Recent Advances in Honey-Based Nanoparticles for Wound Dressing: A Review
Abstract
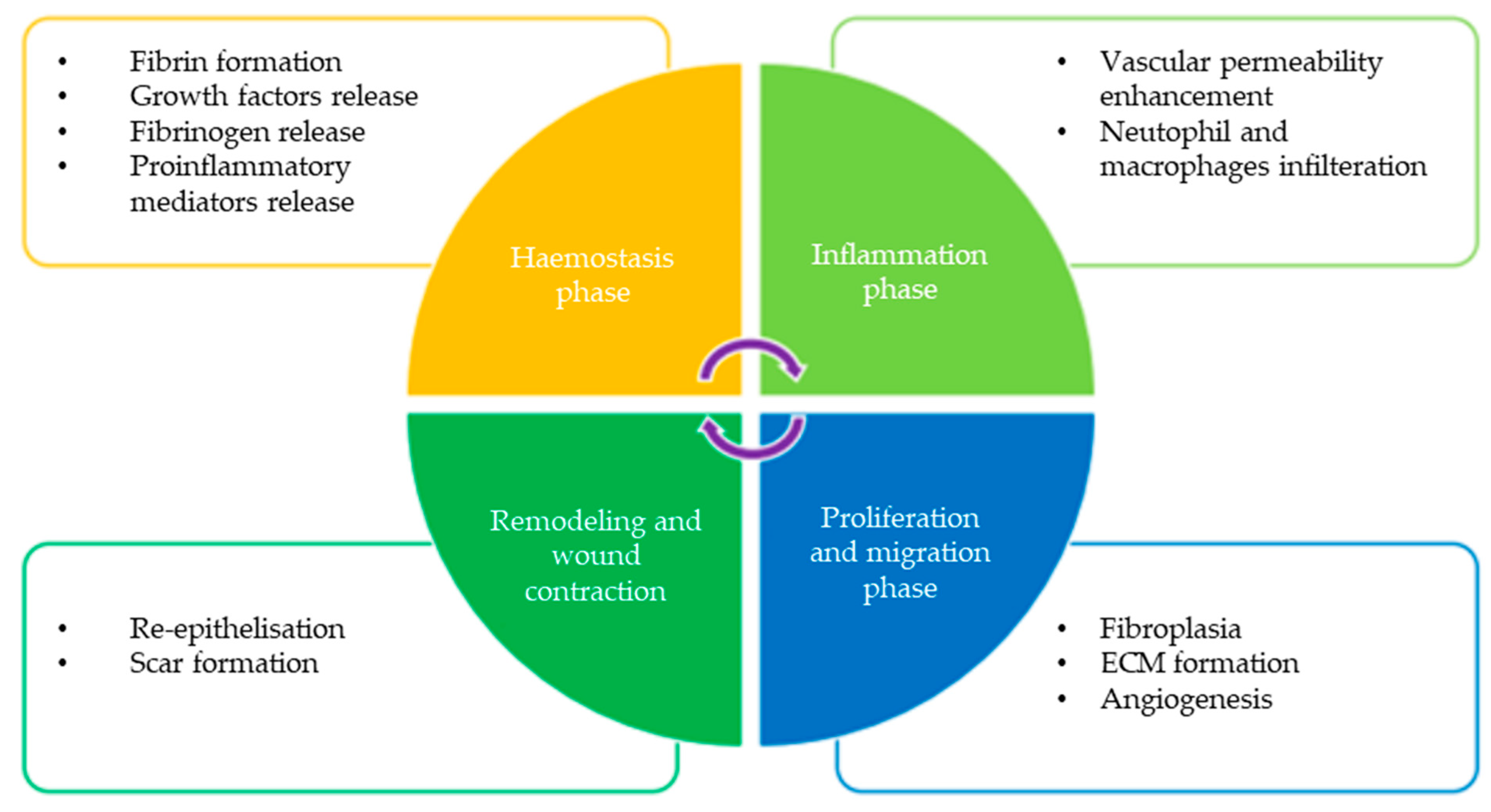
:1. Introduction
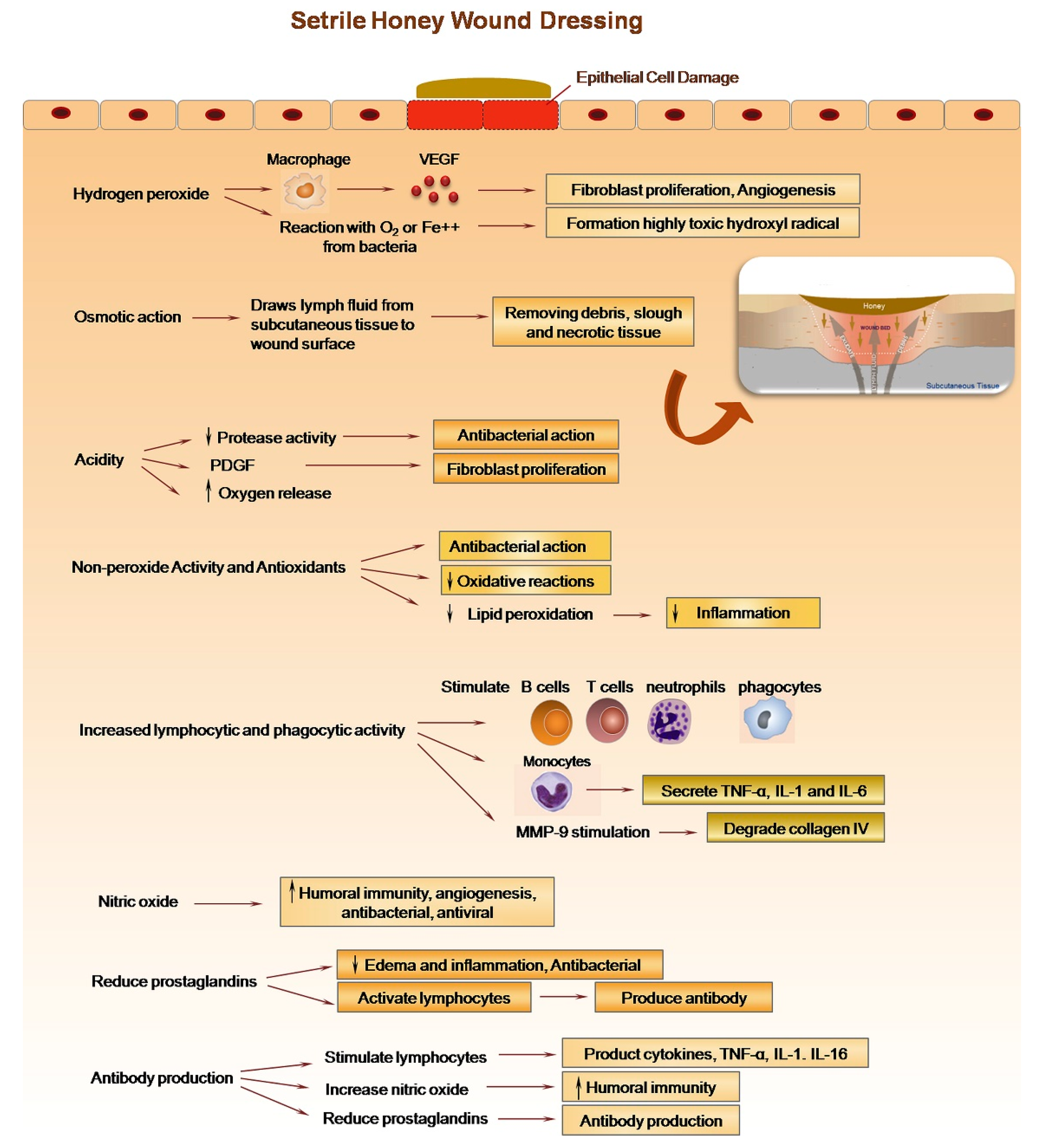
2. Honey for Wound Healing
Potential and Recent Commercial Application of Honey for Wound Healing
| Commercially Available Honey-Based Wound Dressing | Composition | Application | References |
|---|---|---|---|
| Activon® | Manuka Honey or a mixture of Manuka Honey and additives | Protect the wound bed by creating a moist wound healing environment | [35] |
| L-Mesitran™ | Mixture of different percentages of honey, polyethylene, acrylic polymer gel, water, polyurethane film backing, and an adhesive border | Promote healing | [36] |
| MediHoney® | Contains 100% active Leptospermum honey in a hydrocolloid suspension | Protect skin from friction and shear damage, prevent skin damage from frequent hand washing, and promote a moisture-balanced environment | [37] |
| Revamil® | Polyacetate sheet dressing impregnated with pure honey | Heal a wide variety of wounds and skin conditions, including ulcers, dry and damaged skin, infected and chronic wounds, burns, and blistering | [38] |
| Principelle IF® | Made up of an inert acetate substrate mixed with trace elements and a medical-grade honey ointment | Used to treat acute and chronic wounds (particularly those that are contaminated or infected), wounds that are moderately exuding, weakly exuding, or dry; it can also be utilised on wounds that are heavily exuding when a secondary dressing is used | [39] |
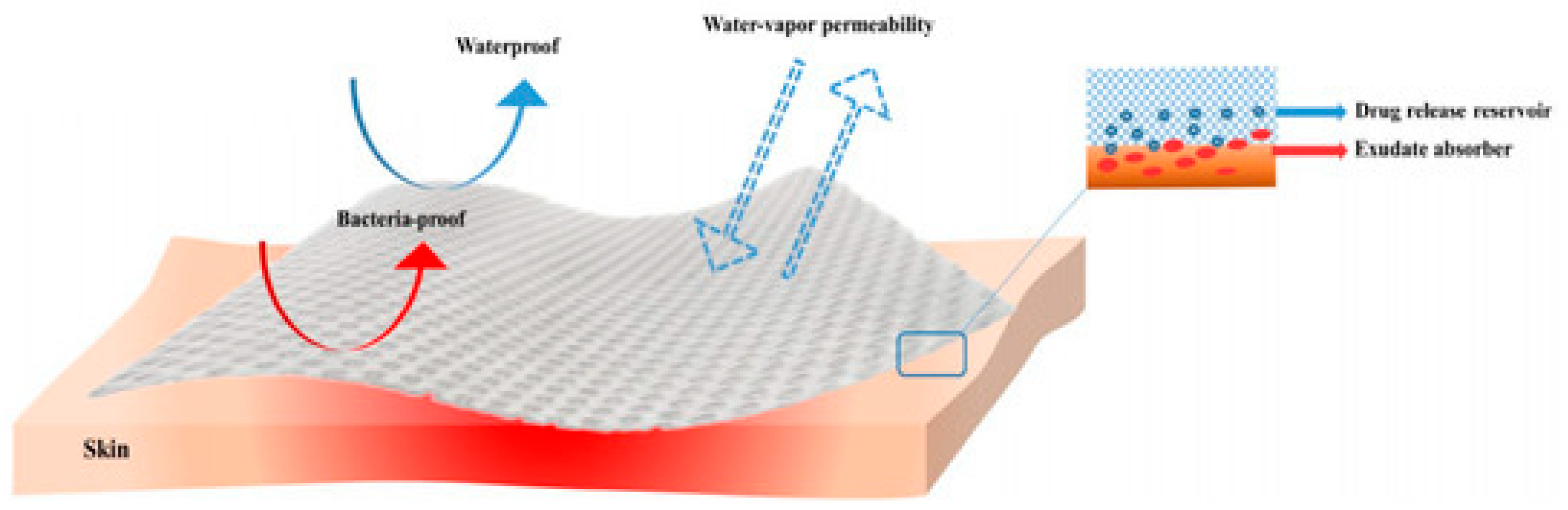
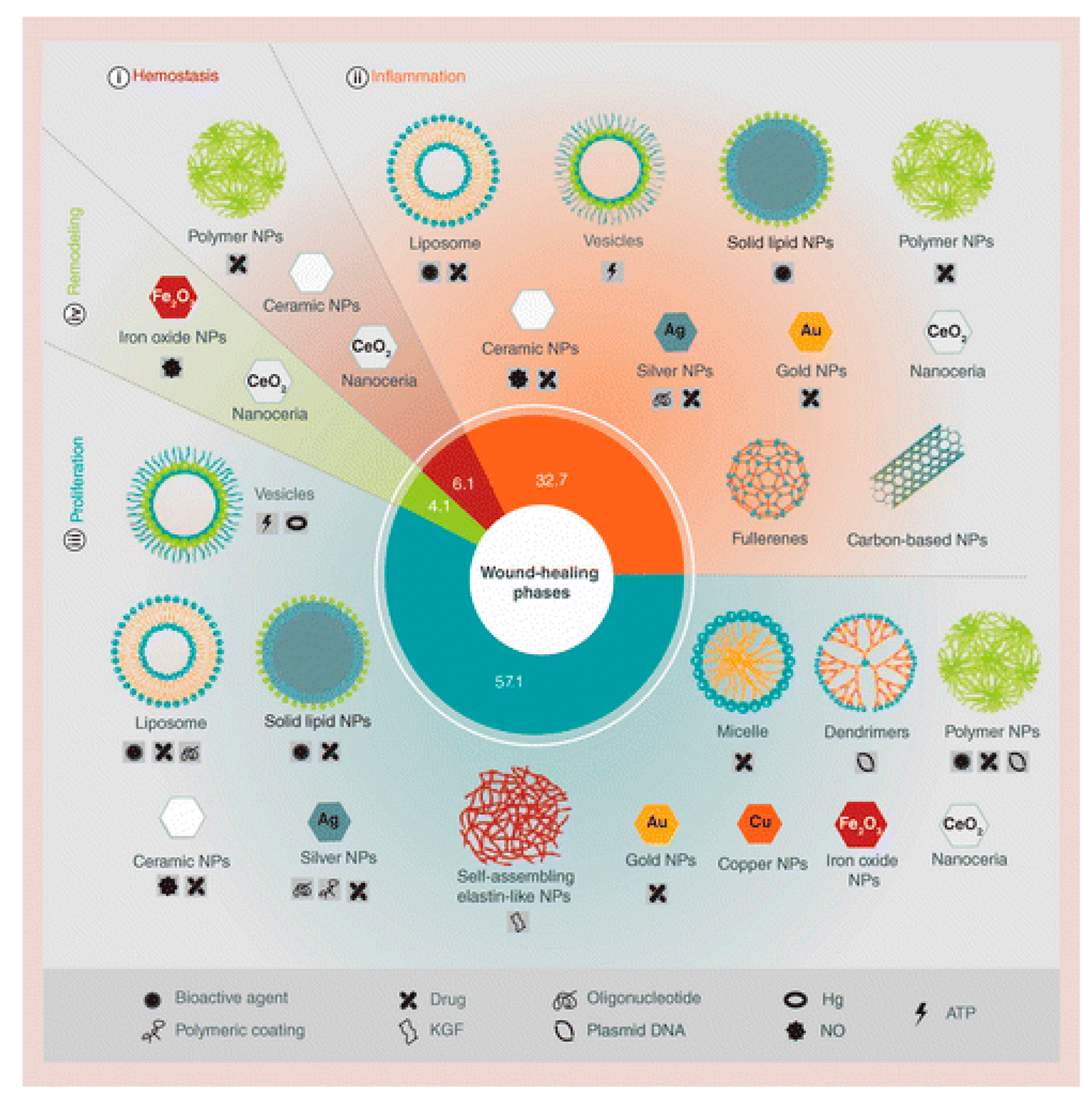
3. Application of Honey-Based Nanoparticles for Wound Dressings
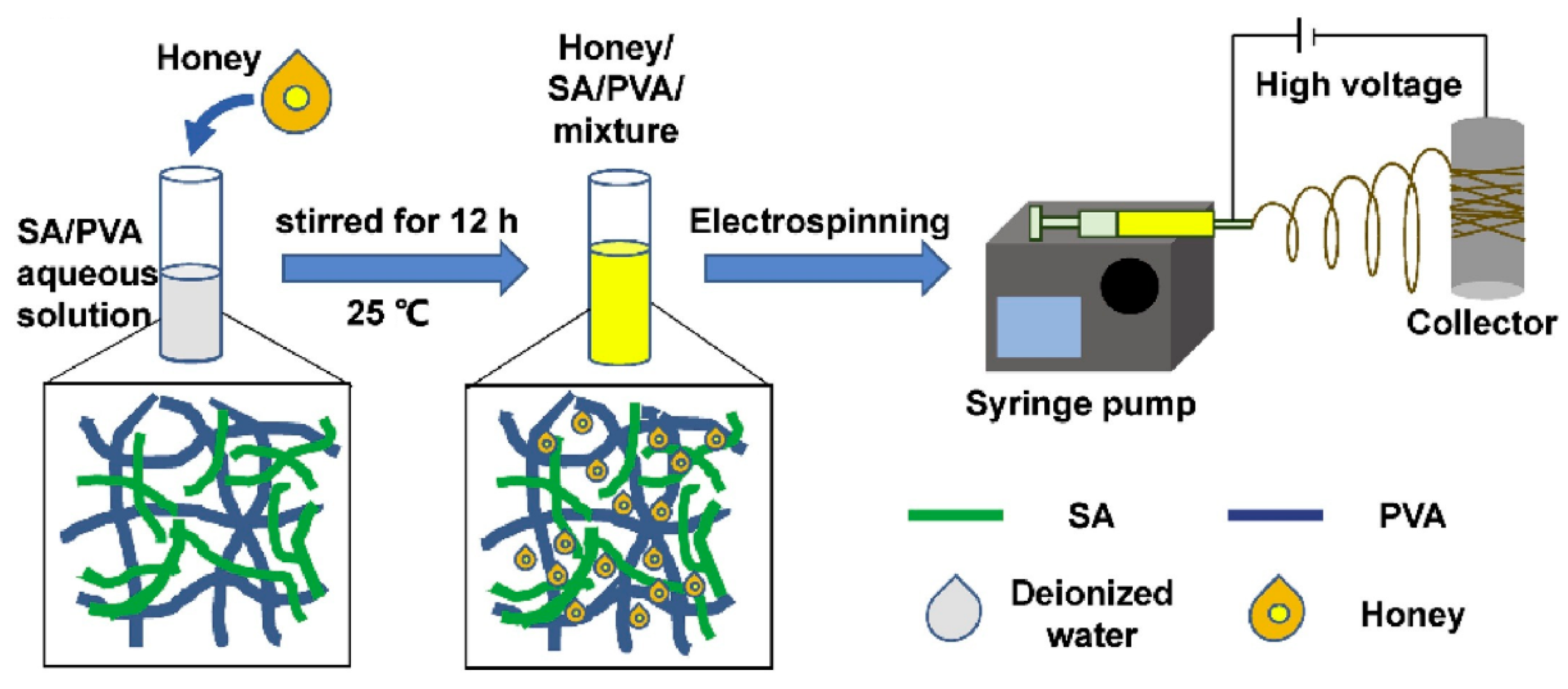
3.1. Honey-Based Nanoparticles
3.2. Application of Honey-Based Nanoparticles
| Intervention | Dressing Form | Findings | Reference |
|---|---|---|---|
| Honey-loaded ethylcellulose/gum tragacanth | Nanofibre | Good antioxidant abilities. High efficacy in preventing bacterial growth on the wound surface. Effective for promoting wound healing. | [59] |
| Honey/cellulose acetate | Nanofibre | Antioxidant properties. Effective for facilitating wound healing. Great efficacy in preventing bacterial growth on the wound surface. | [61] |
| Cellulose/honey composite | Composite sheet | The treated layer with three types of honey (Thyme, Astragalus, and Ziziphus) can absorb water 120 times faster than purified microbial cellulose. | [62] |
| Honey-loaded egg white/poly (vinyl alcohol)/clay | Hydrogel | The treated layer can collect exudate from te surface 1.37 times faster, which can accelerate wound healing by maintaining the moisture environment of the wound as well as preventing the penetration of bacteria into the wound. Decreased the swelling and dehydration capabilities by increasing the content of incorporated clay nanoparticles. Formed and maintained a moist area on the surface of infected and non-infected wounds. Accelerated the healing process of animal wounds. | [63] |
| Honey/chitosan/capsaicin/gold | Nanofibre | HTCs–capsaicin and HTCs-AuNPs are more effective at inhibiting bacterial growth than HTCs and HTCs–capsaicin/AuNP nanofibres and antibiotics. The nanofibrous mats increased cell growth compared to the untreated control. In vivo results show that the developed mats more effectively enhanced the rate of wound closure than the test samples. Has a relatively fast and efficient wound-healing ability. | [58] |
| Honey/PVA | Scaffold | Honey/PVA scaffold showed inhibition activity against proteases in chronic wounds, prevented bacterial infection from occurring over the wound site, and inhibited biofilm formation. | [64] |
| Erythromycin-releasing PVA/sucrose and PVA/honey | Hydrogel | The addition of honey improved the bio-adhesion of PVA/honey hydrogel compared to PVA/sucrose and pure PVA hydrogel. Antibacterial tests revealed that erythromycin-loaded PVA hydrogels inhibited the growth of P. aeruginosa and S. aureus bacteria. | [65] |
| Ag/alginate/honey | Hydrogel | The hydrogels exhibited strong bactericidal activity against the standard (at the total released silver concentration of ~9 μg/mL). | [66] |
| Honey/alginate/PVA | Nanofibre | Increased antioxidant activity, implying the ability to limit reactive oxygen species generation. Strong antibacterial activity against Gram-positive bacterium (S. aureus) and Gram-negative bacterium (E. coli). Non-cytotoxicity and biocompatibility. | [57] |
| Honey/iron oxide NPs | Cotton fabric | It had significant antibacterial properties against E. coli. | [67] |
| DNG/Ch/Manuka Honey | Composite sheet | Antibacterial studies confirmed >99% antibacterial activity against both S. aureus and E. coli. A bacterial adherence test showed that it was effective in preventing microbial invasion. Enhanced epithelialisation in terms of scar prevention and aesthetics. | [68] |
| Honey/PVA | Nanofibre | Scaffold with 1% honey exhibited antibacterial activity. Scaffold with 0.5% exhibited antioxidant and anti-inflammatory properties. | [69] |
| Silver/honey | Solution mixture | Increased the rate of healing in wounds infected by P. aeruginosa. | [70] |
| Poly (vinyl alcohol)/chitosan/honey/clay | Hydrogel | Higher pH values resulted in a faster honey release rate. MTT results revealed no cytotoxicity. Its antibacterial activity demonstrated that the suggested system has antibacterial activity of over 99%. The developed wound-healing capabilities of the system were proven in vivo. | [60] |
| Manuka Honey/silk fibroin | Silk Fibroin | Improved the antimicrobial activity of SF fibrous matrices, without negative effects on the excellent biocompatibility of SF. Good performance in improving wound healing. | [71] |
| Honey/chitosan hydrogel | Hydrogel | Honey plays a positive role in modulating wound healing when incorporated into a chitosan-based hydrogel matrix. | [72] |
| Honey/chitosan | Nanofibre | Improved wound healing compared to untreated controls, as evidenced by increased wound closure rates in mice. | [73] |
| PET/honey | Nanofibre | Cytotoxicity evaluation demonstrated that fibres exhibited no cytotoxic activity. | [56] |
| HA/PEO/Manuka Honey | Nanofibre | Promising results in terms of in vitro cell toxicity, antimicrobial effect, and antioxidant potential. | [74] |
4. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bayrami, M.; Bayrami, A.; Habibi-Yangjeh, A.; Shafeeyan, M.S.; Feizpoor, S.; Arvanagh, F.M.; Nourani, M.R.; Taheri, R.A. Biologically-synthesised ZnO/CuO/Ag nanocomposite using propolis extract and coated on the gauze for wound healing applications. IET Nanobiotechnol. 2020, 14, 548–554. [Google Scholar] [CrossRef]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef]
- Andreu, V.; Mendoza, G.; Arruebo, M.; Irusta, S. Smart dressings based on nanostructured fibers containing natural origin antimicrobial, anti-inflammatory, and regenerative compounds. Materials 2015, 8, 5154–5193. [Google Scholar] [CrossRef]
- Bernal-Chávez, S.; Nava-Arzaluz, M.G.; Quiroz-Segoviano, R.I.Y.; Ganem-Rondero, A. Nanocarrier-based systems for wound healing. Drug Dev. Ind. Pharm. 2019, 45, 1389–1402. [Google Scholar] [CrossRef]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for Wound Healing and Infection Control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Stejskalová, A.; Almquist, B.D. Using biomaterials to rewire the process of wound repair. Biomater. Sci. 2017, 5, 1421–1434. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef]
- Zarei, F.; Soleimaninejad, M. Role of growth factors and biomaterials in wound healing. Artif. Cells Nanomed. Biotechnol. 2018, 46, 906–911. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Fernandez, B.; Castaño, O.; Mateos-Timoneda, M.Á.; Engel, E.; Pérez-Amodio, S. Nanotechnology Approaches in Chronic Wound Healing. Adv. Wound Care 2020, 10, 234–256. [Google Scholar] [CrossRef]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef]
- Salimi, F.; Mohammadipanah, F. Nanomaterials versus the Microbial Compounds with Wound Healing Property. Front. Nanotechnol. 2021, 2, 584489. [Google Scholar] [CrossRef]
- Wang, Y.; Armato, U.; Wu, J. Targeting Tunable Physical Properties of Materials for Chronic Wound Care. Front. Bioeng. Biotechnol. 2020, 8, 584. [Google Scholar] [CrossRef]
- Dalisson, B.; Barralet, J. Bioinorganics and Wound Healing. Adv. Healthc. Mater. 2019, 8, e1900764. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Nanomaterials for Wound Dressings: An Up-to-Date Overview. Molecules 2020, 25, 2699. [Google Scholar] [CrossRef]
- Yadav, V.; Bansal, P.; Mittal, A.; Singh, S.K. Global regulatory aspects of wound care and burn dressings. Asian J. Pharm. Clin. Res. 2018, 11, 516–535. [Google Scholar] [CrossRef]
- Miguel, M.G.; Antunes, M.D.; Faleiro, M.L. Honey as a complementary medicine. Integr. Med. Insights 2017, 12, 1178633717702869. [Google Scholar] [CrossRef] [Green Version]
- Saikaly, S.K.; Khachemoune, A. Honey and Wound Healing: An Update. Am. J. Clin. Dermatol. 2017, 18, 237–251. [Google Scholar] [CrossRef]
- Zulkhairi Amin, F.A.; Sabri, S.; Mohammad, S.M.; Ismail, M.; Chan, K.W.; Ismail, N.; Norhaizan, M.E.; Zawawi, N. Therapeutic properties of stingless bee honey in comparison with european bee honey. Adv. Pharmacol. Sci. 2018, 2018, 6179596. [Google Scholar] [CrossRef]
- Abd Jalil, M.A.; Kasmuri, A.R.; Hadi, H. Stingless bee honey, the natural wound healer: A review. Skin Pharmacol. Physiol. 2017, 30, 66–75. [Google Scholar] [CrossRef]
- Rosli, F.N.; Hazemi, M.H.F.; Akbar, M.A.; Basir, S.; Kassim, H.; Bunawan, H. Stingless Bee Honey: Evaluating Its Antibacterial. Insects 2020, 11, 500. [Google Scholar] [CrossRef]
- Biluca, F.C.; da Silva, B.; Caon, T.; Mohr, E.T.B.; Vieira, G.N.; Gonzaga, L.V.; Vitali, L.; Micke, G.; Fett, R.; Dalmarco, E.M.; et al. Investigation of phenolic compounds, antioxidant and anti-inflammatory activities in stingless bee honey (Meliponinae). Food Res. Int. 2020, 129, 108756. [Google Scholar] [CrossRef]
- Ezhilarasu, H.; Vishalli, D.; Dheen, S.T.; Bay, B.H.; Kumar Srinivasan, D. Nanoparticle-based therapeutic approach for diabetic wound healing. Nanomaterials 2020, 10, 1234. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Hydrogel dressings for the treatment of burn wounds: An up-to-date overview. Materials 2020, 13, 2853. [Google Scholar] [CrossRef]
- Minden-Birkenmaier, B.A.; Bowlin, G.L. Honey-based templates in wound healing and tissue engineering. Bioengineering 2018, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another alternative in the fight against antibiotic-resistant bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef]
- Yilmaz, A.C.; Aygin, D. Honey Dressing in Wound Treatment: A Systematic Review. Complement. Ther. Med. 2020, 51, 102388. [Google Scholar] [CrossRef]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J. Tissue Viability 2016, 25, 98–118. [Google Scholar] [CrossRef]
- Anis, A.; Sharshar, A.; El Hanbally, S.; Sadek, Y. A Novel Organic Composite Accelerates Wound Healing: Experimental and Clinical Study in Equine. J. Equine Vet. Sci. 2021, 99, 103406. [Google Scholar] [CrossRef]
- Rosidi Sujanto, I.S.; Ramly, N.S.; Abd Ghani, A.; Tang Yew Huat, J.; Alias, N.; Ngah, N. Composition and functional properties of stingless bee honey: A review. Malays. J. Appl. Sci. 2021, 6, 111–127. [Google Scholar]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohamed, M.T.M.; Shukor, N.I.A. Phytochemical compositions and antioxidant activities of malaysian stingless bee honey. Pertanika J. Sci. Technol. 2019, 27, 15–28. [Google Scholar]
- Angioi, R.; Morrin, A.; White, B. The rediscovery of honey for skin repair: Recent advances in mechanisms for honey-mediatedwound healing and scaffolded application techniques. Appl. Sci. 2021, 11, 5192. [Google Scholar] [CrossRef]
- Activon—Manuka Honey dressings. Adv. Med. 2021. Available online: http://www.advancis.co.uk/products/activon-manuka-honey/activon-tulle (accessed on 14 August 2021).
- Lukanc, B.; Potokar, T.; Erjavec, V. Observational study of the effect of L-Mesitran® medical honey on wound healing in cats. Vet. Arh. 2018, 88, 59–74. [Google Scholar] [CrossRef]
- Hossain, M.L.; Lim, L.Y.; Hammer, K.; Hettiarachchi, D.; Locher, C. Honey-based medicinal formulations: A critical review. Appl. Sci. 2021, 11, 5159. [Google Scholar] [CrossRef]
- Revamil Honey Gel; Oswell Penda Pharmaceutical Ltd.: Oswestry, UK, 2021; Available online: https://opp-uk.com/shop/revamil-wound-care/revamil-honey-gel/ (accessed on 14 August 2021).
- Principelle IF—Intega Health. 2021. Available online: http://integahealth.com/product/principelle-if/ (accessed on 14 August 2021).
- Deutsch, C.; Edwards, D.; Myers, S. Wound dressings. Br. J. Hosp. Med. 2017, 78, 103–109. [Google Scholar] [CrossRef]
- Axibal, E.; Brown, M. Surgical Dressings and Novel Skin Substitutes. Dermatol. Clin. 2019, 37, 349–366. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef]
- Pan, Z.; Ye, H.; Wu, D. Recent advances on polymeric hydrogels as wound dressings. APL Bioeng. 2021, 5, 011504. [Google Scholar] [CrossRef]
- Shamloo, A.; Aghababaie, Z.; Afjoul, H.; Jami, M.; Bidgoli, M.R.; Vossoughi, M.; Ramazani, A.; Kamyabhesari, K. Fabrication and evaluation of chitosan/gelatin/PVA hydrogel incorporating honey for wound healing applications: An in vitro, in vivo study. Int. J. Pharm. 2021, 592, 120068. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Das, S.; Seal, S. Nanomaterials for wound healing: Scope and advancement. Nanomedicine 2015, 10, 2593–2612. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Rayani Nivethitha, P.; Carolin Jeniba Rachel, D. A study of antioxidant and antibacterial activity using honey mediated Chromium oxide nanoparticles and its characterization. Mater. Today Proc. 2020, 48, 276–281. [Google Scholar] [CrossRef]
- Data, D.; Suresh Kumar, R. A review on wound management with special reference to nanotechnology. Int. J. Res. Pharm. Sci. 2020, 11, 2815–2824. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, K.S. Recent advances in nanomaterial-based wound-healing therapeutics. Pharmaceutics 2020, 12, 499. [Google Scholar] [CrossRef] [PubMed]
- Philip, D. Honey mediated green synthesis of gold nanoparticles. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2009, 73, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Philip, D. Honey mediated green synthesis of silver nanoparticles. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2010, 75, 1078–1081. [Google Scholar] [CrossRef]
- Ismail, N.A.; Shameli, K.; Wong, M.M.T.; Teow, S.Y.; Chew, J.; Sukri, S.N.A.M. Antibacterial and cytotoxic effect of honey mediated copper nanoparticles synthesized using ultrasonic assistance. Mater. Sci. Eng. C 2019, 104, 109899. [Google Scholar] [CrossRef]
- Czernel, G.; Bloch, D.; Matwijczuk, A.; Cieśla, J.; Kędzierska-Matysek, M.; Florek, M.; Gagoś, M. Biodirected synthesis of silver nanoparticles using aqueous honey solutions and evaluation of their antifungal activity against pathogenic Candida spp. Int. J. Mol. Sci. 2021, 22, 7715. [Google Scholar] [CrossRef]
- Haiza, H.; Azizan, A.; Mohidin, A.H.; Halin, D.S.C. Green Synthesis of Silver Nanoparticles Using Local Honey. Nano Hybrids 2013, 4, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Rasouli, E.; Basirun, W.J.; Rezayi, M.; Shameli, K.; Nourmohammadi, E.; Khandanlou, R.; Izadiyan, Z.; Sarkarizi, H.K. Ultrasmall Superparamagnetic Fe3O4 Nanoparticles: Honey-based Green and Facile Synthesis and In Vitro Viability Assay. Int. J. Nanomed. 2018, 13, 6903–6911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arslan, A.; Şimşek, M.; Aldemir, S.D.; Kazaroǧlu, N.M.; Gumuşderelioǧlu, M. Honey-based PET or PET/chitosan fibrous wound dressings: Effect of honey on electrospinning process. J. Biomater. Sci. Polym. Ed. 2014, 25, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lan, X.; Liang, C.; Zhong, Z.; Xie, R.; Zhou, Y.; Miao, X.; Wang, H.; Wang, W. Honey loaded alginate/PVA nanofibrous membrane as potential bioactive wound dressing. Carbohydr. Polym. 2019, 219, 113–120. [Google Scholar] [CrossRef]
- Al-musawi, S.; Albukhaty, S.; Al-karagoly, H. Antibacterial Activity of Honey/Chitosan Nanofibers Loaded with Capsaicin and Gold Nanoparticles for Wound Dressing. Mol. Artic. 2020, 25, 4770. [Google Scholar]
- Ghorbani, M.; Ramezani, S.; Rashidi, M.R. Fabrication of honey-loaded ethylcellulose/gum tragacanth nanofibers as an effective antibacterial wound dressing. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126615. [Google Scholar] [CrossRef]
- Noori, S.; Kokabi, M.; Hassan, Z.M. Poly(vinyl alcohol)/chitosan/honey/clay responsive nanocomposite hydrogel wound dressing. J. Appl. Polym. Sci. 2018, 135, 46311. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, S.; Khan, M.Q.; Hashmi, M.; Nam, P.D.; Kato, Y.; Tamada, Y.; Kim, I.S. Manuka honey incorporated cellulose acetate nanofibrous mats: Fabrication and in vitro evaluation as a potential wound dressing. Int. J. Biol. Macromol. 2020, 155, 479–489. [Google Scholar] [CrossRef]
- Meftahi, A.; Shahriari, H.R.; Khajavi, R.; Rahimi, M.K.; Sharifian, A.; Meftahi, A. Investigation on nano microbial cellulose/honey composite for medical application. Mater. Res. Express 2020, 7, 085003. [Google Scholar] [CrossRef]
- Rafati, Z.; Sirousazar, M.; Hassan, Z.M.; Kheiri, F. Honey-Loaded Egg White/Poly(vinyl alcohol)/Clay Bionanocomposite Hydrogel Wound Dressings: In Vitro and In Vivo Evaluations. J. Polym. Environ. 2020, 28, 32–46. [Google Scholar]
- Kanimozhi, S.; Kathiresan, G.; Kathalingam, A.; Kim, H.S.; Doss, M.N.R. Organic nanocomposite Band-Aid for chronic wound healing: A novel honey-based nanofibrous scaffold. Appl. Nanosci. 2020, 10, 1639–1652. [Google Scholar] [CrossRef]
- Fathollahipour, S.; Koosha, M.; Tavakoli, J.; Maziarfar, S.; Mehrabadi, J.F. Erythromycin releasing PVA/sucrose and PVA/honey hydrogels as wound dressings with antibacterial activity and enhanced bio-adhesion. Iran. J. Pharm. Res. 2020, 19, 448–464. [Google Scholar] [PubMed]
- Stojkovska, J.; Petrovic, P.; Jancic, I.; Milenkovic, M.T.; Obradovic, B. Novel nano-composite hydrogels with honey effective against multi-resistant clinical strains of Acinetobacter baumannii and Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2019, 103, 8529–8543. [Google Scholar] [CrossRef] [PubMed]
- Neupane, B.P.; Chaudhary, D.; Paudel, S.; Timsina, S.; Chapagain, B.; Jamarkattel, N.; Tiwari, B.R. Himalayan honey loaded iron oxide nanoparticles: Synthesis, characterization and study of antioxidant and antimicrobial activities. Int. J. Nanomed. 2019, 14, 3533–3541. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Gupta, A.; Gupta, B. Scar free healing mediated by the release of aloe vera and manuka honey from dextran bionanocomposite wound dressings. Int. J. Biol. Macromol. 2018, 120, 1581–1590. [Google Scholar] [CrossRef]
- Sarkar, R.; Ghosh, A.; Barui, A.; Datta, P. Repositing honey incorporated electrospun nanofiber membranes to provide anti-oxidant, anti-bacterial and anti-inflammatory microenvironment for wound regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 31. [Google Scholar] [CrossRef]
- Rani, G.N.; Rao, B.N.; Shamili, M.; Jyothi Padmaja, I. Combined effect of silver nanoparticles and honey in experimental wound healing process in rats. Biomed. Res. 2018, 29, 3074–3078. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Fan, L.; Ma, L.; Wang, Y.; Lin, S.; Yu, F.; Pan, X.; Luo, G.; Zhang, D.; Wang, H. Green electrospun Manuka honey/silk fibroin fibrous matrices as potential wound dressing. Mater. Des. 2017, 119, 76–84. [Google Scholar] [CrossRef]
- El-Kased, R.F.; Amer, R.I.; Attia, D.; Elmazar, M.M. Honey-based hydrogel: In vitro and comparative in vivo evaluation for burn wound healing. Sci. Rep. 2017, 7, 9692. [Google Scholar] [CrossRef] [Green Version]
- Sarhan, W.A.; Azzazy, H.M.E.; El-Sherbiny, I.M. Honey/Chitosan Nanofiber Wound Dressing Enriched with Allium sativum and Cleome droserifolia: Enhanced Antimicrobial and Wound Healing Activity. ACS Appl. Mater. Interfaces 2016, 8, 6379–6390. [Google Scholar] [CrossRef]
- Tsang, K.K.; Kwong, E.W.Y.; Woo, K.Y.; To, T.S.S.; Chung, J.W.Y.; Wong, T.K.S. The anti-inflammatory and antibacterial action of nanocrystalline silver and manuka honey on the molecular alternation of diabetic foot ulcer: A comprehensive literature review. Evid.-Based Complement. Altern. Med. 2015, 2015, 218283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Research, T.M. Antimicrobial Wound Dressings Market—Global Industry Analysis, Size, Share, Growth, Trends and Forecast 2018–2026. 2021. Available online: https://www.transparencymarketresearch.com/platinum-based-drugs-market.html (accessed on 2 August 2021).


| Type | Examples | Level of Risk | FDA Classification | Regulatory Requirements |
|---|---|---|---|---|
| Fabric dressings | Hydrophilic wound dressings, occlusive wound dressings, and hydrogel wound dressings | Low risk | Class I | Approval not required; the FDA only needs to be informed before marketing. It is the responsibility of the manufacturer to maintain the safety and quality of the product. |
| Advanced wound care dressings | Medihoney, Prisma, and Oasis wound matrix | Intermediate risk | Class II | 510 (k) approval is required. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahari, N.; Hashim, N.; Md Akim, A.; Maringgal, B. Recent Advances in Honey-Based Nanoparticles for Wound Dressing: A Review. Nanomaterials 2022, 12, 2560. https://doi.org/10.3390/nano12152560
Bahari N, Hashim N, Md Akim A, Maringgal B. Recent Advances in Honey-Based Nanoparticles for Wound Dressing: A Review. Nanomaterials. 2022; 12(15):2560. https://doi.org/10.3390/nano12152560
Chicago/Turabian StyleBahari, Norfarina, Norhashila Hashim, Abdah Md Akim, and Bernard Maringgal. 2022. "Recent Advances in Honey-Based Nanoparticles for Wound Dressing: A Review" Nanomaterials 12, no. 15: 2560. https://doi.org/10.3390/nano12152560
APA StyleBahari, N., Hashim, N., Md Akim, A., & Maringgal, B. (2022). Recent Advances in Honey-Based Nanoparticles for Wound Dressing: A Review. Nanomaterials, 12(15), 2560. https://doi.org/10.3390/nano12152560








