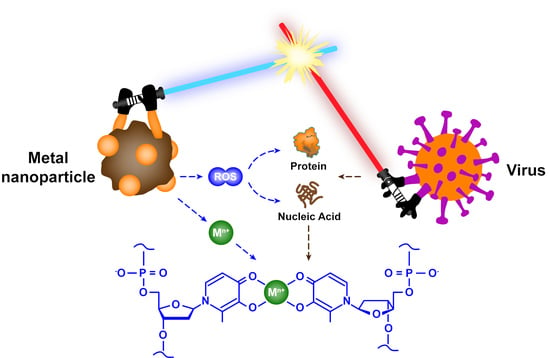
Chemical Nature of Metals and Metal-Based Materials in Inactivation of Viruses
Abstract
1. Introduction
2. Antiviral Performances of Different Metallic Materials
2.1. Metal Nanoparticles
2.1.1. Copper Nanoparticles
2.1.2. Silver Nanoparticles
2.1.3. Nickel Nanoparticles
2.1.4. Gold Nanoparticles
2.1.5. Iron Nanoparticles
2.2. Metal Ions
2.2.1. Copper Ions
2.2.2. Silver Ions
2.2.3. Zinc Ions
2.2.4. Others
2.3. Pure Metals and Alloys
2.3.1. Copper and Copper Alloys
2.3.2. Iron and Iron Alloys
2.4. Metal Compounds
2.4.1. Copper Compounds
2.4.2. Iron Compounds
2.4.3. Titanium Compounds
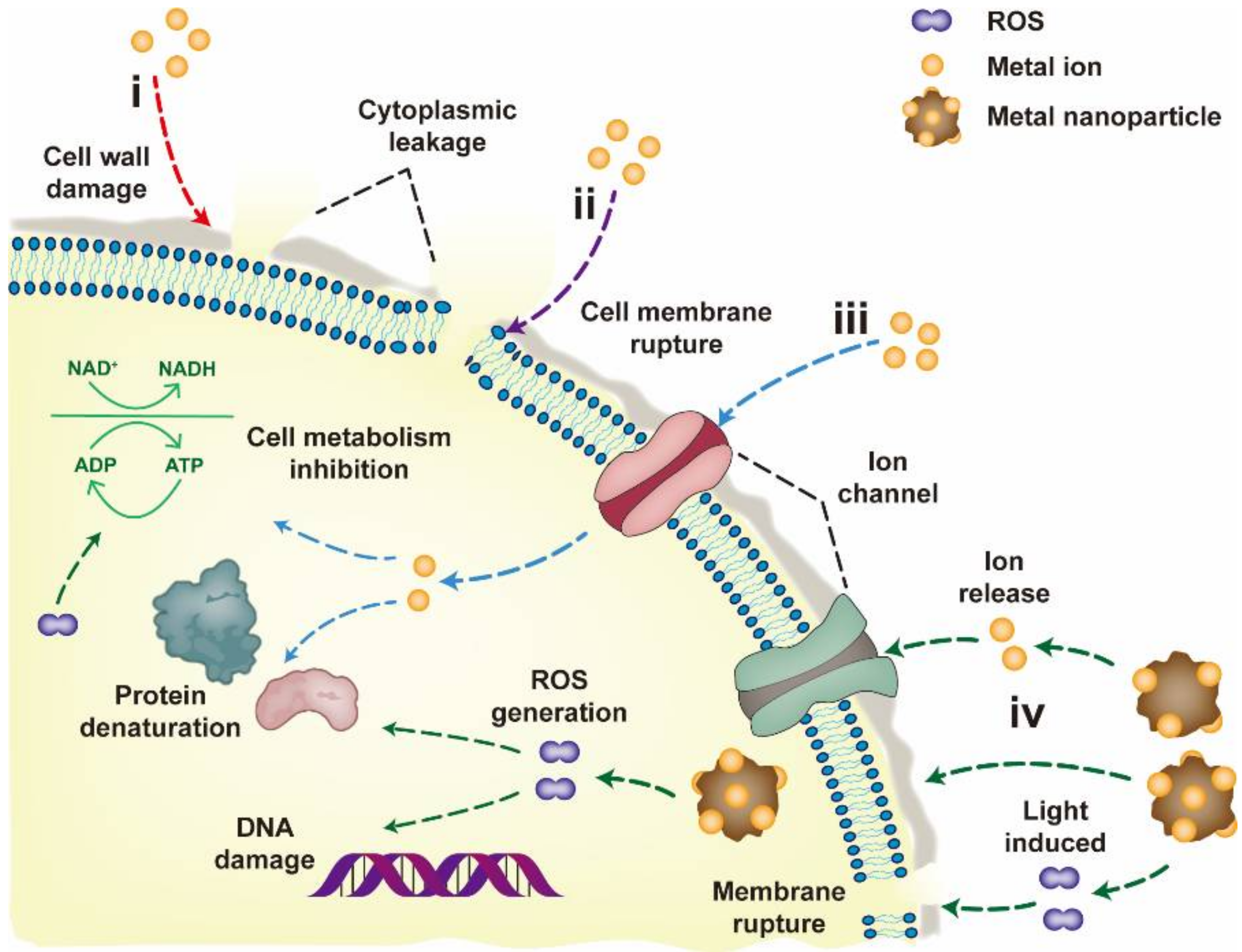
3. Antiviral Mechanisms at Biological Level
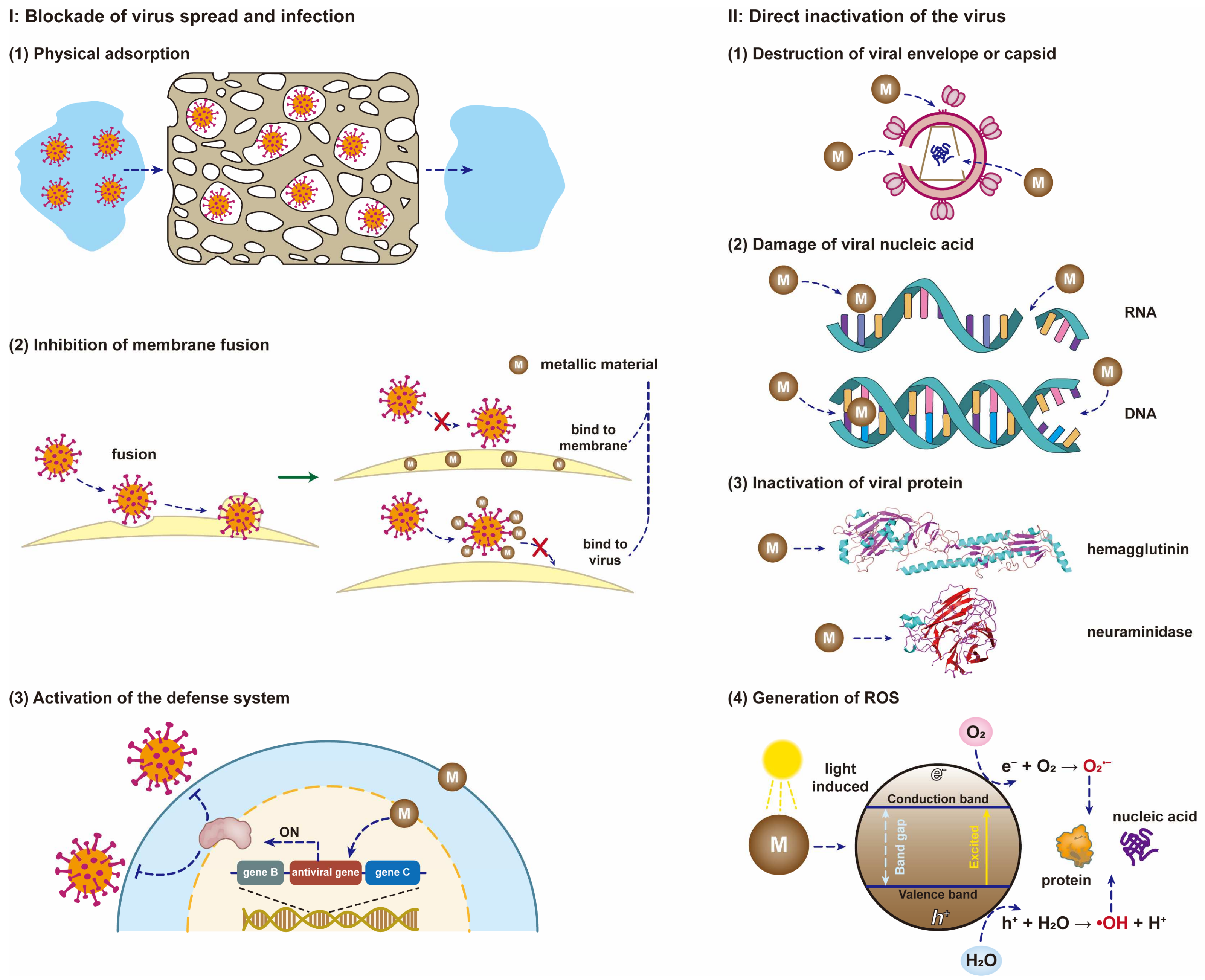
- I.
- Blockade of virus spread and infection
- (1)
- Porous metallic materials or metallic materials with positive charges on the surface can effectively remove viruses through physical adsorption. The virus is only transferred between different phases, and it remains active under certain conditions and can also be released from the surface of the material.
- (2)
- Metal nanoparticles or metal ions can bind to the membrane of the host cell, or attach to the surface of the viral envelope or capsid. Both types of binding inhibit fusion between the virus and the host cell membrane, thereby hindering the spread and infection of the virus.
- (3)
- When the metallic material enters the host cell, the expression of its viral defense-related genes is activated so that the cell develops resistance to the virus, which can also inhibit the spread and infection of the virus.
- II.
- Direct inactivation of the virus
- (1)
- Metallic materials can directly destroy the envelope of the virus after contact with the virus or bind to the glycoprotein on the surface of the virus envelope, resulting in virus inactivation.
- (2)
- Metallic materials can directly damage the genetic material (DNA or RNA) of the virus and prevent the virus from replicating.
- (3)
- Metallic materials can cleave disulfide and thiol bonds of proteins (e.g., hemagglutinin, neuraminidase and RNA polymerase) in the virus, thereby preventing virus replication and inhibiting virus spread and infection. Hemagglutinin is a necessary protein for the virus to enter host cells through endocytosis, and neuraminidase is an essential protein for the virus to be released from the surface of host cells.
- (4)
- Metallic materials can react with oxidants and reductants in the environment to generate reactive oxygen species (e.g., hydroxyl radicals and superoxide anions), which can effectively damage the proteins and genetic material of viruses.
- (5)
- Metallic materials with photocatalytic activity can induce the generation of reactive oxygen species and other oxidants, and then these oxidants promote the peroxidation of phospholipids, resulting in severe destruction of viral functions.
- (6)
- Metallic materials without photocatalytic activity can also act as catalysts for virus inactivation and accelerate the rate of virus inactivation.
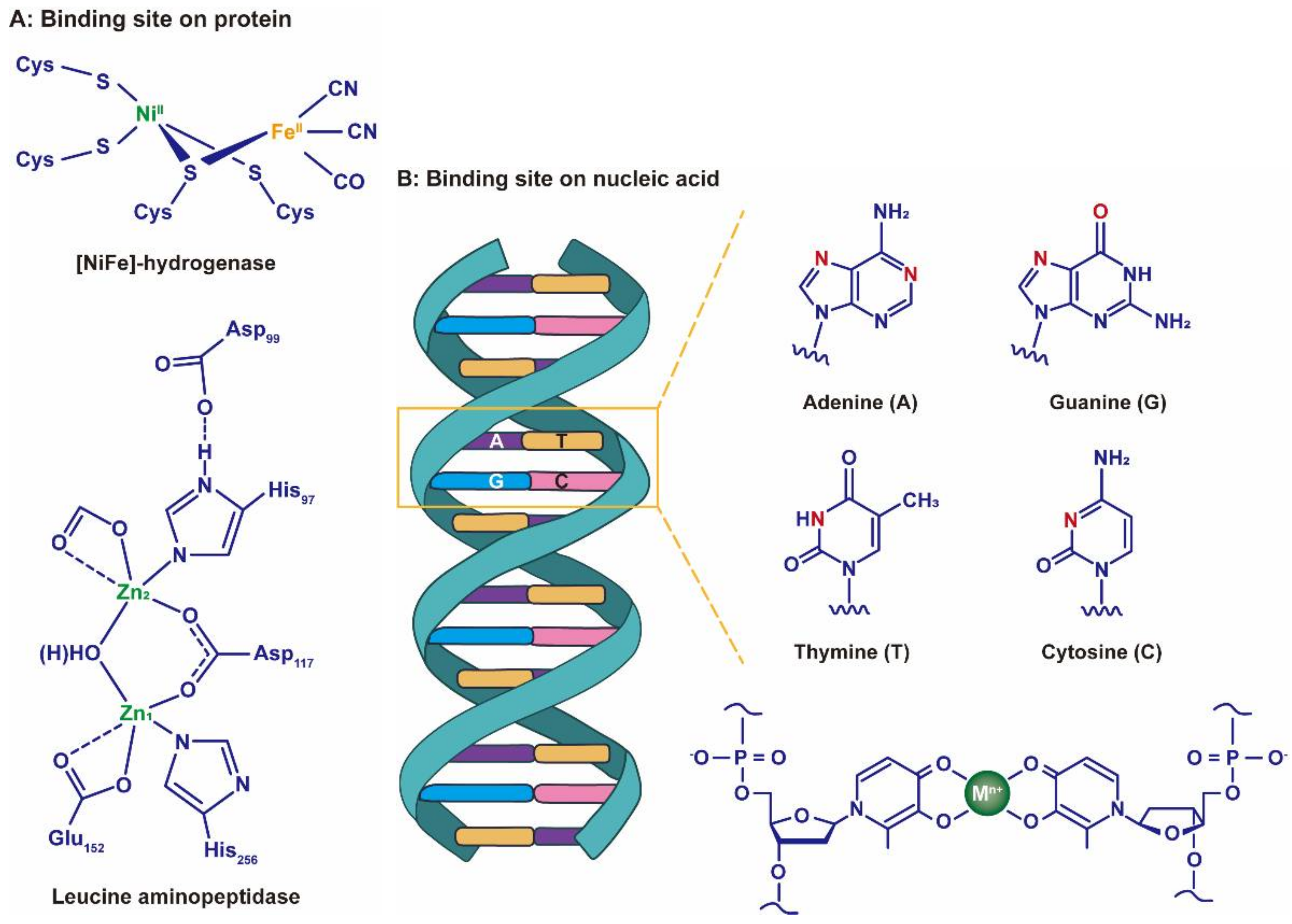
4. Potential Antiviral Mechanisms at Physicochemical Level
4.1. Chelation Reaction Equilibrium Constant
4.2. Hydrate Ion Radius
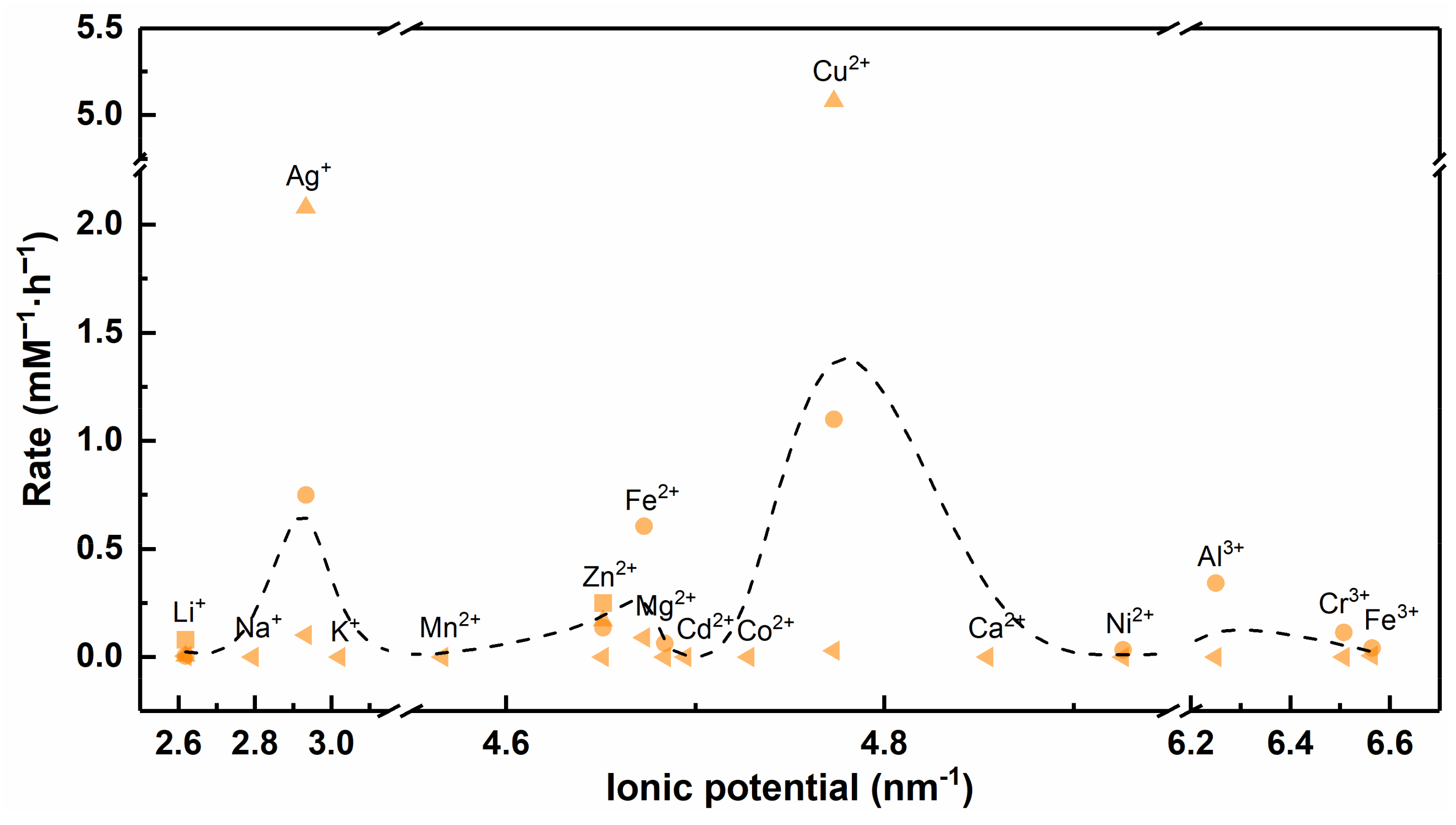
4.3. Ionic Potential
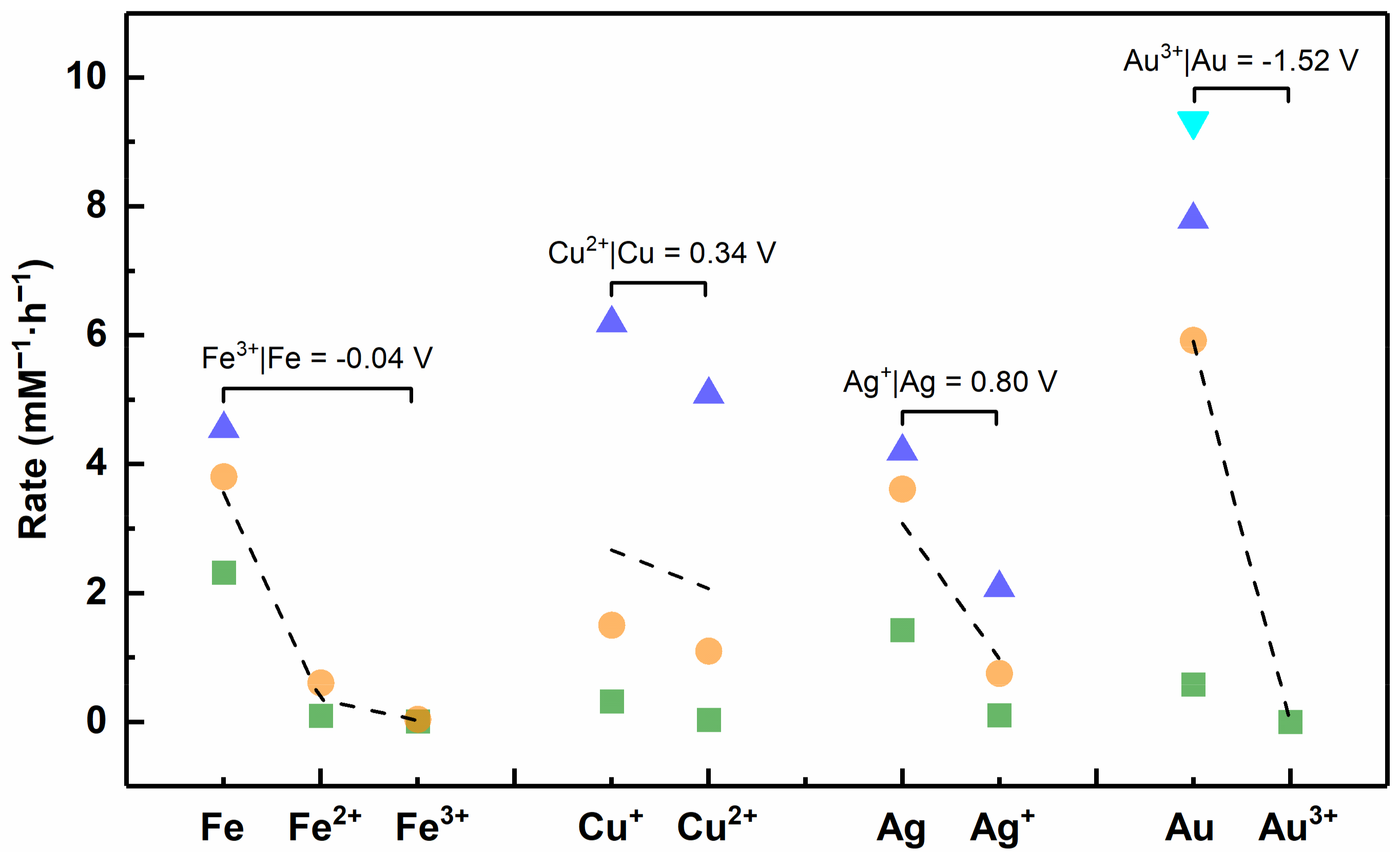
4.4. Standard Electrode Potential
4.5. Others
5. Challenges and Future Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; McGoogan, J. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, H.; Geisbert, T.W. Ebola haemorrhagic fever. Lancet 2011, 377, 849–862. [Google Scholar] [CrossRef]
- Bolles, M.; Donaldson, E.; Baric, R. SARS-CoV and emergent coronaviruses: Viral determinants of interspecies transmission. Curr. Opin. Virol. 2011, 1, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Dolan, K.; Wirtz, A.L.; Moazen, B.; Ndeffo-mbah, M.; Galvani, A.; Kinner, S.A.; Courtney, R.; McKee, M.; Amon, J.J.; Maher, L.; et al. Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. Lancet 2016, 388, 1089–1102. [Google Scholar] [CrossRef]
- Zell, R.; Krumbholz, A.; Wutzler, P. Impact of global warming on viral diseases: What is the evidence? Curr. Opin. Biotechnol. 2008, 19, 652–660. [Google Scholar] [CrossRef]
- Pullano, G.; Valdano, E.; Scarpa, N.; Rubrichi, S.; Colizza, V. Evaluating the effect of demographic factors, socioeconomic factors, and risk aversion on mobility during the COVID-19 epidemic in France under lockdown: A population-based study. Lancet Digit. Health 2020, 2, e638–e649. [Google Scholar] [CrossRef]
- Lyu, M.; Fan, G.; Xiao, G.; Wang, T.; Xu, D.; Gao, J.; Ge, S.; Li, Q.; Ma, Y.; Zhang, H.; et al. Traditional Chinese medicine in COVID-19. Acta Pharm. Sin. B 2021, 11, 3337–3363. [Google Scholar] [CrossRef]
- Delorme, M.M.; Guimarães, J.T.; Coutinho, N.M.; Balthazar, C.F.; Rocha, R.S.; Silva, R.; Margalho, L.P.; Pimentel, T.C.; Silva, M.C.; Freitas, M.Q.; et al. Ultraviolet radiation: An interesting technology to preserve quality and safety of milk and dairy foods. Trends Food Sci. Technol. 2020, 102, 146–154. [Google Scholar] [CrossRef]
- Torii, S.; Hashimoto, T.; Do, A.T.; Furumai, H.; Katayama, H. Repeated pressurization as a potential cause of deterioration in virus removal by aged reverse osmosis membrane used in households. Sci. Total Environ. 2019, 695, 133814. [Google Scholar] [CrossRef]
- Vo, H.T.; Imai, T.; Ho, T.T.; Dang, T.-L.T.; Hoang, S.A. Potential application of high pressure carbon dioxide in treated wastewater and water disinfection: Recent overview and further trends. J. Environ. Sci. 2015, 36, 38–47. [Google Scholar] [CrossRef]
- Torii, S.; Itamochi, M.; Katayama, H. Inactivation kinetics of waterborne virus by ozone determined by a continuous quench flow system. Water Res. 2020, 186, 116291. [Google Scholar] [CrossRef]
- Mazhar, M.A.; Khan, N.A.; Ahmed, S.; Khan, A.H.; Hussain, A.; Rahisuddin; Changani, F.; Yousefi, M.; Ahmadi, S.; Vambol, V. Chlorination disinfection by-products in municipal drinking water—A review. J. Clean. Prod. 2020, 273, 123159. [Google Scholar] [CrossRef]
- Block, P.; Morgan, S.; Bell, K.; Stewart, S. Control Strategies for PAA Wastewater Disinfection at WWTPs with Variable Effluent Quality. Proc. Water Environ. Fed. 2015, 2015, 508–527. [Google Scholar] [CrossRef]
- Yang, X.; Guo, W.; Zhang, X.; Chen, F.; Ye, T.; Liu, W. Formation of disinfection by-products after pre-oxidation with chlorine dioxide or ferrate. Water Res. 2013, 47, 5856–5864. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.-J. Effects of UV intensity and water turbidity on microbial indicator inactivation. J. Environ. Sci. 2006, 18, 650–653. [Google Scholar]
- Booker, E.P.; Jabbour, G.E. Antiviral nanoparticle ligands identified with datamining and high-throughput virtual screening. RSC Adv. 2021, 11, 23136–23143. [Google Scholar] [CrossRef]
- Sahani, S.; Sharma, Y.C. Advancements in applications of nanotechnology in global food industry. Food Chem. 2020, 342, 128318. [Google Scholar] [CrossRef]
- Sarmah, D.; Banerjee, M.; Datta, A.; Kalia, K.; Dhar, S.; Yavagal, D.R.; Bhattacharya, P. Nanotechnology in the diagnosis and treatment of stroke. Drug Discov. Today 2020, 26, 585–592. [Google Scholar] [CrossRef]
- Prakash, S.; Kumbhojkar, N.; Clegg, J.R.; Mitragotri, S. Cell-bound Nanoparticles for Tissue Targeting and Immunotherapy: Engineering of the Particle-Membrane Interface. Curr. Opin. Colloid Interface Sci. 2020, 52, 101408. [Google Scholar] [CrossRef]
- Fujimori, Y.; Sato, T.; Hayata, T.; Nagao, T.; Nakayama, M.; Nakayama, T.; Sugamata, R.; Suzuki, K. Novel antiviral characteristics of nanosized copper(I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl. Env. Microbiol. 2012, 78, 951–955. [Google Scholar] [CrossRef]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 2005, 3, 6. [Google Scholar] [CrossRef]
- Hamed Derbalah, A.S.; Elsharkawy, M.M. A new strategy to control Cucumber mosaic virus using fabricated NiO-nanostructures. J. Biotechnol. 2019, 306, 134–141. [Google Scholar] [CrossRef]
- Vonnemann, J.; Sieben, C.; Wolff, C.; Ludwig, K.; Böttcher, C.; Herrmann, A.; Haag, R. Virus inhibition induced by polyvalent nanoparticles of different sizes. Nanoscale 2014, 6, 2353–2360. [Google Scholar] [CrossRef]
- Cheng, R.; Li, G.; Shi, L.; Xue, X.; Kang, M.; Zheng, X. The mechanism for bacteriophage f2 removal by nanoscale zero-valent iron. Water Res. 2016, 105, 429–435. [Google Scholar] [CrossRef]
- Lin, N.; Verma, D.; Saini, N.; Arbi, R.; Munir, M.; Jovic, M.; Turak, A. Antiviral nanoparticles for sanitizing surfaces: A roadmap to self-sterilizing against COVID-19. Nano Today 2021, 40, 101267. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Lim, Y.Q.; Low, C.Y.; Lee, C.T.; Marilyn, T.C.L.; Loh, H.S.; Lim, Y.P.; Lee, C.F.; Bhattamishra, S.K.; et al. Silver nanoparticles: Advanced and promising technology in diabetic wound therapy. Mater. Sci. Eng. C 2020, 112, 110925. [Google Scholar] [CrossRef]
- Crane, R.A.; Scott, T.B. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211–212, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.C.; Roy, S.; Andrews, P.C.; Gasser, G. Metal Compounds against Neglected Tropical Diseases. Chem. Rev. 2019, 119, 730–796. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.M.; Li, H.; Werrett, M.V.; Andrews, P.C.; Sun, H. Medicinal chemistry and biomedical applications of bismuth-based compounds and nanoparticles. Chem. Soc. Rev. 2021, 50, 12037–12069. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Betts, H.; Keller, S.; Cariou, K.; Gasser, G. Recent developments of metal-based compounds against fungal pathogens. Chem. Soc. Rev. 2021, 50, 10346–10402. [Google Scholar] [CrossRef]
- De Castro, F.; De Luca, E.; Benedetti, M.; Fanizzi, F.P. Platinum compounds as potential antiviral agents. Coord. Chem. Rev. 2022, 451, 214276. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.; Ren, L.; Tang, C. Metal-containing and related polymers for biomedical applications. Chem. Soc. Rev. 2016, 45, 5232–5263. [Google Scholar] [CrossRef]
- Mudd, G.M. The Resources Cycle: Key Sustainability Issues for the Mining of Metals and Minerals. In Encyclopedia of Geology, 2nd ed.; Alderton, D., Elias, S.A., Eds.; Academic Press: Oxford, UK, 2021; pp. 607–620. [Google Scholar]
- Spooren, J.; Binnemans, K.; Björkmalm, J.; Breemersch, K.; Dams, Y.; Folens, K.; González-Moya, M.; Horckmans, L.; Komnitsas, K.; Kurylak, W.; et al. Near-zero-waste processing of low-grade, complex primary ores and secondary raw materials in Europe: Technology development trends. Resour. Conserv. Recycl. 2020, 160, 104919. [Google Scholar] [CrossRef]
- Elshkaki, A.; Reck, B.K.; Graedel, T.E. Anthropogenic nickel supply, demand, and associated energy and water use. Resour. Conserv. Recycl. 2017, 125, 300–307. [Google Scholar] [CrossRef]
- Leonel, A.G.; Mansur, A.A.P.; Mansur, H.S. Advanced Functional Nanostructures based on Magnetic Iron Oxide Nanomaterials for Water Remediation: A Review. Water Res. 2021, 190, 116693. [Google Scholar] [CrossRef]
- He, J.; Kumar, A.; Khan, M.; Lo, I.M.C. Critical review of photocatalytic disinfection of bacteria: From noble metals- and carbon nanomaterials-TiO2 composites to challenges of water characteristics and strategic solutions. Sci. Total Environ. 2021, 758, 143953. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Env. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef]
- Li, Y.P.; Ben Fekih, I.; Chi Fru, E.; Moraleda-Munoz, A.; Li, X.; Rosen, B.P.; Yoshinaga, M.; Rensing, C. Antimicrobial Activity of Metals and Metalloids. Annu. Rev. Microbiol. 2021, 75, 175–197. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Ahmed, K.B.A.; Raman, T.; Veerappan, A. Future prospects of antibacterial metal nanoparticles as enzyme inhibitor. Mater. Sci. Eng. C 2016, 68, 939–947. [Google Scholar] [CrossRef]
- Sinha, R.; Karan, R.; Sinha, A.; Khare, S.K. Interaction and nanotoxic effect of ZnO and Ag nanoparticles on mesophilic and halophilic bacterial cells. Bioresour. Technol. 2011, 102, 1516–1520. [Google Scholar] [CrossRef]
- Wyszogrodzka, G.; Marszałek, B.; Gil, B.; Dorożyński, P. Metal-organic frameworks: Mechanisms of antibacterial action and potential applications. Drug Discov. Today 2016, 21, 1009–1018. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Krupovic, M.; Cvirkaite-Krupovic, V.; Iranzo, J.; Prangishvili, D.; Koonin, E.V. Viruses of archaea: Structural, functional, environmental and evolutionary genomics. Virus Res. 2018, 244, 181–193. [Google Scholar] [CrossRef]
- Zerbib, S.; Vallet, L.; Muggeo, A.; de Champs, C.; Lefebvre, A.; Jolly, D.; Kanagaratnam, L. Copper for the Prevention of Outbreaks of Health Care–Associated Infections in a Long-term Care Facility for Older Adults. J. Am. Med. Dir. Assoc. 2020, 21, 68–71.e61. [Google Scholar] [CrossRef]
- Shi, C.; Wei, J.; Jin, Y.; Kniel, K.E.; Chiu, P.C. Removal of viruses and bacteriophages from drinking water using zero-valent iron. Sep. Purif. Technol. 2012, 84, 72–78. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, D.; Wang, Z.; Lei, Y.; Cheng, R. Nano-TiO2 membrane adsorption reactor (MAR) for virus removal in drinking water. Chem. Eng. J. 2013, 230, 180–187. [Google Scholar] [CrossRef]
- Park, S.; Ko, Y.-S.; Jung, H.; Lee, C.; Woo, K.; Ko, G. Disinfection of waterborne viruses using silver nanoparticle-decorated silica hybrid composites in water environments. Sci. Total Environ. 2018, 625, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl. Environ. Microbiol. 2007, 73, 2748–2750. [Google Scholar] [CrossRef]
- Sengupta, D.; Timilsina, U.; Mazumder, Z.H.; Mukherjee, A.; Ghimire, D.; Markandey, M.; Upadhyaya, K.; Sharma, D.; Mishra, N.; Jha, T.; et al. Dual activity of amphiphilic Zn(II) nitroporphyrin derivatives as HIV-1 entry inhibitors and in cancer photodynamic therapy. Eur. J. Med. Chem. 2019, 174, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Ogawa, H.; Bui, V.N.; Inoue, H.; Fukuda, J.; Ohba, M.; Yamamoto, Y.; Nakamura, K. Inactivation of high and low pathogenic avian influenza virus H5 subtypes by copper ions incorporated in zeolite-textile materials. Antivir. Res. 2012, 93, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31. [Google Scholar] [CrossRef]
- Choi, J.; Reddy, D.A.; Islam, M.J.; Seo, B.; Joo, S.H.; Kim, T.K. Green synthesis of the reduced graphene oxide–CuI quasi-shell–core nanocomposite: A highly efficient and stable solar-light-induced catalyst for organic dye degradation in water. Appl. Surf. Sci. 2015, 358, 159–167. [Google Scholar] [CrossRef]
- Amalina, M.N.; Rusop, M. Investigation on the I2:CuI thin films and its stability over time. Microelectron. Eng. 2013, 108, 106–111. [Google Scholar] [CrossRef]
- Das, K.; Aramini, J.M.; Ma, L.-C.; Krug, R.M.; Arnold, E. Structures of influenza A proteins and insights into antiviral drug targets. Nat. Struct. Mol. Biol. 2010, 17, 530–538. [Google Scholar] [CrossRef]
- Matrosovich, M.N.; Matrosovich, T.Y.; Gray, T.; Roberts, N.A.; Klenk, H.D. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J. Virol. 2004, 78, 12665–12667. [Google Scholar] [CrossRef]
- Cross, J.B.; Currier, R.P.; Torraco, D.J.; Vanderberg, L.A.; Wagner, G.L.; Gladen, P.D. Killing of bacillus spores by aqueous dissolved oxygen, ascorbic acid, and copper ions. Appl. Env. Microbiol. 2003, 69, 2245–2252. [Google Scholar] [CrossRef]
- Mazurkow, J.M.; Yüzbasi, N.S.; Domagala, K.W.; Pfeiffer, S.; Kata, D.; Graule, T. Nano-Sized Copper (Oxide) on Alumina Granules for Water Filtration: Effect of Copper Oxidation State on Virus Removal Performance. Environ. Sci. Technol. 2020, 54, 1214–1222. [Google Scholar] [CrossRef]
- Green, M.V.; Thayer, S.A. HIV gp120 upregulates tonic inhibition through α5-containing GABAARs. Neuropharmacology 2019, 149, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Y.-C.; Zhang, X.-L.; Deng, L.; Sang, P.; Yang, L.-Q.; Liu, S.-Q. CD4-binding obstacles in conformational transitions and allosteric communications of HIV gp120. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183217. [Google Scholar] [CrossRef] [PubMed]
- Chen, B. Molecular Mechanism of HIV-1 Entry. Trends Microbiol. 2019, 27, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Lu, J.; Liu, N.; Lu, W.; Li, Y.; Shang, C.; Li, X.; Hu, L.; Jiang, G. Antiviral Properties of Silver Nanoparticles against SARS-CoV-2: Effects of Surface Coating and Particle Size. Nanomaterials 2022, 12, 990. [Google Scholar] [CrossRef]
- Abulikemu, M.; Tabrizi, B.E.A.; Ghobadloo, S.M.; Mofarah, H.M.; Jabbour, G.E. Silver Nanoparticle-Decorated Personal Protective Equipment for Inhibiting Human Coronavirus Infectivity. ACS Appl. Nano Mater. 2022, 5, 309–317. [Google Scholar] [CrossRef]
- Argueta-Figueroa, L.; Morales-Luckie, R.A.; Scougall-Vilchis, R.J.; Olea-Mejía, O.F. Synthesis, characterization and antibacterial activity of copper, nickel and bimetallic Cu–Ni nanoparticles for potential use in dental materials. Prog. Nat. Sci. Mater. Int. 2014, 24, 321–328. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, X.; Yuan, N.; Ding, J. A novel nickel-copper alternating-deposition coating with excellent tribological and antibacterial property. J. Alloys Compd. 2020, 849, 156222. [Google Scholar] [CrossRef]
- Karthikeyan, V.; Padmanaban, A.; Dhanasekaran, T.; Kumar, S.; Gnanamoorthy, G.; Narayanan, V. Synthesis and Characterization of ZnO/NiO and Its Photocatalytic Activity. Mech. Mater. Sci. Eng. J. 2017, 9. [Google Scholar] [CrossRef]
- Nakano, R.; Hara, M.; Ishiguro, H.; Yao, Y.; Ochiai, T.; Nakata, K.; Murakami, T.; Kajioka, J.; Sunada, K.; Hashimoto, K.; et al. Broad Spectrum Microbicidal Activity of Photocatalysis by TiO2. Catalysts 2013, 3, 310. [Google Scholar] [CrossRef]
- Cheng, R.; Kang, M.; Zhuang, S.; Wang, S.; Zheng, X.; Pan, X.; Shi, L.; Wang, J. Removal of bacteriophage f2 in water by Fe/Ni nanoparticles: Optimization of Fe/Ni ratio and influencing factors. Sci. Total Environ. 2019, 649, 995–1003. [Google Scholar] [CrossRef]
- Zhang, X.-m.; He, D.-N.; Zhou, B.; Pang, R.; Liu, K.; Zhao, J.; Chen, P.-Y. In vitro inhibition of vesicular stomatitis virus replication by purified porcine Mx1 protein fused to HIV-1 Tat protein transduction domain (PTD). Antivir. Res. 2013, 99, 149–157. [Google Scholar] [CrossRef]
- Halder, A.; Das, S.; Ojha, D.; Chattopadhyay, D.; Mukherjee, A. Highly monodispersed gold nanoparticles synthesis and inhibition of herpes simplex virus infections. Mater. Sci. Eng. C 2018, 89, 413–421. [Google Scholar] [CrossRef]
- Marcocci, M.E.; Napoletani, G.; Protto, V.; Kolesova, O.; Piacentini, R.; Li Puma, D.D.; Lomonte, P.; Grassi, C.; Palamara, A.T.; De Chiara, G. Herpes Simplex Virus-1 in the Brain: The Dark Side of a Sneaky Infection. Trends Microbiol. 2020, 28, 808–820. [Google Scholar] [CrossRef]
- Hübschen, J.M.; Gouandjika-Vasilache, I.; Dina, J. Measles. Lancet 2022, 399, 678–690. [Google Scholar] [CrossRef]
- Meléndez-Villanueva, M.A.; Morán-Santibañez, K.; Martínez-Sanmiguel, J.J.; Rangel-López, R.; Garza-Navarro, M.A.; Rodríguez-Padilla, C.; Zarate-Triviño, D.G.; Trejo-Ávila, L.M. Virucidal Activity of Gold Nanoparticles Synthesized by Green Chemistry Using Garlic Extract. Viruses 2019, 11, 1111. [Google Scholar] [CrossRef]
- Kim, J.; Yeom, M.; Lee, T.; Kim, H.O.; Na, W.; Kang, A.; Lim, J.W.; Park, G.; Park, C.; Song, D.; et al. Porous gold nanoparticles for attenuating infectivity of influenza A virus. J. Nanobiotechnol. 2020, 18, 54. [Google Scholar] [CrossRef]
- Chen, J.; Lee, K.H.; Steinhauer, D.A.; Stevens, D.J.; Skehel, J.J.; Wiley, D.C. Structure of the Hemagglutinin Precursor Cleavage Site, a Determinant of Influenza Pathogenicity and the Origin of the Labile Conformation. Cell 1998, 95, 409–417. [Google Scholar] [CrossRef]
- Krawczyk, K.; Wacławek, S.; Silvestri, D.; Padil, V.V.T.; Řezanka, M.; Černík, M.; Jaroniec, M. Surface modification of zero-valent iron nanoparticles with β-cyclodextrin for 4-nitrophenol conversion. J. Colloid Interface Sci. 2021, 586, 655–662. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Duan, W.; Zhang, W.; Zhao, Y.; Liu, J. Inactivation of sulfonamide antibiotic resistant bacteria and control of intracellular antibiotic resistance transmission risk by sulfide-modified nanoscale zero-valent iron. J. Hazard. Mater. 2020, 400, 123226. [Google Scholar] [CrossRef]
- Yuan, D.; Zhai, L.; Zhang, X.; Cui, Y.; Wang, X.; Zhao, Y.; Xu, H.; He, L.; Yan, C.; Cheng, R.; et al. Study on the characteristics and mechanism of bacteriophage MS2 inactivated by bacterial cellulose supported nanoscale zero-valent iron. J. Clean. Prod. 2020, 270, 122527. [Google Scholar] [CrossRef]
- Cheng, R.; Li, G.; Cheng, C.; Liu, P.; Shi, L.; Ma, Z.; Zheng, X. Removal of bacteriophage f2 in water by nanoscale zero-valent iron and parameters optimization using response surface methodology. Chem. Eng. J. 2014, 252, 150–158. [Google Scholar] [CrossRef]
- Soliman, M.Y.M.; Medema, G.; Bonilla, B.E.; Brouns, S.J.J.; van Halem, D. Inactivation of RNA and DNA viruses in water by copper and silver ions and their synergistic effect. Water Res. X 2020, 9, 100077. [Google Scholar] [CrossRef]
- Benli, B.; Yalın, C. The influence of silver and copper ions on the antibacterial activity and local electrical properties of single sepiolite fiber: A conductive atomic force microscopy (C-AFM) study. Appl. Clay Sci. 2017, 146, 449–456. [Google Scholar] [CrossRef]
- Horie, M.; Ogawa, H.; Yoshida, Y.; Yamada, K.; Hara, A.; Ozawa, K.; Matsuda, S.; Mizota, C.; Tani, M.; Yamamoto, Y.; et al. Inactivation and morphological changes of avian influenza virus by copper ions. Arch. Virol. 2008, 153, 1467. [Google Scholar] [CrossRef]
- Kim, N.S.; Numan, M.; Nam, S.C.; Park, S.-E.; Jo, C. Dynamic adsorption/desorption of p-xylene on nanomorphic MFI zeolites: Effect of zeolite crystal thickness and mesopore architecture. J. Hazard. Mater. 2021, 403, 123659. [Google Scholar] [CrossRef]
- Dutta, P.; Wang, B. Zeolite-supported silver as antimicrobial agents. Coord. Chem. Rev. 2019, 383, 1–29. [Google Scholar] [CrossRef]
- Minoshima, M.; Lu, Y.; Kimura, T.; Nakano, R.; Ishiguro, H.; Kubota, Y.; Hashimoto, K.; Sunada, K. Comparison of the antiviral effect of solid-state copper and silver compounds. J. Hazard. Mater. 2016, 312, 1–7. [Google Scholar] [CrossRef]
- Gevondyan, N.M.; Volynskaia, A.M.; Gevondyan, V.S. Four free cysteine residues found in human IgG1 of healthy donors. Biochemistry. Biokhimiia 2006, 71, 279–284. [Google Scholar] [CrossRef]
- Ahn, A.J.; Ahn, K.S.; Suh, G.H.; Noh, J.H.; Kim, Y.H.; Yoo, M.S.; Kang, S.W.; Shin, S.S. Efficacy of silver ions against Sacbrood virus infection in the Eastern honey bee Apis cerana. J. Vet. Sci. 2015, 16, 289–295. [Google Scholar] [CrossRef][Green Version]
- Joo, Y.S.; Kim, H.W.; Lee, S.; Nam, K.H.; Yun, H.-R.; Jhee, J.H.; Han, S.H.; Yoo, T.-H.; Kang, S.-W.; Park, J.T. Dietary zinc intake and incident chronic kidney disease. Clin. Nutr. 2020, 40, 1039–1045. [Google Scholar] [CrossRef]
- Wessels, I.; Fischer, H.J.; Rink, L. Update on the multi-layered levels of zinc-mediated immune regulation. Semin. Cell Dev. Biol. 2020, 115, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Cao, H.; Qiao, Y.; Meng, F.; Zhu, H.; Liu, X. Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids Surf. B Biointerfaces 2014, 117, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.M.J.; Robins, B.; Bugert, J.J.; Denyer, S.P.; Heard, C.M. In vitro permeation and biological activity of punicalagin and zinc (II) across skin and mucous membranes prone to Herpes simplex virus infection. Eur. J. Pharm. Sci. 2017, 96, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Burwinkel, M.; Palissa, C.; Ephraim, E.; Schmidt, M.F.G. Antiviral activity of zinc salts against transmissible gastroenteritis virus in vitro. Vet. Microbiol. 2012, 160, 468–472. [Google Scholar] [CrossRef]
- Ding, Z.; An, K.; Xie, L.; Wu, W.; Zhang, R.; Wang, D.; Fang, Y.; Chen, H.; Xiao, S.; Fang, L. Transmissible gastroenteritis virus infection induces NF-κB activation through RLR-mediated signaling. Virology 2017, 507, 170–178. [Google Scholar] [CrossRef]
- Hodek, J.; Zajícová, V.; Lovětinská-Šlamborová, I.; Stibor, I.; Müllerová, J.; Weber, J. Protective hybrid coating containing silver, copper and zinc cations effective against human immunodeficiency virus and other enveloped viruses. BMC Microbiol. 2016, 16, 56. [Google Scholar] [CrossRef]
- Swain, N.; Mishra, S. A review on the recovery and separation of rare earths and transition metals from secondary resources. J. Clean. Prod. 2019, 220, 884–898. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, F.; Liu, X. Protection of magnesium alloys: From physical barrier coating to smart self-healing coating. J. Alloys Compd. 2021, 853, 157010. [Google Scholar] [CrossRef]
- Kabir, H.; Munir, K.; Wen, C.; Li, Y. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: Biomechanical and biocorrosion perspectives. Bioact. Mater. 2021, 6, 836–879. [Google Scholar] [CrossRef]
- Heller, L.; Mota, C.R.; Greco, D.B. COVID-19 faecal-oral transmission: Are we asking the right questions? Sci. Total Environ. 2020, 729, 138919. [Google Scholar] [CrossRef]
- Hinsa-Leasure, S.M.; Nartey, Q.; Vaverka, J.; Schmidt, M.G. Copper alloy surfaces sustain terminal cleaning levels in a rural hospital. Am. J. Infect. Control 2016, 44, e195–e203. [Google Scholar] [CrossRef]
- Warnes, S.L.; Little, Z.R.; Keevil, C.W. Human Coronavirus 229E Remains Infectious on Common Touch Surface Materials. mBio 2015, 6, e01697. [Google Scholar] [CrossRef]
- Anju Rose Puthukkara, P.; Sunil Jose, T.; Dinoop lal, S. Plant mediated synthesis of zero valent iron nanoparticles and its application in water treatment. J. Environ. Chem. Eng. 2020, 9, 104569. [Google Scholar] [CrossRef]
- You, Y.; Han, J.; Chiu, P.C.; Jin, Y. Removal and inactivation of waterborne viruses using zerovalent iron. Env. Sci Technol 2005, 39, 9263–9269. [Google Scholar] [CrossRef]
- Terio, V.; Bottaro, M.; Di Pinto, A.; Fusco, G.; Barresi, T.; Tantillo, G.; Martella, V. Occurrence of Aichi virus in retail shellfish in Italy. Food Microbiol. 2018, 74, 120–124. [Google Scholar] [CrossRef]
- Goikhman, Y.; Drori, Y.; Friedman, N.; Sherbany, H.; Keller, N.; Mendelson, E.; Pando, R.; Mandelboim, M. Adenovirus load correlates with respiratory disease severity among hospitalized pediatric patients. Int. J. Infect. Dis. 2020, 97, 145–150. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Q.; Yang, H.; Shi, D.; Qian, J. Photocatalytic antibacterial properties of copper doped TiO2 prepared by high-energy ball milling. Ceram. Int. 2020, 46, 16716–16724. [Google Scholar] [CrossRef]
- Baig, U.; Ansari, M.A.; Gondal, M.A.; Akhtar, S.; Khan, F.A.; Falath, W.S. Single step production of high-purity copper oxide-titanium dioxide nanocomposites and their effective antibacterial and anti-biofilm activity against drug-resistant bacteria. Mater. Sci. Eng. C 2020, 113, 110992. [Google Scholar] [CrossRef]
- Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A novel anti-influenza copper oxide containing respiratory face mask. PLoS ONE 2010, 5, e11295. [Google Scholar] [CrossRef]
- Sunada, K.; Minoshima, M.; Hashimoto, K. Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J. Hazard. Mater. 2012, 235–236, 265–270. [Google Scholar] [CrossRef]
- Fan, X.; Huang, R.; Chen, H. Application of ultraviolet C technology for surface decontamination of fresh produce. Trends Food Sci. Technol. 2017, 70, 9–19. [Google Scholar] [CrossRef]
- Ruales-Lonfat, C.; Barona, J.F.; Sienkiewicz, A.; Bensimon, M.; Vélez-Colmenares, J.; Benítez, N.; Pulgarín, C. Iron oxides semiconductors are efficients for solar water disinfection: A comparison with photo-Fenton processes at neutral pH. Appl. Catal. B Environ. 2015, 166–167, 497–508. [Google Scholar] [CrossRef]
- Pecson, B.M.; Decrey, L.; Kohn, T. Photoinactivation of virus on iron-oxide coated sand: Enhancing inactivation in sunlit waters. Water Res. 2012, 46, 1763–1770. [Google Scholar] [CrossRef]
- Fidalgo de Cortalezzi, M.M.; Gallardo, M.V.; Yrazu, F.; Gentile, G.J.; Opezzo, O.; Pizarro, R.; Poma, H.R.; Rajal, V.B. Virus removal by iron oxide ceramic membranes. J. Environ. Chem. Eng. 2014, 2, 1831–1840. [Google Scholar] [CrossRef]
- Giannakis, S.; Liu, S.; Carratalà, A.; Rtimi, S.; Talebi Amiri, M.; Bensimon, M.; Pulgarin, C. Iron oxide-mediated semiconductor photocatalysis vs. heterogeneous photo-Fenton treatment of viruses in wastewater. Impact of the oxide particle size. J. Hazard. Mater. 2017, 339, 223–231. [Google Scholar] [CrossRef]
- Feizpoor, S.; Habibi-Yangjeh, A. Ternary TiO2/Fe3O4/CoWO4 nanocomposites: Novel magnetic visible-light-driven photocatalysts with substantially enhanced activity through p-n heterojunction. J. Colloid Interface Sci. 2018, 524, 325–336. [Google Scholar] [CrossRef]
- Poulopoulos, S.G.; Philippopoulos, C.J. Photo-assisted oxidation of chlorophenols in aqueous solutions using hydrogen peroxide and titanium dioxide. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2004, 39, 1385–1397. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, Z.; Khan, A.U.; Mastoi, N.R.; Aslam, M.; Kim, J. Photocatalytic systems as an advanced environmental remediation: Recent developments, limitations and new avenues for applications. J. Environ. Chem. Eng. 2016, 4, 4143–4164. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, Z.-p.; Cheng, C.; Shi, L.; Cheng, R.; Yuan, D.-h. Photocatalytic disinfection performance in virus and virus/bacteria system by Cu-TiO2 nanofibers under visible light. Environ. Pollut. 2018, 237, 452–459. [Google Scholar] [CrossRef]
- Wang, N.; Ferhan, A.R.; Yoon, B.K.; Jackman, J.A.; Cho, N.-J.; Majima, T. Chemical design principles of next-generation antiviral surface coatings. Chem. Soc. Rev. 2021, 50, 9741–9765. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Dyson, P.J.; Gasser, G. Classification of Metal-Based Drugs according to Their Mechanisms of Action. Chem 2020, 6, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Rumlová, M.; Ruml, T. In vitro methods for testing antiviral drugs. Biotechnol. Adv. 2018, 36, 557–576. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chang, L.; Liu, W.; Xiong, Z.; Zhao, Y.; Zhang, J. Advances in mercury removal from coal-fired flue gas by mineral adsorbents. Chem. Eng. J. 2020, 379, 122263. [Google Scholar] [CrossRef]
- Holz, R.C. The aminopeptidase from Aeromonas proteolytica: Structure and mechanism of co-catalytic metal centers involved in peptide hydrolysis. Coord. Chem. Rev. 2002, 232, 5–26. [Google Scholar] [CrossRef]
- Finney Lydia, A.; O’Halloran Thomas, V. Transition Metal Speciation in the Cell: Insights from the Chemistry of Metal Ion Receptors. Science 2003, 300, 931–936. [Google Scholar] [CrossRef]
- Schatz, M.; Tong, P.B.V.; Beaumelle, B. Unconventional secretion of viral proteins. Semin. Cell Dev. Biol. 2018, 83, 8–11. [Google Scholar] [CrossRef]
- Abe, S.; Maity, B.; Ueno, T. Functionalization of protein crystals with metal ions, complexes and nanoparticles. Curr. Opin. Chem. Biol. 2018, 43, 68–76. [Google Scholar] [CrossRef]
- Samuel, M.S.; Datta, S.; Khandge, R.S.; Selvarajan, E. A state of the art review on characterization of heavy metal binding metallothioneins proteins and their widespread applications. Sci. Total Environ. 2021, 775, 145829. [Google Scholar] [CrossRef]
- Ghosh, A.C.; Duboc, C.; Gennari, M. Synergy between metals for small molecule activation: Enzymes and bio-inspired complexes. Coord. Chem. Rev. 2021, 428, 213606. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, C.; Cao, F.; Ren, J.; Qu, X. DNA metallization: Principles, methods, structures, and applications. Chem. Soc. Rev. 2018, 47, 4017–4072. [Google Scholar] [CrossRef]
- Saunders, A.M.; DeRose, V.J. Beyond Mg2+: Functional interactions between RNA and transition metals. Curr. Opin. Chem. Biol. 2016, 31, 153–159. [Google Scholar] [CrossRef]
- May, P.; Rowland, D. JESS, a Joint Expert Speciation System–VI: Thermodynamically-consistent Standard Gibbs Energies of Reaction for Aqueous Solutions. New J. Chem. 2018, 42, 7617–7629. [Google Scholar] [CrossRef]
- Appleby, T.; Perry, J.; Murakami, E.; Barauskas, O.; Feng, J.; Cho, A.; Iii, D.; Wetmore, D.; McGrath, M.; Ray, A.; et al. Structural basis for RNA replication by the hepatitis C virus polymerase. Science 2015, 347, 771. [Google Scholar] [CrossRef]
- Gao, Y.; Liming, Y.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L.; et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 2020, 368, eabb7498. [Google Scholar] [CrossRef]
- Nieva, J.; Madan, V.; Carrasco, L. Viroporins: Structure and biological functions. Nat. Rev. Microbiol. 2012, 10, 563–574. [Google Scholar] [CrossRef]
- Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects. Sep. Purif. Technol. 2012, 86, 119–126. [Google Scholar] [CrossRef]
- De Baaij, J.; Hoenderop, J.; Bindels, R. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Fernández-Calderón, M.C.; Pacha-Olivenza, M.A.; Pérez-Giraldo, C.; Gallardo-Moreno, A.M.; González-Martín, M.L. The role of magnesium in biomaterials related infections. Colloids Surf. B Biointerfaces 2020, 191, 110996. [Google Scholar] [CrossRef]
- Michen, B.; Graule, T. Isoelectric point of viruses. J. Appl. Microbiol. 2010, 109, 388–397. [Google Scholar] [CrossRef]
- Traeger, J.C.; Schwartz, D.K. Interplay of electrostatic repulsion and surface grafting density on surface-mediated DNA hybridization. J. Colloid Interface Sci. 2020, 566, 369–374. [Google Scholar] [CrossRef]
- Kralchevsky, P.; Danov, K.; Denkov, N. Chapter 5: Chemical physics of colloid systems and interfaces. In Handbook of Surface and Colloidal Chemistry; Taylor & Francis Group: Oxfordshire, UK, 2003; pp. 137–344. [Google Scholar]
- Langlet, J.; Gaboriaud, F.; Gantzer, C.; Duval, J.F.L. Impact of Chemical and Structural Anisotropy on the Electrophoretic Mobility of Spherical Soft Multilayer Particles: The Case of Bacteriophage MS2. Biophys. J. 2008, 94, 3293–3312. [Google Scholar] [CrossRef]
- Brett, C.M.A. Standard Electrode Potentials and Application to Characterization of Corrosion Phenomena. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Oxford, UK, 2018; pp. 511–516. [Google Scholar]
- Hou, W.-C.; Stuart, B.; Howes, R.; Zepp, R. Sunlight-Driven Reduction of Silver Ions by Natural Organic Matter: Formation and Transformation of Silver Nanoparticles. Environ. Sci. Technol. 2013, 47, 7713–7721. [Google Scholar] [CrossRef]
- Xiao, R.; Liu, K.; Bai, L.; Minakata, D.; Seo, Y.; Kaya Göktaş, R.; Dionysiou, D.D.; Tang, C.-J.; Wei, Z.; Spinney, R. Inactivation of pathogenic microorganisms by sulfate radical: Present and future. Chem. Eng. J. 2019, 371, 222–232. [Google Scholar] [CrossRef]
- Chen, Y.-D.; Duan, X.; Zhou, X.; Wang, R.; Wang, S.; Ren, N.-Q.; Ho, S.-H. Advanced oxidation processes for water disinfection: Features, mechanisms and prospects. Chem. Eng. J. 2021, 409, 128207. [Google Scholar] [CrossRef]
- Murakami, T.; Ono, A. HIV-1 entry: Duels between Env and host antiviral transmembrane proteins on the surface of virus particles. Curr. Opin. Virol. 2021, 50, 59–68. [Google Scholar] [CrossRef]
- Bosshard, F.; Riedel, K.; Schneider, T.; Geiser, C.; Bucheli, M.; Egli, T. Protein oxidation and aggregation in UVA-irradiated Escherichia coli cells as signs of accelerated cellular senescence. Env. Microbiol. 2010, 12, 2931–2945. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Wang, D. Progress and challenges in photocatalytic disinfection of waterborne Viruses: A review to fill current knowledge gaps. Chem. Eng. J. 2019, 355, 399–415. [Google Scholar] [CrossRef]
- Kumar, A.; Pottiboyina, V.; Sevilla, M. Hydroxyl Radical (OH center dot) Reaction with Guanine in an Aqueous Environment: A DFT Study. J. Phys. Chem. B 2011, 115, 15129–15137. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 2008, 77, 755–776. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, X.; Wu, Y.; Hou, J.; Zhang, S.; Zhou, N.; Wang, X. Toxicity responses of different organs of zebrafish (Danio rerio) to silver nanoparticles with different particle sizes and surface coatings. Environ. Pollut. 2019, 246, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Mosselhy, D.A.; Virtanen, J.; Kant, R.; He, W.; Elbahri, M.; Sironen, T. COVID-19 Pandemic: What about the Safety of Anti-Coronavirus Nanoparticles? Nanomaterials 2021, 11, 796. [Google Scholar] [CrossRef] [PubMed]
- Ramskov, T.; Thit, A.; Croteau, M.-N.; Selck, H. Biodynamics of copper oxide nanoparticles and copper ions in an oligochaete–Part I: Relative importance of water and sediment as exposure routes. Aquat. Toxicol. 2015, 164, 81–91. [Google Scholar] [CrossRef]
- Gong, N.; Shao, K.; Che, C.; Sun, Y. Stability of nickel oxide nanoparticles and its influence on toxicity to marine algae Chlorella vulgaris. Mar. Pollut. Bull. 2019, 149, 110532. [Google Scholar] [CrossRef]
- Kaloyianni, M.; Dimitriadi, A.; Ovezik, M.; Stamkopoulou, D.; Feidantsis, K.; Kastrinaki, G.; Gallios, G.; Tsiaoussis, I.; Koumoundouros, G.; Bobori, D. Magnetite nanoparticles effects on adverse responses of aquatic and terrestrial animal models. J. Hazard. Mater. 2020, 383, 121204. [Google Scholar] [CrossRef]
- Rehman, A.U.; Nazir, S.; Irshad, R.; Tahir, K.; ur Rehman, K.; Islam, R.U.; Wahab, Z. Toxicity of heavy metals in plants and animals and their uptake by magnetic iron oxide nanoparticles. J. Mol. Liq. 2021, 321, 114455. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, S.; Yadav, K.K.; Shrivastava, M.; Gupta, N.; Nagar, S.; Bach, Q.-V.; Kamyab, H.; Khan, S.A.; Yadav, S.; et al. Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches—A review. Environ. Res. 2019, 179, 108792. [Google Scholar] [CrossRef]
- Rahim, M.F.; Pal, D.; Ariya, P.A. Physicochemical studies of aerosols at Montreal Trudeau Airport: The importance of airborne nanoparticles containing metal contaminants. Environ. Pollut. 2019, 246, 734–744. [Google Scholar] [CrossRef]
- Li, C.; Quan, Q.; Gan, Y.; Dong, J.; Fang, J.; Wang, L.; Liu, J. Effects of heavy metals on microbial communities in sediments and establishment of bioindicators based on microbial taxa and function for environmental monitoring and management. Sci. Total Environ. 2020, 749, 141555. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.-F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Chen, P.-J.; Wu, W.-L.; Wu, K.C.-W. The zerovalent iron nanoparticle causes higher developmental toxicity than its oxidation products in early life stages of medaka fish. Water Res. 2013, 47, 3899–3909. [Google Scholar] [CrossRef]
- Shaw, B.J.; Handy, R.D. Physiological effects of nanoparticles on fish: A comparison of nanometals versus metal ions. Environ. Int. 2011, 37, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Farzin, L.; Shamsipur, M.; Sheibani, S. A review: Aptamer-based analytical strategies using the nanomaterials for environmental and human monitoring of toxic heavy metals. Talanta 2017, 174, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Ken, D.S.; Sinha, A. Recent developments in surface modification of nano zero-valent iron (nZVI): Remediation, toxicity and environmental impacts. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100344. [Google Scholar] [CrossRef]
- Sheng, Z.; Liu, Y. Potential impacts of silver nanoparticles on bacteria in the aquatic environment. J. Environ. Manag. 2017, 191, 290–296. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, D.; Ma, J.; Chen, X. Fluidization of nanoparticle agglomerates assisted by combining vibration and stirring methods. Chem. Eng. J. 2020, 388, 124213. [Google Scholar] [CrossRef]
- Adewunmi, A.A.; Kamal, M.S.; Solling, T.I. Application of magnetic nanoparticles in demulsification: A review on synthesis, performance, recyclability, and challenges. J. Pet. Sci. Eng. 2021, 196, 107680. [Google Scholar] [CrossRef]
- Ren, Y.; Abbood, H.A.; He, F.; Peng, H.; Huang, K. Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: Preparation, characterization, and application in heavy metal adsorption. Chem. Eng. J. 2013, 226, 300–311. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, H.; Sharma, A.K.; Hong, W.G.; Shin, K.; Song, H.; Kim, H.Y.; Hong, Y.J. Recyclable aqueous metal adsorbent: Synthesis and Cu(II) sorption characteristics of ternary nanocomposites of Fe3O4 nanoparticles@graphene–poly-N-phenylglycine nanofibers. J. Hazard. Mater. 2021, 401, 123283. [Google Scholar] [CrossRef]
- Wetzel, C.; Lonneman, M.; Wu, C. Polypharmacological drug actions of recently FDA approved antibiotics. Eur. J. Med. Chem. 2021, 209, 112931. [Google Scholar] [CrossRef]
- Dybul, M.; Attoye, T.; Baptiste, S.; Cherutich, P.; Dabis, F.; Deeks, S.G.; Dieffenbach, C.; Doehle, B.; Goodenow, M.M.; Jiang, A.; et al. The case for an HIV cure and how to get there. Lancet HIV 2021, 8, e51–e58. [Google Scholar] [CrossRef]
- Lin, M.; Dong, H.-Y.; Xie, H.-Z.; Li, Y.-M.; Jia, L. Why do we lack a specific magic anti-COVID-19 drug? Analyses and solutions. Drug Discov. Today 2020, 26, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.J.; Peng, K.W.; Bell, J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef]
- Roy, D.G.; Bell, J.C.; Bourgeois-Daigneault, M.-C. Magnetic targeting of oncolytic VSV-based therapies improves infection of tumor cells in the presence of virus-specific neutralizing antibodies in vitro. Biochem. Biophys. Res. Commun. 2020, 526, 641–646. [Google Scholar] [CrossRef]
- Pan, C.; Liu, Y.; Zhou, M.; Wang, W.; Shi, M.; Xing, M.; Liao, W. Theranostic pH-sensitive nanoparticles for highly efficient targeted delivery of doxorubicin for breast tumor treatment. Int. J. Nanomed. 2018, 13, 1119–1137. [Google Scholar] [CrossRef]
- Shokeen, K.; Srivathsan, A.; Kumar, S. Lithium chloride functions as Newcastle disease virus-induced ER-stress modulator and confers anti-viral effect. Virus Res. 2021, 292, 198223. [Google Scholar] [CrossRef]
- Li, H.-J.; Gao, D.-S.; Li, Y.-T.; Wang, Y.-S.; Liu, H.-Y.; Zhao, J. Antiviral effect of lithium chloride on porcine epidemic diarrhea virus in vitro. Res. Vet. Sci. 2018, 118, 288–294. [Google Scholar] [CrossRef]
- Ren, X.; Meng, F.; Yin, J.; Li, G.; Li, X.; Wang, C.; Herrler, G. Action mechanisms of lithium chloride on cell infection by transmissible gastroenteritis coronavirus. PLoS ONE 2011, 6, e18669. [Google Scholar] [CrossRef]
- Sui, X.; Yin, J.; Ren, X. Antiviral effect of diammonium glycyrrhizinate and lithium chloride on cell infection by pseudorabies herpesvirus. Antivir. Res. 2010, 85, 346–353. [Google Scholar] [CrossRef]
- Molan, K.; Rahmani, R.; Krklec, D.; Brojan, M.; Stopar, D. Phi 6 Bacteriophage Inactivation by Metal Salts, Metal Powders, and Metal Surfaces. Viruses 2022, 14, 204. [Google Scholar] [CrossRef]
- Takeda, Y.; Jamsransuren, D.; Nagao, T.; Fukui, Y.; Matsuda, S.; Ogawa, H.; Johnson Karyn, N. Application of Copper Iodide Nanoparticle-Doped Film and Fabric To Inactivate SARS-CoV-2 via the Virucidal Activity of Cuprous Ions (Cu+). Appl. Environ. Microbiol. 2021, 87, e01824-21. [Google Scholar] [CrossRef] [PubMed]
- Lukasik, J.; Scott Troy, M.; Andryshak, D.; Farrah Samuel, R. Influence of Salts on Virus Adsorption to Microporous Filters. Appl. Environ. Microbiol. 2000, 66, 2914–2920. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Deng, S.; Zhang, X.; Fang, L.; Liang, J.; Xiao, S. Inhibitory effect and mechanism of gelatin stabilized ferrous sulfide nanoparticles on porcine reproductive and respiratory syndrome virus. J. Nanobiotechnol. 2022, 20, 70. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Subramani, C.; Anang, S.; Muthumohan, R.; Shalimar, n.; Nayak, B.; Ranjith-Kumar, C.T.; Surjit, M.; Ou, J.H.J. Zinc Salts Block Hepatitis E Virus Replication by Inhibiting the Activity of Viral RNA-Dependent RNA Polymerase. J. Virol. 2017, 91, e00754-17. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chang, C.-H. In vitro inhibition of enterovirus 71 infection with a nickel ion/chitosan microcomposite. Virus Res. 2014, 190, 17–24. [Google Scholar] [CrossRef]
- Terpiłowska, S.; Siwicki, A.K. Chromium(III) and iron(III) inhibits replication of DNA and RNA viruses. BioMetals 2017, 30, 565–574. [Google Scholar] [CrossRef]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Cheng, R.; Zhang, Y.; Zhang, T.; Hou, F.; Cao, X.; Shi, L.; Jiang, P.; Zheng, X.; Wang, J. The inactivation of bacteriophages MS2 and PhiX174 by nanoscale zero-valent iron: Resistance difference and mechanisms. Front. Environ. Sci. Eng. 2022, 16, 108. [Google Scholar] [CrossRef]
- Morris, D.; Ansar, M.; Speshock, J.; Ivanciuc, T.; Qu, Y.; Casola, A.; Garofalo, R.P. Antiviral and Immunomodulatory Activity of Silver Nanoparticles in Experimental RSV Infection. Viruses 2019, 11, 732. [Google Scholar] [CrossRef]
- Lv, X.; Wang, P.; Bai, R.; Cong, Y.; Suo, S.; Ren, X.; Chen, C. Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections. Biomaterials 2014, 35, 4195–4203. [Google Scholar] [CrossRef]
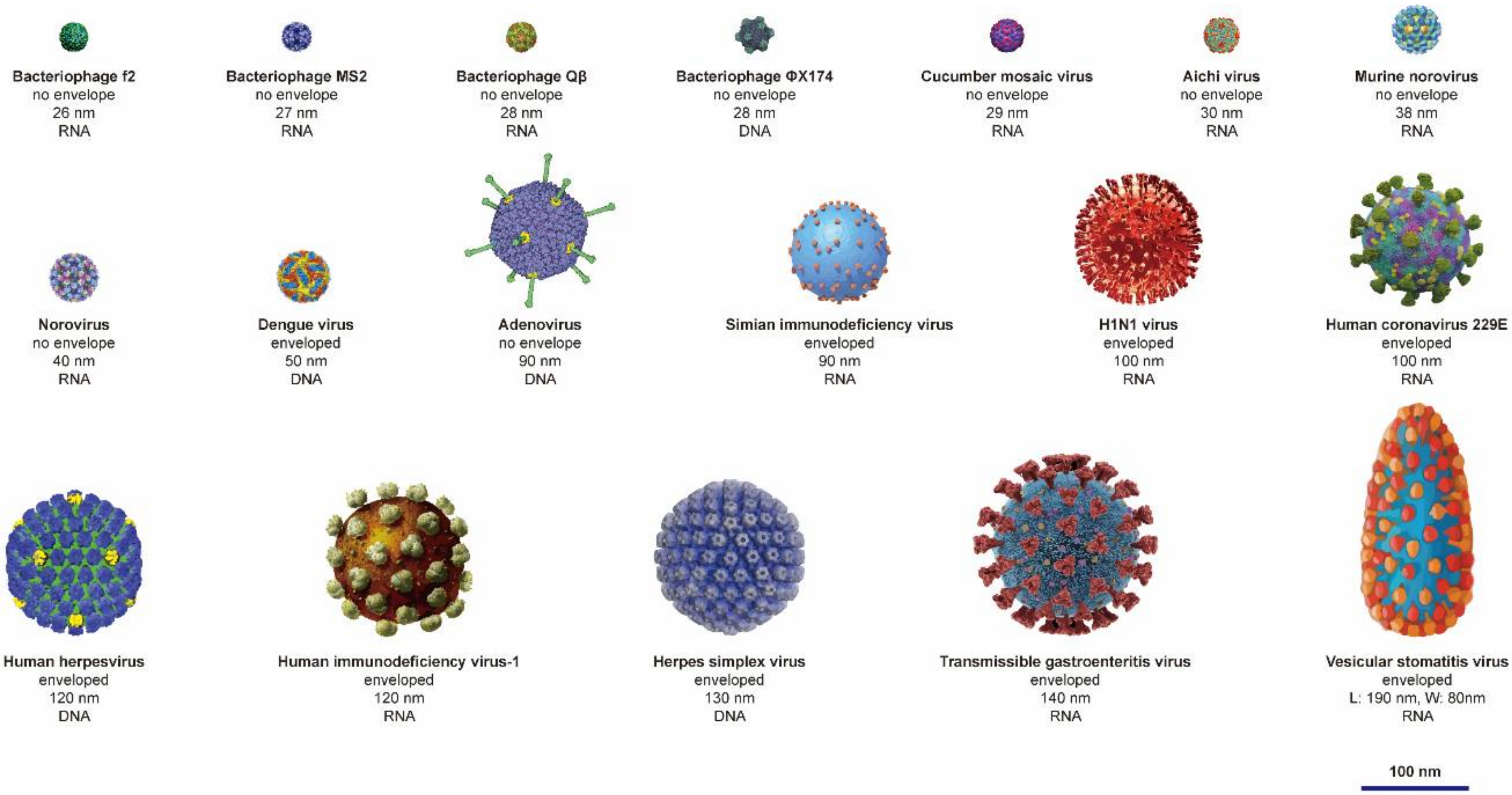




| Metallic Material | Size | Viruses | Mechanism | Reference |
|---|---|---|---|---|
| CuI NPs | D50 = 160 nm | H1N1 virus | Cu+ dissolved from NPs induced ROS production to destroy viral proteins (e.g., hemagglutinin and neuraminidase). | [20] |
| CuxOy-Al2O3 | 30~50 nm | Bacteriophage MS2 | Electrostatic adsorption, positively charged NPs bound negatively charged viruses. | [60] |
| Ag NPs | 21 ± 18 nm | HIV-1 virus | The combination of Ag NPs and HIV-1 glycoprotein gp120. | [21] |
| Ag NPs | 2~15 nm | SARS-CoV-2 | Ag NPs damaged the surface proteins to affect the structural integrity of virions. | [64] |
| Ag NPs | 5 nm | SARS-CoV-2 | [65] | |
| Ag30-SiO2 NPs | ≈30 nm | Murine norovirus, Bacteriophage MS2 | Ag+ dissolved from NPs bound to the thiol group of viral proteins. *,a | [50] |
| Ag NPs | 27 ± 4 nm | HcoV-229E | [66] | |
| NiO NPs | 15~20 nm | Cucumber mosaic virus | NiO NPs activated the expression of defense-related genes in cells to resist CMV. | [22] |
| Photocatalytic NiO NPs induced the production of ROS to destroy the virus structure. * | ||||
| Ni/Fe NPs | 92.6 ± 3.5 nm | Bacteriophage f2 | Ni as a catalyst for inactivation. | [71] |
| Viruses were damaged by ROS which was generated during Fe oxidation. | ||||
| Au NPs | 19~110 nm | Vesicular stomatitis virus | Au NPs attached to VSV and prevented VSV binding to host cells. | [23] |
| Au NPs | ≈18.27 nm | Herpes simplex virus | Au NPs attached to the surface of HSV to eliminate the infectivity of the virus. | [73] |
| Au NPs entered the host cells and interfered with viral replication. | ||||
| Au NPs | 11 nm | Measles virus | High affinity between Au NPs and disulfide bonds prevented viral infection of host cells. | [76] |
| Au NPs | ≈150 nm | Influenza virus | The disulfide bonds were cleaved by Au NPs to block membrane fusion. | [77] |
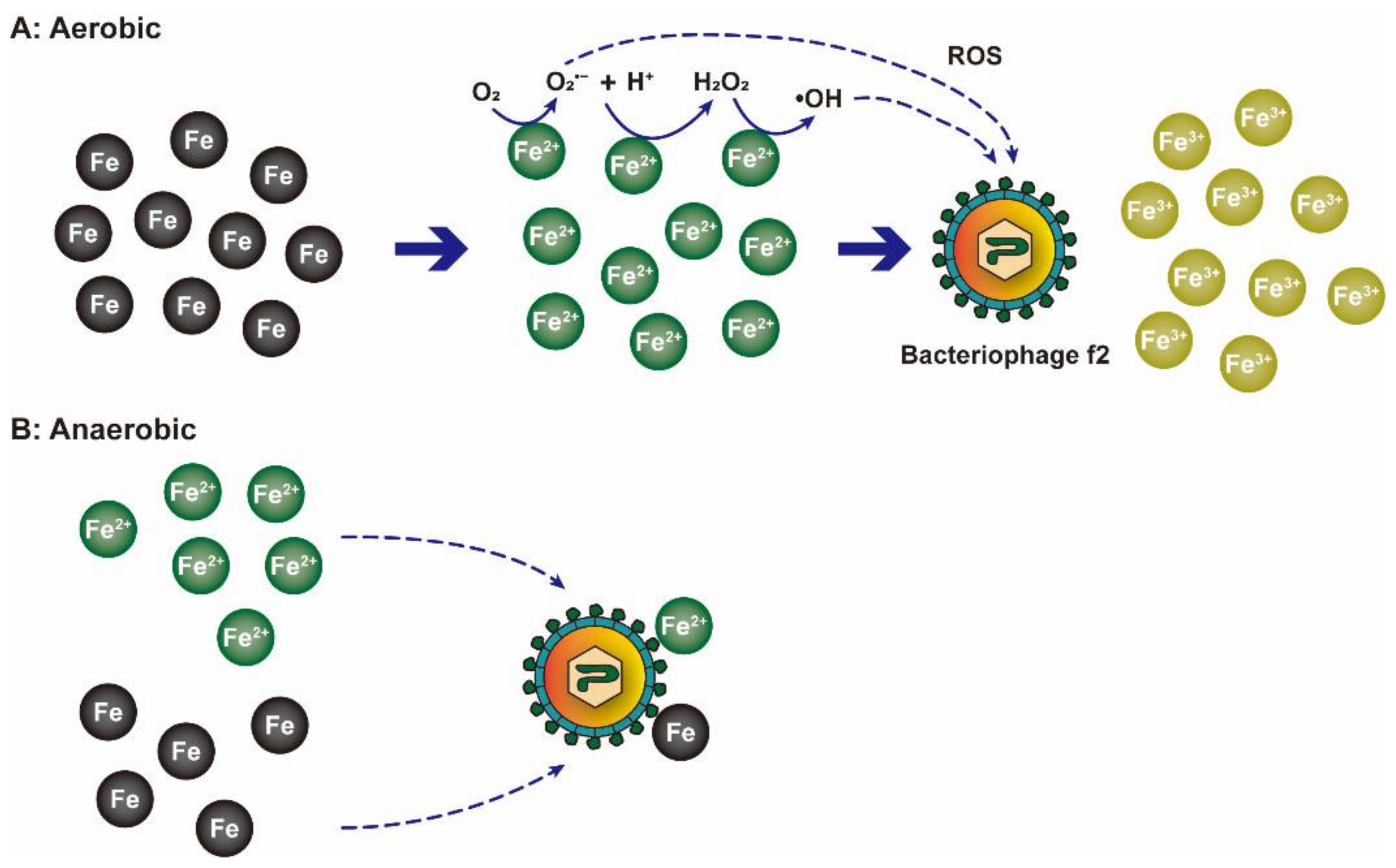
| NZVI | ≈200 nm | Bacteriophage MS2 | O2•− played the major role in phase I and ·OH played the major role in phase II. | [81] |
| NZVI | <100 nm | Bacteriophage f2 | [82] | |
| NZVI | ≈50 nm | Bacteriophage f2 | NZVI were oxidized to Fe3O4 or Fe2O3 which adsorbed viruses in the initial stage. | [24] |
| Fe2+ dissolved from NZVI generated ROS to inactivate viruses in the late stage. |
| Metal Ion | Source | Viruses | Mechanism | Reference |
|---|---|---|---|---|
| Cu2+ | CuCl2, CuSO4 | H9N2 virus | Cu2+ destroyed the structure of viruses. | [85] |
| Cu2+ | CuZeo | H5N1 virus, H5N3 virus | Cu2+ destroyed the structure of viruses. | [53] |
| Ag+ | AgNO3, Ag2O | Influenza A virus, bacteriophage Qβ | Ag+ broke disulfide and thiol bonds of viral proteins. | [88] |
| Ag+ | Silver electrode | Sacbrood virus | [90] | |
| Zn2+ | ZnCl2, ZnSO4 | Transmissible gastroenteritis virus | Zn2+ destroyed the RNA polymerase of viruses to shorten their life cycle. a,* | [95] |
| Zn2+ | Nitroporphyrin-zinc complexes | HIV-1 virus, SIVmac virus | [52] | |
| Ag+, Cu2+, Zn2+ | Hybrid coating | HIV-1 virus, H1N1 virus, Human herpesvirus, Dengue virus | Ag+ and Cu2+ ruptured the viral envelope or were bound to the thiol group of the viral proteins. | [97] |
| Metallic Material | Viruses | Mechanism | Reference |
|---|---|---|---|
| Copper | Adenovirus, Norovirus | [47] | |
| Copper | Influenza A Virus | Copper directly damaged the RNA of viruses to prevent viral replication. a,* | [51] |
| Copper, copper-nickel alloys, brass | Human coronavirus 229E | Cu2+ and Cu+ dissolved from copper alloy directly inactivated viruses. | [103] |
| O2•− generated on the surface of the alloy enhanced the inactivation effect. | |||
| Zero-valent iron | Aichi virus, Adenovirus 41, Bacteriophage ΦX174 and MS2 | [105] |
| Metallic Material | Viruses | Mechanism | Reference |
|---|---|---|---|
| Cu2O | Influenza A virus, Bacteriophage Qβ | Cu2O damaged the function of viral hemagglutinin and neuraminidase. | [88] |
| Cu2O, Cu2S, CuI | Bacteriophage Qβ | Cuprous compounds adsorbed viral proteins. | [111] |
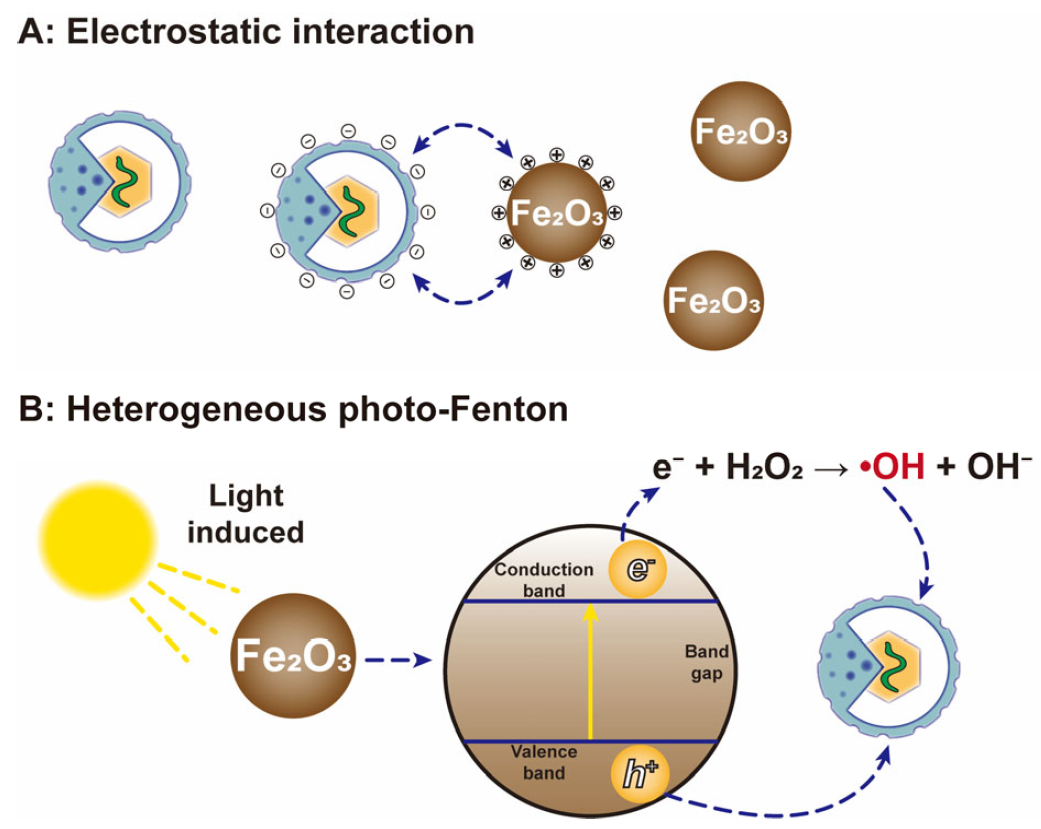
| Fe2O3 | Bacteriophage MS2 and ΦX174 | Fe2O3 adsorbed viruses. | [114] |
| Light induced Fe2O3 to inactivate viruses. | |||
| Fe2O3 | Bacteriophage P22 | Fe2O3 combined with viruses through electrostatic adsorption. | [115] |
| Fe2O3 | Bacteriophage MS2 | Fe2O3 directly bound to viruses through electrostatic interaction. | [116] |
| Dissolved iron generated ROS which destroyed viral capsid or envelope. | |||
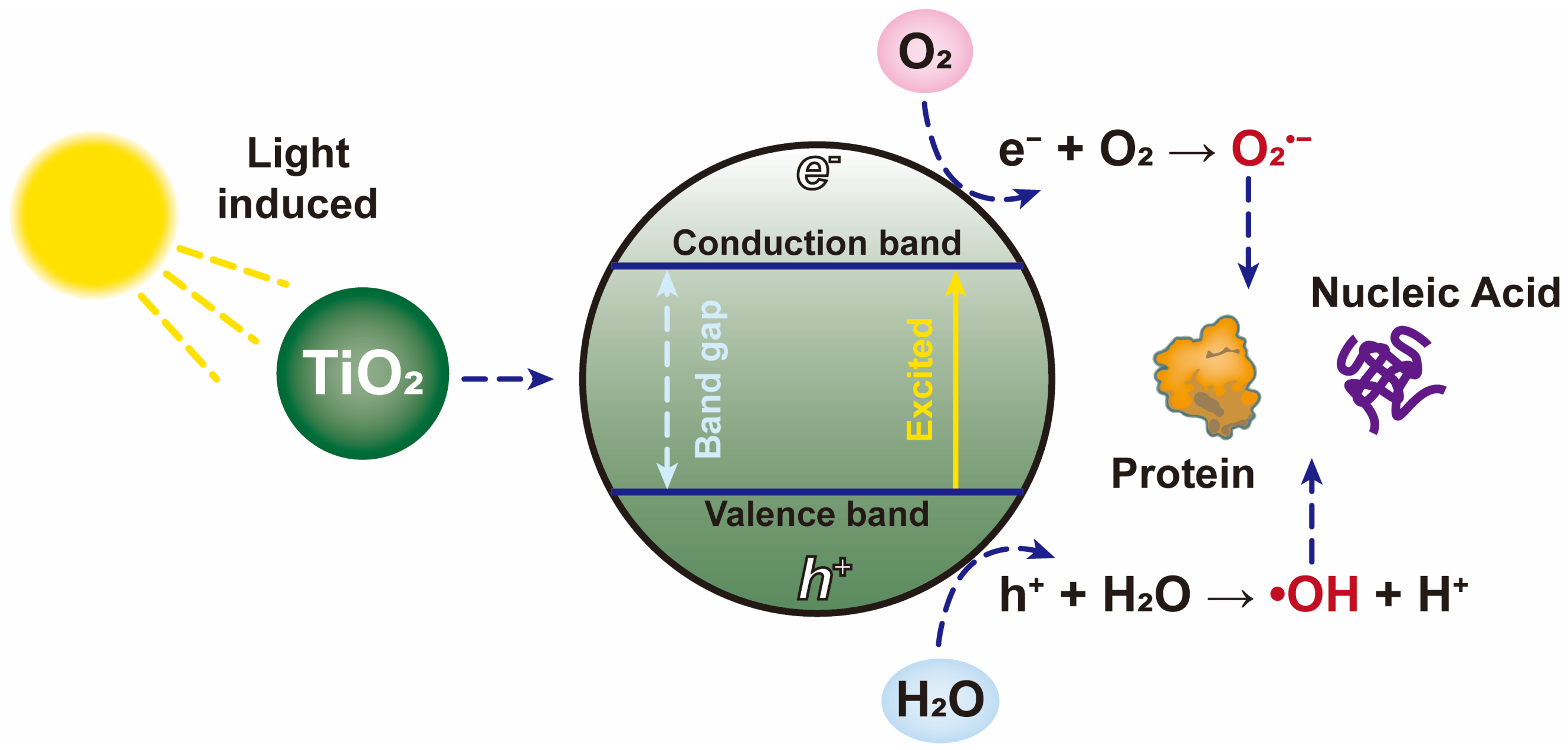
| Cu-TiO2 | Bacteriophage f2 | Light induced Cu-TiO2 to generate ROS. | [49] |
| HA/TiO2 | H1N1 virus | TiO2 was activated by UV lamps to produce ROS which destroyed the viral envelope, nucleic acids and proteins. | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, H.; He, B.; Yin, Y.; Liu, L.; Shi, J.; Hu, L.; Jiang, G. Chemical Nature of Metals and Metal-Based Materials in Inactivation of Viruses. Nanomaterials 2022, 12, 2345. https://doi.org/10.3390/nano12142345
Tian H, He B, Yin Y, Liu L, Shi J, Hu L, Jiang G. Chemical Nature of Metals and Metal-Based Materials in Inactivation of Viruses. Nanomaterials. 2022; 12(14):2345. https://doi.org/10.3390/nano12142345
Chicago/Turabian StyleTian, Haozhong, Bin He, Yongguang Yin, Lihong Liu, Jianbo Shi, Ligang Hu, and Guibin Jiang. 2022. "Chemical Nature of Metals and Metal-Based Materials in Inactivation of Viruses" Nanomaterials 12, no. 14: 2345. https://doi.org/10.3390/nano12142345
APA StyleTian, H., He, B., Yin, Y., Liu, L., Shi, J., Hu, L., & Jiang, G. (2022). Chemical Nature of Metals and Metal-Based Materials in Inactivation of Viruses. Nanomaterials, 12(14), 2345. https://doi.org/10.3390/nano12142345