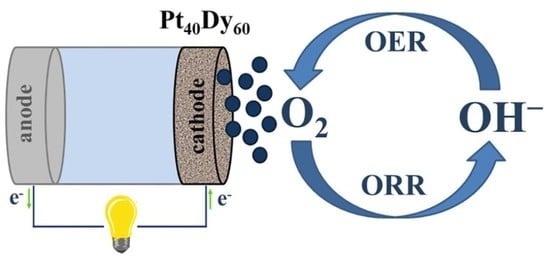
Platinum–Dysprosium Alloys as Oxygen Electrodes in Alkaline Media: An Experimental and Theoretical Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
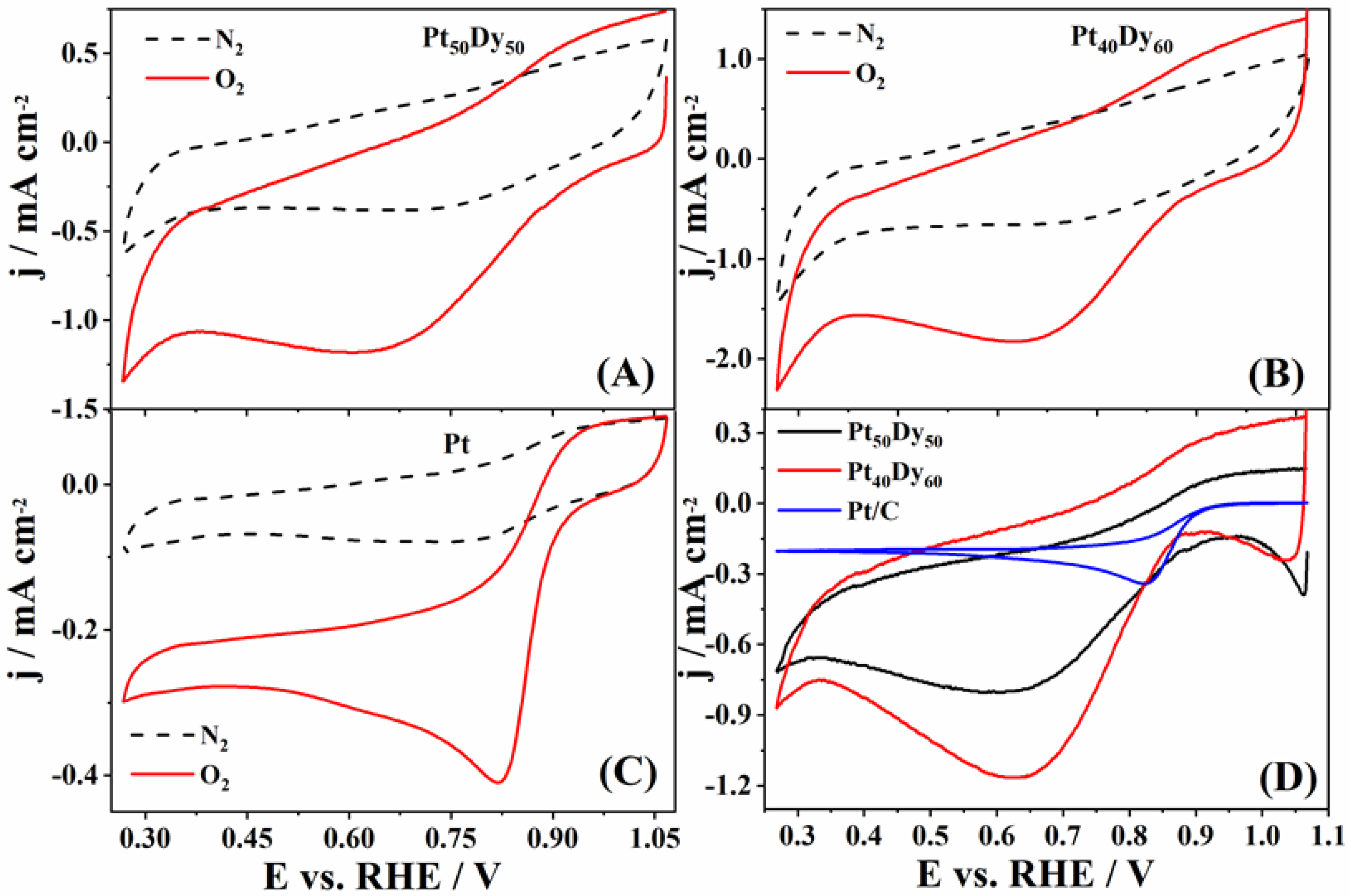
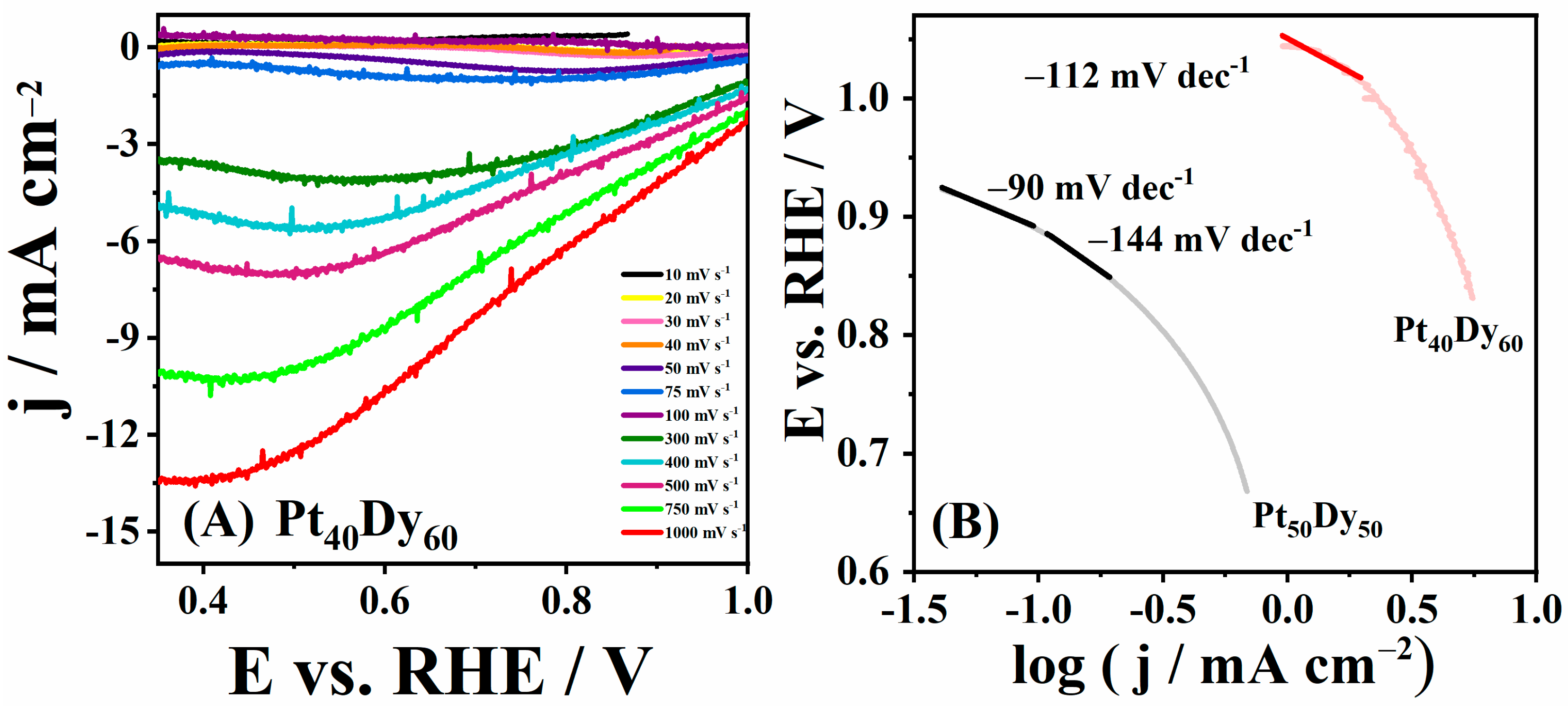
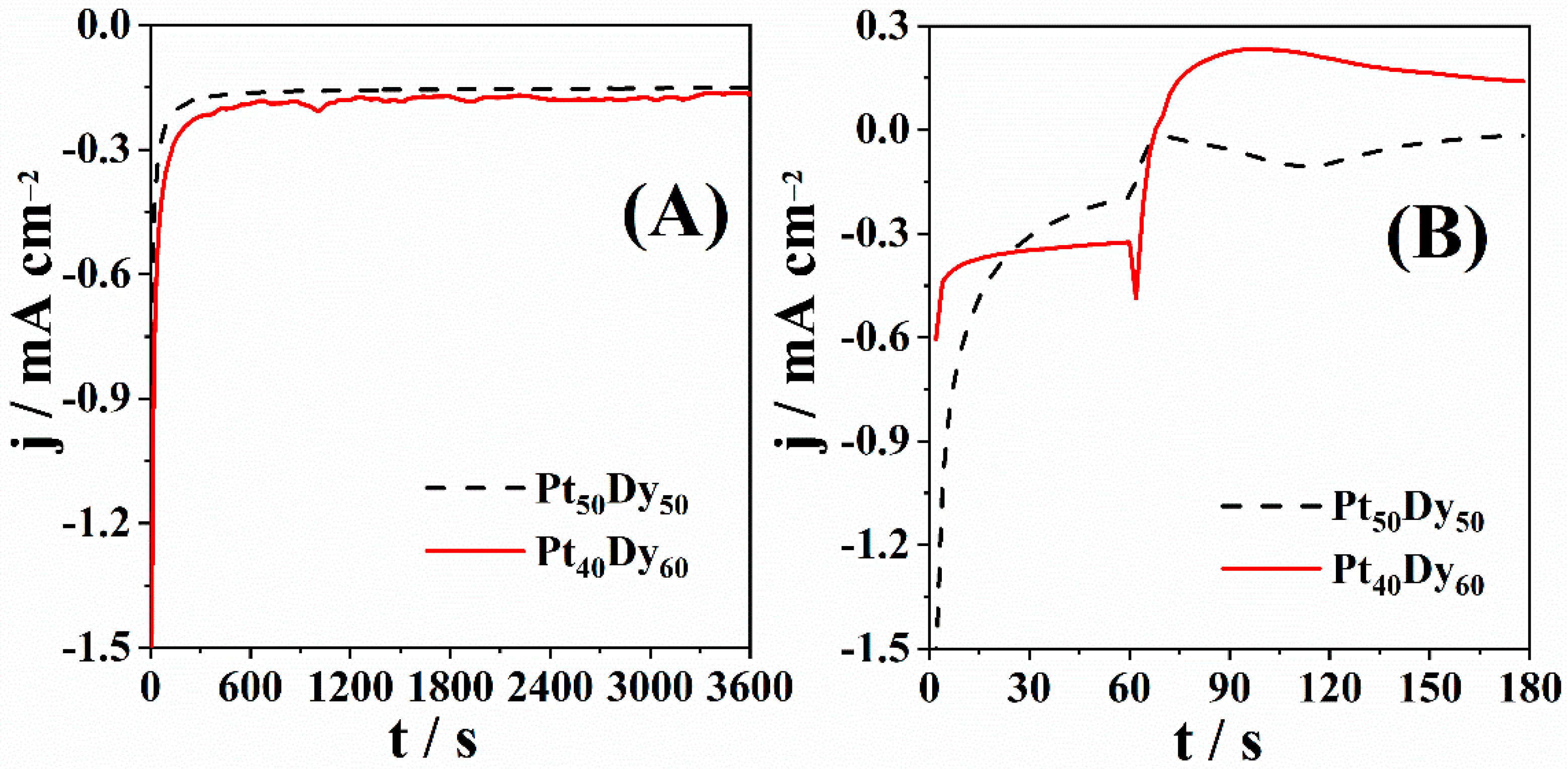
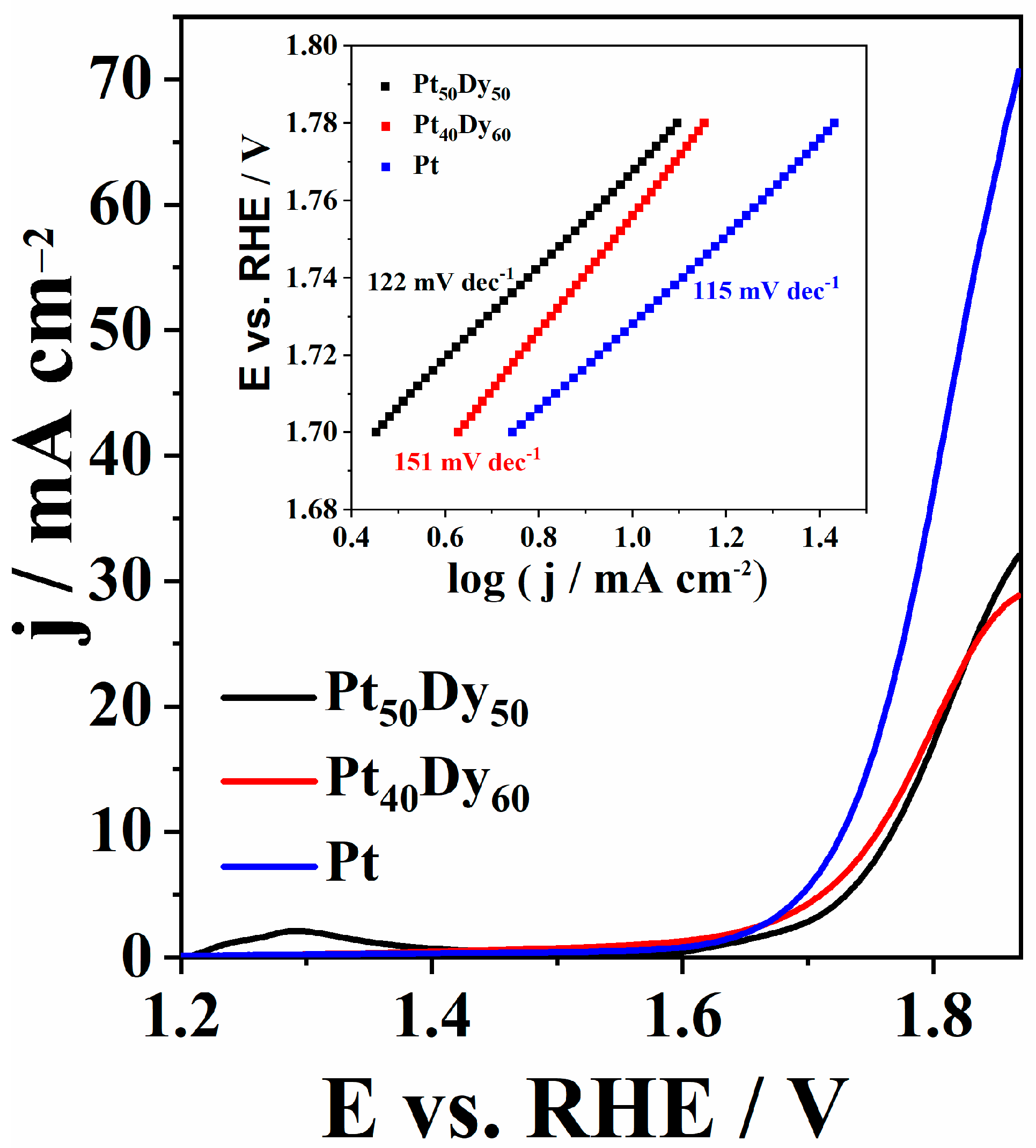
3.1. ORR Experimental Study
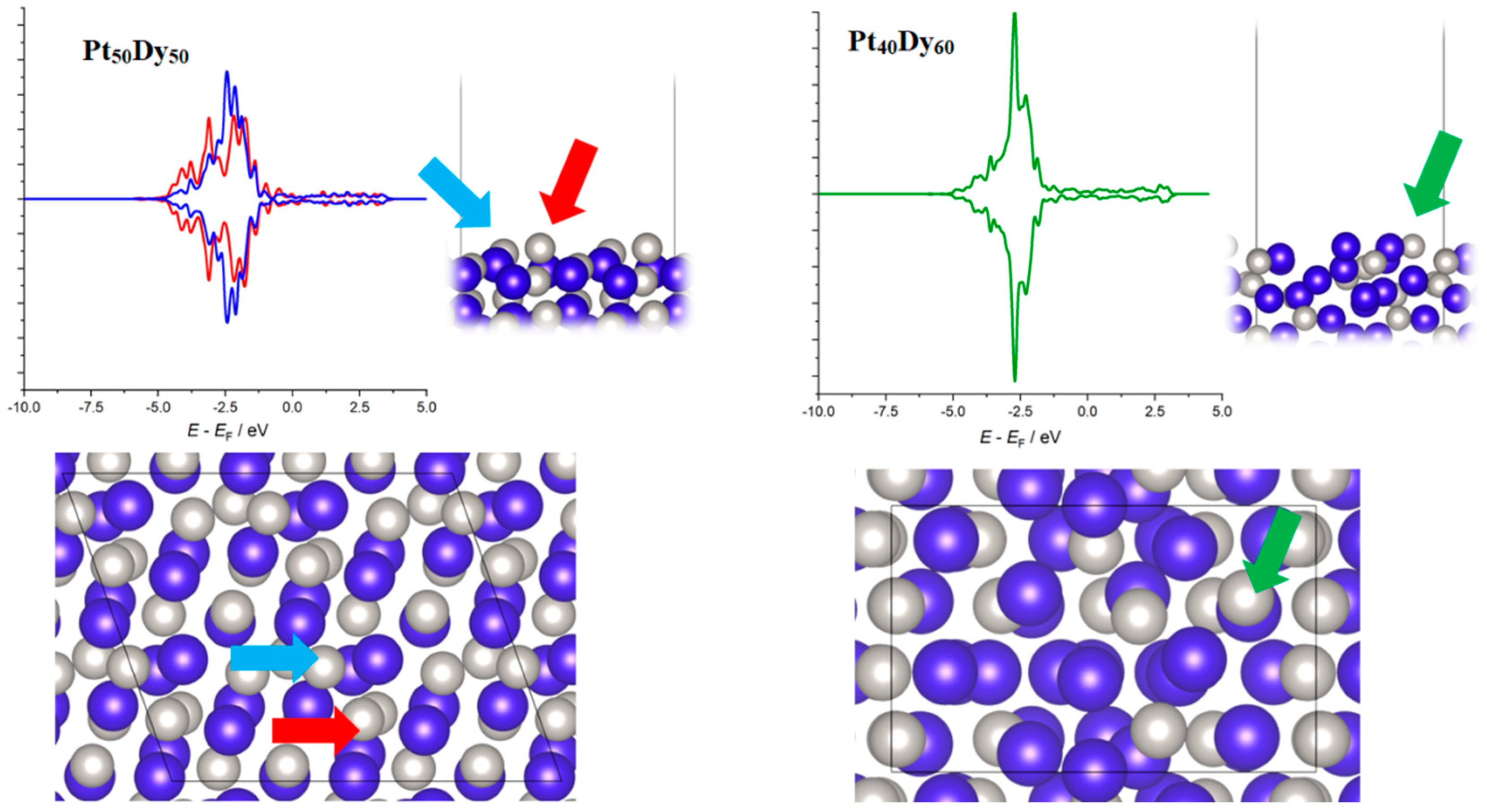
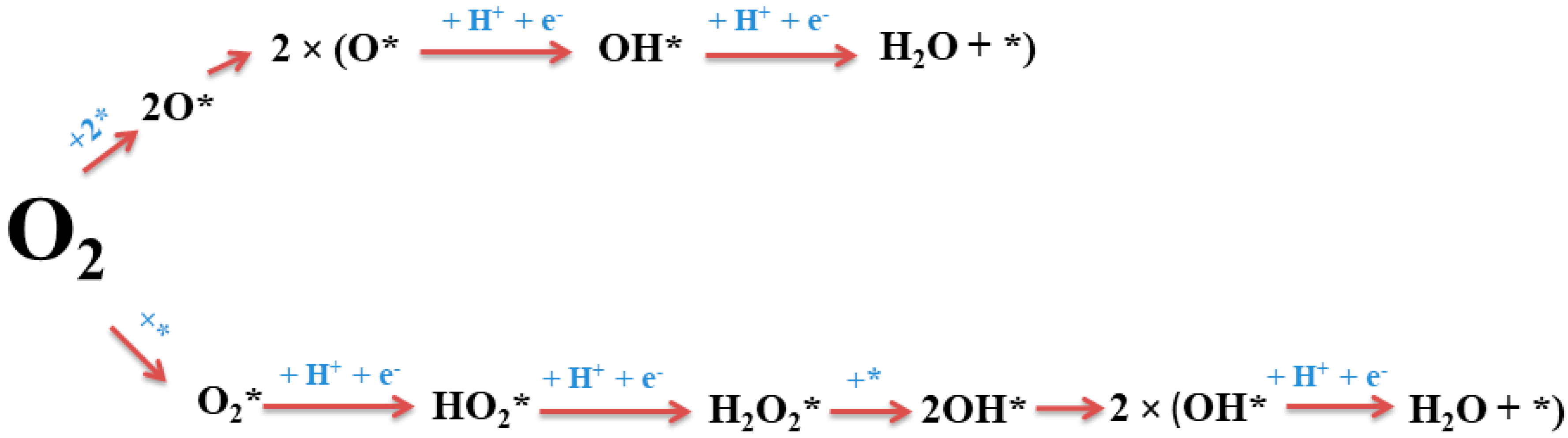
3.2. DFT Analysis of the Compositional Dependence of ORR Activity of Pt–Dy Alloys
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.; Jeong, B.; Ocon, J.D. Oxygen electrocatalysis in chemical energy conversion and storage technologies. Curr. Appl. Phys. 2013, 13, 309–321. [Google Scholar] [CrossRef]
- Yang, H.; Han, X.; Douka, A.I.; Huang, L.; Gong, L.; Xia, C.; Park, H.S.; Xia, B.Y. Advanced Oxygen Electrocatalysis in Energy Conversion and Storage. Adv. Funct. Mater. 2021, 31, 2007602. [Google Scholar] [CrossRef]
- Garche, J.; Dyer, C.; Moseley, P.; Ogumi, Z.; Rand, D.; Scrosati, B. (Eds.) Encyclopedia of Electrochemical Power Sources, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Šljukić, B.; Santos, D.M.F.; Sequeira, C.A.C. Manganese dioxide electrocatalysts for borohydride fuel cell cathodes? J. Electroanal. Chem. 2013, 694, 77–83. [Google Scholar] [CrossRef]
- Gavrilov, N.; Dašić-Tomić, M.; Pašti, I.; Ćirić-Marjanović, G.; Mentus, S. Carbonized polyaniline nanotubes/nanosheets-supported Pt nanoparticles: Synthesis, characterization and electrocatalysis. Mater. Lett. 2011, 65, 962–965. [Google Scholar] [CrossRef]
- Spendelow, J.S.; Wieckowski, A. Electrocatalysis of oxygen reduction and small alcohol oxidation in alkaline media. Phys. Chem. Chem. Phys. 2007, 9, 2654–2675. [Google Scholar] [CrossRef] [PubMed]
- Ferriday, T.B.; Middleton, P.H. Alkaline fuel cell technology—A review. Int. J. Hydrogen Energy 2021, 46, 18489–18510. [Google Scholar] [CrossRef]
- Markovic, N.M.; Ross, P.N., Jr. Surface science studies of model fuel cell electrocatalysts. Surf. Sci. Rep. 2002, 45, 117–229. [Google Scholar] [CrossRef]
- Lim, D.H.; Wilcox, J. Mechanisms of the oxygen reduction reaction on defective graphene-supported Pt nanoparticles from first-principles. J. Phys. Chem. C 2012, 116, 3653–3660. [Google Scholar] [CrossRef] [Green Version]
- Anderson, A.B. O2 reduction and CO oxidation at the Pt-electrolyte interface. The role of H2O and OH adsorption bond strengths. Electrochim. Acta 2002, 47, 3759–3763. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Mun, B.S.; Mayrhofer, K.J.J.; Ross, P.N.; Markovic, N.M.; Rossmeisl, J.; Greeley, J.; Nørskov, J.K. Changing the Activity of Electrocatalysts for Oxygen Reduction by Tuning the Surface Electronic Structure. Angew. Chem. 2006, 118, 2963–2967. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Qu, Y.; Yuan, T.; Wang, W.; Wu, Y.; Li, Y. Review of Metal Catalysts for Oxygen Reduction Reaction: From Nanoscale Engineering to Atomic Design. Chem 2019, 5, 1486–1511. [Google Scholar] [CrossRef]
- Jalan, V.M. Noble Metal/Vanadium Alloy Catalyst and Method for Making. U.S. Patent US4202934A, 13 May 1980. [Google Scholar]
- Lee, C.L.; Syu, C.M.; Huang, C.H.; Chiou, H.P.; Chao, Y.J.; Yang, C.C. Cornered silver and silverplatinum nanodisks: Preparation and promising activity for alkaline oxygen reduction catalysis. Appl. Catal. B Environ. 2013, 132–133, 229–236. [Google Scholar] [CrossRef]
- Altamirano-Gutierrez, A.; Fernandez, A.M.; Rodriguez Varela, F.J. Preparation and characterization of Pt-CeO2 and Pt-Pd electrocatalysts for the oxygen reduction reaction in the absence and presence of methanol in alkaline medium. Int. J. Hydrogen Energy 2013, 38, 12657–12666. [Google Scholar] [CrossRef]
- Mladenović, D.; Santos, D.M.F.; Bozkurt, G.; Soylu, G.S.P.; Yurtcan, A.B.; Miljanić, Š.; Šljukić, B. Electrochemistry Communications Tailoring metal-oxide-supported PtNi as bifunctional catalysts of superior activity and stability for unitised regenerative fuel cell applications. Electrochem. Commun. 2021, 124, 106963. [Google Scholar] [CrossRef]
- Lima, F.H.B.; Ticianelli, E.A. Oxygen electrocatalysis on ultra-thin porous coating rotating ring/disk platinum and platinum-cobalt electrodes in alkaline media. Electrochim. Acta 2004, 49, 4091–4099. [Google Scholar] [CrossRef]
- Arico, A.S.; Shukla, A.K.; Kim, H.; Park, S.; Min, M.; Antonucci, V. XPS study on oxidation states of Pt and its alloys with Co and Cr and its relevance to electroreduction of oxygen. Appl. Surf. Sci. 2001, 172, 33–40. [Google Scholar] [CrossRef]
- Toda, T.; Igarashi, H.; Watanabe, M. Enhancement of the electrocatalytic O2 reduction on Pt–Fe alloys. J. Electroanal. Chem. 1999, 460, 258–262. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Marković, N.M. Improved Oxygen Reduction Activity on Pt₃Ni(111) via Increased Surface Site Availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef] [Green Version]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef]
- dos Santos, R.B.; Rivelino, R.; Gueorguiev, G.K.; Kakanakova-Georgieva, A. Exploring 2D structures of indium oxide of different stoichiometry. CrystEngComm 2021, 23, 6661–6667. [Google Scholar] [CrossRef]
- Kakanakova-Georgieva, A.; Ivanov, I.G.; Suwannaharn, N.; Hsu, C.W.; Cora, I.; Pécz, B.; Giannazzo, F.; Sangiovanni, D.G.; Gueorguiev, G.K. MOCVD of AlN on epitaxial graphene at extreme temperatures. CrystEngComm 2021, 23, 385–390. [Google Scholar] [CrossRef]
- Pašti, I.A.; Gavrilov, N.M.; Baljozović, M.; Mitrić, M.; Mentus, S.V. Oxygen reduction reaction of Pt-In alloy: Combined theoretical and experimental investigations. Electrochim. Acta 2013, 114, 706–712. [Google Scholar] [CrossRef]
- Bezerra, L.S.; Maia, G. Developing efficient catalysts for the OER and ORR using a combination of Co, Ni, and Pt oxides along with graphene nanoribbons and NiCo2O4. J. Mater. Chem. A 2020, 8, 17691–17705. [Google Scholar] [CrossRef]
- Pierozynski, B.; Mikolajczyk, T.; Luba, M.; Zolfaghari, A. Kinetics of oxygen evolution reaction on nickel foam and platinum-modified nickel foam materials in alkaline solution. J. Electroanal. Chem. 2019, 847, 113194. [Google Scholar] [CrossRef]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and pt catalysts: A comparative study of nanoparticles and bulk materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Buy Gold, Silver and Platinum at These Live Prices. Available online: https://www.bullionvault.com/ (accessed on 15 April 2022).
- Santos, D.M.F.; Šljukić, B.; Sequeira, C.A.C.; Macciò, D.; Saccone, A.; Figueiredo, J.L. Electrocatalytic approach for the efficiency increase of electrolytic hydrogen production: Proof-of-concept using platinum—Dysprosium alloys. Energy 2013, 50, 486–492. [Google Scholar] [CrossRef]
- Macciò, D.; Rosalbino, F.; Saccone, A.; Delfino, S. Partial phase diagrams of the Dy-Pt and Ho-Pt systems and electrocatalytic behaviour of the DyPt and HoPt phases. J. Alloys Compd. 2005, 391, 60–66. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B-Condens. Matter Mater. Phys. 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillonin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Novčić, K.A.; Dobrota, A.S.; Petković, M.; Johansson, B.; Skorodumova, N.V.; Mentus, S.V.; Pašti, I.A. Theoretical analysis of doped graphene as cathode catalyst in Li-O2 and Na-O2 batteries—The impact of the computational scheme. Electrochim. Acta 2020, 354, 136735. [Google Scholar] [CrossRef]
- Ponra, A.; Etindele, A.J.; Motapon, O.; Casida, M.E. Practical treatment of singlet oxygen with density-functional theory and the multiplet-sum method. Theor. Chem. Acc. 2021, 140, 154. [Google Scholar] [CrossRef]
- Obradović, M.D.; Babić, B.M.; Krstajić, N.V.; Gojković, S.L. Uticaj prisustva volfram-karbida u ugljeničnom nosaču Pt nanočestica na elektrohemijsku redukciju kiseonika u kiselom rastvoru. Hem. Ind. 2013, 67, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Parthasarathy, A.; Srinivasan, S.; Appleby, A.J.; Martin, C.R. Electrode kinetics of oxygen reduction at carbon-supported and unsupported platinum microcrystallite/Nafion® interfaces. J. Electroanal. Chem. 1992, 339, 101–121. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Takeichi, M.; Itagaki, M.; Uchida, H.; Watanabe, M. Temperature-dependence of oxygen reduction activity at a platinum electrode in an acidic electrolyte solution investigated with a channel flow double electrode. J. Electroanal. Chem. 2005, 574, 339–346. [Google Scholar] [CrossRef]
- Anderson, A.B.; Albu, T.V. Catalytic Effect of Platinum on Oxygen Reduction. J. Electrochem. Soc. 2000, 147, 4229–4238. [Google Scholar] [CrossRef]
- Song, C.; Tang, Y.; Zhang, J.L.; Zhang, J.; Wang, H.; Shen, J.; McDermid, S.; Li, J.; Kozak, P. PEM fuel cell reaction kinetics in the temperature range of 23–120 °C. Electrochim. Acta 2007, 52, 2552–2561. [Google Scholar] [CrossRef]
- Šljukić, B.; Milikić, J.; Santos, D.M.F.; Sequeira, C.A.C.; Maccio, D.; Saccone, A. Electrocatalytic performance of Pt–Dy alloys for direct borohydride fuel cells. J. Power Sources 2014, 272, 335–343. [Google Scholar] [CrossRef]
- Hunter, M.A.; Fischer, J.M.T.A.; Yuan, Q.; Hankel, M.; Searles, D.J. Evaluating the Catalytic Efficiency of Paired, Single-Atom Catalysts for the Oxygen Reduction Reaction. ACS Catal. 2019, 9, 7660–7667. [Google Scholar] [CrossRef] [Green Version]
- Yeager, E. Electrocatalysts for O2 reduction. Electrochim. Acta 1984, 29, 1527–1537. [Google Scholar] [CrossRef]
- Marković, N.M.; Schmidt, T.J.; Stamenković, V.R.P.N. Oxygen Reduction Reaction on Pt and Pt Bimetallic Surfaces: A Selective Review Oxygen Reduction Reaction on Pt and Pt Bimetallic Surfaces: A Selective Review. Fuel Cells 2001, 1, 105–116. [Google Scholar] [CrossRef]
- Lamas, E.J.; Balbuena, P.B. Oxygen Reduction on Pd0.75Co0.25 (111) and Pt0.75Co0.25 (111) Surfaces: An ab Initio Comparative Study. J. Chem. Theory Comput. 2006, 2, 1388–1394. [Google Scholar] [CrossRef]
- Liu, J.; Jiao, M.; Lu, L.; Barkholtz, H.M.; Li, Y.; Jiang, L.; Wu, Z.; Liu, D.J.; Zhuang, L.; Ma, C.; et al. High performance platinum single atom electrocatalyst for oxygen reduction reaction. Nat. Commun. 2017, 8, 15938. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milikić, J.; Nikolić, N.; Santos, D.M.F.; Macciò, D.; Saccone, A.; Alsaiari, M.; Jalalah, M.; Faisal, M.; Harraz, F.A.; Li, Y.; et al. Platinum–Dysprosium Alloys as Oxygen Electrodes in Alkaline Media: An Experimental and Theoretical Study. Nanomaterials 2022, 12, 2318. https://doi.org/10.3390/nano12142318
Milikić J, Nikolić N, Santos DMF, Macciò D, Saccone A, Alsaiari M, Jalalah M, Faisal M, Harraz FA, Li Y, et al. Platinum–Dysprosium Alloys as Oxygen Electrodes in Alkaline Media: An Experimental and Theoretical Study. Nanomaterials. 2022; 12(14):2318. https://doi.org/10.3390/nano12142318
Chicago/Turabian StyleMilikić, Jadranka, Nikola Nikolić, Diogo M. F. Santos, Daniele Macciò, Adriana Saccone, Mabkhoot Alsaiari, Mohammed Jalalah, M. Faisal, Farid A. Harraz, Yizhao Li, and et al. 2022. "Platinum–Dysprosium Alloys as Oxygen Electrodes in Alkaline Media: An Experimental and Theoretical Study" Nanomaterials 12, no. 14: 2318. https://doi.org/10.3390/nano12142318
APA StyleMilikić, J., Nikolić, N., Santos, D. M. F., Macciò, D., Saccone, A., Alsaiari, M., Jalalah, M., Faisal, M., Harraz, F. A., Li, Y., Nassr, A. B., Pašti, I., & Šljukić, B. (2022). Platinum–Dysprosium Alloys as Oxygen Electrodes in Alkaline Media: An Experimental and Theoretical Study. Nanomaterials, 12(14), 2318. https://doi.org/10.3390/nano12142318