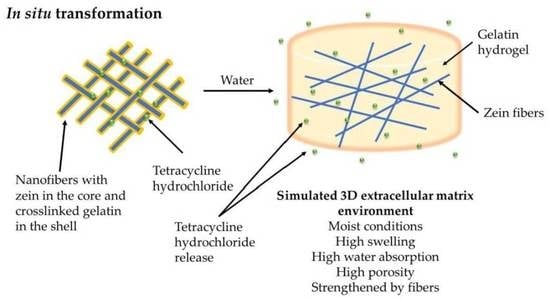
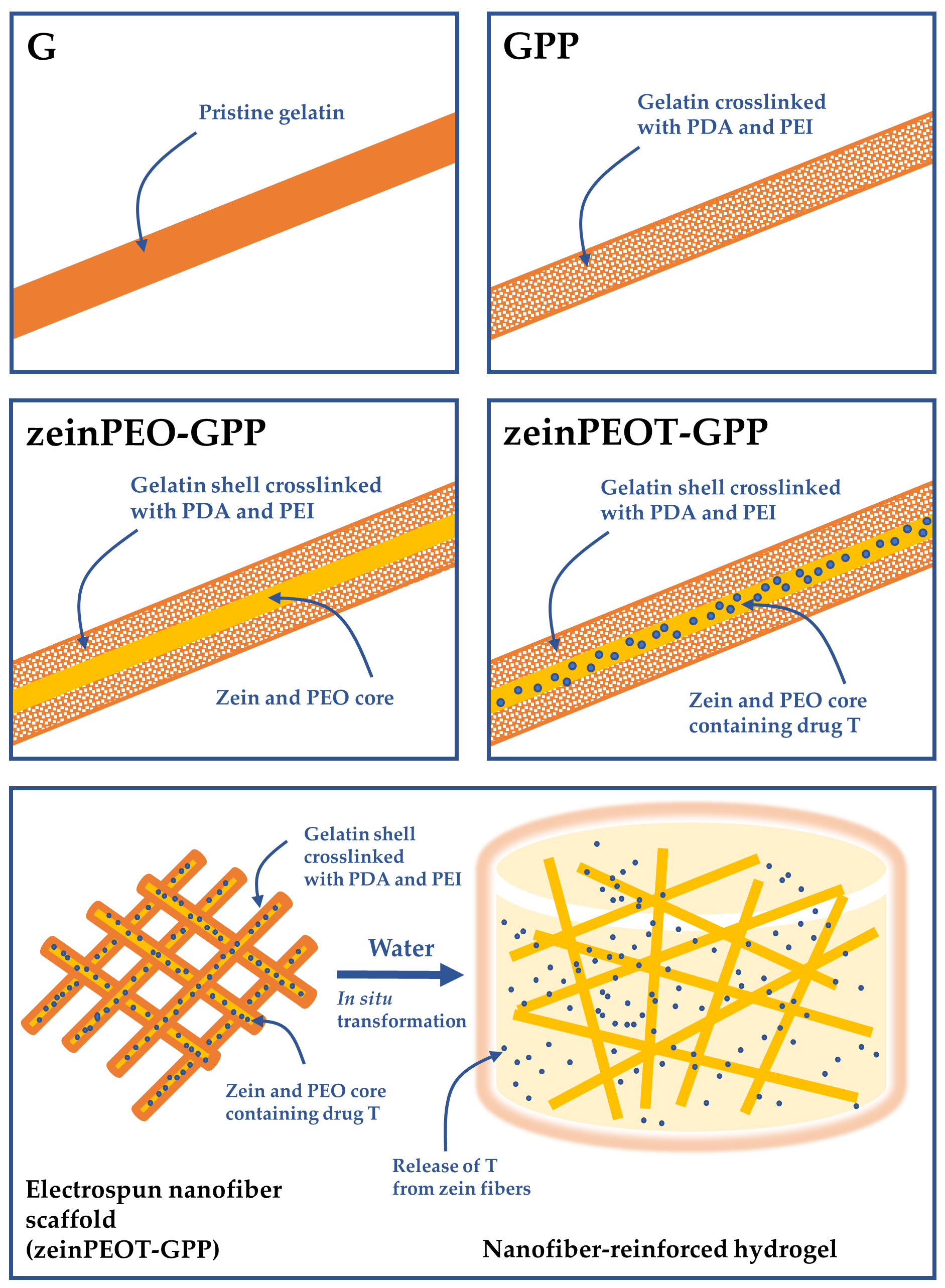
In Situ Transformation of Electrospun Nanofibers into Nanofiber-Reinforced Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning of Nanofiber Scaffolds
2.3. Morphological Characterization of Nanofiber Scaffolds
2.4. Interactions of Nanofiber Scaffolds with Water
2.5. Mechanical Characterization of Nanofiber Scaffolds
2.6. Solid-State Characterization of Nanofiber Scaffolds
2.7. Drug Loading and Release
2.8. Antimicrobial Study
2.9. In Vitro Cell Cultures
2.10. Cell Viability Assays
2.11. Statistical Analysis
3. Results
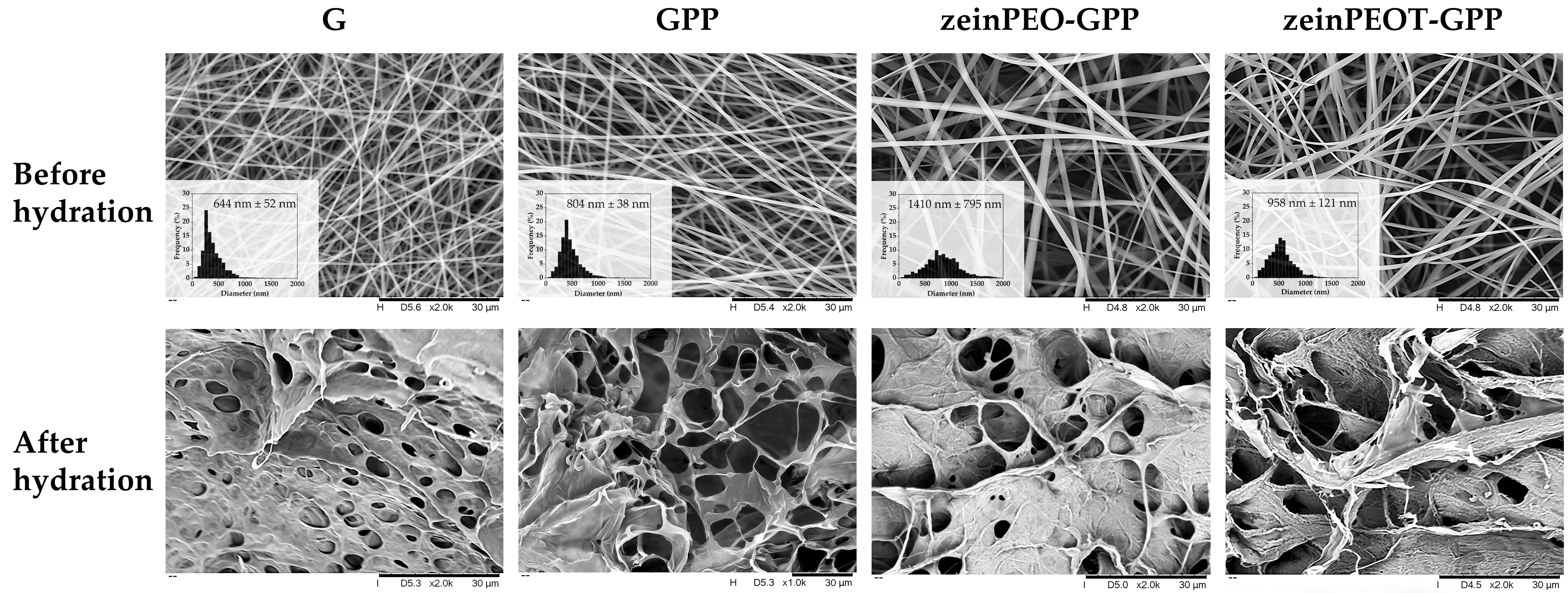
3.1. Morphological Characterization of Nanofiber Scaffolds
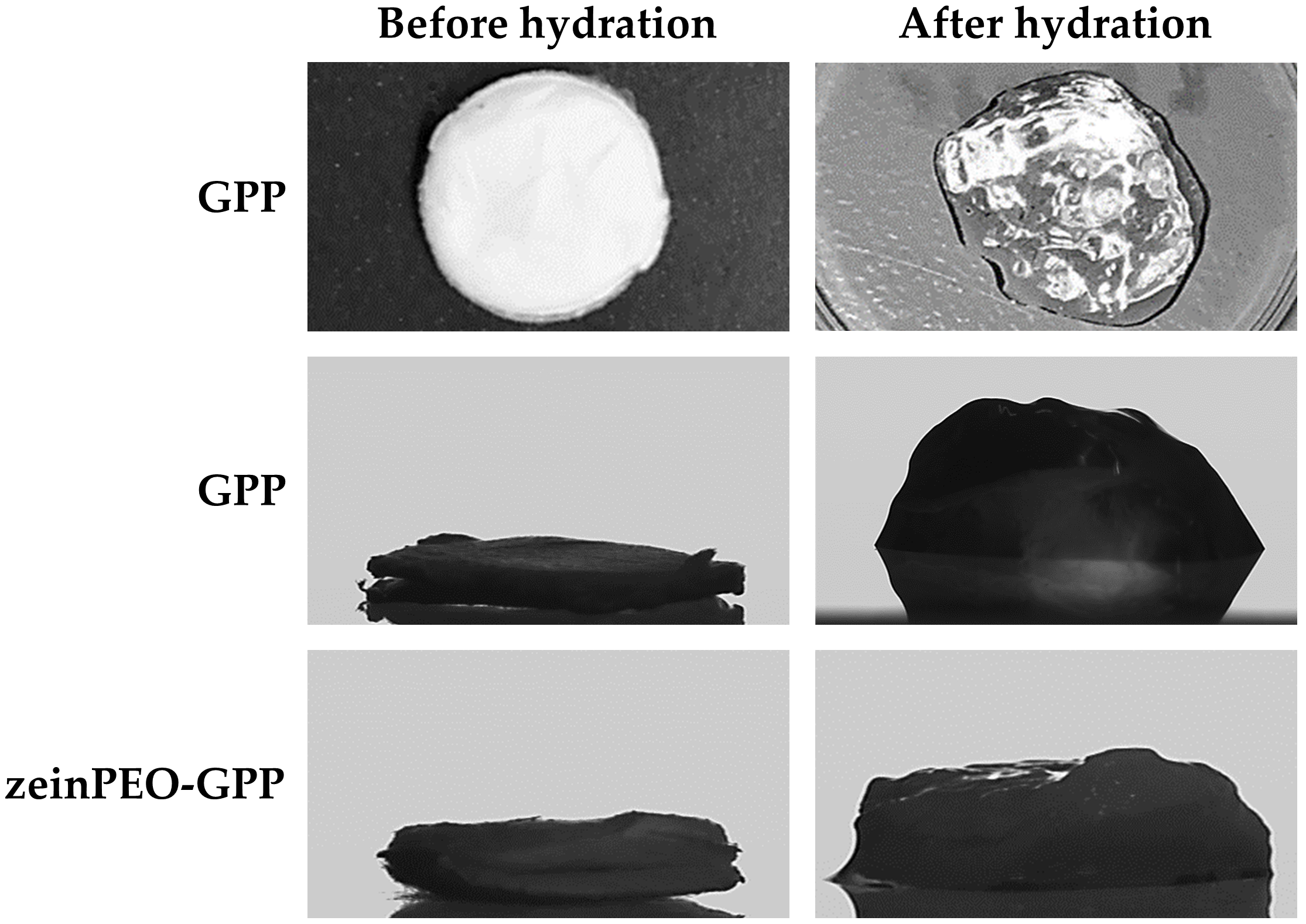
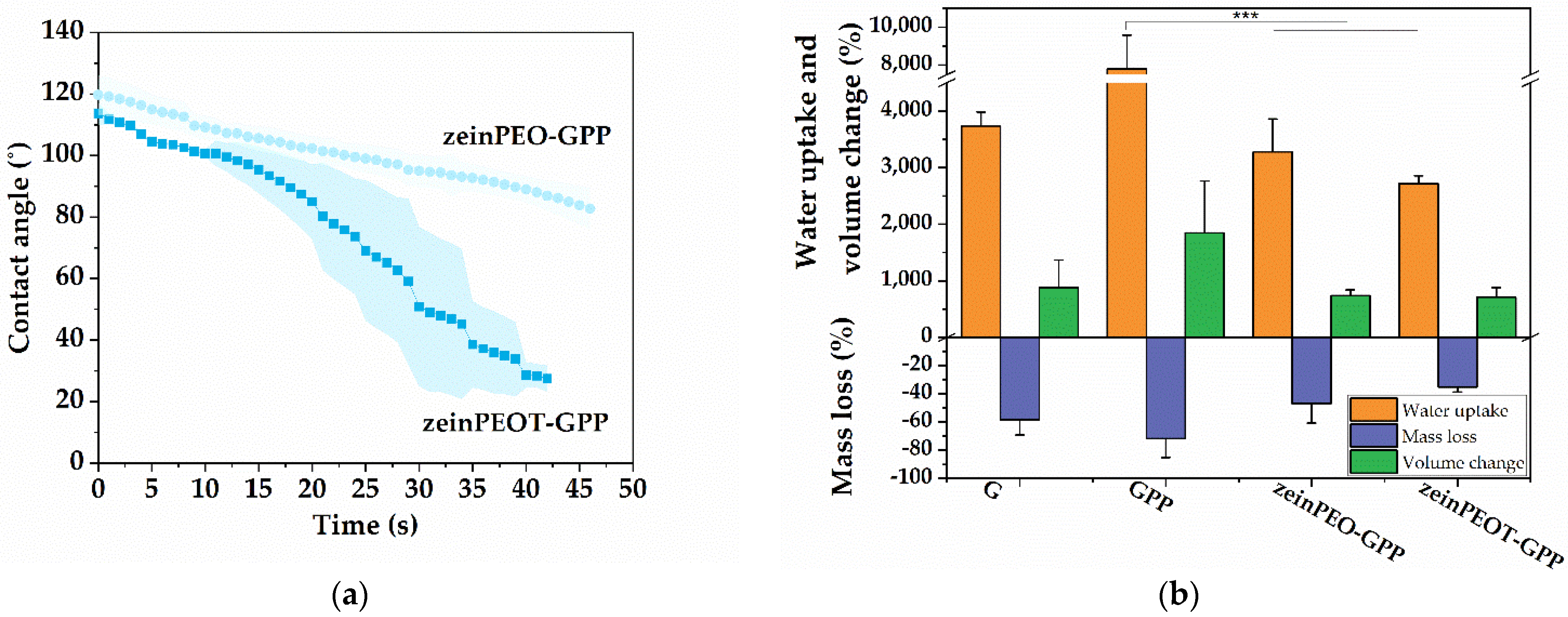
3.2. Interaction of Nanofiber Scaffolds with Water
3.3. Mechanical Characterization
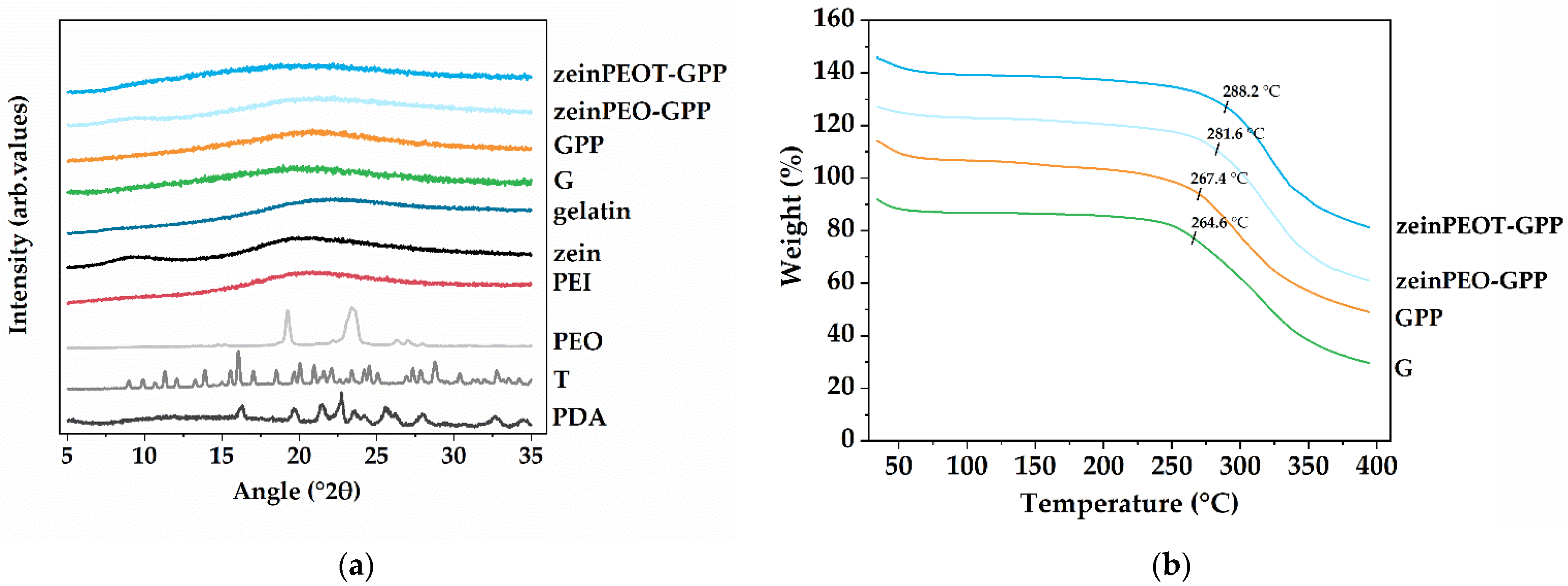
3.4. Solid-State Characterization
3.5. Drug Loading and Release
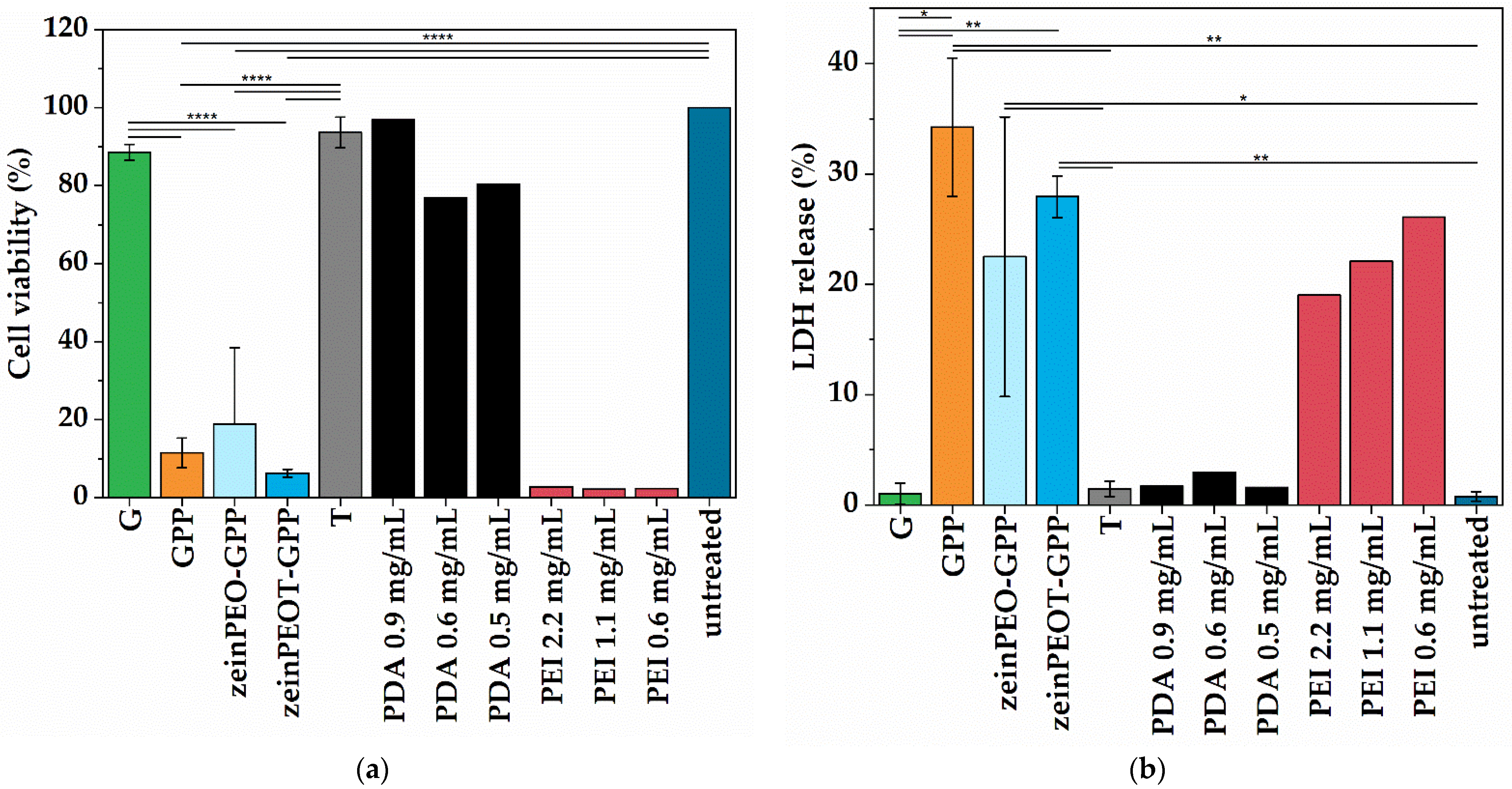
3.6. Biological Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A

| Sample | Onset of Degradation (°C) |
|---|---|
| G | 273.0 ± 2.7 |
| GPP | 266.6 ± 0.9 |
| zeinPEO-GPP | 282.9 ± 2.1 **** (vs. G and GPP) |
| zeinPEOT-GPP | 286.5 ± 1.5 **** (vs. G and GPP) |
| Sample | Model Fitting | |||
|---|---|---|---|---|
| Zero Order R2 | First Order R2 | Higuchi R2 | Korsmeyer–Peppas R2 | |
| zeinPEOT-GPP | 0.901 | 0.708 | 0.983 | 0.991 (n = 0.48) |
References
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- Op’t Veld, R.C.; Walboomers, X.F.; Jansen, J.A.; Wagener, F. Design Considerations for Hydrogel Wound Dressings: Strategic and Molecular Advances. Tissue Eng. Part B Rev. 2020, 26, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Azimi, B.; Maleki, H.; Zavagna, L.; De la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-Based Electrospun Fibers for Wound Healing. J. Funct. Biomater. 2020, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.G. Electrospun Medicated Nanofibers for Wound Healing: Review. Membranes 2021, 11, 770. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Chen, W.; Chen, S.; Morsi, Y.; El-Hamshary, H.; El-Newhy, M.; Fan, C.; Mo, X. Superabsorbent 3D Scaffold Based on Electrospun Nanofibers for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 24415–24425. [Google Scholar] [CrossRef]
- Keit, E.; Chen, S.; Wang, H.; Xie, J. Expansion of Two-dimension Electrospun Nanofiber Mats into Three-dimension Scaffolds. J. Vis. Exp. 2019, 143, e58918. [Google Scholar] [CrossRef] [Green Version]
- Oyen, M.L. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 2014, 59, 44–59. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Dziadek, M.; Gorodzha, S.; Liskova, J.; Brackman, G.; Vanhoorne, V.; Vervaet, C.; Balcaen, L.; Del Rosario Florez Garcia, M.; Boccaccini, A.R.; et al. Novel injectable gellan gum hydrogel composites incorporating Zn- and Sr-enriched bioactive glass microparticles: High-resolution X-ray microcomputed tomography, antibacterial and in vitro testing. J. Tissue Eng. Regen. Med. 2018, 12, 1313–1326. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Chen, X.; Meng, T.; Liu, L. Multi-network granular hydrogel with enhanced strength for 3D bioprinting. J. Biomater. Appl. 2022, 36, 1852–1862. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Li, Z.; Luo, Y.; Gong, J.; Li, Q.; Zhang, J.; Zhang, Q.; Qiao, C. Bio-based poly (gamma-glutamic acid)-gelatin double-network hydrogel with high strength for wound healing. Int. J. Biol. Macromol. 2022, 202, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Beckett, L.E.; Lewis, J.T.; Tonge, T.K.; Korley, L.T.J. Enhancement of the Mechanical Properties of Hydrogels with Continuous Fibrous Reinforcement. ACS Biomater. Sci. Eng. 2020, 6, 5453–5473. [Google Scholar] [CrossRef]
- Bosworth, L.A.; Turner, L.A.; Cartmell, S.H. State of the art composites comprising electrospun fibres coupled with hydrogels: A review. Nanomedicine 2013, 9, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Rnjak-Kovacina, J.; Weiss, A.S. Increasing the pore size of electrospun scaffolds. Tissue Eng. Part B Rev. 2011, 17, 365–372. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; Wise, S.G.; Li, Z.; Maitz, P.K.; Young, C.J.; Wang, Y.; Weiss, A.S. Tailoring the porosity and pore size of electrospun synthetic human elastin scaffolds for dermal tissue engineering. Biomaterials 2011, 32, 6729–6736. [Google Scholar] [CrossRef]
- Memic, A.; Abudula, T.; Mohammed, H.S.; Joshi Navare, K.; Colombani, T.; Bencherif, S.A. Latest Progress in Electrospun Nanofibers for Wound Healing Applications. ACS Appl. Biol. Mater. 2019, 2, 952–969. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Lu, Z.; Zhang, H.; Huang, J.; Yan, K.; Wang, D. Nanofiber-reinforced bulk hydrogel: Preparation and structural, mechanical, and biological properties. J. Mater. Chem. B 2020, 8, 9794–9803. [Google Scholar] [CrossRef]
- Joshi, M.K.; Lee, S.; Tiwari, A.P.; Maharjan, B.; Poudel, S.B.; Park, C.H.; Kim, C.S. Integrated design and fabrication strategies for biomechanically and biologically functional PLA/beta-TCP nanofiber reinforced GelMA scaffold for tissue engineering applications. Int. J. Biol. Macromol. 2020, 164, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Prabhakaran, M.P.; Stahl, B.; Eblenkamp, M.; Wintermantel, E.; Ramakrishna, S. Mechanical properties and in vitro behavior of nanofiber-hydrogel composites for tissue engineering applications. Nanotechnology 2012, 23, 095705. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cha, C. Enhanced mechanical and electrical properties of heteroscaled hydrogels infused with aqueous-dispersible hybrid nanofibers. Biofabrication 2019, 12, 015020. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, S.; Huang, J.; Fu, H.; Ning, X.; He, Y.; Zhang, Z. HBC-nanofiber hydrogel scaffolds with 3D printed internal microchannels for enhanced cartilage differentiation. J. Mater. Chem. B 2020, 8, 6115–6127. [Google Scholar] [CrossRef]
- Maharjan, B.; Park, J.; Kaliannagounder, V.K.; Awasthi, G.P.; Joshi, M.K.; Park, C.H.; Kim, C.S. Regenerated cellulose nanofiber reinforced chitosan hydrogel scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 251, 117023. [Google Scholar] [CrossRef]
- Wang, L.; Lv, H.; Liu, L.; Zhang, Q.; Nakielski, P.; Si, Y.; Cao, J.; Li, X.; Pierini, F.; Yu, J.; et al. Electrospun nanofiber-reinforced three-dimensional chitosan matrices: Architectural, mechanical and biological properties. J. Colloid Interface Sci. 2020, 565, 416–425. [Google Scholar] [CrossRef]
- Gunes, O.C.; Albayrak, A.Z.; Tasdemir, S.; Sendemir, A. Wet-electrospun PHBV nanofiber reinforced carboxymethyl chitosan-silk hydrogel composite scaffolds for articular cartilage repair. J. Biomater. Appl. 2020, 35, 515–531. [Google Scholar] [CrossRef]
- Jang, J.; Lee, J.; Seol, Y.-J.; Jeong, Y.H.; Cho, D.-W. Improving mechanical properties of alginate hydrogel by reinforcement with ethanol treated polycaprolactone nanofibers. Compos. Part B Eng. 2013, 45, 1216–1221. [Google Scholar] [CrossRef]
- Pang, L.; Sun, P.; Dong, X.; Tang, T.; Chen, Y.; Liu, Q.; Qi, M. Shear viscoelasticity of electrospinning PCL nanofibers reinforced alginate hydrogels. Mater. Res. Express 2021, 8, 055402. [Google Scholar] [CrossRef]
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef]
- Stan, D.; Tanase, C.; Avram, M.; Apetrei, R.; Mincu, N.B.; Mateescu, A.L.; Stan, D. Wound healing applications of creams and “smart” hydrogels. Exp. Dermatol. 2021, 30, 1218–1232. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Steckl, A.J. Coaxial Electrospinning Formation of Complex Polymer Fibers and their Applications. Chempluschem 2019, 84, 1453–1497. [Google Scholar] [CrossRef] [PubMed]
- EMA. Q3C (R8): Impurities: Guideline for Residual Solvents; EMA: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Akhmetova, A.; Lanno, G.M.; Kogermann, K.; Malmsten, M.; Rades, T.; Heinz, A. Highly Elastic and Water Stable Zein Microfibers as a Potential Drug Delivery System for Wound Healing. Pharmaceutics 2020, 12, 458. [Google Scholar] [CrossRef]
- Labib, G. Overview on zein protein: A promising pharmaceutical excipient in drug delivery systems and tissue engineering. Expert Opin. Drug Deliv. 2018, 15, 65–75. [Google Scholar] [CrossRef]
- Sajkiewicz, P.; Kolbuk, D. Electrospinning of gelatin for tissue engineering—Molecular conformation as one of the overlooked problems. J. Biomater. Sci. Polym. Ed. 2014, 25, 2009–2022. [Google Scholar] [CrossRef]
- Akhmetova, A.; Heinz, A. Electrospinning Proteins for Wound Healing Purposes: Opportunities and Challenges. Pharmaceutics 2020, 13, 4. [Google Scholar] [CrossRef]
- Dhand, C.; Venkatesh, M.; Barathi, V.A.; Harini, S.; Bairagi, S.; Goh Tze Leng, E.; Muruganandham, N.; Low, K.Z.W.; Fazil, M.; Loh, X.J.; et al. Bio-inspired crosslinking and matrix-drug interactions for advanced wound dressings with long-term antimicrobial activity. Biomaterials 2017, 138, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Inal, M.; Mulazimoglu, G. Production and characterization of bactericidal wound dressing material based on gelatin nanofiber. Int. J. Biol. Macromol. 2019, 137, 392–404. [Google Scholar] [CrossRef]
- Mayandi, V.; Wen Choong, A.C.; Dhand, C.; Lim, F.P.; Aung, T.T.; Sriram, H.; Dwivedi, N.; Periayah, M.H.; Sridhar, S.; Fazil, M.; et al. Multifunctional Antimicrobial Nanofiber Dressings Containing epsilon-Polylysine for the Eradication of Bacterial Bioburden and Promotion of Wound Healing in Critically Colonized Wounds. ACS Appl. Mater. Interfaces 2020, 12, 15989–16005. [Google Scholar] [CrossRef]
- Fox, S.J.; Fazil, M.H.; Dhand, C.; Venkatesh, M.; Goh, E.T.; Harini, S.; Eugene, C.; Lim, R.R.; Ramakrishna, S.; Chaurasia, S.S.; et al. Insight into membrane selectivity of linear and branched polyethylenimines and their potential as biocides for advanced wound dressings. Acta Biomater. 2016, 37, 155–164. [Google Scholar] [CrossRef]
- Zheng, Z.X.; Zhang, K.H.; Wu, B.; Yang, H.Y.; Wang, M.Q.; Dong, T.H.; Zhang, J.Y.; He, Y. Green electrospun nanocuprous oxide-poly(ethylene oxide)-silk fibroin composite nanofibrous scaffolds for antibacterial dressings. J. Appl. Polym. Sci. 2019, 136, 47730. [Google Scholar] [CrossRef]
- Singh, I.; Dhawan, G.; Gupta, S.; Kumar, P. Recent Advances in a Polydopamine-Mediated Antimicrobial Adhesion System. Front. Microbiol. 2020, 11, 607099. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Bauer, M.; Hipler, U.C.; Fischer, D. Poly(ethyleneimines) in dermal applications: Biocompatibility and antimicrobial effects. Int. J. Pharm. 2013, 456, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zuo, F.; Liao, Z.; Qin, Z.; Du, S.; Zhao, Z. Mussel-inspired one-pot synthesis of a fluorescent and water-soluble polydopamine-polyethyleneimine copolymer. Macromol. Rapid Commun. 2015, 36, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Andoy, N.M.O.; Patel, M.; Lui, C.L.J.; Sullan, R.M.A. Immobilization of Polyethyleneimine (PEI) on Flat Surfaces and Nanoparticles Affects Its Ability to Disrupt Bacterial Membranes. Microorganisms 2021, 9, 2176. [Google Scholar] [CrossRef] [PubMed]
- Taranejoo, S.; Liu, J.; Verma, P.; Hourigan, K. A review of the developments of characteristics of PEI derivatives for gene delivery applications. J. Appl. Polym. Sci. 2015, 132, 42096. [Google Scholar] [CrossRef]
- Torkamani, A.E.; Syaharizaa, Z.A.; Norziaha, M.H.; Wanb, A.K.M.; Juliano, P. Encapsulation of polyphenolic antioxidants obtained from Momordica charantia fruit within zein/gelatin shell core fibers via coaxial electrospinning. Food Biosci. 2018, 21, 60–71. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Kim, S.H.; Sharker, S.M.; Lee, H.; In, I.; Lee, K.D.; Park, S.Y. Photothermal conversion upon near-infrared irradiation of fluorescent carbon nanoparticles formed from carbonized polydopamine. RSC Adv. 2016, 6, 68412. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, L.; Zhou, Z.; Wu, X.; Wang, Y. Preparation and properties of electrospun soy protein isolate/polyethylene oxide nanofiber membranes. ACS Appl. Mater. Interfaces 2012, 4, 4331–4337. [Google Scholar] [CrossRef]
- Martin, A.; Cai, J.; Schaedel, A.-L.; van der Plas, M.; Malmsten, M.; Rades, T.; Heinz, A. Zein-polycaprolactone core-shell nanofibers for wound healing. Int. J. Pharmaceut. 2022, in press. [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative Study of Gelatin Hydrogels Modified by Various Cross-Linking Agents. Materials 2021, 14, 396. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, L.; Li, J.; Yang, C.; Frenkel, N.; Welle, A.; Heissler, S.; Nefedov, A.; Grunze, M.; Levkin, P.A. UV-triggered dopamine polymerization: Control of polymerization, surface coating, and photopatterning. Adv. Mater. 2014, 26, 8029–8033. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Suk Na, Y.; Choi, S.; Taek Song, I.; Youn Kim, W.; Lee, H. Non-Covalent Self-Assembly and Covalent Polymerization Co-Contribute to Polydopamine Formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Muthuselvi, L.; Dhathathreyan, A. Contact angle hysteresis of liquid drops as means to measure adhesive energy of zein on solid substrates. Pramana 2006, 66, 563–574. [Google Scholar] [CrossRef]
- Park, S.; Tao, J.; Sun, L.; Fan, C.M.; Chen, Y. An Economic, Modular, and Portable Skin Viscoelasticity Measurement Device for In Situ Longitudinal Studies. Molecules 2019, 24, 907. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, I.; Rosolen, M. Thermal transitions of gelatin evaluated using DSC sample pans of various seal integrities. J. Therm. Anal. Calorim. 2013, 114, 1161–1166. [Google Scholar] [CrossRef]
- Barreto, P.L.M.; Pires, A.T.N.; Soldi, V. Thermal degradation of edible films based on milk proteins and gelatin in inert atmosphere. Polym. Degrad. Stab. 2003, 79, 147–152. [Google Scholar] [CrossRef]
- Ali, S.; Khatri, Z.; Wha, K.O.; Kim, I.-S.; Kim, S.H. Zein/Cellulose Acetate Hybrid Nanofibers: Electrospinning and Characterization. Macromol. Res. 2014, 22, 971–977. [Google Scholar] [CrossRef]
- Gough, C.R.; Bessette, K.; Xue, Y.; Mou, X.; Hu, X. Air-Jet Spun Corn Zein Nanofibers and Thin Films with Topical Drug for Medical Applications. Int. J. Mol. Sci. 2020, 21, 5780. [Google Scholar] [CrossRef] [PubMed]
- Morsy, R.; Hosny, M.; Reicha, F.; Elnimr, T. Developing and physicochemical evaluation of cross-linked electrospun gelatin–glycerol nanofibrous membranes for medical applications. J. Mol. Struct. 2017, 1135, 222–227. [Google Scholar] [CrossRef]
- Lin, M.; Sun, J. Antimicrobial peptide-inspired antibacterial polymeric materials for biosafety. Biosaf. Health 2022, in press. [CrossRef]
- Xi, Y.; Ge, J.; Guo, Y.; Lei, B.; Ma, P.X. Biomimetic Elastomeric Polypeptide-Based Nanofibrous Matrix for Overcoming Multidrug-Resistant Bacteria and Enhancing Full-Thickness Wound Healing/Skin Regeneration. ACS Nano 2018, 12, 10772–10784. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, Z.; Huang, Z.; Tang, X.; Zhang, X. Antimicrobial cationic polymers: From structural design to functional control. Polym. J. 2018, 50, 33–44. [Google Scholar] [CrossRef]
- Campiglio, C.E.; Contessi Negrini, N.; Fare, S.; Draghi, L. Cross-Linking Strategies for Electrospun Gelatin Scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef] [Green Version]


| Sample | Core | Shell | Flow Rate Core, µL h−1 | Flow Rate Shell, µL h−1 | Injector Voltage, kV | Collector Voltage, kV | Distance, cm |
|---|---|---|---|---|---|---|---|
| G | Gelatin | - | 250 | - | 8 | −1 | 12 |
| GPP | Gelatin, PEI, PDA | - | 250 | - | 9 | 0 | 12 |
| zeinPEO-GPP | Zein, PEO | Gelatin, PEI, PDA | 400 | 400 | 9 | −7.5 | 21.5 |
| zeinPEOT-GPP | Zein, PEO, T | Gelatin, PEI, PDA | 400 | 400 | 9 | −6.5 | 21.5 |
| Sample | Young’s Modulus, kPa | Tensile Strength, kPa | Elongation at Break, % | Vapor Sorption, % | Hardness, N | Cohesiveness, % | Springiness, % |
|---|---|---|---|---|---|---|---|
| G | 18.9 ± 13.5 | 490.3 ± 286.8 | 4.2 ± 0.2 | 48.4 | - | - | - |
| GPP | 31.6 ± 1.2 | 527.7 ± 182.2 | 2.6 ± 0.4 | 45.4 | - | - | - |
| zeinPEO-GPP | 4.8 ± 4.9 | 131.1 ± 126.6 | 5.2 ± 2.2 | 33.3 | 0.65 ± 0.17 | 65.0 ± 8.6 | 99.9 ± 1.3 |
| zeinPEOT-GPP | 4.9 ± 3.0 | 97.5± 63.9 | 3.2 ± 0.4 | 33.9 | 0.46 ± 0.17 | 55.6 ± 4.3 | 100.3 ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, A.; Nyman, J.N.; Reinholdt, R.; Cai, J.; Schaedel, A.-L.; van der Plas, M.J.A.; Malmsten, M.; Rades, T.; Heinz, A. In Situ Transformation of Electrospun Nanofibers into Nanofiber-Reinforced Hydrogels. Nanomaterials 2022, 12, 2437. https://doi.org/10.3390/nano12142437
Martin A, Nyman JN, Reinholdt R, Cai J, Schaedel A-L, van der Plas MJA, Malmsten M, Rades T, Heinz A. In Situ Transformation of Electrospun Nanofibers into Nanofiber-Reinforced Hydrogels. Nanomaterials. 2022; 12(14):2437. https://doi.org/10.3390/nano12142437
Chicago/Turabian StyleMartin, Alma, Jenny Natalie Nyman, Rikke Reinholdt, Jun Cai, Anna-Lena Schaedel, Mariena J. A. van der Plas, Martin Malmsten, Thomas Rades, and Andrea Heinz. 2022. "In Situ Transformation of Electrospun Nanofibers into Nanofiber-Reinforced Hydrogels" Nanomaterials 12, no. 14: 2437. https://doi.org/10.3390/nano12142437
APA StyleMartin, A., Nyman, J. N., Reinholdt, R., Cai, J., Schaedel, A.-L., van der Plas, M. J. A., Malmsten, M., Rades, T., & Heinz, A. (2022). In Situ Transformation of Electrospun Nanofibers into Nanofiber-Reinforced Hydrogels. Nanomaterials, 12(14), 2437. https://doi.org/10.3390/nano12142437