Abstract
In recent times, upconversion nanomaterials with mesoporous hollow structures have gained significant interest as a prospective nano-platform for cancer imaging and therapeutic applications. In this study, we report a highly biocompatible YVO4:1Er3+/10Yb3+ upconversion mesoporous hollow nanospheriods (YVO4:Er3+/Yb3+ UC-MHNSPs) by a facile and rapid self-sacrificing template method. The Rietveld analysis confirmed their pure phase of tetragonal zircon structure. Nitrogen adsorption–desorption isotherms revealed the mesoporous nature of these UC-MHNSPs and the surface area is found to be ~87.46 m2/g. Under near-infrared excitation (980 nm), YVO4:Er3+/Yb3+ UC-MHNSPs showed interesting color tunability from red to green emission. Initially (at 0.4 W), energy back transfer from Er3+ to Yb3+ ions leads to the strong red emission. Whereas at high pump powers (1 W), a fine green emission is observed due to the dominant three-photon excitation process and traditional energy transfer route from Er3+ to Yb3+ ions. The bright red light from the membrane of HeLa cells confirmed the effective cellular uptake of YVO4:Er3+/Yb3+ UC-MHNSPs. The resonant decrease in cell viability on increasing the concentration of curcumin conjugated YVO4:Er3+/Yb3+ UC-MHNSPs established their excellent antitumor activity. Therefore, the acquired results indicate that these YVO4:Er3+/Yb3+ UC-MHNSPs are promising drug carriers for bioimaging and various therapeutic applications.
1. Introduction
Currently, the aid of mesoporous hollow nanomaterials in multimodal imaging and drug delivery has gained substantial attention owing to its high drug loading efficiency, long circulation times, and sustained drug release behavior [1,2,3]. So far, several research groups have focused on and established the potential of surface functionalized mesoporous silicates for drug delivery applications because of their higher surface area and straight narrow channels, which allows the high adsorption of drugs into their texture [4,5,6,7]. However, there are still considerable disadvantages, including toxicity and weak permeability into the cellular nucleus [8]. It is also a noticeable fact that, as compared to near-infrared (NIR) light, ultraviolet (UV) light source damages human organs. In addition, the cell culture media and some of the drugs show autofluorescence under UV–vis excitations [9,10]. In addition, the upconversion (UC) nanomaterials, which converts NIR light (808, 915, and 980 nm) to UV and visible light, exhibit innovative pharmacokinetics without a background autofluorescence [11,12,13]. Therefore, in order to avoid the mentioned shortcomings and enable the safest and most efficient drug delivery carriers, the current research is focused on the integration of UC luminescence properties with mesoporous hollow nanoparticles.
Among different rare-earth-based host matrices, yttrium vanadate (YVO4) has received abundant interest as an ideal host material owing to its exceptional characteristics such as excellent chemical and thermal stability, outstanding optical enactment, and high luminescence quantum yield [14,15]. Since the first production of the YVO4 host matrix, extensive studies have been conducted to establish its optical properties using various dopant ions because the D2d point symmetry of Y3+ ions provides an idle doping site for lanthanide ions [16]. For instance, YVO4:Eu3+ is established as a viable red phosphor for cathode ray tubes and an inorganic scintillator, whereas its single crystals are proven as an excellent laser host materials [17,18,19,20,21]. Likewise, there are few reports available on the UC luminescence properties of YVO4 material and its application as a temperature sensor [22]. Until now, many researchers focused on the synthesis procedure and photoluminescence studies of YVO4:Eu3+ nanophosphors. However, no reports were available on biocompatibility and antitumor activity of YVO4:Er3+/Yb3+ mesoporous nanoparticles so far.
In the present study, we developed a highly stable and extremely biocompatible YVO4:1Er3+/10Yb3+ UC mesoporous hollow nanospheriods (YVO4:Er3+/Yb3+ UC-MHNSPs) as potential drug carriers by a simple and rapid self-sacrificing template method. The formation mechanism and the crystalline nature of YVO4:Er3+/Yb3+ UC-MHNSPs were explained through morphological and structural studies. Depending on the input laser pump power (980 nm), these UC-MHNSPs exhibited red and green emissions. The reason behind this color tunability was discussed with the help of a number of excited photons and the energy transfer pathways among Er3+ and Yb3+ ions. HeLa cells were used to assess the cytotoxicity, permeability, and cellular uptake of these YVO4:Er3+/Yb3+ UC-MHNSPs, and curcumin was used as a modal drug to evaluate their antitumor activity.
2. Materials and Methods
2.1. Synthesis of Monodisperse Y(OH)CO3:1Er3+/10Yb3+ Nanospheres
Y(1−x−y)(OH)CO3:xEr3+/yYb3+ nanospheres (YC:Er3+/Yb3+ NSs) were fabricated by a modified urea-based homogeneous precipitation (UHP) method according to our earlier report [23]. All the reagents (analytical grade) were from Sigma-Aldrich (Gangnam-gu, Seoul, South Korea) and used without any additional purification. In a typical process, required amounts of yttrium nitrate [Y(NO3)3∙6H2O], erbium nitrate [Eu(NO3)3∙5H2O], ytterbium nitrate [Yb(NO3)3∙5H2O], and urea (10 mg/mL) were dissolved in 100 mL of de-ionized (DI) water. After 2 h of vigorous stirring, 20 µL of tetrapropylammonium hydroxide (TPAOH) was added and the magnetic stirring was continued for 30 min. Eventually, the colloidal solution was moved to silicone oil bath, and the reaction temperature was fixed to 90 °C for 10 h. After that, the YC:Er3+/Yb3+ NSs were gathered by using centrifugation (6000× g rpm/5 min) technique and washed multiple times with ethanol and DI water.
2.2. Fabrication of YVO4:1Er3+/10Yb3+ UC-MHNSPs
To fabricate the UC-MHNSPs, the obtained precursor (YC:Er3+/Yb3+ NSs) was dispersed into 40 mL of triple-distilled DI water and sonicated for 30 min to obtain the homogeneous solution. In another beaker, required amount of ammonium metavanadate [NH4VO3], ammonium fluoride [NH4F], and 120 µL of 2 M hydrochloric acid (HCl) were dissolved in 30 mL DI water. Then, the YC:Er3+/Yb3+ NSs were added dropwise to the vanadium solution under continuous stirring. After 30 min of stirring, the mixture was transformed into a Teflon liner and the autoclave temperature was fixed to 180 °C for 10 h. Once the autoclave temperature was cooled down to normal room temperature, the residue was gathered by centrifugal separator and washed numerous times with DI water and ethanol. After drying the sample in an oven (75 °C) overnight, the final form of the YVO4:Er3+/Yb3+ UC-MHNSPs was ready for further characterizations. All the characterization techniques were presented in Supplementary Materials.
2.3. Cell Culture and Cytotoxicity Test
HeLa cell lines (ATCC, Manassas, VA, USA) were grown in 24 well plates (at a density of 5 × 104 cells/well) and cultured for 24 h and then incubated with different quantities (50, 25, 12.5, 6.25, and 3.125 ug/mL) of YVO4:Er3+/Yb3+ UC-MHNSPs in a CO2 incubator at 37 °C for another 24 h. After that, HeLa cell lines were washed with Dulbecco’s phosphate-buffered saline (DPBS) 5 times, and then the cell viability was measured by cell count kit-8 (CCK-8) based on the manufacturer’s protocol.
2.4. Drug Loading and In Vitro Releasing Protocol
For drug loading, 9 mg curcumin was dissolved in ethanol (9 mg/900 µL), and 45 mg YVO4:Er3+/Yb3+ UC-MHNSPs were dissolved in 2 mL DI water. The curcumin and UC-MHNSPs were used in 1:5 ratio. After 20 min of sonication, the curcumin solution was added dropwise to YVO4:Er3+/Yb3+ UC-MHNSPs and allowed to mix using a rotator for 24 h. The curcumin conjugated sample was collected by centrifugation (5000× g rpm/5 min) after washed with DI water several times.
3. Results and Discussion
3.1. Morphological Evolution
Herein, the nontoxic and highly water soluble YVO4:Er3+/Yb3+ UC-MHNSPs were produced by a rapid self-sacrificing template method. Initially, the YC:Er3+/Yb3+ NSs of around 50 nm size were synthesized by a well-established UHP route where TPAOH was used as a size controlling agent. At high temperatures (about 90 °C), urea decomposes into OH− and CO32− ions, and then combines with Y3+ ions to produce the uniform YC:Er3+/Yb3+ NSs and the respective scanning and transmission electron microscope (SEM and TEM) images are shown in Figure 1a–d. Here, the addition of TPAOH (20 µL) encourages the nucleation process rather than crystal growth and thus the NSs size was limited to 50 nm. After that, these YC:Er3+/Yb3+ NSs were allowed to react with NH4VO3 under hydrothermal conditions (180 °C for 1 h), where the Y3+ cations react with VO43− anions and produces the YVO4:Er3+/Yb3+ UC-MHNSPs as a result of the Kirkendall effect. Figure 1e–h displays the corresponding SEM and TEM images of YVO4:Er3+/Yb3+ UC-MHNSPs and the possible chemical reactions that happened during the synthesis were given here:
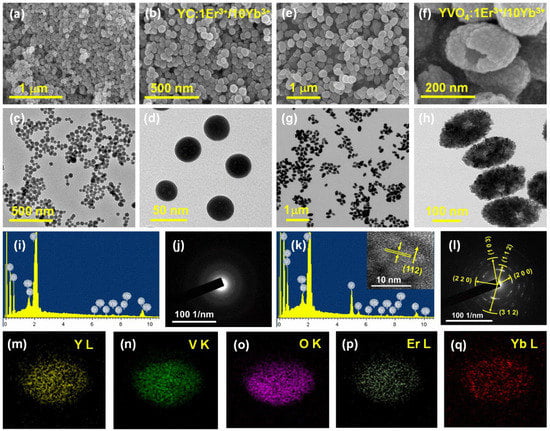
Figure 1.
FE-SEM and FE-TEM images of (a–d) YC:Er3+/Yb3+ NSs and (e–h) YVO4:Er3+/Yb3+ UC-MHNSPs. (i,k) EDS spectrum and (j,l) SAED patterns of NSs and UC-HMNSPs, respectively, and the inset of (k) shows the corresponding HR-TEM image. (m–q) Elemental mapping of an individual YVO4:Er3+/Yb3+ UC-MHNSPs based on EDS spectrum.
The high-magnification SEM and TEM images (Figure 1f,h) unveils the mesoporous hollow nature of the YVO4 nanoparticles with a uniform size of around 150 nm. The high-resolution TEM (HRTEM) image of YVO4:Er3+/Yb3+ UC-MHNSPs (inset of Figure 1k) displayed clear lattice fringes with d-spacing of 2.676 Å, which is well synchronized with the theoretical d-spacing value of 2.668 Å corresponding to (112) plane of YVO4 host matrix. The selected area electron diffraction (SAED) patterns presented in Figure 1j,l reveals that the YC:Er3+/Yb3+ NSs were amorphous in nature whereas the ring pattern overlaid with bright spots confirms the nanocrystalline feature of YVO4:Er3+/Yb3+ UC-MHNSPs, respectively. The d-spacings 2.518, 2.012, 2.676, 3.568, and 1.823 Å obtained from the SAED pattern were analogous to the (220), (103), (112), (200), and (312) planes of tetragonal structured YVO4 matrix, respectively.
Energy-dispersive X-ray spectroscopy (EDS) was conducted to study the chemical characterization of YC:Er3+/Yb3+ NSs and YVO4:Er3+/Yb3+ UC-MHNSPs, and the resulting EDS spectra are produced in Figure 1i,k. The X-ray peaks that appeared in the energy spectrum of YC:Er3+/Yb3+ NSs (Figure 1i) confirmed the presence of host element (Y) together with dopant elements (Er, and Yb), while the energy spectrum of YVO4:Er3+/Yb3+ UC-MHNSPs established the existence of V along with Y, Er, and Yb elements as shown in Figure 1k. The position of X-ray peaks in both the spectra suggests that most of the Y, Er, and Yb ions occupied the L-shell, and V and O elements engaged the K-shell of the energy spectrum, respectively. Figure 1m–q shows the elemental mapping of a single UC-MHNSP and the acquired images signifying that all the elements (host and dopant) were uniformly distributed within the particle, which will help to enhance the luminescence properties of the YVO4 matrix.
Further, the formation mechanism of UC-MHNSPs can be explained through the Kirkendall effect, which describes the dissimilar diffusion rates of two elements in a multi-component system that leads to the formation of hollow and/or porous nanostructures [24,25]. When the YC:Er3+/Yb3+ NSs were added to the NH4VO3 solution, initially (within 5 min) the Y3+ ions at the surface of YC:Er3+/Yb3+ NSs will react with VO43− anions and forms a layer of YVO4 rod-like particles at the outer surface of the NSs and the corresponding chemical reactions are presented in Equations (3)–(5). As the reaction proceeds, the inner dissolved Y3+ cations tend to diffuse outwards, and simultaneously the VO43− anions in the solution try to diffuse inwards through NSs. When the outward diffusion rate of Y3+ cations was much quicker compared to the inward diffusion rate of VO43− anions, the voids will form within the NSs and the reaction will occur on or near the surface (or interface) which leads to the outward growth of the YVO4 hollow shell. Thus, the YVO4:Er3+/Yb3+ UC-MHNSPs were formed as a result of the Kirkendall effect and the schematic representation of the formation mechanism is displayed in Figure 2. Interestingly, all these reactions occurred within 1 h of time duration, which is very rapid, and this morphology continued even after 24 h. The corresponding low and high magnification SEM and TEM images of YVO4:Er3+/Yb3+ UC-MHNSPs at different reaction timings (4, 6, 12, and 24 h) were presented in the Figures S1 and S2, respectively.
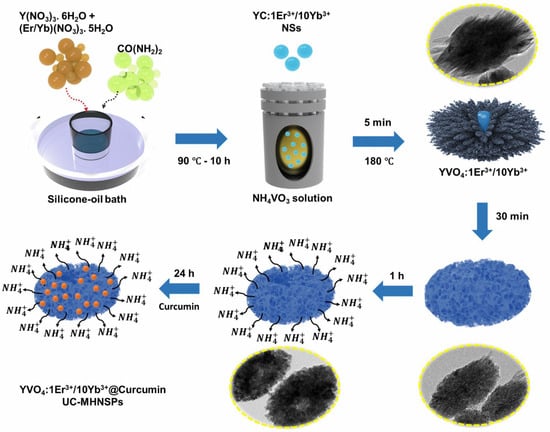
Figure 2.
Schematic of the synthetic route and the transformation of YC:Er3+/Yb3+ NSs into YVO4:Er3+/Yb3+ UC-MHNSPs.
3.2. Structural Analysis
The X-ray diffraction (XRD) technique was used to determine the crystalline nature of YC:Er3+/Yb3+ NSs and YVO4:Er3+/Yb3+ UC-MHNSPs, and the resulting diffraction patterns are presented in Figure 3a. The XRD profile of the precursor sample exhibited two broad bands with maxima at around 29° and 46°, indicating the amorphous nature of the YC:Er3+/Yb3+ NSs. Whereas a well indexed tetragonal phase was attained for YVO4:Er3+/Yb3+ UC-MHNSPs, which exactly matches with the previously reported reference pattern on the YVO4 host matrix with JCPDS card no. 76-1649 and the space group of I41/amdz. Because of the similar ionic radii, no other impurity peaks were detected after the doping of Er3+ (0.89) and Yb3+ (0.868) ions in the Y (0.9) site, indicating that the crystalline structure of the YVO4 host lattice did not effected by the current doping level. Likewise, the effect of flux (NH4F) on the crystal structure of YVO4:Er3+/Yb3+ UC-MHNSPs was also verified. At 4 mmol concentration of NH4F, a pure tetragonal phase without any impurity peaks was observed whereas a mixed phase was observed for 8 mmol concentration of flux and the corresponding diffraction patterns are illustrated in Figure S3. In general, the excess of flux leads to the formation of mixed phases and thus 4 mmol NH4F was used as flux during the synthesis of YVO4:Er3+/Yb3+ UC-MHNSPs [26]. Figure 3b displays the diffraction patterns of YVO4:Er3+/Yb3+ UC-MHNSPs synthesized at various reaction timings from 5 min to 24 h. Interestingly, the diffraction pattern exactly matched the reference pattern (JCPDS No. 76-1649) and the witnessed increase in diffraction pattern intensity signified the increasing crystallinity of the particles. The well-known Scherrer equation was employed to evaluate the mean crystallite size (D):
where k and λ represents the Scherrer constant and X-ray beam wavelength, while θ and β indicates the diffraction angle and full width at half maximum of the diffraction peak, respectively. The average crystallite sizes were calculated to be 23.6, 24.9, 25.7, 26.9, 32.9, 34.5, 35.5, and 37.4 nm for 5 min, 15 min, 30 min, 1 h, 4 h, 6 h, 12 h, and 24 h of reaction time, respectively. After the addition of flux (4 mmol NH4F), the mean crystallite size increased from 26.8 to 41.7 nm for YVO4:Er3+/Yb3+ UC-MHNSPs at 1 h of reaction time, signifying the increased crystallinity of the material. General Structure Analysis System (GSAS) software was used to execute the Rietveld refinement of the XRD pattern to validate the tetragonal zircon-type structure of YVO4:Er3+/Yb3+ UC-MHNSPs. Figure 3c presents the Rietveld fit of the YVO4:Er3+/Yb3+ UC-MHNSPs where the difference profile appeared to be nearly flat signifying a good quality fit between calculated and experimental profiles with decent agreement values (Rp = 2.32, Rwp = 2.96, and χ2 = 2.682). The obtained crystallographic data including atomic positions and occupancies are presented in Table S1. The obtained unit cell parameters were: a and b = 7.1095 Å, c = 6.2844 Å, and volume (V) = 317.65 Å3 for YVO4:Er3+/Yb3+ UC-MHNSPs. Diamond 3.2, a crystal structure visualization software, was used to sketch the crystal structure of YVO4:Er3+/Yb3+ UC-MHNSPs and the resultant ideal unit cell model is displayed in Figure 3d. Generally, the YVO4 host lattice belongs to rare-earth orthovanadates (MVO4) with tetragonal zircon structure with space group I41/amdz (where Z = 4) [27]. This zircon-type structure is assembled from the chains of alternative edge-sharing [VO4]3− tetrahedra and [YO8] bisdisphenoids which are growing parallel to the c-axis and laterally linked through edge-sharing [YO8] bisdisphenoids to build “zigzag” chains parallel to a-axis as shown in Figure 3d.
Dhkl = κλ/β cos θ
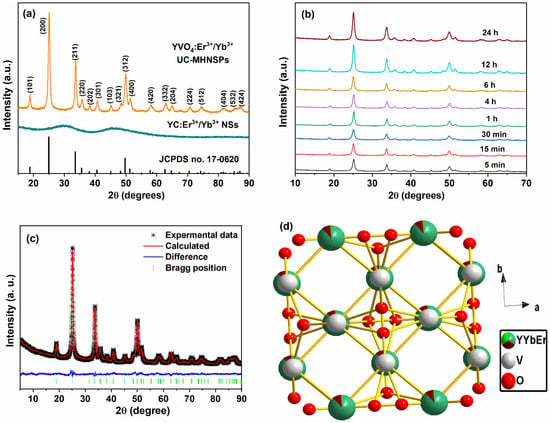
Figure 3.
(a) XRD patterns of YC:Er3+/Yb3+ NSs and YVO4:Er3+/Yb3+ UC-MHNSPs, (b) diffraction patterns of YVO4:Er3+/Yb3+ UC-MHNSPs at various reaction timings, (c) Rietveld fit and (d) ideal unit cell of YVO4:Er3+/Yb3+ UC-MHNSPs.
Brunauer–Emmett–Teller (BET) approach was adopted to quantify the surface area of YC:Er3+/Yb3+ NSs and YVO4:Er3+/Yb3+ UC-MHNSPs and the resultant N2 adsorption–desorption isotherms are shown in Figure 4. The acquired adsorption–desorption profiles of both the samples resembles the type IV isotherm accompanied by H3 type hysteresis loop, based on the IUPAC classifications [28,29]. The hysteresis loop appeared at high pressure region (ρ/ρ0 = 0.8 to 0.99) shown in Figure 4a indicates the presence of large pores (mesopores to macropores) in the YC:Er3+/Yb3+ NSs, whereas the wider hysteresis loop at partial relative pressure region (ρ/ρ0 = 0.4 to 0.8) illustrated in Figure 4b signifies the existence of mesoporous nature of the YVO4:Er3+/Yb3+ UC-MHNSPs with narrow sized pore distribution. The specific surface area of YC:Er3+/Yb3+ NSs and YVO4:Er3+/Yb3+ UC-MHNSPs were found to be 25.74 and 87.46 m2/g, respectively. Compared to the NSs, the surface area is 3.4-fold increased for MHNSPs. According to the Barrett–Joyner–Halenda (BJH) plot shown in Figure 4c, the pore size distribution was depicted prominently at around 3.8 nm along with average pore volume of 0.16 cm3/g for YVO4:Er3+/Yb3+ UC-MHNSPs, and the broad peak obtained at around 82 nm region belongs to the hollow structure of the particle. Thus, the BET analysis infers the mesoporous nature of our material.
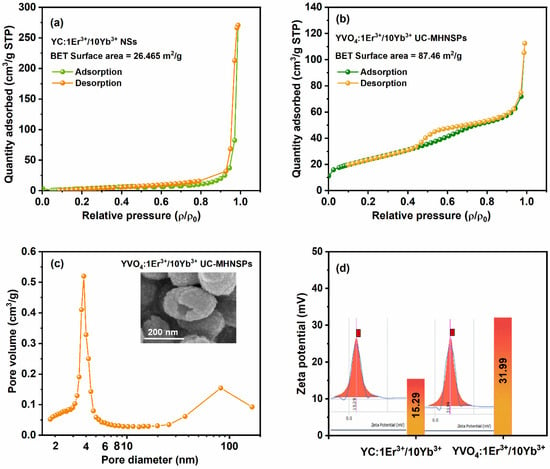
Figure 4.
(a,b) Nitrogen adsorption desorption isotherms of YC:Er3+/Yb3+ NSs and YVO4:Er3+/Yb3+ UC-MHNSPs, (c) pore size distribution plot of YVO4:Er3+/Yb3+ UC-MHNSPs, and (d) particle zeta potential value and the related distribution graphs of NSs and UC-MHNSPs.
Zeta potential was measured to study the dispersion stability of the YC:Er3+/Yb3+ NSs and YVO4:Er3+/Yb3+ UC-MHNSPs in DI water and the acquired results are shown in Figure 4d. As expected, both the NSs and UC-MHNSPs exhibited positive surface charge of 15.29 and 31.99 mV, respectively. Because YC:Er3+/Yb3+ NSs were synthesized by the well-known UHP method (where urea (CO(NH)2) produces NH4+ ions) and for the synthesis of YVO4:Er3+/Yb3+ UC-MHNSPs, NH4VO3 was taken as vanadium source and NH4F was used as flux. Thus, the excess of NH4+ ions present in the solvent causes the positive charge density on the surface of the UC-MHNSPs. Typically, the nanoparticles with Zeta potential lower than −30 mV or higher than +30 mV are considered as highly dispersible and are suitable for biomedical applications. Hence, our synthesized YVO4:Er3+/Yb3+ UC-MHNSPs with 31.99 mV Zeta potential can be used as an efficient drug carrier for biomedical applications.
3.3. Upconversion Luminescence Studies
It has been well established that, the Yb3+ ions absorb most of the incident NIR light and then resonantly donates their energy to the adjacent Er3+ ions. Because of the spectral overlap between the 4I15/2 → 4I11/2 transition of Er3+ ions and 2F7/2 → 2F5/2 transition of Yb3+ ions, the co-doping of Er3+/Yb3+ ions forms the most attractive activator/sensitizer pair to achieve the UC luminescence [30]. Figure 5a illustrates the luminescence spectra of YVO4:Er3+/Yb3+ UC-MHNSPs as a function of excitation pump power from 0.4 to 1 W under 980 nm laser excitation. All the emission spectra displayed a weak green emission band between 516 to 560 nm region with maxima at 522 and 546 nm along with an intense red emission band around 642 to 690 nm region with band maxima at 670 nm, which are analogous to the transitions from the emitting excited levels 2H11/2, 4S3/2, and 4F9/2 to the ground level 4I15/2 of Er3+ ions. It is worthwhile to mention that, at low pump powers of 0.4 W, the UC emission spectrum displayed only the red emission band and the green emission band intensity increased along with the red emission band by rising the input laser pump power from 0.5 to 1 W (Figure 5a). The comparison between emission spectra of YVO4:Er3+/Yb3+ UC-MHNSPs at low (0.4 W) and high (1 W) pump powers are presented in Figure 5b. The digital photos of red and green emissions at low and high pump powers are displayed as insets of Figure 5b. According to the literature, energy back transfer (EBT) from Er3+ ions to Yb3+ ions and cross-relaxation (CR) among adjacent Er3+ ions might be the reasons for the occurrence of a strong red emission band as compared to the green emission band [31,32].
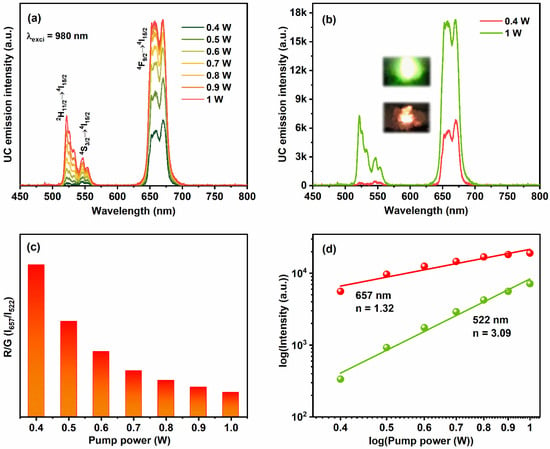
Figure 5.
(a) UC luminescence spectra of YVO4:Er3+/Yb3+ UC-MHNSPs as a function of incident pump power under 980 nm excitation, (b) comparison between the emission spectra of YVO4:Er3+/Yb3+ UC-MHNSPs at low and high excitation powers and the insets show the corresponding digital photos. (c) R/G versus input pump power and (d) logarithmic plots of input laser pump power versus green and red emission intensities.
The dependency of the integrated intensity ratio of red and green (R/G) emission bands versus laser pump power is presented in Figure 5c. The R/G values were decreased continuously (from 16.65 to 2.66) by rising the excitation power from 0.4 to 1 W. In general, the intensities of red and green emission bands depend on the amount of population in the excited states of Er3+ ions (2H11/2, 4S3/2, and 4F9/2). In the present case of YVO4:Er3+/Yb3+ UC-MHNSPs, the intensities of both red and green emission bands constantly increased up to 1 W laser pump power without quenching. Compared to the red emission intensity, a strong increase was observed for green emission intensity due to the traditional energy transfer (ET) and non-radiative (NR) transitions, and hence the R/G ratio was decreased at high excitation pump powers. To understand the UC excitation process, the study of UC emission intensity (I) versus excitation pump power (P) is important and their relation is denoted by the following Equation: [33]
where n represents the number of pumping photons needed to populate the desired emitting states. Figure 5d shows the plots of log (I) vs log (Pn) for green (2H11/2 → 4I15/2) and red (4F9/2 → 4I15/2) emission peaks, which formed a straight line. The slope values were calculated to be 1.32 and 3.09 for red and green emissions, suggesting the involvement of two-photon and three-photon excitation processes for red and green emissions, respectively.
The population process of emitting excited states and the proposed energy transfer routes in YVO4:Er3+/Yb3+ UC-MHNSPs are schematically visualized in Figure 6. Typically, ground state absorption (GSA), ET, and excited state absorption (ESA) are the three basic possible ways that are responsible to populate the excited levels in the UC mechanism [34]. Under 980 nm laser excitation, the Yb3+ and Er3+ ions in 2F7/2 and 4I15/2 states absorb the incident photons and are excited to 2F5/2 and 4I11/2 states as a result of GSA. As compared to Er3+ ions, Yb3+ ions absorb more NIR radiation and transfer their energy to 4I11/2 and 4F7/2 levels of Er3+ ions through the ET process. At the same time, the Er3+ ions in the 4I11/2 level are further excited to the 4F7/2 level by grabbing one more photon of 980 nm and this method is acknowledged as ESA. Hence, the 4F7/2 level is populated by GSA, ET, and ESA routes. Then, the excited Er3+ ions in the 4F7/2 level undergo NR transition to populate 2H11/2, 4S3/2, and then 4F9/2 levels. As a result of radiative transitions 2H11/2, 4S3/2 → 4I15/2, the relatively low intense green emission bands around 522 and 546 nm are achieved. However, the proposed three-photon excitation process for green emission occurred at high pump powers due to CR between adjacent Er3+ ions. Some of the Er3+ ions in the emitting excited levels (2H11/2, 4S3/2) can further be excited to the 2G7/2 level through the ET process and then relax to the 4G11/2 level by NR transition. Further, the emitting excited levels 2H11/2 and 4S3/2 were populated through the CR processes (4G11/2 → 2H11/2, 4S3/2, and 4I15/2 → 4I13/2)) between Er3+ ions [35,36,37].
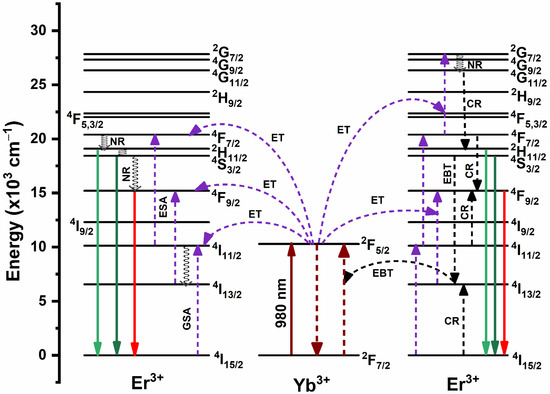
Figure 6.
Schematic energy level illustration of Er3+ and Yb3+ ions, and the suggested UC excitation mechanism in YVO4:Er3+/Yb3+ UC-MHNSPs.
In the case of strong red emission, other than NR transition from 2H11/2, 4S3/2 levels to 4F9/2 level, there are two more possible ways to populate the4F9/2 level. Among them, the first one is ESA (4I13/2 + a photon (980 nm) → 4F9/2) or ET (2F5/2 + 4I13/2 → 2F7/2 + 4F9/2) process, where the level 4I13/2 is populated by NR transition from 4I11/2 level [38]. An another way to populate 4F9/2 level is CR process (4F7/2 → 4F9/2 + 4I11/2 → 4F9/2) among Er3+ ions and EBT process from Er3+ to Yb3+ ions (4S3/2 + 2F7/2 → 4I13/2 + 2F5/2) as shown in Figure 6 [39]. Therefore, the green emitting excited levels (2H11/2 and 4S3/2) are more populated when compared to the red emitting level (4F9/2), and thus an intense red emission band achieved for YVO4:1Er3+/10Yb3+ UC-MHNSPs. In addition, during the synthesis of YVO4:Er3+/Yb3+ UC-MHNSPs, (4 mmol) NH4F was used as a flux to enrich the emission properties. It is well established that a small amount of suitable flux enhances the crystallization of the material which eventually leads to the enhancement of its luminescence properties [26,40].
Figure 7a displays the temperature-dependent emission spectra of YVO4:Er3+/Yb3+ UC-MHNSPs under 980 nm laser excitation. On rising the temperature from 25 to 160 °C, the profile and band positions of green and red emissions did not alter, but the intensities were slightly decreased due to increased lattice vibrations. The inset of Figure 7a shows the luminescence intensity ratio (LIR) versus the temperature graph for thermally coupled red emission levels (I657/I670). Usually, the LIR is calculated for thermally coupled levels (2H11/2 and 4S3/2) of green emission to estimate the sensing behavior of the material. However, in the present case of YVO4:Er3+/Yb3+ UC-MHNSPs, red emission was more prominent than the green emission and thus we calculated LIR for stark levels of red emission band, which is almost flat [41,42]. Surprisingly, at an elevated temperature of 160 °C, around 87.5% emission intensity was acquired as compared to the room-temperature (25 °C) emission intensity, signifying the high thermal stability of these YVO4:Er3+/Yb3+ UC-MHNSPs. Likewise, the photostability was evaluated for these UC-MHNSPs under continuous laser irradiation for 20 min, and the resultant emission spectra under high pump power of 1 W are displayed in Figure 7b. Interestingly, 90% of the emission intensity was attained even after 20 min of continuous laser irradiation at the high input pump power of 1 W. Therefore, the obtained results suggest that our synthesized YVO4:Er3+/Yb3+ UC-MHNSPs are highly stable at elevated temperatures and longer irradiation times.
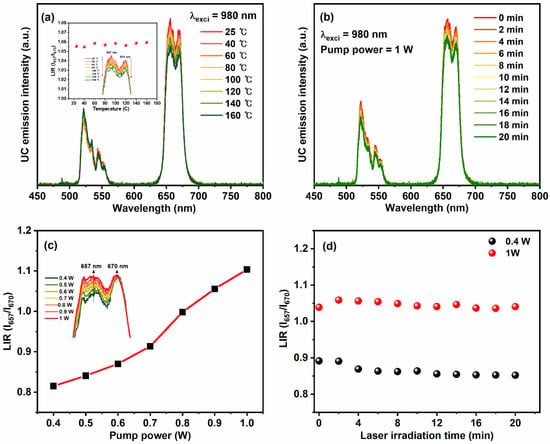
Figure 7.
(a) Temperature-dependent emission spectra of YVO4:Er3+/Yb3+ UC-MHNSPs under 980 nm excitation and the inset displays the corresponding LIR (I657/I670) versus temperature plot. (b) UC emission spectra of YVO4:Er3+/Yb3+ UC-MHNSPs as a series of laser irradiation time, (c) a plot of LIR (I657/I670) versus incident laser pump power, and (d) LIR (I657/I670) versus laser irradiation time plots at low and high pump powers.
In addition, the LIR (I657/I670) increased gradually on rising the pump power from 0.4 to 1 W (Figure 7c), indicating the presence of laser-induced heat effect on the emission spectra of YVO4:Er3+/Yb3+ UC-MHNSPs. Generally, laser irradiation causes an increase in the local temperature of the sample as a result the emission intensity increases. Therefore, the laser-induced heat effect was estimated for YVO4:Er3+/Yb3+ UC-MHNSPs at low and high pump powers of 0.4 and 1 W, and the corresponding LIR (I657/I670) versus irradiation time plots are presented in Figure 7d. As shown in the figure, the change in LIR (I657/I670) was negligible at the low pump power of 0.4 W, as compared to the change in LIR (I657/I670) at the high pump power of 1 W, suggesting that the laser-induced heat effect was insignificant at low pump powers. Therefore, the input laser pump power was set to 0.4 W for biomedical applications to avoid the laser-induced heat effect.
3.4. In Vitro Imaging, Cell Viability, and Antitumor Activity
In vitro imaging studies were performed to study the permeability and translocation of YVO4:Er3+/Yb3+ UC-MHNSPs using human cervical carcinoma (HeLa) cell lines. Figure 8a–d shows the bright field and dark field images of the HeLa cells under 980 nm excitation with a fixed pump power of 0.4 W. Figure 8a,b shows the confocal microscope images of the nuclear stain (Hoechst 33342) and the red emission from YVO4:Er3+/Yb3+ UC-MHNSPs, respectively. The merged images displayed in Figure 8c,d confirmed the successful uptake of YVO4:Er3+/Yb3+ UC-MHNSPs by the cell lines. The bright red luminescence spots observed in the cytoplasm and nucleoplasm of the HeLa cell lines revealed the penetration capability of these UC-MHNSPs. The absence of background autofluorescence and the effective cellular uptake signify that these YVO4:Er3+/Yb3+ UC-MHNSPs can be used as promising live cell imaging agents for cancer imaging and therapeutic applications.
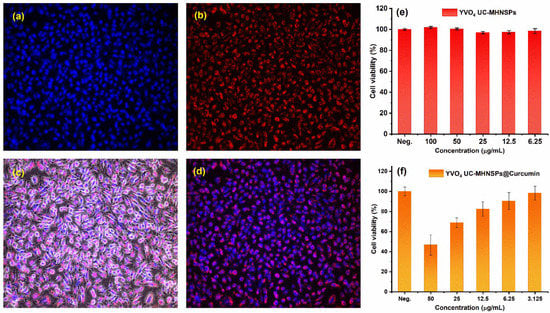
Figure 8.
In vitro fluorescence images of YVO4:Er3+/Yb3+ UC-MHNSPs uptake by HeLa cell lines, (a) nuclear stain (Hoechst 33342), (b) red emission from YVO4:Er3+/Yb3+ UC-MHNSPs, (c,d) merged fluorescence bright-field and dark-field pictures of HeLa cells under 980 nm excitation. (e) Cell viability assessed by CCK-8 assay after incubating with various quantities of YVO4:Er3+/Yb3+ UC-MHNSPs for 24 h and (f) antitumor activity of YVO4:Er3+/Yb3+ UC-MHNSPs after conjugation with different quantities of curcumin.
Likewise, the cytotoxicity of UC nanoparticles is one of the most important concerns for their use in biomedical applications. The HeLa cells’ feasibility was examined using Kit-8 cell count kit (CCK-8) assay after incubation with YVO4:Er3+/Yb3+ UC-MHNSPs for 24 h. Figure 8e shows the viabilities of HeLa cell lines treated with various concentrations (100, 50, 25, 12.5, and 6.25 µg/mL) of YVO4:Er3+/Yb3+ UC-MHNSPs. After 24 h of incubation, our synthesized UC-HMNSPs showed negligible effect on the activation and proliferation of HeLa cells even at higher concentrations (100 µg/mL), suggesting the non-toxic nature of these YVO4:Er3+/Yb3+ UC-MHNSPs. We also calculated the drug loading efficiency of YVO4:Er3+/Yb3+ UC-MHNSPs and the amount of curcumin loaded on YVO4:Er3+/Yb3+ UC-MHNSPs was estimated to be about 12%. Most of the time, the direct loading of drugs does not yield high loading efficiencies and surface functionalization is needed to improve their drug loading efficiency. In the present study, the curcumin was loaded on YVO4:Er3+/Yb3+ UC-MHNSPs particles without any surface functionalization and thus the acquired amount of curcumin loading was somewhat less.
Furthermore, the antitumor activity of curcumin conjugated YVO4:Er3+/Yb3+ UC-MHNSPs was studied on HeLa cells. The YVO4:Er3+/Yb3+@Curcumin composite showed a substantial effect on HeLa cells viability as shown in Figure 8f. Upon rising the concentration of YVO4:Er3+/Yb3+@Curcumin composite from 3.125 to 50 µg/mL, the HeLa cells’ viability decreased from 98.3 to 46.7%, indicating the drug releasing capability of YVO4:Er3+/Yb3+ UC-MHNSPs on HeLa cell lines. Generally, the drug carrier needs to protect the drug from degradation by enzymes and bio-environment before reaching the targeted tumor site and these YVO4:Er3+/Yb3+@Curcumin UC-MHNSPs exhibited an abundant antitumor effect on HeLa cell lines. Therefore, the obtained results indicate that these YVO4:Er3+/Yb3+ UC-MHNSPs have great potential as drug carriers due to their excellent dispersion stability, biocompatibility, and UC luminescence properties.
4. Conclusions
Highly stable, aqueous dispersible, and biocompatible YVO4:Er3+/Yb3+ UC-MHNSPs were successfully synthesized by a facile and rapid self-sacrificing templet method. The pure phase of the tetragonal zircon-type structure was confirmed by performing the Rietveld refinement analysis on the XRD patterns of YVO4:Er3+/Yb3+ UC-MHNSPs. The transformation of YC:Er3+/Yb3+ NSs into YVO4:Er3+/Yb3+ UC-MHNSPs was explained with the help of FE-SEM and FE-TEM images. The N2 adsorption–desorption isotherms confirmed the mesoporous nature of these materials and the specific surface area was found to be 87.46 m2/g. Interestingly, these YVO4:Er3+/Yb3+ UC-MHNSPs exhibited red emission at low pump powers and a fine green emission at high pump powers under NIR excitation. At low pump powers, EBT from Er3+ to Yb3+ ions and CR among Er3+ ions result in strong red emission. While at high pump powers, along with ET from Er3+ to Yb3+ ions, the three-photon excitation process takes place to populate the green emitting excited states (2H11/2, 4S3/2). These YVO4:Er3+/Yb3+ UC-MHNSPs exhibited around 87.5 and 90% emission intensity at an elevated temperature of 160 °C and continuous laser irradiation of 20 min, demonstrating the high thermal stability and photostability of these UC-MHNSPs. The bright red spots from the membrane of HeLa cells confirmed the effective cellular uptake of these YVO4:Er3+/Yb3+ UC-MHNSPs. After conjugating with curcumin, the HeLa cells’ viability was significantly decreased, demonstrating the excellent antitumor activity of these UC-MHNSPs. Based on these results, it is quite clear that these YVO4:Er3+/Yb3+ UC-MHNSPs have the potential to be used as bioimaging agents and drug delivery systems in various therapeutic applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano12152520/s1, Characterizations, Figure S1: FE-SEM images of YVO4:Er3+/Yb3+ UC-MHNSPs at different reaction timings of (a) 4 h, (b) 6 h, (c) 12 h, (d) 24 h, respectively; Figure S2: FE-TEM images of YVO4:Er3+/Yb3+ UC-MHNSPs at different reaction timings of (a) 4 h, (b) 6 h, (c) 12 h, (d) 24 h, respectively; Figure S3: XRD patterns of YVO4:Er3+/Yb3+ UC-MHNSPs with two different concentrations of NH4F flux; Table S1: Atomic parameters, occupancy, and atomic displacement parameters (Uiso [Å2]) of YVO4:Er3+/Yb3+ UC-MHNSPs.
Author Contributions
Conceptualization and methodology, E.P. and G.S.R.R.; data curation, investigation, and validation, E.P., G.S.R.R., H.L. and J.Y.P.; writing—original draft preparation, E.P. and G.S.R.R.; writing—review and editing, G.S.R.R., Y.-K.H. and Y.S.H.; formal analysis and software, J.Y.P. and S.K.H.; supervision, G.S.R.R., Y.-K.H. and Y.S.H.; funding acquisition and project administration, E.P. and Y.S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Research Foundation of Korea (NRF) grant sponsored by the Korea Government (2022R1A6A1A0305170511, 2021R1I1A1A01061115).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, C.; Lei, C.; Wang, Y.; Yu, C. Dendritic Mesoporous Nanoparticles: Structure, Synthesis and Properties. Angew. Chem. Int. Ed. 2022, 61, e202112752. [Google Scholar] [CrossRef]
- Zhao, T.; Elzatahry, A.; Li, X.; Zhao, D. Single-micelle-directed synthesis of mesoporous materials. Nat. Rev. Mater. 2019, 4, 775–791. [Google Scholar] [CrossRef]
- Jin, T.; Wu, D.; Liu, X.-M.; Xu, J.-T.; Ma, B.-J.; Ji, Y.; Jin, Y.-Y.; Wu, S.-Y.; Wu, T.; Ma, K. Intra-articular delivery of celastrol by hollow mesoporous silica nanoparticles for pH-sensitive anti-inflammatory therapy against knee osteoarthritis. J. Nanobiotechnol. 2020, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Janjua, T.I.; Cao, Y.; Yu, C.; Popat, A. Clinical translation of silica nanoparticles. Nat. Rev. Mater. 2021, 6, 1072–1074. [Google Scholar] [CrossRef]
- Kankala, R.K.; Han, Y.-H.; Na, J.; Lee, C.-H.; Sun, Z.; Wang, S.-B.; Kimura, T.; Ok, Y.S.; Yamauchi, Y.; Chen, A.-Z.; et al. Nanoarchitectured Structure and Surface Biofunctionality of Mesoporous Silica Nanoparticles. Adv. Mater. 2020, 32, 1907035. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Song, H.; Yu, C. Silica-Based Nanoparticles for Biomedical Applications: From Nanocarriers to Biomodulators. Acc. Chem. Res. 2020, 53, 1545–1556. [Google Scholar] [CrossRef]
- Yang, B.; Shi, J. Defect Engineering of Mesoporous Silica Nanoparticles for Biomedical Applications. Acc. Mater. Res. 2021, 2, 581–593. [Google Scholar] [CrossRef]
- Huang, L.; Feng, J.; Fan, W.; Tang, W.; Rong, X.; Liao, W.; Wei, Z.; Xu, Y.; Wu, A.; Chen, X.; et al. Intelligent Pore Switch of Hollow Mesoporous Organosilica Nanoparticles for High Contrast Magnetic Resonance Imaging and Tumor-Specific Chemotherapy. Nano Lett. 2021, 21, 9551–9559. [Google Scholar] [CrossRef]
- Liu, R.; Peng, Y.; Lu, L.; Peng, S.; Chen, T.; Zhan, M. Near-infrared light-triggered nano-prodrug for cancer gas therapy. J. Nanobiotechnol. 2021, 19, 443. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, G. NIR light-responsive nanocarriers for controlled release. J. Photochem. Photobiol. C Photochem. Rev. 2021, 47, 100420. [Google Scholar] [CrossRef]
- Liao, J.; Yang, L.; Wu, S.; Yang, Z.; Zhou, J.; Jin, D.; Guan, M. NIR-II emissive properties of 808 nm-excited lanthanide-doped nanoparticles for multiplexed in vivo imaging. J. Lumin. 2022, 242, 118597. [Google Scholar] [CrossRef]
- Premcheska, S.; Lederer, M.; Kaczmarek, A.M. The importance, status, and perspectives of hybrid lanthanide-doped upconversion nanothermometers for theranostics. Chem. Commun. 2022, 58, 4288–4307. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-M.; Conde, J.; Lipiński, T.; Bednarkiewicz, A.; Huang, C.-C. Revisiting the classification of NIR-absorbing/emitting nanomaterials for in vivo bioapplications. NPG Asia Mater. 2016, 8, e295. [Google Scholar] [CrossRef]
- Kolesnikov, I.E.; Kalinichev, A.A.; Kurochkin, M.A.; Golyeva, E.V.; Terentyeva, A.S.; Kolesnikov, E.Y.; Lähderanta, E. Structural, luminescence and thermometric properties of nanocrystalline YVO4:Dy3+ temperature and concentration series. Sci. Rep. 2019, 9, 2043. [Google Scholar] [CrossRef]
- Kshetri, Y.K.; Regmi, C.; Kim, H.-S.; Lee, S.W.; Kim, T.-H. Microwave hydrothermal synthesis and upconversion properties of Yb3+/Er3+ doped YVO4 nanoparticles. Nanotechnology 2018, 29, 204004. [Google Scholar] [CrossRef] [PubMed]
- Fuks, H.; Kaczmarek, S.M.; Macalik, L.; Macalik, B.; Hanuza, J. EPR and vibrational studies of YVO4:Tm3+, Yb3+ single crystal. Opt. Mater. 2009, 31, 1883–1887. [Google Scholar] [CrossRef]
- Waritanant, T.; Major, A. Diode-pumped Nd:YVO4 laser with discrete multi-wavelength tunability and high efficiency. Opt. Lett. 2017, 42, 1149–1152. [Google Scholar] [CrossRef]
- Fields, R.A.; Birnbaum, M.; Fincher, C.L. Highly efficient Nd:YVO4 diode-laser end-pumped laser. Appl. Phys. Lett. 1987, 51, 1885–1886. [Google Scholar] [CrossRef]
- Levine, A.K.; Palilla, F.C. A New, highly efficient red-emitting cathodoluminescent phosphor (YVO4:Eu) for color television. Appl. Phys. Lett. 1964, 5, 118–120. [Google Scholar] [CrossRef]
- Yu, H.; Song, Y.; Li, Y.; Wu, Y.; Chen, B.; Li, P.; Sheng, C. Preparation and luminescent properties of one-dimensional YVO4:Eu nanocrystals. J. Mater. Sci. Mater. Electron. 2016, 27, 2608–2613. [Google Scholar] [CrossRef]
- Ding, L.; Wu, Q.; Wang, Q.; Li, Y.; Perks, R.M.; Zhao, L. Advances on inorganic scintillator-based optic fiber dosimeters. EJNMMI Phys. 2020, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, I.E.; Mamonova, D.V.; Kurochkin, M.A.; Kolesnikov, E.Y.; Lähderanta, E. Optical Thermometry by Monitoring Dual Emissions from YVO4 and Eu3+ in YVO4:Eu3+ Nanoparticles. ACS Appl. Nano Mater. 2021, 4, 1959–1966. [Google Scholar] [CrossRef]
- Pavitra, E.; Seeta Rama Raju, G.; Nagaraju, G.P.; Nagaraju, G.; Han, Y.-K.; Huh, Y.S.; Yu, J.S. TPAOH assisted size-tunable Gd2O3@mSi core–shell nanostructures for multifunctional biomedical applications. Chem. Commun. 2018, 54, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, Y.; Wang, D. Design of Hollow Nanostructures for Energy Storage, Conversion and Production. Adv. Mater. 2019, 31, 1801993. [Google Scholar] [CrossRef]
- Anderson, B.D.; Tracy, J.B. Nanoparticle conversion chemistry: Kirkendall effect, galvanic exchange, and anion exchange. Nanoscale 2014, 6, 12195–12216. [Google Scholar] [CrossRef]
- Guo, H.; Wang, X.; Zhang, X.; Tang, Y.; Chen, L.; Ma, C. Effect of NH4F Flux on Structural and Luminescent Properties of Sr2SiO4:Eu2+ Phosphors Prepared by Solid-State Reaction Method. J. Electrochem. Soc. 2010, 157, J310. [Google Scholar] [CrossRef]
- Chakoumakos, B.C.; Abraham, M.M.; Boatner, L.A. Crystal Structure Refinements of Zircon-Type MVO4 (M = Sc, Y, Ce, Pr, Nd, Tb, Ho, Er, Tm, Yb, Lu). J. Solid State Chem. 1994, 109, 197–202. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Teng, Z.; Wang, S.; Su, X.; Chen, G.; Liu, Y.; Luo, Z.; Luo, W.; Tang, Y.; Ju, H.; Zhao, D.; et al. Facile Synthesis of Yolk–Shell Structured Inorganic–Organic Hybrid Spheres with Ordered Radial Mesochannels. Adv. Mater. 2014, 26, 3741–3747. [Google Scholar] [CrossRef]
- Dong, H.; Sun, L.-D.; Yan, C.-H. Energy transfer in lanthanide upconversion studies for extended optical applications. Chem. Soc. Rev. 2015, 44, 1608–1634. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, Z.; Li, J.; Zhang, X.; Luo, Y.; Pan, G. Observation of efficient population of the red-emitting state from the green state by non-multiphonon relaxation in the Er3+–Yb3+ system. Light Sci. Appl. 2015, 4, e239. [Google Scholar] [CrossRef]
- Berry, M.T.; May, P.S. Disputed Mechanism for NIR-to-Red Upconversion Luminescence in NaYF4:Yb3+,Er3+. J. Phys. Chem. A 2015, 119, 9805–9811. [Google Scholar] [CrossRef] [PubMed]
- Pavitra, E.; Lee, H.; Hwang, S.K.; Park, J.Y.; Varaprasad, G.L.; Rao, M.V.B.; Han, Y.-K.; Raju, G.S.R.; Huh, Y.S. Cooperative ligand fields enriched luminescence of AgGd(MoO4)2:Er3+/Yb3+@mSi core–shell upconversion nanoplates for optical thermometry and biomedical applications. Appl. Surf. Sci. 2022, 579, 152166. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhong, H.; Xu, S.; Cheng, L.; Sun, J.; Zhang, J.; Li, L.; Chen, B. Up-conversion luminescence, temperature sensing properties and laser-induced heating effect of Er3+/Yb3+ co-doped YNbO4 phosphors under 1550 nm excitation. Sci. Rep. 2018, 8, 5736. [Google Scholar] [CrossRef]
- Mai, H.-X.; Zhang, Y.-W.; Sun, L.-D.; Yan, C.-H. Highly Efficient Multicolor Up-Conversion Emissions and Their Mechanisms of Monodisperse NaYF4:Yb,Er Core and Core/Shell-Structured Nanocrystals. J. Phys. Chem. C 2007, 111, 13721–13729. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Chen, L.; Huang, Y.; Peng, Y.; Luo, Y.; Zhang, L.; Mu, J. Hydrothermal Synthesis and Upconversion Properties of About 19 nm Sc2O3: Er3+, Yb3+ Nanoparticles with Detailed Investigation of the Energy Transfer Mechanism. Nanoscale Res. Lett. 2018, 13, 372. [Google Scholar] [CrossRef]
- Xiang, G.; Zhang, J.; Hao, Z.; Zhang, X.; Pan, G.-H.; Luo, Y.; Zhao, H. Decrease in particle size and enhancement of upconversion emission through Y3+ ions doping in hexagonal NaLuF4:Yb3+/Er3+ nanocrystals. CrystEngComm 2015, 17, 3103–3109. [Google Scholar] [CrossRef]
- Suo, H.; Guo, C.; Li, T. Broad-Scope Thermometry Based on Dual-Color Modulation up-Conversion Phosphor Ba5Gd8Zn4O21:Er3+/Yb3+. J. Phys. Chem. C 2016, 120, 2914–2924. [Google Scholar] [CrossRef]
- Mi, C.; Wu, J.; Yang, Y.; Han, B.; Wei, J. Efficient upconversion luminescence from Ba5Gd8Zn4O21:Yb3+, Er3+ based on a demonstrated cross-relaxation process. Sci. Rep. 2016, 6, 22545. [Google Scholar] [CrossRef]
- Li, B.; Zhou, K.; Chen, Z.; Song, Z.; Zhang, D.; Fang, G. NH4F-assisted one-pot solution synthesis of hexagonal ZnO microdiscs for efficient ultraviolet photodetection. R. Soc. Open Sci. 2018, 5, 180822. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Marques-Hueso, J.; Yan, X. Improving Optical Temperature Sensing Performance of Er3+ Doped Y2O3 Microtubes via Co-doping and Controlling Excitation Power. Sci. Rep. 2017, 7, 758. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wu, J.; Wang, X.; He, Y.; Feng, Z.; Dong, B. Multiple Temperature-Sensing Behavior of Green and Red Upconversion Emissions from Stark Sublevels of Er3+. Sensors 2015, 15, 30981–30990. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).