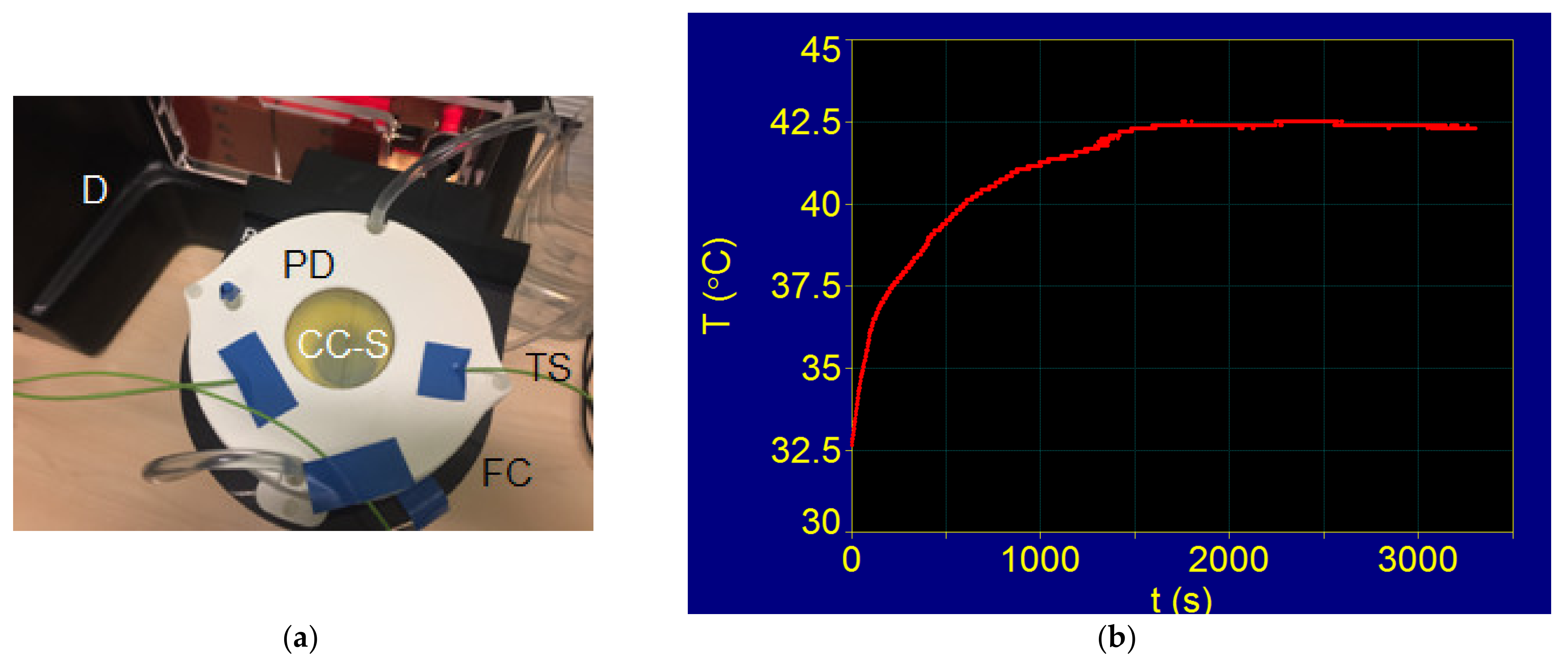
3.5.1. Experimental Superparamagnetic Hyperthermia with Fe3O4-PAA Nanoparticles: T–t Temperature Diagram in Calorimetric Conditions
3.5.2. Superparamagnetic Hyperthermia with Fe3O4-PAA–(HP-γ-CDs) Nanobioconjugates in Cells Culture: T–t Temperature Diagram of In Vitro Conditions
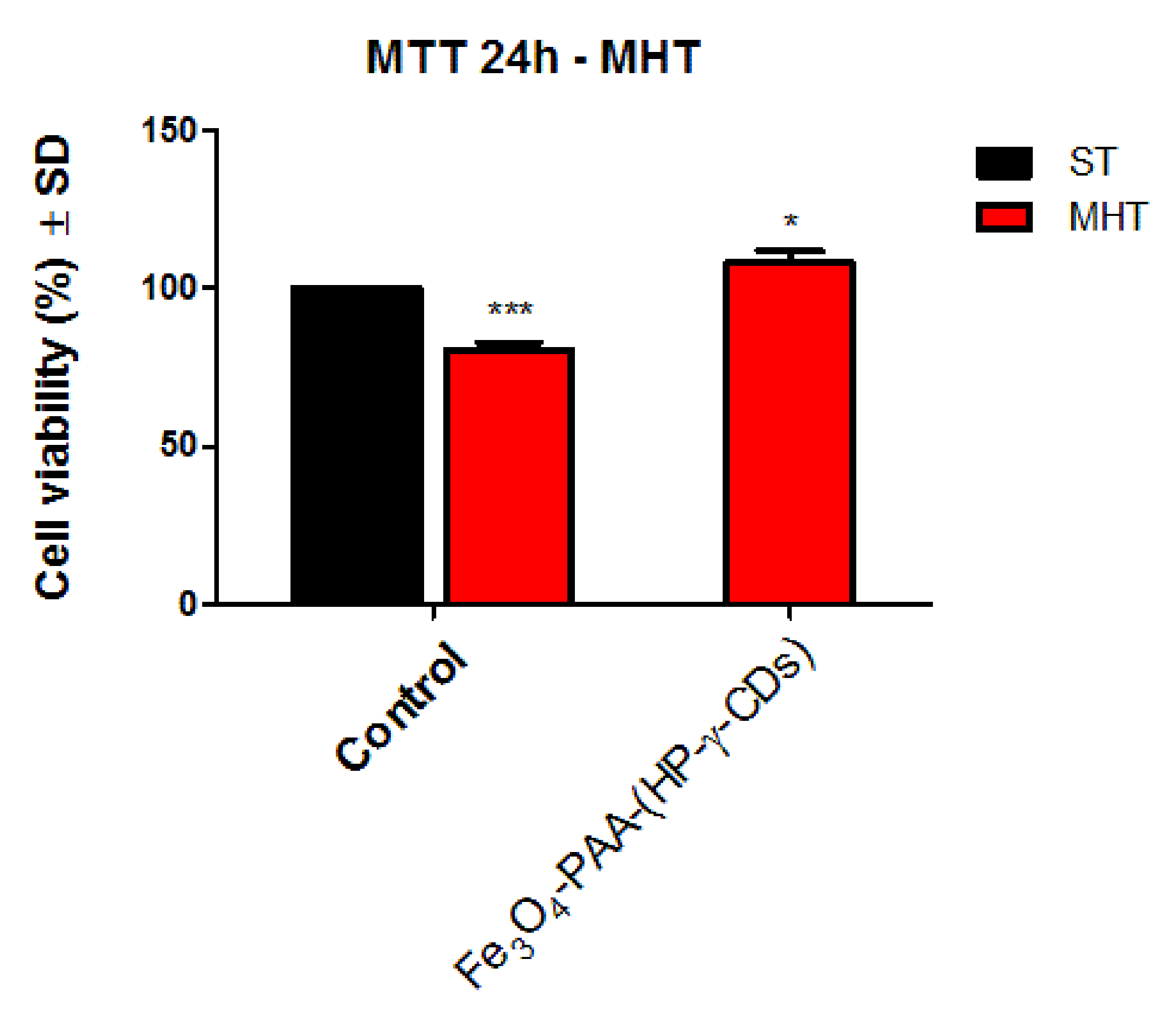
3.5.3. Cell Viability Assessment of HaCaT Cells under Magnetically Induced Hyperthermia Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spirou, S.V.; Costa Lima, S.A.; Bouziotis, P.; Vranješ-Djurić, S.; Efthimiadou, E.K.; Laurenzana, A.; Barbosa, A.I.; Garcia-Alonso, I.; Jones, C.; Jankovic, D.; et al. Recommendations for in vitro and in vivo testing of magnetic nanoparticle hyperthermia combined with radiation therapy. Nanomaterials 2018, 8, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Sahu, N.K. Review on magnetic nanoparticle-mediated hyperthermia for cancer therapy. J. Nanoparticle Res. 2020, 22, 1–25. [Google Scholar] [CrossRef]
- Jose, J.; Kumar, R.; Harilal, S.; Mathew, G.E.; Parambi, D.G.T.; Prabhu, A.; Uddin, M.S.; Aleya, L.; Kim, H.; Mathew, B. Magnetic nanoparticles for hyperthermia in cancer treatment: An emerging tool. Environ. Sci. Pollut. Res. 2020, 27, 19214–19225. [Google Scholar] [CrossRef]
- Iacovita, C.; Fizeșan, I.; Pop, A.; Scorus, L.; Dudric, R.; Stiufiuc, G.; Vedeanu, N.; Tetean, R.; Loghin, F.; Stiufiuc, R.; et al. In vitro intracellular hyperthermia of iron oxide magnetic nanoparticles, synthesized at high temperature by a polyol process. Pharmaceutics 2020, 12, 424. [Google Scholar] [CrossRef]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Chen, L.; Zang, F.; Wu, H.; Li, J.; Xie, J.; Ma, M.; Gu, N.; Zhang, Y. Using PEGylated magnetic nanoparticles to describe the EPR effect in tumor for predicting therapeutic efficacy of micelle drugs. Nanoscale 2018, 10, 1788–1797. [Google Scholar] [CrossRef]
- Hinderliter, P.M.; Minard, K.R.; Orr, G.; Chrisler, W.B.; Thrall, B.D.; Pounds, J.G.; Teeguarden, J.G. ISDD: A computational model of particle sedimentation, diffusion and target cell dosimetry for in vitro toxicity studies. Part. Fibre Toxicol. 2010, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Fraguas-Sánchez, A.I.; Martín-Sabroso, C.; Fernández-Carballido, A.; Torres-Suárez, A.I. Current status of nanomedicine in the chemotherapy of breast cancer. Cancer Chemother. Pharmacol. 2019, 84, 689–706. [Google Scholar] [CrossRef]
- Bakhtiary, Z.; Saei, A.A.; Hajipour, M.J.; Raoufi, M.; Vermesh, O.; Mahmoudi, M. Targeted superparamagnetic iron oxide nanoparticles for early detection of cancer: Possibilities and challenges. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 287–307. [Google Scholar] [CrossRef]
- Xie, L.; Jin, W.; Zuo, X.; Ji, S.; Nan, W.; Chen, H.; Gao, S.; Zhang, Q. Construction of small-sized superparamagnetic Janus nanoparticles and their application in cancer combined chemotherapy and magnetic hyperthermia. Biomater. Sci. 2020, 8, 1431–1441. [Google Scholar] [CrossRef]
- Shen, L.; Li, B.; Qiao, Y. Fe3O4 nanoparticles in targeted drug/gene delivery systems. Materials 2018, 11, 324. [Google Scholar] [CrossRef] [Green Version]
- Shinkai, M.; Ito, A. Functional magnetic particles for medical application. In Recent Progress of Biochemical and Biomedical Engineering in Japan II. Advances in Biochemical Engineering; Springer: Berlin/Heidelberg, Germany, 2004; Volume 91, pp. 191–220. [Google Scholar]
- Tanaka, K.; Ito, A.; Kobayashi, T.; Kawamura, T.; Shimada, S.; Matsumoto, K.; Saida, T.; Honda, H. Intratumoral injection of immature dendritic cells enhances antitumor effect of hyperthermia using magnetic nanoparticles. Int. J. Cancer 2005, 116, 624–633. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Maier-Hauff, K.; van Landeghem, F.K.H.; Waldoefner, N.; Teichgraeber, U.; Pinkernelle, J.; Bruhn, H.; Neumann, F.; Thiesen, B.; et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J. Neurooncol. 2006, 78, 7–14. [Google Scholar] [CrossRef]
- Gazeau, F.; Lévy, M.; Wilhelm, C. Optimizing magnetic nanoparticle design for nanothermotherapy. Nanomedicine 2008, 3, 831–844. [Google Scholar] [CrossRef]
- Alphandéry, E.; Faure, S.; Seksek, O.; Guyot, F.; Chebbi, I. Chains of magnetosomes extracted from AMB-1 magnetotactic bacteria for application in alternative magnetic field cancer therapy. ACS Nano 2011, 5, 6279–6296. [Google Scholar] [CrossRef]
- Di Corato, R.; Béalle, G.; Kolosnjaj-Tabi, J.; Espinosa, A.; Clément, O.; Silva, A.; Ménager, C.; Wilhelm, C. Combining magnetic hyperthermia and photodynamic therapy for tumor ablation with photoresponsive magnetic liposomes. ACS Nano 2015, 9, 2904–2916. [Google Scholar] [CrossRef]
- Wang, F.; Yang, Y.; Ling, Y.; Liu, J.; Cai, X.; Zhou, X.; Tang, X.; Liang, B.; Chen, Y.; Chen, H.; et al. Injectable and thermally contractible hydroxypropyl methyl cellulose/Fe3O4 for magnetic hyperthermia ablation of tumors. Biomaterials 2017, 128, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Shang, W.; Sun, X.; Zhao, L.; Wang, J.; Xiong, Z.; Yuan, J.; Zhang, R.; Huang, Q.; Wang, K.; et al. “All-in-One” Nanoparticles for trimodality imaging-guided intracellular photo-magnetic hyperthermia therapy under intravenous administration. Adv. Funct. Mater. 2018, 28, 1705710. [Google Scholar] [CrossRef]
- Caizer, C. Magnetic/Superparamagnetic hyperthermia as an effective noninvasive alternative method for therapy of malignant tumors. In Nanotheranostics: Applications and Limitations; Rai, M., Jamil, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 297–335. [Google Scholar]
- Caizer, C. Optimization study on specific loss power in superparamagnetic hyperthermia with magnetite nanoparticles for high efficiency in alternative cancer therapy. Nanomaterials 2021, 11, 40. [Google Scholar] [CrossRef]
- Pradhan, P.; Giri, J.; Samanta, G.; Sarma, H.D.; Mishra, K.P.; Bellare, J.; Banerjee, R.; Bahadur, D. Comparative evaluation of heating ability and biocompatibility of different ferrite-based magnetic fluids for hyperthermia application. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81B, 12–22. [Google Scholar] [CrossRef]
- Kumar, C.S.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef] [Green Version]
- Saldıvar-Ramırez, M.M.G.; Sanchez-Torres, C.G.; Cortés-Hernández, D.A.; Escobedo-Bocardo, J.C.; Almanza-Robles, J.M.; Larson, A.; Reséndiz-Hernández, P.J.; Acuña-Gutiérrez, I.O. Study on the efficiency of nanosized magnetite and mixed ferrites in magnetic hyperthermia. J. Mater. Sci. Mater. Med. 2014, 25, 2229–2236. [Google Scholar] [CrossRef]
- Almaki, J.H.; Nasiri, R.; Idris, A.; Majid, F.A.A.; Salouti, M.; Wong, T.S.; Dabagh, S.; Marvibaigi, M.; Amini, N. Synthesis, characterization and in vitro evaluation of exquisite targeting SPIONs–PEG–HER in HER2+ human breast cancer cells. Nanotechnology 2016, 27, 105601. [Google Scholar] [CrossRef]
- Liu, X.L.; Ng, C.T.; Chandrasekharan, P.; Yang, H.T.; Zhao, L.Y.; Peng, E.; Lv, Y.B.; Xiao, W.; Fang, J.; Yi, J.B.; et al. Synthesis of ferromagnetic Fe0.6Mn0.4O nanofl owers as a new class of magnetic theranostic platform for in vivo T1-T2 Dual-Mode magnetic resonance imaging and magnetic hyperthermia therapy. Adv. Healthc. Mater. 2016, 5, 2092–2104. [Google Scholar] [CrossRef]
- Caizer, C. Theoretical study on specific loss power and heating temperature in CoFe2O4 nanoparticles as possible candidate for alternative cancer therapy by superparamagnetic hyperthemia. Appl. Sci. 2021, 11, 5505. [Google Scholar] [CrossRef]
- Caizer, C. Computational study regarding CoxFe3-xO4 ferrite nanoparticles with tunable magnetic properties in superparamagnetic hyperthermia for effective alternative cancer therapy. Nanomaterials 2021, 11, 3294. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2919. [Google Scholar] [CrossRef]
- Caizer, C. Magnetic hyperthermia-using magnetic metal/oxide nanoparticles with potential in cancer therapy. In Metal Nanoparticles in Pharma; Rai, M., Shegokar, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Valenzuela, R. Magnetic Ceramics; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Caizer, C.; Rai, M. Magnetic nanoparticles in alternative tumors therapy: Biocompatibility, toxicity and safety compared with classical methods. In Magnetic Nanoparticles in Human Health and Medicine: Current Medical Applications and Alternative Therapy of Cancer; Caizer, C., Rai, M., Eds.; Wiley: Oxford, UK, 2022. [Google Scholar]
- Caizer, C.; Buteica, A.S.; Mindrila, I. Biocompatible magnetic oxide nanoparticles with metal ions coated with organic shell as potential therapeutic agents in cancer. In Metal Nanoparticles in Pharma; Rai, M., Shegokar, R., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef]
- Caizer, C.; Dehelean, C.; Soica, C. Classical magnetoliposomes vs current magnetociclodextrins with ferrimagnetic nanoparticle for high efficiency and low toxicity in alternative therapy of cancer by magnetic/superparamagnetic hyperthermia. In Magnetic Nanoparticles in Human Health and Medicine: Current Medical Applications and Alternative Therapy of Cancer; Caizer, C., Rai, M., Eds.; Wiley: Oxford, UK, 2022. [Google Scholar]
- Wimmer, T.C. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Kaneto, U.; Fumitoshi, H.; Tetsumi, I. Cyclodextrin Drug Carrier Systems. Chem. Rev. 1998, 98, 2045–2076. [Google Scholar]
- Alice, G.; Nial, W. Macrocycles as drug-enhancing excipients in pharmaceutical formulations. J. Incl. Phenom. Macrocycl. Chem. 2021, 100, 55–69. [Google Scholar]
- Caizer, C. Magnetic behavior of Mn0.6Fe0.4Fe2O4 nanopartciles in ferrofluid at low temperatures. J. Magn. Magn. Mater. 2002, 251, 304–315. [Google Scholar] [CrossRef]
- Caizer, C. Magnetic properties of the novel nanocomposite (Zn0.15Ni0.85Fe2O4)0.15/(SiO2)0.85 at room temperature. J. Magn. Magn. Mater. 2008, 320, 1056–1062. [Google Scholar] [CrossRef]
- Caizer, C.; Caizer, I.S. Study on maximum specific loss power in Fe3O4 nanoparticles decorated with biocompatible gamma-cyclodextrins for cancer therapy with superparamagnetic hyperthermia. Int. J. Molec. Sci. 2021, 22, 10071. [Google Scholar] [CrossRef]
- Trott, O.; Arthur, J.O. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization and Multithreading. J. Comput. Chem. 2010, 31, 455. [Google Scholar] [CrossRef] [Green Version]
- Smit, J.; Wijin, H.P.J. Les Ferites; Bibl. Tehn. Philips.: Paris, France, 1961. [Google Scholar]
- Purushotham, S.; Ramanujan, R.V. Modeling the performance of magnetic nanoparticles in multimodal cancer therapy. J. Appl. Phys. 2010, 107, 114701. [Google Scholar] [CrossRef]
- Bean, C.P.; Livingston, L.D. Superparamagnetism. J. Appl. Phys. 1959, 30, S120–S129. [Google Scholar] [CrossRef]
- Langevin, P. Magnétisme et théorie des électrons. Ann. Chim. Phys. 1905, 5, 70–127. [Google Scholar]
- Jacobs, I.S.; Bean, C.P. Fine particles, thin films and exchange anisotropy. In Magnetism; Rado, G.T., Suhl, H., Eds.; Academic Press: Cambridge, MA, USA, 1963; Volume III, pp. 271–350. [Google Scholar]
- Néel, L. Théorie du traînage magnétique des ferromagnétiques en grains fins avec application aux terres cuites. Ann. Geophys. 1949, 5, 99–136. [Google Scholar]
- Brown, W.F., Jr. Thermal fluctuations of a single-domain particle. Phys. Rev. 1963, 130, 1677–1686. [Google Scholar] [CrossRef]
- Back, C.H.; Weller, D.; Heidmann, J.; Mauri, D.; Guarisco, D.; Garwin, E.L.; Siegmann, H.C. Magnetization reversal in ultrashort magnetic field pulses. Phys. Rev. Lett. 1998, 81, 3251. [Google Scholar] [CrossRef] [Green Version]
- Hamidreza, S. Synthesis of Nanocomposition of Poly Acrylic Acid/Chitosan Coated-Magnetite Nanoparticles to Investigation of Interaction with BSA and IGG Proteins. Int. J. Nanomater. Nanotechnol. Nanomed. 2017, 3, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.D.; Sung, S.K.; Yong-ho, C.; Hee, T.K. Formation and Surface Modification of Fe3O4 Nanoparticles by Co-Precipitation and Sol-Gel Method. J. Ind. Eng. Chem. 2007, 13, 1137–1141. [Google Scholar]
- Caizer, C. T2 law for magnetite-based ferrofluids. J. Phys. Condens. Matter 2003, 15, 765–776. [Google Scholar] [CrossRef]
- Farcas, C.G.; Dehelean, C.; Pinzaru, I.A.; Mioc, M.; Socoliuc, V.; Moaca, E.A.; Avram, S.; Ghiulai, R.; Coricovac, D.; Pavel, I.; et al. Thermosensitive betulinic acid-loaded magnetoliposomes: A promising antitumor potential for highly aggressive human breast adenocarcinoma cells under hyperthermic conditions. Int. J. Nanomed. 2020, 15, 8175–8200. [Google Scholar] [CrossRef]
- Nahar, Y.; Abdur, R.; Kawsar, H.; Mostafa, K.S.; Rabiul, K.; Abdelhamid, E.; Bungo, O.; Hasan, A.; Mahbubor, R. A Facile One-Pot Synthesis of Poly(Acrylic Acid)-Functionalized Magnetic Iron Oxide Nanoparticles for Suppressing Reactive Oxygen Species Generation and Adsorption of Biocatalyst. Mater. Res. Express 2019, 7, 016102. [Google Scholar] [CrossRef]
- Du, N.; Yanfang, X.; Hui, Z.; Chuanxin, Z.; Deren, Y. Selective Synthesis of Fe2O3 and Fe3O4 Nanowires via a Single Precursor: A General Method for Metal Oxide Nanowires. Nanoscale Res. Lett. 2010, 5, 1295–1300. [Google Scholar] [CrossRef] [Green Version]
- Mahdavi, M.; Mansor, B.A.; Jelas, H.; Farideh, N.; Behzad, N.; Mohamad, Z.A.R.; Jamileh, A. Synthesis, Surface Modification and Characterisation of Biocompatible Magnetic Iron Oxide Nanoparticles for Biomedical Applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef] [Green Version]
- Moacă, E.-A.; Ciprian-Valentin, M.; Ioana-Gabriela, M.; Roxana, R.; Codruţa, Ş.; Cristina-Adriana, D.; Cornelia, P.; Sorin, F. Fe3O4@C Matrix with Tailorable Adsorption Capacities for Paracetamol and Acetylsalicylic Acid: Synthesis, Characterization, and Kinetic Modeling. Molecules 2019, 24, 1727. [Google Scholar] [CrossRef] [Green Version]
- Cursaru, L.M.; Roxana, M.P.; Dumitru, V.D.; Robert, M.; Caroline, T.; Marie, C.; Hélène, J.; Bernard, D. One-step Soft Chemical Synthesis of Magnetite Nanoparticles under Inert Gas Atmosphere. Magnetic Properties and In Vitro Study. Nanomaterials 2020, 10, 1500. [Google Scholar] [CrossRef] [PubMed]
- Bordbar, A.K.; Rastegari, A.A.; Amiri, R.; Ranjbakhsh, E.; Abbasi, M.; Khosropour, A.R. Characterization of Modified Magnetite Nanoparticles for Albumin Immobilization. Biotechnol. Res. Int. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirwan, L.J.; Phillip, D.F.; Van Bronswijk, W. In Situ FTIR-ATR Examination of Poly(Acrylic Acid) Adsorbed onto Hematite at Low PH. Langmuir 2003, 19, 5802–5807. [Google Scholar] [CrossRef]
- Kneller, E. Ferromagnetismus; Springer-Verlag: Berlin/Heidelberg, Germany, 1962. [Google Scholar]
- Oroujenia, M.; Kaboudin, B.; Xia, W.; Jönsson, P.; Ossipov, D.A. Conjugation of cyclodextrin to magnetic Fe3O4 nanoparticles via polydopamine coating for drug delivery. Prog. Org. Coat. 2018, 114, 154–161. [Google Scholar] [CrossRef]
- Coey, J.M.D. Noncollinear spin srrangement in ultrafine ferrimagnetic crystallites. Phys. Rev. Lett. 1971, 27, 1140. [Google Scholar] [CrossRef] [Green Version]
- Kodama, R.H.; Berkowitz, A.E.; McNiff, E.J., Jr.; Foner, S. Surface spin disorder in NiFe2O4 nanoparticles. Phys. Rev. Lett. 1996, 77, 394. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, A.E.; Kodama, R.H.; Makhlouf, S.A.; Parker, F.T.; Spada, F.E.; McNiff, E.J., Jr.; Foner, S. Anomalous properties of magnetic nanoparticles. J. Magn. Magn. Mater. 1999, 196–197, 591. [Google Scholar] [CrossRef]
- Alonso, E.C.P.; Riccomini, K.; Antônio, D.; Silva, L.; Galter, D.; Lima, E.M.; Durig, T.; Taveira, S.F.; Martins, F.T.; Marcílio, S.S.C.-F.; et al. Development of Carvedilol-Cyclodextrin Inclusion Complexes Using Fluid-Bed Granulation: A Novel Solid-State Complexation Alternative with Technological Advantages. J. Pharm. Pharmacol. 2016, 68, 1299–1309. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Jiang, K.-M.; An, K.; Ren, S.-H.; Xie, X.-G.; Jin, Y.; Lin, J. Novel Water-Soluble Fisetin/Cyclodextrins Inclusion Complexes: Preparation, Characterization, Molecular Docking and Bioavailability. Carbohydr. Res. 2015, 418, 20–28. [Google Scholar] [CrossRef]
- Atav, R.; Aylin, Y.; Derman, V.B.; Ahmet, Ö.A. Inclusion Complexes of β-Cyclodextrine with Fe3O4@HA@Ag Part I: Preparation and Characterization. Ind. Text. 2019, 70, 255–258. [Google Scholar] [CrossRef]
- Singh, S.V.B. Water Soluble Complexes of Curcumin and Hydroxypropyl Cyclodextrins: Preparation and Characterization. SREE Chitra Tirunal Institute for Medical Sciences and Technology. Available online: http://docplayer.net/58922173-Water-soluble-complexes-of-curcumin-and-hydroxypropyl-cyclodextrins-preparation-and-characterization-s-v-berwin-singh (accessed on 10 March 2022).
- ISO 10993-5:2009; Reviewed and Confirmed in 2017, Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO Catalogue, Edition 3. International Standard Organization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 22 December 2021).
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia—biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Vilas-Boas, V.; Carvalho, F.; Espiña, B. Magnetic hyperthermia for cancer treatment: Main parameters affecting the outcome of in vitro and in vivo studies. Molecules 2020, 25, 2874. [Google Scholar] [CrossRef]
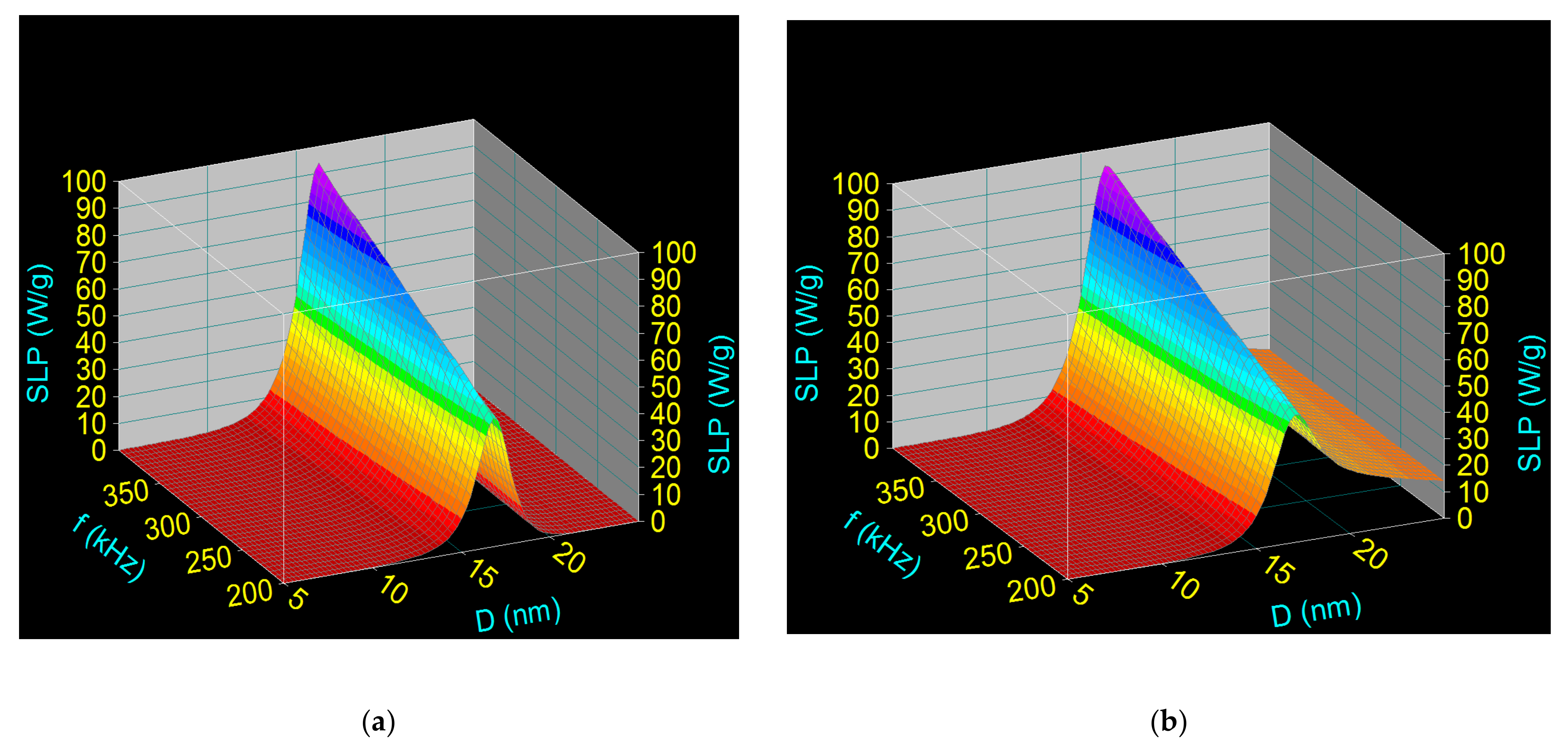




| Observables | H (kA/m) | f (kHz) | Ms (kA/m) | K (kJ/m3) | ρ (×103 kg/m3) | 2d (nm) | η (kg/ms) | ε | D (nm) |
|---|---|---|---|---|---|---|---|---|---|
| Values | 16 | 200–400 | 480 | 11 | 5.24 | 3.6 | 7 × 10−4 | 0.1 | 5–25 |
| Ligand | CD | ∆G (kcal/mol) |
|---|---|---|
| PA20n | β-CD | −4.0 |
| γ-CD | −4.4 | |
| HPBCD | −4.9 | |
| HPGCD | −5.0 | |
| Mono-OH-SGM | −4.9 |
| H (kA/m) | f (kHz) | (SLP)M (W/g) | Do (nm) |
|---|---|---|---|
| 16 | 200 | 47.95 | 16.9 |
| 16 | 321 | 75.77 | 16.5 |
| 16 | 400 | 93.69 | 16.3 |
| H (kA/m) | f (kHz) | (SLP)M (W/g) | Do (nm) |
|---|---|---|---|
| 16 | 200 | 48.44 | 17.1 |
| 16 | 321 | 76.31 | 16.6 |
| 16 | 400 | 94.18 | 16.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caizer, C.; Caizer, I.S.; Racoviceanu, R.; Watz, C.G.; Mioc, M.; Dehelean, C.A.; Bratu, T.; Soica, C. Fe3O4-PAA–(HP-γ-CDs) Biocompatible Ferrimagnetic Nanoparticles for Increasing the Efficacy in Superparamagnetic Hyperthermia. Nanomaterials 2022, 12, 2577. https://doi.org/10.3390/nano12152577
Caizer C, Caizer IS, Racoviceanu R, Watz CG, Mioc M, Dehelean CA, Bratu T, Soica C. Fe3O4-PAA–(HP-γ-CDs) Biocompatible Ferrimagnetic Nanoparticles for Increasing the Efficacy in Superparamagnetic Hyperthermia. Nanomaterials. 2022; 12(15):2577. https://doi.org/10.3390/nano12152577
Chicago/Turabian StyleCaizer, Costica, Isabela Simona Caizer, Roxana Racoviceanu, Claudia Geanina Watz, Marius Mioc, Cristina Adriana Dehelean, Tiberiu Bratu, and Codruța Soica. 2022. "Fe3O4-PAA–(HP-γ-CDs) Biocompatible Ferrimagnetic Nanoparticles for Increasing the Efficacy in Superparamagnetic Hyperthermia" Nanomaterials 12, no. 15: 2577. https://doi.org/10.3390/nano12152577
APA StyleCaizer, C., Caizer, I. S., Racoviceanu, R., Watz, C. G., Mioc, M., Dehelean, C. A., Bratu, T., & Soica, C. (2022). Fe3O4-PAA–(HP-γ-CDs) Biocompatible Ferrimagnetic Nanoparticles for Increasing the Efficacy in Superparamagnetic Hyperthermia. Nanomaterials, 12(15), 2577. https://doi.org/10.3390/nano12152577





.jpg)






