Insight into a Fenton-like Reaction Using Nanodiamond Based Relaxometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Relaxometry
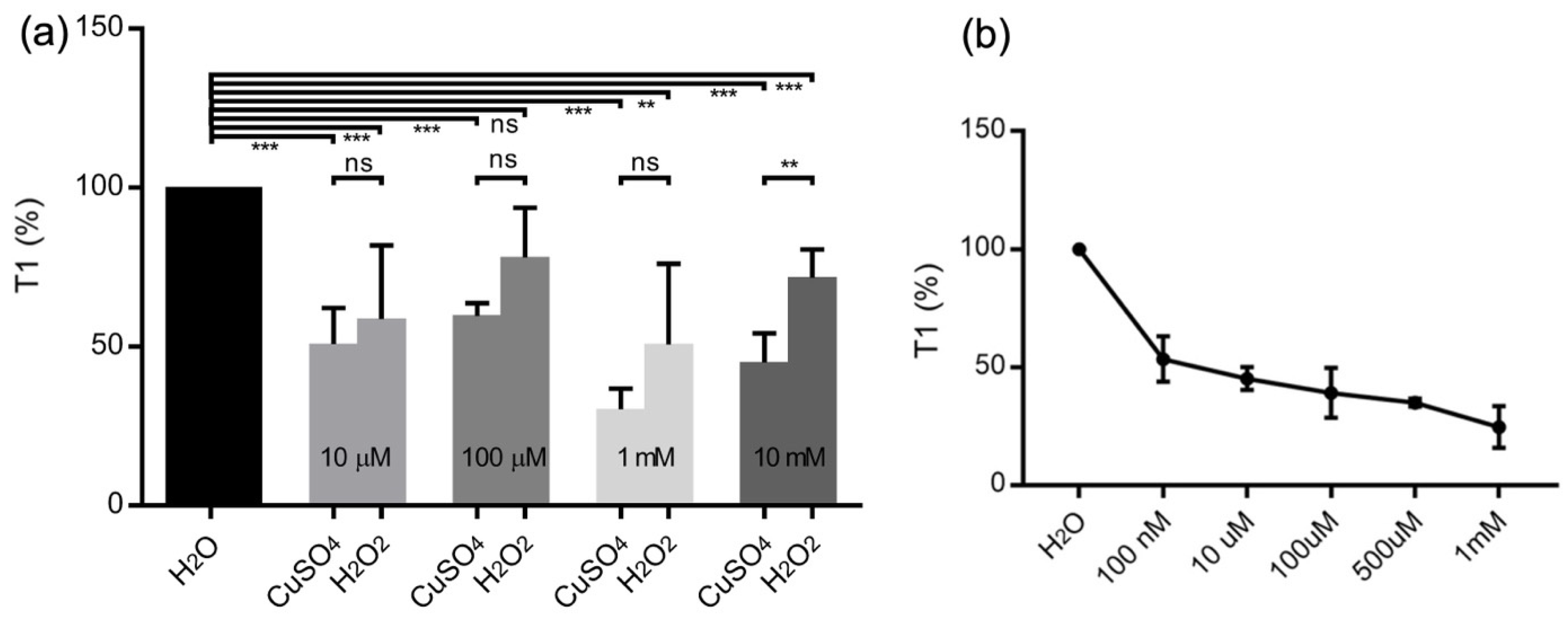
2.3. CuSO4 Calibration Curve Using T1
2.4. UV–Vis Absorption Spectroscopy Measurements
2.5. Measuring the Concentration of Hydroxyl Radicals by HTA
2.6. Raman Spectroscopy
2.7. Oxygen Sensor Measurements
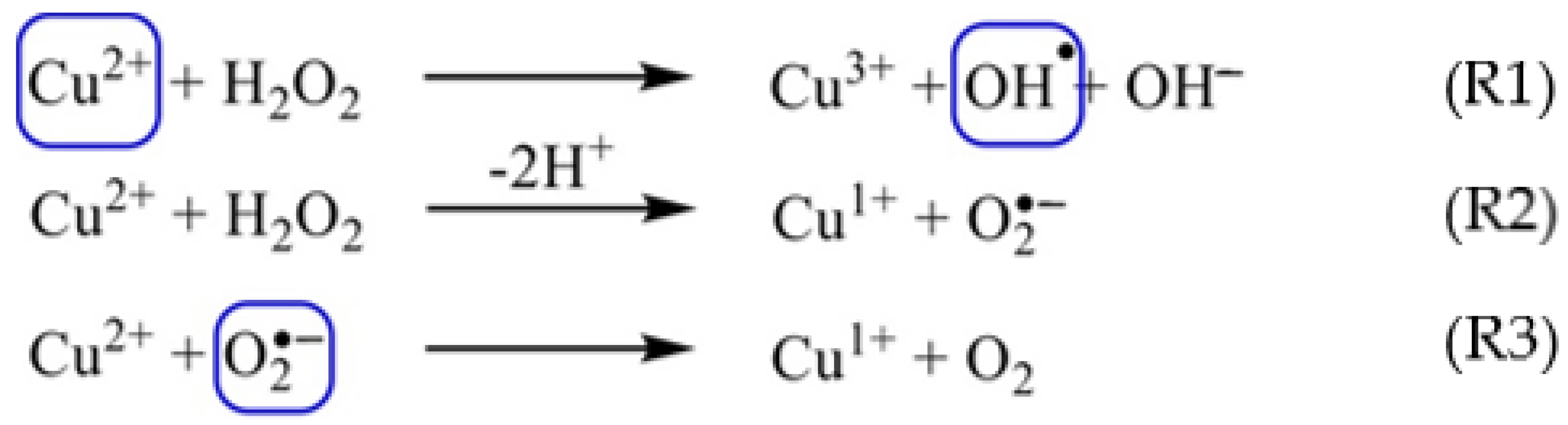
3. Results and Discussion
3.1. T1-Relaxation Measurements:
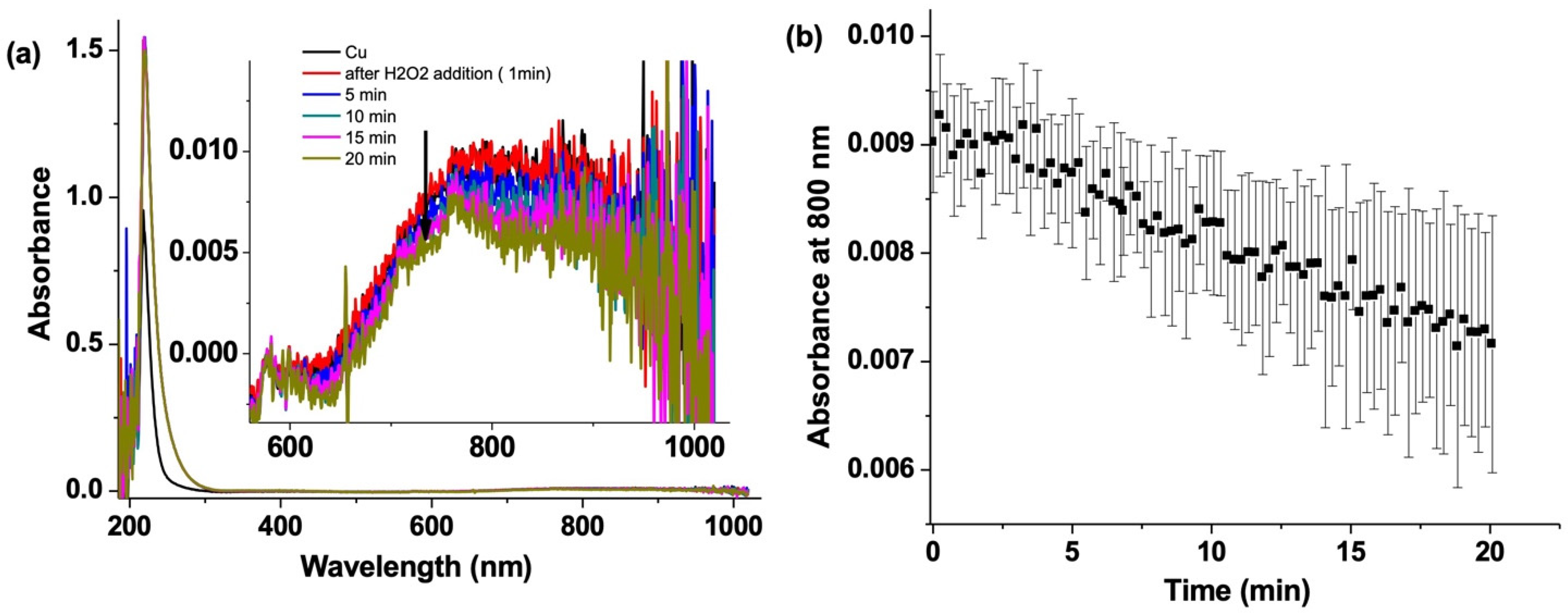
3.2. CuSO4 Decay by UV–Vis Absorption Spectroscopy
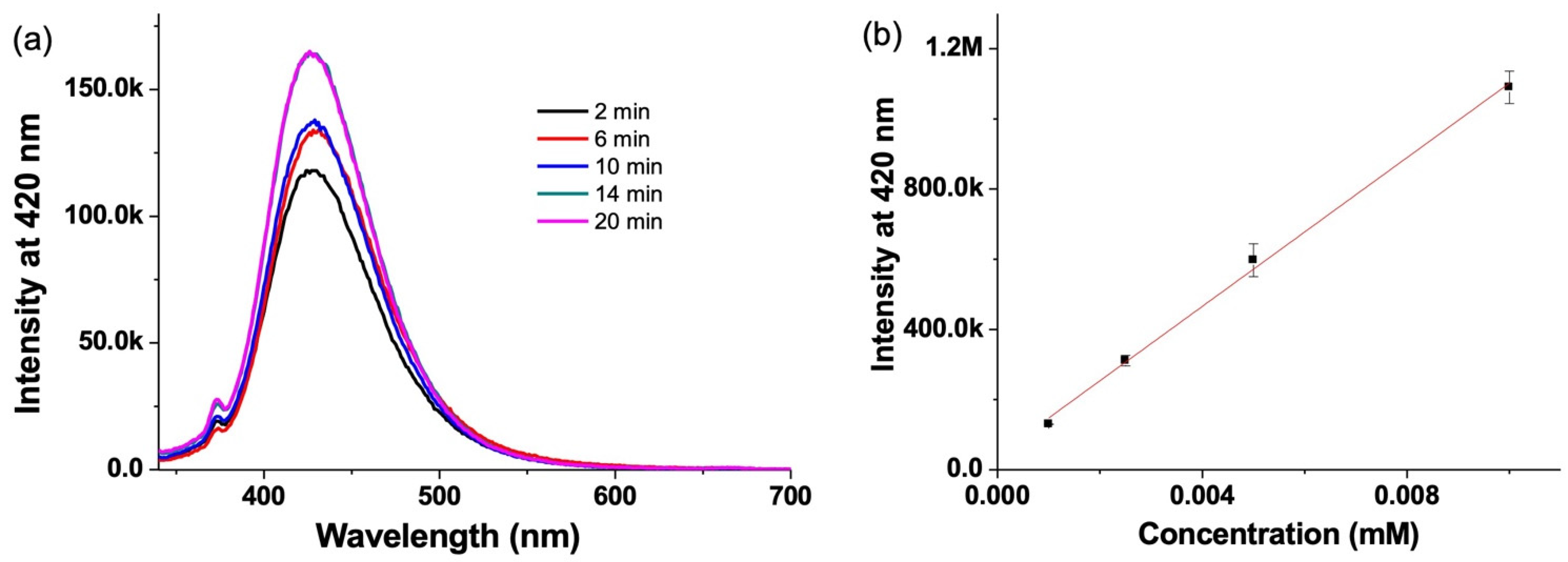
3.3. Hydroxyl Radical Detection by Emission Spectroscopy
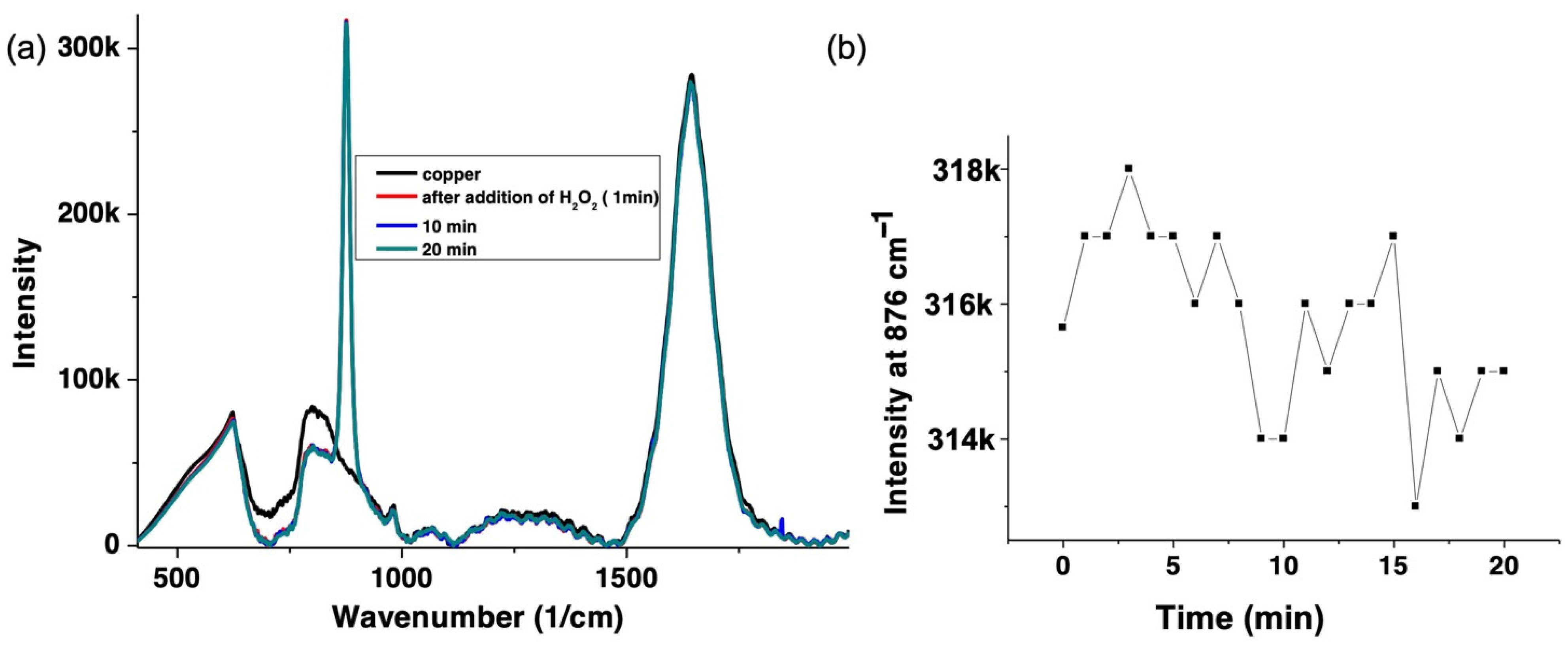
3.4. H2O2 Decay by Raman Spectroscopy
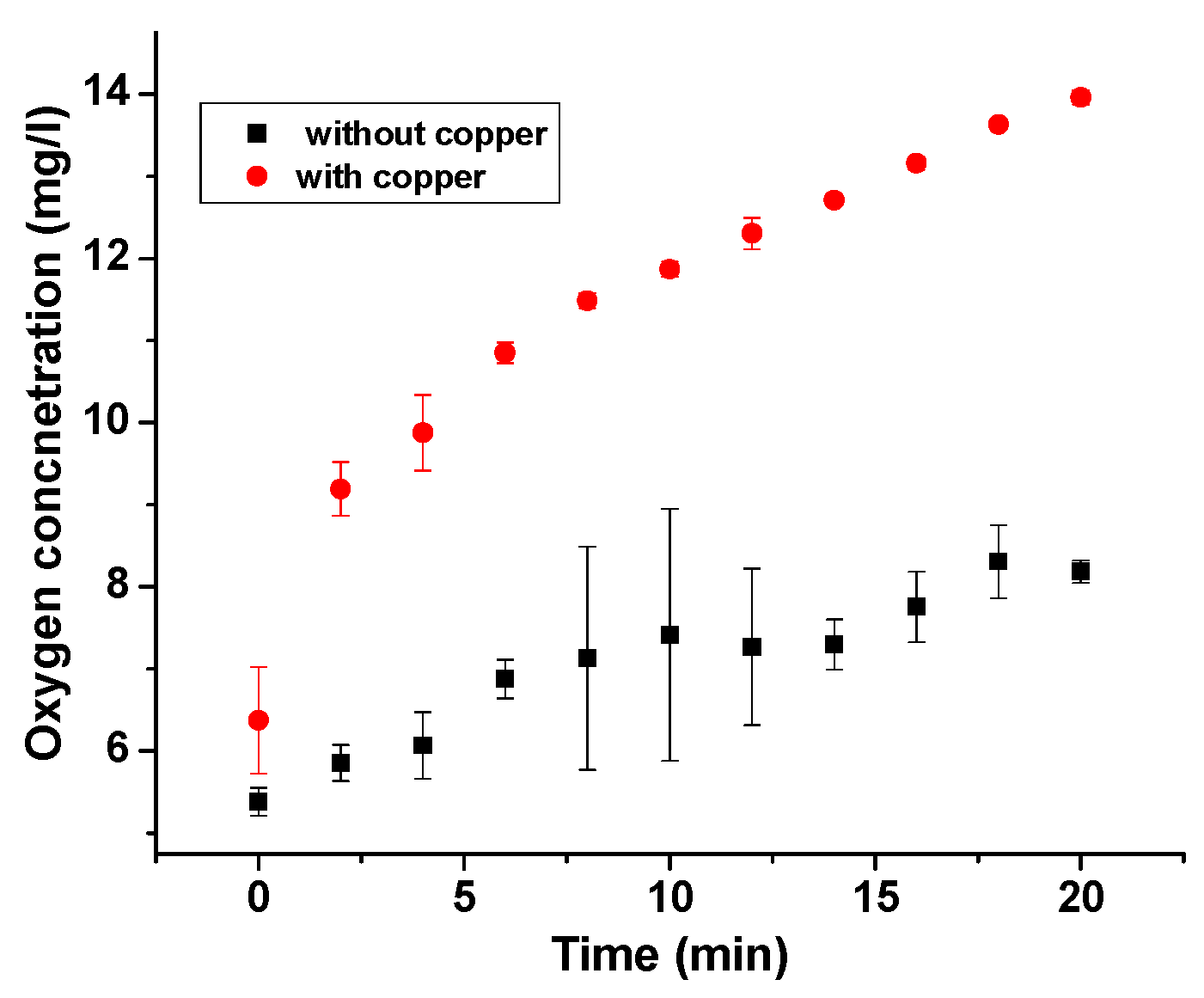
3.5. Oxygen Sensor Measurements:
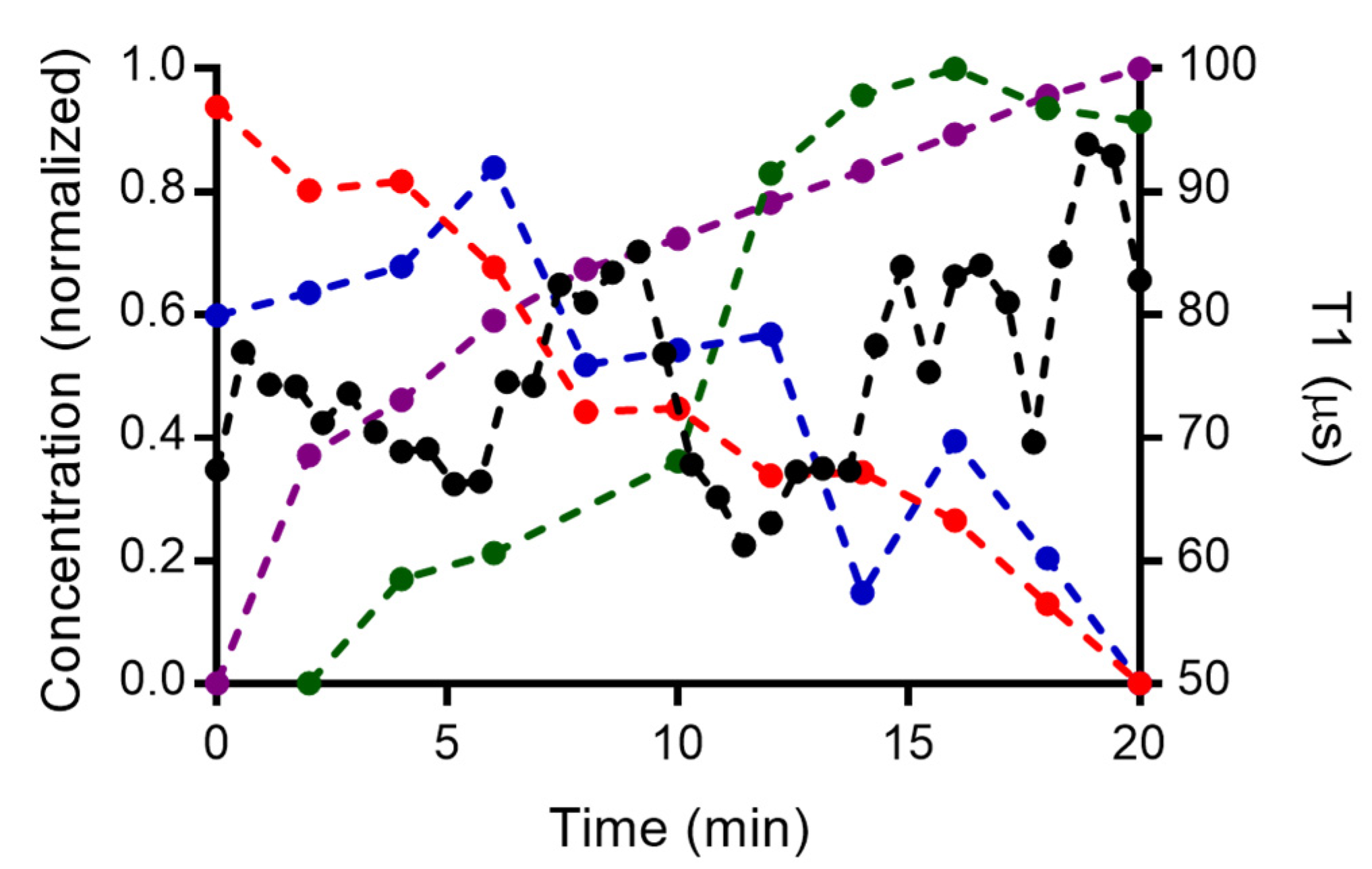
3.6. Combined Measurements from Spectroscopic Techniques
3.7. Copper Detection by Existing Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clavadetscher, J.; Hoffmann, S.; Lilienkampf, A.; Mackay, L.; Yusop, R.M.; Rider, S.A.; Mullins, J.J.; Bradley, M. Copper Catalysis in Living Systems and In Situ Drug Synthesis. Angew. Chem. Int. Ed. 2016, 55, 15662–15666. [Google Scholar] [CrossRef] [PubMed]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, P.S.; Xiao, Z.; Wedd, A.G. Copper and Alzheimer’s disease. Curr. Opin. Chem. Biol. 2007, 11, 128–133. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.J.; Sedlak, D.L.; Lee, C. pH-Dependent reactivity of oxidants formed by iron and copper-catalyzed decomposition of hydrogen peroxide. Chemosphere 2013, 92, 652–658. [Google Scholar] [CrossRef]
- Ranji-Burachaloo, H.; Fu, Q.; Gurr, P.A.; Dunstan, D.E.; Qiao, G.G. Improved Fenton Therapy Using Cancer Cell Hydrogen Peroxide. Aust. J. Chem. 2018, 71, 826. [Google Scholar] [CrossRef] [Green Version]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Chudal, L.; Pandey, N.K.; Phan, J.; Johnson, O.; Lin, L.; Yu, H.; Shu, Y.; Huang, Z.; Xing, M.; Liu, J.P.; et al. Copper-cysteamine nanoparticles as a heterogeneous Fenton-like catalyst for highly selective cancer treatment. ACS Appl. Bio Mater. 2020, 3, 1804–1814. [Google Scholar] [CrossRef]
- Freinbichler, W.; Colivicchi, M.A.; Fattori, M.; Ballini, C.; Tipton, K.F.; Linert, W.; Della Corte, L. Validation of a robust and sensitive method for detecting hydroxyl radical formation together with evoked neurotransmitter release in brain microdialysis. J. Neurochem. 2008, 105, 738–749. [Google Scholar] [CrossRef]
- Nesmelov, Y.E.; Gopinath, A.; Thomas, D.D. Aqueous sample in an EPR cavity: Sensitivity considerations. J. Magn. Reson. 2004, 167, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Le Sage, D.; Arai, K.; Glenn, D.R.; DeVience, S.J.; Pham, L.M.; Rahn-Lee, L.; Lukin, M.D.; Yacoby, A.; Komeili, A.; Walsworth, R.L. Optical magnetic imaging of living cells. Nature 2013, 496, 486–489. [Google Scholar] [CrossRef] [Green Version]
- Juraschek, D.M.; Meier, Q.N.; Trassin, M.; Trolier-McKinstry, S.E.; Degen, C.L.; Spaldin, N.A. Dynamical Magnetic Field Accompanying the Motion of Ferroelectric Domain Walls. Phys. Rev. Lett. 2019, 123, 127601. [Google Scholar] [CrossRef] [Green Version]
- Martínez, F.P.; Nusantara, A.C.; Chipaux, M.; Padamati, S.K.; Schirhagl, R. Nanodiamond Relaxometry-Based Detection of Free-Radical Species When Produced in Chemical Reactions in Biologically Relevant Conditions. ACS Sens. 2020, 5, 3862–3869. [Google Scholar] [CrossRef]
- Ermakova, A.; Pramanik, G.; Cai, J.M.; Algara-Siller, G.; Kaiser, U.; Weil, T.; Tzeng, Y.K.; Chang, H.C.; Mcguinness, L.P.; Plenio, M.B.; et al. Detection of a Few Metallo-Protein Molecules Using Color Centers in Nanodiamonds. Nano Lett. 2013, 13, 3305–3309. [Google Scholar] [CrossRef]
- Davis, H.C.; Ramesh, P.; Bhatnagar, A.; Lee-Gosselin, A.; Barry, J.F.; Glenn, D.R.; Walsworth, R.L.; Shapiro, M.G. Mapping the microscale origins of magnetic resonance image contrast with subcellular diamond magnetometry. Nat. Commun. 2018, 9, 131. [Google Scholar] [CrossRef] [Green Version]
- Morita, A.; Nusantara, A.C.; Martinez, F.P.P.; Hamoh, T.; Damle, V.G.; van der Laan, K.J.; Sigaeva, A.; Vedelaar, T.; Chang, M.; Chipaux, M.; et al. Quantum monitoring the metabolism of individual yeast mutant strain cells when aged, stressed or treated with antioxidant. arXiv 2020, arXiv:2007.16130. [Google Scholar]
- Nie, L.; Nusantara, A.C.; Damle, V.G.; Sharmin, R.; Evans, E.P.P.; Hemelaar, S.R.; Van der Laan, K.J.; Li, R.; Perona Martinez, F.P.; Vedelaar, T.; et al. Quantum monitoring of cellular metabolic activities in single mitochondria. Sci. Adv. 2021, 7, eabf0573. [Google Scholar] [CrossRef]
- Nie, L.; Nusantara, A.C.; Damle, V.G.; Baranov, M.V.; Chipaux, M.; Reyes-San-Martin, C.; Hamoh, T.; Epperla, C.P.; Guricova, M.; Cigler, P.; et al. Quantum Sensing of Free Radicals in Primary Human Dendritic Cells. Nano Lett. 2021, 22, 1818–1825. [Google Scholar] [CrossRef]
- Wu, K.; Vedelaar, T.A.; Damle, V.G.; Morita, A.; Mougnaud, J.; Martin, C.R.S.; Zhang, Y.; van der Pol, D.P.; Ende-Metselaar, H.; Rodenhuis-Zybert, I.; et al. Applying NV center-based quantum sensing to study intracellular free radical response upon viral infections. Redox Biol. 2022, 52, 102279. [Google Scholar] [CrossRef]
- Sharmin, R.; Hamoh, T.; Sigaeva, A.; Mzyk, A.; Damle, V.G.; Morita, A.; Vedelaar, T.; Schirhagl, R. Fluorescent Nanodiamonds for Detecting Free-Radical Generation in Real Time during Shear Stress in Human Umbilical Vein Endothelial Cells. ACS Sens. 2021, 6, 4349–4359. [Google Scholar] [CrossRef]
- Tetienne, J.P.; Hingant, T.; Rondin, L.; Cavaillès, A.; Mayer, L.; Dantelle, G.; Gacoin, T.; Wrachtrup, J.; Roch, J.F.; Jacques, V. Spin relaxometry of single nitrogen-vacancy defects in diamond nanocrystals for magnetic noise sensing. Phys. Rev. B 2013, 87, 235436. [Google Scholar] [CrossRef] [Green Version]
- Schirhagl, R.; Chang, K.; Loretz, M.; Degen, C.L. Nitrogen-Vacancy Centers in Diamond: Nanoscale Sensors for Physics and Biology. Annu. Rev. Phys. Chem. 2014, 65, 83–105. [Google Scholar] [CrossRef] [Green Version]
- Korotkova, E.I.; Misini, B.; Dorozhko, E.V.; Bukkel, M.V.; Plotnikov, E.V.; Linert, W. Study of OH radicals in human serum blood of healthy individuals and those with pathological schizophrenia. Int. J. Mol. Sci. 2011, 12, 401–409. [Google Scholar] [CrossRef]
- Morita, A.; Martinez, F.P.P.; Chipaux, M.; Jamot, N.; Hemelaar, S.R.; van der Laan, K.J.; Schirhagl, R. Cell Uptake of Lipid-Coated Diamond. Part. Part. Syst. Charact. 2019, 36, 1900116. [Google Scholar] [CrossRef]
- Shenderova, O.A.; Shames, A.I.; Nunn, N.A.; Torelli, M.D.; Vlasov, I.; Zaitsev, A. Review Article: Synthesis, properties, and applications of fluorescent diamond particles. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2019, 37, 030802. [Google Scholar] [CrossRef] [Green Version]
- Hemelaar, S.R.; de Boer, P.; Chipaux, M.; Zuidema, W.; Hamoh, T.; Martinez, F.P.; Nagl, A.; Hoogenboom, J.P.; Giepmans, B.N.G.; Schirhagl, R. Nanodiamonds as multi-purpose labels for microscopy. Sci. Rep. 2017, 7, 720. [Google Scholar] [CrossRef]
- Ofori-Okai, B.K.; Pezzagna, S.; Chang, K.; Loretz, M.; Schirhagl, R.; Tao, Y.; Moores, B.A.; Groot-Berning, K.; Meijer, J.; Degen, C.L. Spin properties of very shallow nitrogen vacancy defects in diamond. Phys. Rev. B 2012, 86, 081406. [Google Scholar] [CrossRef]
- Ong, S.Y.; Chipaux, M.; Nagl, A.; Schirhagl, R. Shape and crystallographic orientation of nanodiamonds for quantum sensing. Phys. Chem. Chem. Phys. 2017, 19, 10748–10752. [Google Scholar] [CrossRef]
- Pham, A.N.; Xing, G.; Miller, C.J.; Waite, T.D. Fenton-like copper redox chemistry revisited: Hydrogen peroxide and superoxide mediation of copper-catalyzed oxidant production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- Bauhn, L.; Hansson, N.; Ekberg, C.; Fors, P.; Spahiu, K. The fate of hydroxyl radicals produced during H2O2 decomposition on a SIMFUEL surface in the presence of dissolved hydrogen. J. Nucl. Mater. 2018, 507, 38–43. [Google Scholar] [CrossRef]
- Shames, A.I.; Panich, A.M.; Osipov, V.Y.; Aleksenskiy, A.E.; Vul’, A.Y.; Enoki, T.; Takai, K. Structure and magnetic properties of detonation nanodiamond chemically modified by copper. J. Appl. Phys. 2010, 107, 014318. [Google Scholar] [CrossRef]
- Jancsó, G. Effect of D and 18O isotope substitution on the absorption spectra of aqueous copper sulfate solutions. Radiat. Phys. Chem. 2005, 74, 168–171. [Google Scholar] [CrossRef]
- Aravinda, C.L.; Mayanna, S.M.; Muralidharan, V.S. Electrochemical behaviour of alkaline copper complexes. J. Chem. Sci. 2000, 112, 543–550. [Google Scholar] [CrossRef]
- Padamati, S.K.; Draksharapu, A.; Unjaroen, D.; Browne, W.R. Conflicting role of water in the activation of H2O2 and the formation and reactivity of non-heme FeIII–OOH and FeIII–O–FeIII complexes at room temperature. Inorg. Chem. 2016, 55, 4211–4222. [Google Scholar] [CrossRef]
- Bagherian, G.; Arab Chamjangali, M.; Shariati Evari, H.; Ashrafi, M. Determination of copper (II) by flame atomic absorption spectrometry after its perconcentration by a highly selective and environmentally friendly dispersive liquid–liquid microextraction technique. J. Anal. Sci. Technol. 2019, 10, 3. [Google Scholar] [CrossRef]
- Acar, O. Determination of cadmium, copper and lead in soils, sediments and sea water samples by ETAAS using a Sc + Pd + NH4NO3 chemical modifier. Talanta 2005, 65, 672–677. [Google Scholar] [CrossRef]
- Ferreira, S.L.; Bezerra, M.A.; Santos, A.S.; dos Santos, W.N.; Novaes, C.G.; de Oliveira, O.M.; Oliveira, M.L.; Garcia, R.L. Atomic absorption spectrometry—A multi element technique. TrAC Trends Anal. Chem. 2018, 100, 1–6. [Google Scholar] [CrossRef]
- De la Calle, I.; Perez-Rodriguez, P.; Soto-Gomez, D.; López-Periago, J.E. Detection and characterization of Cu-bearing particles in throughfall samples from vine leaves by DLS, AF4-MALLS (-ICP-MS) and SP-ICP-MS. Microchem. J. 2017, 133, 293–301. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Zhang, Y.; Qin, X.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Hydrothermal treatment of grass: A low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer nanodots as an effective fluorescent sensing platform for label-free detection of Cu (II) ions. Adv. Mater. 2012, 24, 2037–2041. [Google Scholar] [CrossRef]
- Abdulazeez, I.; Basheer, C.; Al-Saadi, A.A. A selective detection approach for copper (ii) ions using a hydra-zone-based colorimetric sensor: Spectroscopic and DFT study. RSC Adv. 2018, 8, 39983–39991. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Mamin, H.J.; Sherwood, M.H.; Ohno, K.; Awschalom, D.D.; Rugar, D. Decoherence of near-surface nitrogen-vacancy centers due to electric field noise. Phys. Rev. Lett. 2015, 115, 087602. [Google Scholar] [CrossRef] [Green Version]
- He, E.; Cai, L.; Zheng, F.; Zhou, Q.; Guo, D.; Zhou, Y.; Zhang, X.; Li, Z. Rapid quantitative fluorescence detection of copper ions with disposable microcapsule arrays utilizing functional nucleic acid strategy. Sci. Rep. 2019, 9, 36. [Google Scholar] [CrossRef] [Green Version]
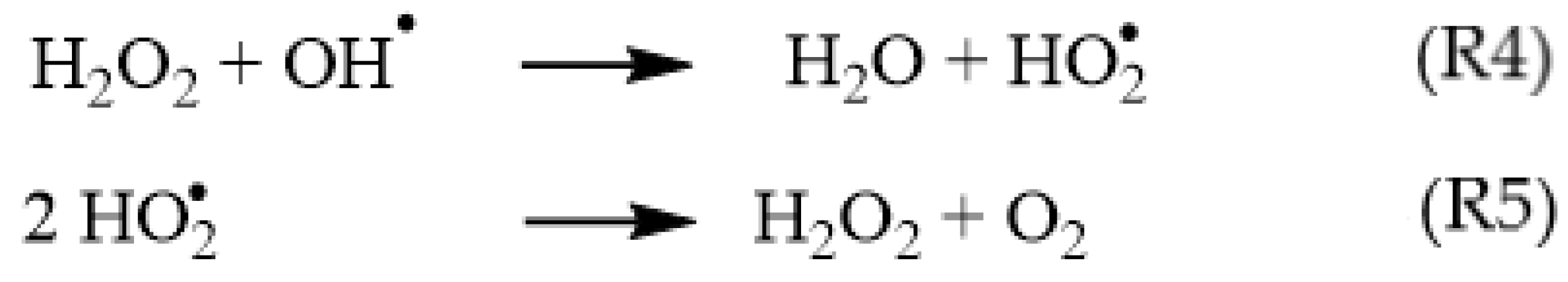
| Technique | Concentration | Pros and Cons | Mechanism and Information Obtained | Reference |
|---|---|---|---|---|
| FAAS (flame atomic absorption spectrometry) | 47–1888 nM | -destructive -spatial resolution +relatively simple | Atomic absorption on the sample is recorded. It is an invasive technique, but is highly sensitive to the element of choice. | [33] |
| ETAAS (Electrothermal atomic absorption spectrometry) | 8 nM | -destructive -spatial resolution +high sensitivity | [34] | |
| ICP OES (Inductively coupled plasma optical emission spectrometry) | 19 nM | -destructive -destructive +relatively simple +high sensitivity | The mass spectra were recorded by emission spectroscopy. It is an invasive technique, and highly selective to copper. | [35] |
| ICP-MS (inductively coupled plasma mass spectrometry) | 1 nM | -Expensive equipment -destructive +high sensitivity | The solution mass spectra were recorded, the method is highly specific to the particular element and highly sensitive to copper. | [36] |
| Cu(II)-DNAzyme | 100 nM | +Non-destructive +spatial resolution | Cu(II) binds to DNA, which leads to the release of fluorophore (6-carboxyfluorescein). Hence a fluorescence signal from the fluorophore is detected. | [41] |
| FNDs (fluorescent nanodiamonds) | 100 nM | -specialised equipment +Non-destructive +spatial resolution | Change in T1 time in presence of paramagnetic species. Copper(II) can be quantified down to nanomolar concentrations. | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padamati, S.K.; Vedelaar, T.A.; Perona Martínez, F.; Nusantara, A.C.; Schirhagl, R. Insight into a Fenton-like Reaction Using Nanodiamond Based Relaxometry. Nanomaterials 2022, 12, 2422. https://doi.org/10.3390/nano12142422
Padamati SK, Vedelaar TA, Perona Martínez F, Nusantara AC, Schirhagl R. Insight into a Fenton-like Reaction Using Nanodiamond Based Relaxometry. Nanomaterials. 2022; 12(14):2422. https://doi.org/10.3390/nano12142422
Chicago/Turabian StylePadamati, Sandeep Kumar, Thea Annie Vedelaar, Felipe Perona Martínez, Anggrek Citra Nusantara, and Romana Schirhagl. 2022. "Insight into a Fenton-like Reaction Using Nanodiamond Based Relaxometry" Nanomaterials 12, no. 14: 2422. https://doi.org/10.3390/nano12142422
APA StylePadamati, S. K., Vedelaar, T. A., Perona Martínez, F., Nusantara, A. C., & Schirhagl, R. (2022). Insight into a Fenton-like Reaction Using Nanodiamond Based Relaxometry. Nanomaterials, 12(14), 2422. https://doi.org/10.3390/nano12142422







