Crystallization of Nano-Sized Macromolecules by the Example of Hexakis-[4-{(N-Allylimino)methyl}phenoxy]cyclotriphosphazene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
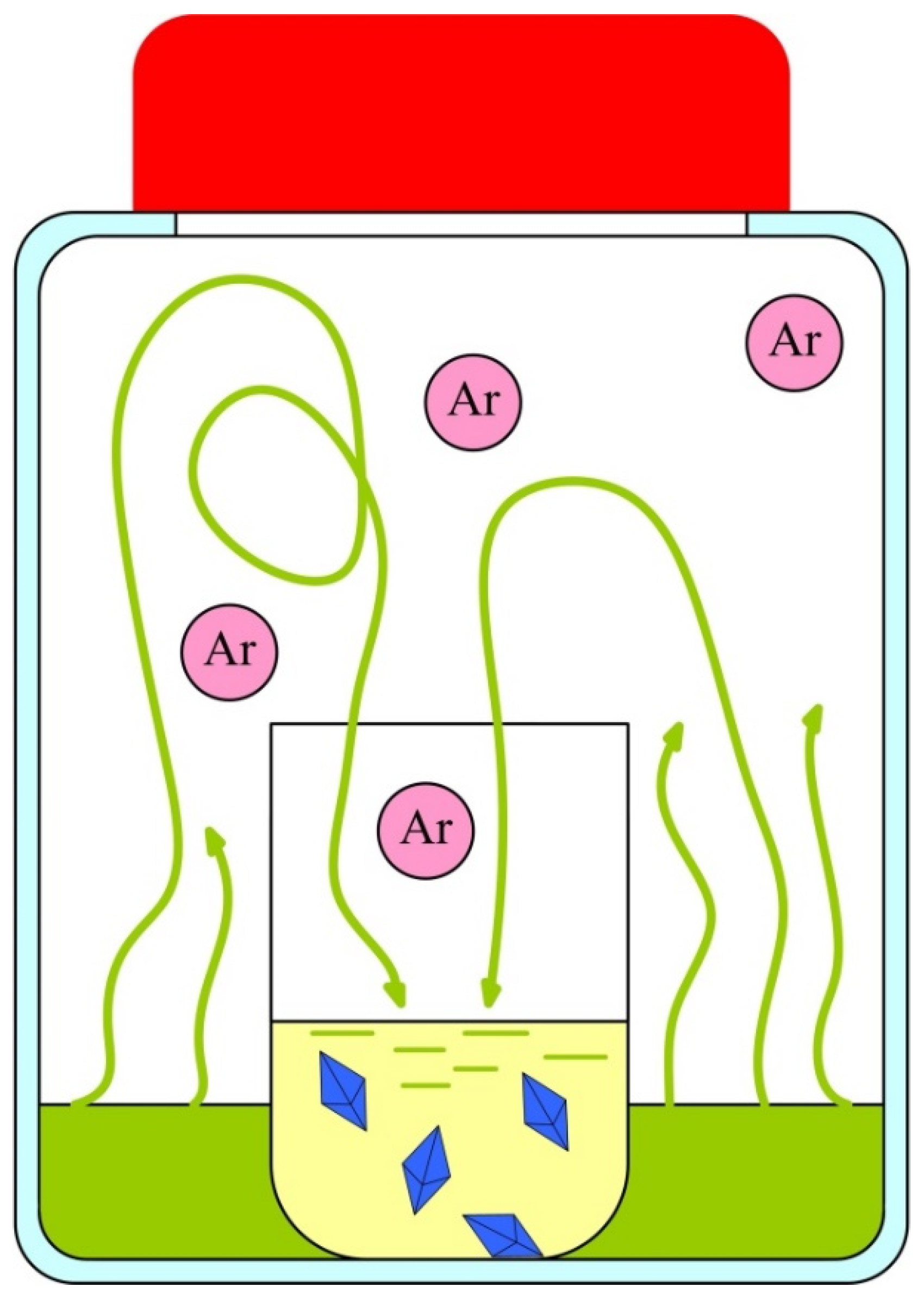
2.2. Methods
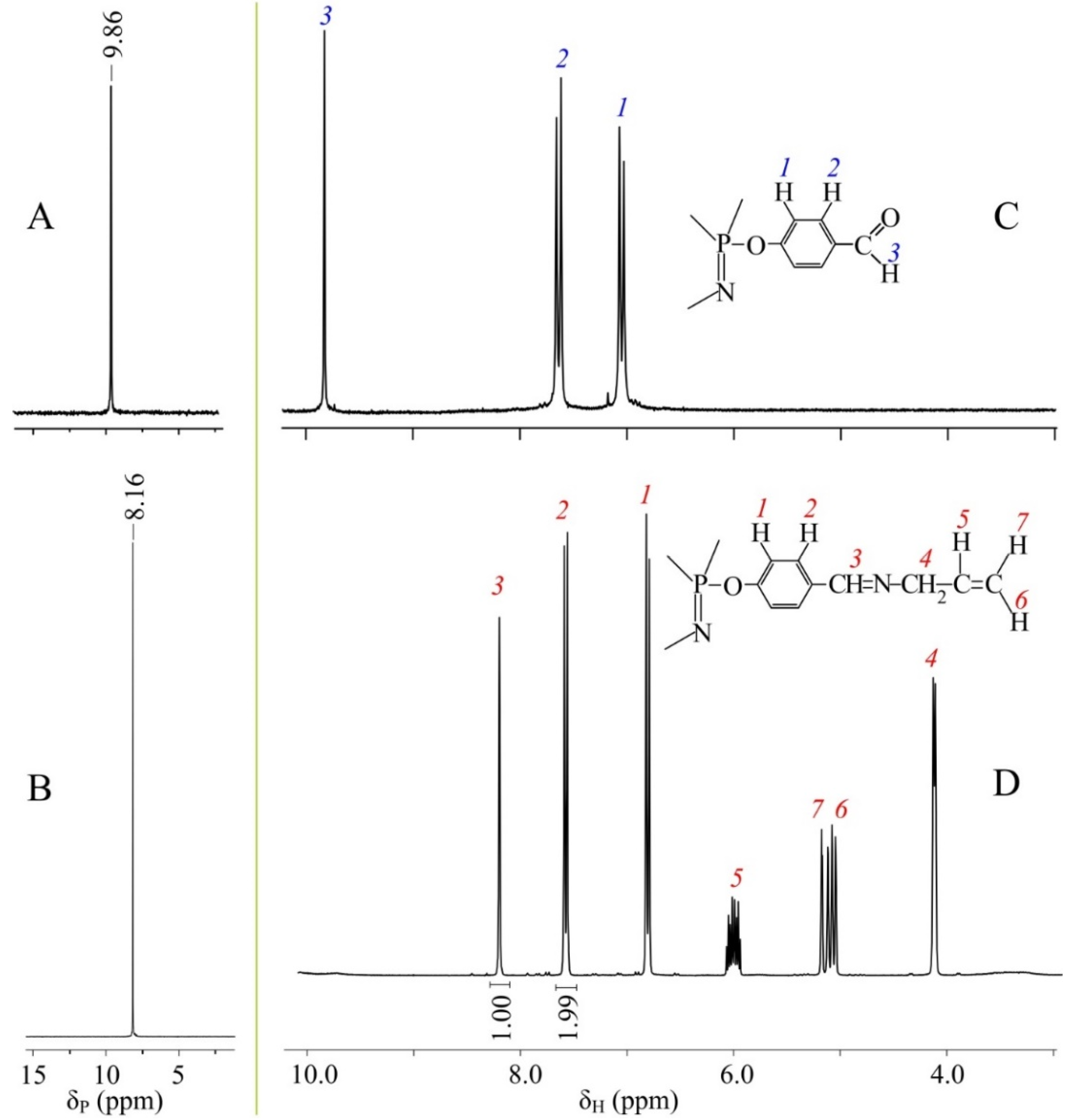
2.3. Synthesis of Hexakis-[(4-formyl)phenoxy]cyclotriphosphazene (FPP)
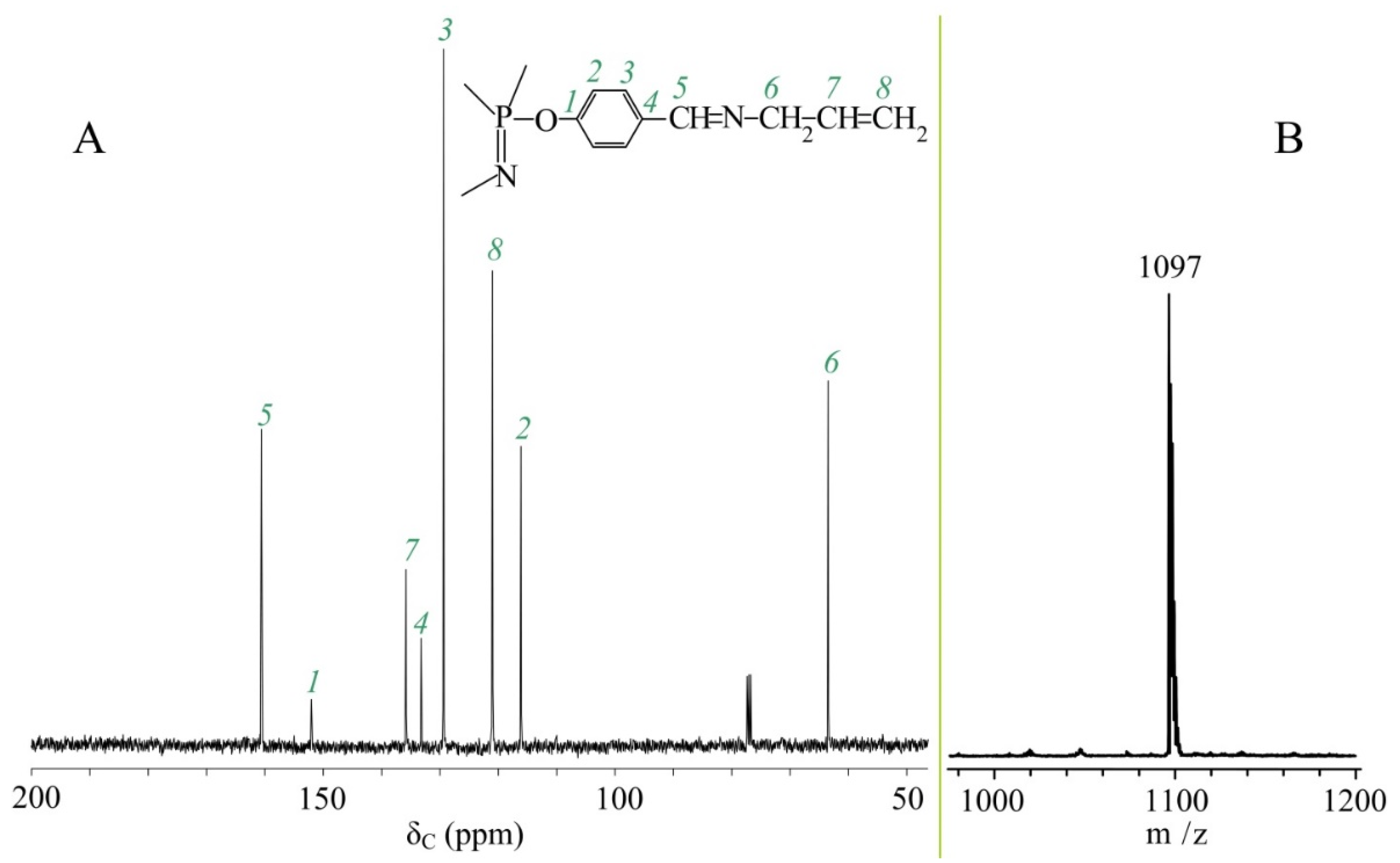
2.4. Synthesis of Hexakis-[4-{(N-allylimino)methyl}phenoxy]cyclotriphosphazene (APP)
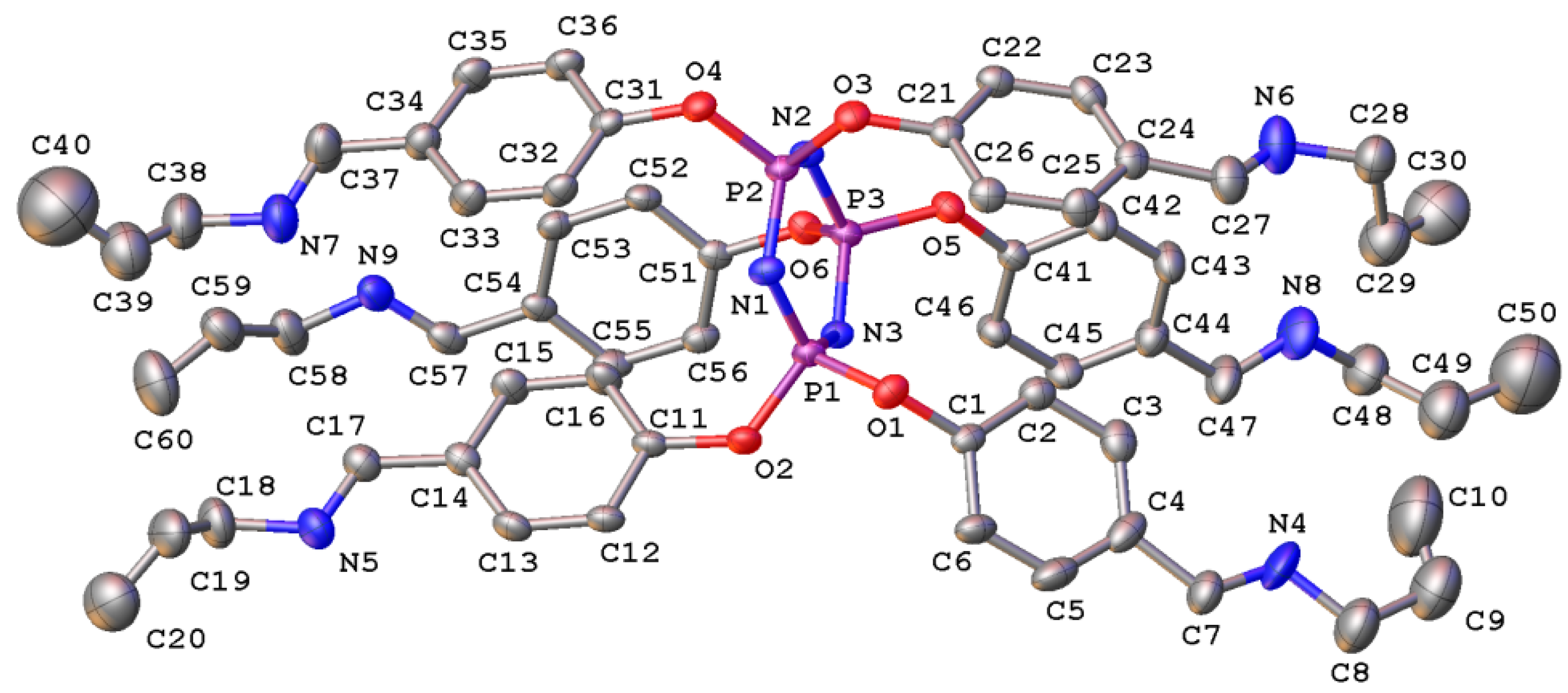
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whitty-Léveillé, L.; Tremblay-Cantin, J.C.; Picard-Lafond, A.; Boudreau, D.; Reynier, N.; Larivière, D. Core-shell nanoparticles bearing Schiff base ligand for the selective extraction of uranium from REE leach liquors. Hydrometallurgy 2022, 208, 105780. [Google Scholar] [CrossRef]
- Chang, I.J.; Choi, M.G.; Jeong, Y.A.; Lee, S.H.; Chang, S.K. Colorimetric determination of Cu2+ in simulated wastewater using naphthalimide-based Schiff base. Tetrahedron Lett. 2017, 58, 474–477. [Google Scholar] [CrossRef]
- Taniguchi, T.; Urayama, K. Linear dynamic viscoelasticity of dual cross-link poly (vinyl alcohol) hydrogel with determined borate ion concentration. Gels 2021, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ponce, L.; Galindo-Riaño, M.D.; Casanueva-Marenco, M.J.; Granado-Castro, M.D.; Díaz-de-Alba, M. Sensing Cd (II) Using a Disposable Optical Sensor Based on a Schiff Base Immobilisation on a Polymer-Inclusion Membrane. Applications in Water and Art Paint Samples. Polymers 2021, 13, 4414. [Google Scholar] [CrossRef]
- El-Hiti, G.A.; Ahmed, D.S.; Yousif, E.; Al-Khazrajy, O.S.; Abdallh, M.; Alanazi, S.A. Modifications of polymers through the addition of ultraviolet absorbers to reduce the aging effect of accelerated and natural irradiation. Polymers 2021, 14, 20. [Google Scholar] [CrossRef]
- Hashim, N.Z.N.; Kahar, M.A.M.; Kassim, K.; Embong, Z. Experimental and theoretical studies of azomethines derived from benzylamine as corrosion inhibitors of mild steel in 1 M HCl. J. Mol. Struct. 2020, 1222, 128899. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Rashwan, S.M.; Meleek, S.; Kamel, M.M. Synthesis, characterization and mitigation action of innovative Schiff base on steel disintegration in sulfuric acid solution. Mater. Chem. Phys. 2021, 267, 124697. [Google Scholar] [CrossRef]
- Abu-Yamin, A.A.; Jbarah, A.A.Q.M.; Al Khalyfeh, K.; Matar, S.A.; Alqasaimeh, M.; Rüffer, T.; Lang, H. Crystal structure, spectroscopic studies, DFT calculations, and biological activity of 5-bromosalicylaldehyde–based Schiff bases. J. Mol. Struct. 2022, 1262, 132976. [Google Scholar] [CrossRef]
- Restrepo-Acevedo, A.; Osorio, N.; Giraldo-López, L.E.; D’Vries, R.F.; Zacchino, S.; Abonia, R.; Le Lagadec, R.; Cuenú-Cabezas, F. Synthesis and antifungal activity of nitrophenyl-pyrazole substituted Schiff bases. J. Mol. Struct. 2022, 1253, 132289. [Google Scholar] [CrossRef]
- Ermiş, E.; Durmuş, K. Novel thiophene-benzothiazole derivative azomethine and amine compounds: Microwave assisted synthesis, spectroscopic characterization, solvent effects on UV–Vis absorption and DFT studies. J. Mol. Struct. 2020, 1217, 128354. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, Y.; Zhen, X.; Yang, E.; Zhao, Y. Synthesis and Characterization of Nitrogen Heterocyclic Derivatives Containing Sulfur–Ether and Schiff Base. Phosphorus Sulfur Silicon Relat. Elem. 2013, 188, 1564–1575. [Google Scholar] [CrossRef]
- Korkmaz, A.; Bursal, E. Benzothiazole sulfonate derivatives bearing azomethine: Synthesis, characterization, enzyme inhibition, and molecular docking study. J. Mol. Struct. 2022, 1257, 132641. [Google Scholar] [CrossRef]
- Vildanova, R.; Lobov, A.; Spirikhin, L.; Kolesov, S. Hydrogels on the Base of Modified Chitosan and Hyaluronic Acid Mix as Polymer Matrices for Cytostatics Delivery. Gels 2022, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Donia, A.M.; Atia, A.A.; Elwakeel, K.Z. Recovery of gold (III) and silver (I) on a chemically modified chitosan with magnetic properties. Hydrometallurgy 2007, 87, 197–206. [Google Scholar] [CrossRef]
- Pudovik, M.A.; Kibardina, L.; Trifonov, A.; Dobrynin, A.; Katsyuba, S.; Burilov, A. Phosphorylation of pyridoxal azomethines. Synthesis of phosphorus containing azomethines and furopyridines. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 120–126. [Google Scholar] [CrossRef]
- Sünbül, A.B.; Inan, A.; Köse, M.; Evren, E.; Gürbüz, N.; Ozdemir, İ.; Ikiz, M.; Dag, A.K.; Ispir, E. Azo-azomethine based palladium (II) complexes as catalysts for the Suzuki-Miyaura cross-coupling reaction. J. Mol. Struct. 2020, 1216, 128279. [Google Scholar] [CrossRef]
- Vibhute, S.P.; Mhaldar, P.M.; Shejwal, R.V.; Rashinkar, G.S.; Pore, D.M. Palladium Schiff base complex immobilized on magnetic nanoparticles: An efficient and recyclable catalyst for Mizoroki and Matsuda-Heck coupling. Tetrahedron Lett. 2020, 61, 151801. [Google Scholar] [CrossRef]
- Osypiuk, D.; Cristóvão, B.; Mazur, L. New heteronuclear complexes of PdII–LnIII–PdII with Schiff base ligand: Synthesis, crystal structures and chemical properties. J. Mol. Struct. 2022, 1261, 132924. [Google Scholar] [CrossRef]
- Dehghani-Firouzabadi, A.A.; Morovati, F.; Notash, B. Metal complexes with thioether containing unsymmetrical Ni2S donor Schiff base ligand: Crystal and molecular structure of nickel (II) complex. Phosphorus Sulfur Relat. Elem. 2020, 195, 644–650. [Google Scholar] [CrossRef]
- Burlov, A.S.; Lyssenko, K.A.; Koshchienko, Y.V.; Nikolaevskii, S.A.; Vasilchenko, I.S.; Garnovskii, D.A.; Uraev, A.I.; Garnovskii, A.D. Mixed-ligand azomethine–benzimidazole palladium complex. Mendeleev Commun. 2008, 4, 198–199. [Google Scholar] [CrossRef]
- Bazhin, D.N.; Kudyakova, Y.S.; Slepukhin, P.A.; Burgart, Y.V.; Malysheva, N.N.; Kozitsina, A.N.; Ivanova, A.V.; Bogomyakov, A.S.; Saloutin, V.I. Dinuclear copper (ii) complex with novel N, N’, N”, O-tetradentate Schiff base ligand containing trifluoromethylpyrazole and hydrazone moieties. Mendeleev Commun. 2018, 28, 202–204. [Google Scholar] [CrossRef]
- Uraev, A.I.; Popov, L.D.; Levchenkov, S.I.; Shcherbakov, I.N.; Suponitsky, K.Y.; Garnovskii, D.A.; Lukov, V.V.; Kogan, V.A. Crystal structure and magnetic properties of a tetranuclear carbonate-bridged CuII complex with a Schiff base compartmental ligand with the N2OS2 donor set. Mendeleev Commun. 2015, 25, 62–64. [Google Scholar] [CrossRef]
- Yek, S.M.G.; Nasrollahzadeh, M.; Azarifar, D.; Rostami-Vartooni, A.; Ghaemi, M.; Shokouhimehr, M. Grafting Schiff base Cu (II) complex on magnetic graphene oxide as an efficient recyclable catalyst for the synthesis of 4H-pyrano [2, 3-b] pyridine-3-carboxylate derivatives. Mater. Chem. Phys. 2022, 284, 126053. [Google Scholar]
- Gurina, G.A.; Kissel, A.A.; Ob’edkov, A.M.; Cherkasov, A.V.; Trifonov, A.A. Alkyl scandium complexes coordinated by dianionic O, N, N-and O, N, O-ligands derived from Schiff bases. Mendeleev Commun. 2021, 31, 631–634. [Google Scholar] [CrossRef]
- Borisova, N.E.; Ustynyuk, Y.A.; Reshetova, M.D.; Aleksandrov, G.G.; Eremenko, I.L.; Moiseev, I.I. New polydentate Schiff bases and their cobalt complexes. Mendeleev Commun. 2003, 13, 202–204. [Google Scholar] [CrossRef]
- Krylova, I.V.; Saverina, E.A.; Rynin, S.S.; Lalov, A.V.; Minyaev, M.E.; Nikolaevskaya, E.N.; Syroeshkin, M.A.; Egorov, M.P. Synthesis, characterization and redox properties of Ar–C= N→ Ge← N= C–Ar containing system. Mendeleev Commun. 2020, 30, 563–566. [Google Scholar] [CrossRef]
- Aljohani, F.S.; Abu-Dief, A.M.; El-Khatib, R.M.; Al-Abdulkarim, H.A.; Alharbi, A.; Mahran, A.; Khalifa, M.E.; El-Metwaly, N.M. Structural inspection for novel Pd (II), VO (II), Zn (II) and Cr (III)-azomethine metal chelates: DNA interaction, biological screening and theoretical treatments. J. Mol. Struct. 2021, 1246, 131139. [Google Scholar] [CrossRef]
- Arafath, M.A.; Adam, F.; Hassan, M.Z. Synthesis, characterization, X-ray crystal structure and antibacterial activity of nickel, palladium and platinum complexes with Schiff base derived from N-cyclohexylhydrazinecarbothioamide and 5-(tert-butyl)-2-hydroxybenzaldehyde. Phosphorus Sulfur Silicon Relat. Elem. 2021, 196, 530–537. [Google Scholar] [CrossRef]
- Garnovskii, A.D.; Ponomarenko, A.G.; Burlov, A.S.; Bicherov, A.V.; Konoplev, B.G.; Ageev, O.A.; Kolomiitsev, A.S.; Chetverikova, V.A.; Borodkina, I.G.; Chigarenko, G.G.; et al. Tribologically active azomethine metal complexes. Russ. J. Gen. Chem. 2010, 80, 982–986. [Google Scholar] [CrossRef]
- Milutka, M.S.; Burlov, A.S.; Vlasenko, V.G.; Koshchienko, Y.V.; Makarova, N.I.; Metelitsa, A.V.; Korshunova, E.V.; Trigub, A.L.; Zubenko, A.A.; Klimenko, A.I. Synthesis, Structure, Spectral-Luminescent Properties, and Biological Activity of Chlorine-Substituted Azomethines and Their Zinc (II) Complexes. Russ. J. Gen. Chem. 2021, 91, 1706–1716. [Google Scholar] [CrossRef]
- Alamro, F.S.; Ahmed, H.A.; Bedowr, N.S.; Khushaim, M.S.; El-Atawy, M.A. New Advanced Liquid Crystalline Materials Bearing Bis-Azomethine as Central Spacer. Polymers 2022, 14, 1256. [Google Scholar] [CrossRef] [PubMed]
- Cozan, V.; Ardeleanu, R.; Airinei, A.; Timpu, D. Crystalline smectic E phase revisited in case of symmetrical dibenzo-18-crown-6-ether azomethine dimers. J. Mol. Struct. 2018, 1156, 22–29. [Google Scholar] [CrossRef]
- Shienok, A.I.; Krutius, O.N.; Mardaleishvili, I.R.; Kol’tsova, L.S.; Popov, L.D.; Shepelenko, E.N.; Zaichenko, N.L. Synthesis and Spectral-Luminescent Properties of Fluorescent Dyads Based on Tetraarylimidazole and Azomethines of Various Nature. Russ. J. Gen. Chem. 2021, 91, 2101–2109. [Google Scholar] [CrossRef]
- Moussallem, C.; Gohier, F.; Frère, P. Extended benzodifuran–thiophene systems connected with azomethine junctions: Synthesis and electronic properties. Tetrahedron Lett. 2015, 56, 5116–5119. [Google Scholar] [CrossRef]
- Sęk, D.; Szlapa-Kula, A.; Siwy, M.; Fabiańczyk, A.; Janeczek, H.; Szalkowski, M.; Mackowski, S.; Schab-Balcerzak, E. Branched azomethines based on tris (2-aminoethyl) amine: Impact of imine core functionalization on thermal, electrochemical and luminescence properties. Mater. Chem. Phys. 2020, 240, 122246. [Google Scholar] [CrossRef]
- Alam, M.S.; Lee, D.U. Molecular structure, spectral (FT-IR, FT-Raman, Uv-Vis, and fluorescent) properties and quantum chemical analyses of azomethine derivative of 4-aminoantipyrine. J. Mol. Struct. 2020, 1227, 129512. [Google Scholar] [CrossRef]
- Gondia, N.K.; Sharma, S.K. Comparative optical studies of naphthalenebased Schiff base complexes for colour tunable application. Mater. Chem. Phys. 2019, 224, 314–319. [Google Scholar] [CrossRef]
- Otaibi, A.A.A.; Alsukaibi, A.K.D.; Rahman, M.A.; Mushtaque, M.; Haque, A. From waste to Schiff base: Upcycling of aminolysed poly (ethylene terephthalate) product. Polymers 2022, 14, 1861. [Google Scholar] [CrossRef]
- Chelike, D.K.; Alagumalai, A.; Acharya, J.; Kumar, P.; Sarkar, K.; Thangavelu, S.A.G.; Chandrasekhar, V. Functionalized iron oxide nanoparticles conjugate of multi-anchored Schiff’s base inorganic heterocyclic pendant groups: Cytotoxicity studies. Appl. Surf. Sci. 2020, 501, 143963. [Google Scholar] [CrossRef]
- DoĞan, S.; TÜmay, S.O.; Balci, C.M.; YeŞİlot, S.; BeŞlİ, S. Synthesis of new cyclotriphosphazene derivatives bearing Schiff bases and their thermal and absorbance properties. Turk. J. Chem. 2020, 44, 31. [Google Scholar] [CrossRef]
- Doğan, S.; Balcı, C.M.; Şenocak, A.; Beşli, S. Cu (II) complexes of cyclotriphosphazene bearing Schiff bases: Synthesis, structural characterization, DFT calculations, absorbance and thermal properties. Polyhedron 2020, 183, 114541. [Google Scholar] [CrossRef]
- İbişoğlu, H.; Ün, Ş.Ş.; Erdemir, E.; Tümay, S.O. Synthesis, characterization, and photophysical properties of cyclotriphosphazenes containing quinoline-4-aldehyde-p-oxyanil moieties. Phosphorus Sulfur Silicon Relat. Elem. 2021, 196, 760–768. [Google Scholar] [CrossRef]
- Aslan, F.; Öztürk, A.İ.; Söylemez, B. Synthesis of fluorescence organocyclotriphosphazene derivatives having functional groups such as formyl, Schiff base and both formyl and Schiff base without using Ar or N2 atmosphere. J. Mol. Struct. 2017, 1137, 387–395. [Google Scholar] [CrossRef]
- Aslan, F.; Demirpence, Z.; Tatsiz, R.; Turkmen, H.; Ozturk, A.I.; Arslan, M. The synthesis, characterization and photophysical properties of some new cyclotriphosphazene derivatives bearing Schiff base. Z. Anorg. Allg. Chem. 2008, 634, 1140–1144. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Chistyakov, E.M.; Kireev, V.V.; Filatov, S.N.; Terekhov, I.V.; Buzin, M.I.; Komarova, L.I. Thermal polycondensation of hexa-p-hydroxymethylphenoxycyclotriphosphazene. Polym. Sci. Ser. B 2012, 54, 407–412. [Google Scholar] [CrossRef]
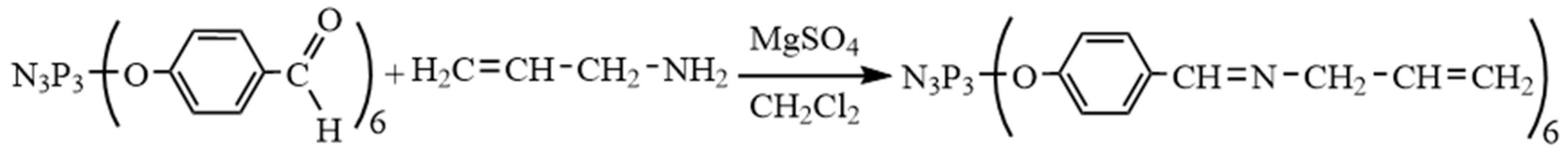

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chistyakov, E.; Yudaev, P.; Nelyubina, Y. Crystallization of Nano-Sized Macromolecules by the Example of Hexakis-[4-{(N-Allylimino)methyl}phenoxy]cyclotriphosphazene. Nanomaterials 2022, 12, 2268. https://doi.org/10.3390/nano12132268
Chistyakov E, Yudaev P, Nelyubina Y. Crystallization of Nano-Sized Macromolecules by the Example of Hexakis-[4-{(N-Allylimino)methyl}phenoxy]cyclotriphosphazene. Nanomaterials. 2022; 12(13):2268. https://doi.org/10.3390/nano12132268
Chicago/Turabian StyleChistyakov, Evgeniy, Pavel Yudaev, and Yulia Nelyubina. 2022. "Crystallization of Nano-Sized Macromolecules by the Example of Hexakis-[4-{(N-Allylimino)methyl}phenoxy]cyclotriphosphazene" Nanomaterials 12, no. 13: 2268. https://doi.org/10.3390/nano12132268
APA StyleChistyakov, E., Yudaev, P., & Nelyubina, Y. (2022). Crystallization of Nano-Sized Macromolecules by the Example of Hexakis-[4-{(N-Allylimino)methyl}phenoxy]cyclotriphosphazene. Nanomaterials, 12(13), 2268. https://doi.org/10.3390/nano12132268






