Self-Assembled Nanoscale Materials for
Abstract
:1. Introduction
2. Biomolecule Delivery in Neuroregeneration Strategies
2.1. Neuroprotective Biomolecules and Nucleic Acids under Current Investigation
2.1.1. Neurotrophic Factor Protein-Based Therapies
2.1.2. siRNA-Based Therapy
2.2. Therapeutic Delivery Approaches for Neuroprotective Biomacromolecules
2.2.1. Invasive versus Noninvasive Administration of Carrier-Free Biomolecules
2.2.2. Gene Delivery
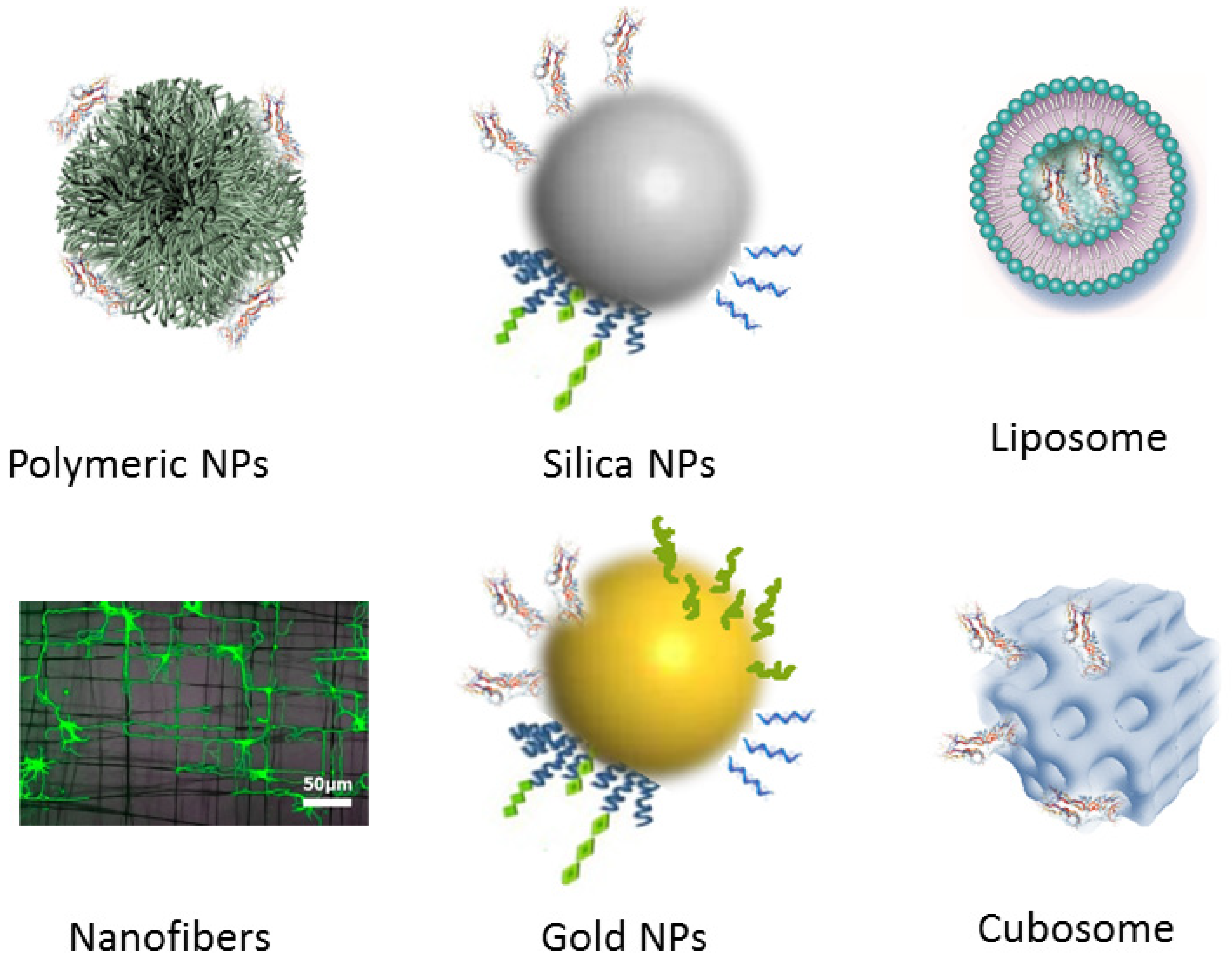
2.2.3. Carrier-Mediated Delivery Employing Different Nanoscale Materials
3. Nanoscale Materials for Stimulation of Neurogenesis and Neuroregeneration
3.1. Functionalized Nanoparticles for Brain-Targeted Drug Delivery
3.2. Neuron-Targeted Biomolecule Delivery by Nanocarriers
3.2.1. Nanoparticles for Protein Delivery
3.2.2. Nanoparticles for Gene Delivery
3.3. Nanomaterials Promote Neuroregeneration by Targeting the Extracellular Environment
3.4. Multifunctional Nanomaterials Promoting Neuroregeneration
4. BDNF Delivery by Nanocarriers and Nanoscale Materials in Neuronal Diseases
4.1. BDNF Protein Delivery by Nanocarriers to Neurons
4.2. Nanoparticles Modified by BDNF-Derived Peptides for Drug Delivery to Neurons
4.3. BDNF Gene Delivery by Nanocarriers
4.4. BDNF Delivery by Hybrid Systems and Scaffolds for Tissue Engineering
5. Nanoscale Assemblies of Bioactive Lipids Offering Therapeutic Opportunities
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Karlawish, J.; Jack, C.R., Jr.; Rocca, W.A.; Snyder, H.M.; Carrillo, M.C. Alzheimer’s Disease: The next frontier—Special report. Alzheimer Dement. 2017, 13, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimer Dement. 2016, 12, 459–509. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weiutraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 2017, 13, 217–231. [Google Scholar] [CrossRef] [Green Version]
- Titova, N.; Padmakumar, C.; Lewis, S.J.G.; Chaudhuri, K.R. Parkinson’s: A syndrome rather than a disease? J. Neural Transm. 2017, 124, 907–914. [Google Scholar] [CrossRef] [Green Version]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Jha, N.K.; Ojha, S.; Jha, S.K.; Dureja, H.; Singh, S.K.; Shukla, S.D.; Chellappan, D.K.; Gupta, G.; Bhardwaj, S.; Kumar, N.; et al. Evidence of coronavirus (CoV) pathogenesis and emerging pathogen SARS-CoV-2 in the nervous system: A review on neurological impairments and manifestations. J. Mol. Neurosci. 2021, 71, 2192–2209. [Google Scholar] [CrossRef]
- Liu, J.M.; Tan, B.H.; Wu, S.; Gui, Y.; Suo, J.L.; Li, Y.C. Evidence of central nervous system infection and neuroinvasive routes, as well as neurological involvement, in the lethality of SARS-CoV-2 infection. J. Med. Virol. 2020, 93, 1304–1313. [Google Scholar] [CrossRef]
- Tancheva, L.; Petralia, M.C.; Miteva, S.; Dragomanova, S.; Solak, A.; Kalfin, R.; Lazarova, M.; Yarkov, D.; Ciurleo, R.; Cavalli, E.; et al. Emerging neurological and psychobiological aspects of COVID-19 infection. Brain Sci. 2020, 10, 852. [Google Scholar] [CrossRef]
- Nuzzo, D.; Cambula, G.; Bacile, I.; Rizzo, M.; Galia, M.; Mangiapane, P.; Scalisi, L. Long-term brain disorders in post Covid-19 neurological syndrome (PCNS) patient. Brain Sci. 2021, 11, 454. [Google Scholar] [CrossRef]
- Jakhmola, S.; Indari, O.; Chatterjee, S.; Jha, H.C. SARS-CoV-2, an underestimated pathogen of the nervous system. SN Compr. Clin. Med. 2020, 2, 2137–2146. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Gong, E.; Zhang, B.; Zheng, J.; Gao, Z.; Zhong, Y.; Zou, W.; Zhan, J.; Wang, S.; Xie, Z.; et al. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005, 202, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Al Saiegh, F.; Ghosh, R.; Leibold, A.; Avery, M.B.; Schmidt, R.F.; Theofanis, T.; Miuchtouris, N.; Philipp, L.; Peiper, S.C.; Wang, Z.-X. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J. Neurol. Neurosurg. Psychiatry 2020, 91, 846–848. [Google Scholar] [CrossRef]
- Zubair, A.S.; Mcalpine, L.S.; Gardin, T.; Farhadian, S.; Kuruvilla, D.E.; Spudich, S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: A review. JAMA Neurol. 2020, 77, 1018–1027. [Google Scholar] [CrossRef]
- Nuzzo, D.; Picone, P. Potential neurological effects of severe COVID-19 infection. Neurosci. Res. 2020, 158, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Anrather, J.; Kamel, H. Effects of COVID-19 on the nervous system. Cell 2020, 183, 16–27. [Google Scholar] [CrossRef]
- Pacheco-Herrero, M.; Soto-Rojas, L.O.; Harrington, C.R.; Flores-Martinez, Y.M.; Villegas-Rojas, M.M.; León-Aguilar, A.M.; Martínez-Gómez, P.A.; Campa-Córdoba, B.B.; Apátiga-Pérez, R.; Corniel-Taveras, C.N.; et al. Elucidating the neuropathologic mechanisms of SARS-CoV-2 infection. Front. Neurol. 2021, 12, 660087. [Google Scholar] [CrossRef]
- Dziedzic, A.; Saluk-Bijak, J.; Miller, E.; Niemcewicz, M.; Bijak, M. The impact of SARS-CoV-2 infection on the development of neurodegeneration in multiple sclerosis. Int. J. Mol. Sci. 2021, 22, 1804. [Google Scholar] [CrossRef]
- Chaudhry, Z.L.; Klenja, D.; Janjua, N.; Cami-Kobeci, G.; Ahmed, B.Y. COVID-19 and Parkinson’s disease: Shared inflammatory pathways under oxidative stress. Brain Sci. 2020, 10, 807. [Google Scholar] [CrossRef]
- Chaná-Cuevas, P.; Salles-Gándara, P.; Rojas-Fernandez, A.; Salinas-Rebolledo, C.; Milán-Solé, A. The potential role of SARS-COV-2 in the pathogenesis of Parkinson’s disease. Front. Neurol. 2020, 11, 1044. [Google Scholar] [CrossRef]
- Abate, G.; Memo, M.; Uberti, D. Impact of COVID-19 on Alzheimer’s disease risk: Viewpoint for research action. Healthcare 2020, 8, 30286. [Google Scholar] [CrossRef] [PubMed]
- Varahachalam, S.P.; Lahooti, B.; Chamaneh, M.; Bagchi, S.; Chhibber, T.; Morris, K.; Bolanos, J.F.; Kim, N.Y.; Kaushik, A. Nanomedicine for the SARS-CoV-2: State-of-the-art and future prospects. Int. J. Nanomed. 2021, 16, 539–560. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Mostafavi, E.; Vincent, S.; Negash, H.; Andavar, R.; Perumal, V.; Barabadi, H. Nanotechnology-based approaches for emerging and re-emerging viruses: Special emphasis on COVID-19. Microb. Pathog. 2021, 156, 104908. [Google Scholar] [CrossRef]
- Cummings, J.L.; Morstorf, T.; Zhong, K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimer Res. Ther. 2014, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Maiti, P.; Manna, J.; Dunbar, G.L. Current understanding of the molecular mechanisms in Parkinson’s disease: Targets for potential treatments. Transl. Neurodegener. 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capriotti, T.; Terzakis, K. Parkinson disease. Home Healthc. Now 2016, 34, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Asil, S.M.; Ahlawat, J.; Barroso, G.G.; Narayan, M. Nanomaterial based drug delivery systems for the treatment of neurodegenerative diseases. Biomater. Sci. 2020, 8, 4109–4128. [Google Scholar] [CrossRef]
- Donaghue, I.E.; Tam, R.; Sefton, M.V.; Shoichet, M.S. Cell and biomolecule delivery for tissue repair and regeneration in the central nervous system. J. Control. Release 2014, 190, 219–227. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B. Dual and multi-drug delivery nanoparticles towards neuronal survival and synaptic repair. Neural Regen. Res. 2017, 12, 886–889. [Google Scholar] [CrossRef]
- Wong, K.H.; Riaz, M.K.; Xie, Y.; Zhang, X.; Liu, Q.; Chen, H.; Bian, Z.; Chen, X.; Lu, A.; Yang, Z. Review of current strategies for delivering Alzheimer’s disease drugs across the blood-brain barrier. Int. J. Mol. Sci. 2019, 20, 381. [Google Scholar] [CrossRef] [Green Version]
- Géral, C.; Angelova, A.; Lesieur, S. From molecular to nanotechnology strategies for delivery of neurotrophins: Emphasis on brain-derived neurotrophic factor (BDNF). Pharmaceutics 2013, 5, 127–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelova, A.; Angelov, B.; Drechsler, M.; Lesieur, S. Neurotrophin delivery using nanotechnology. Drug Discov. Today 2013, 18, 1263–1271. [Google Scholar] [CrossRef]
- Huttunen, H.J.; Saarma, M. CDNF protein therapy in Parkinson’s disease. Cell Transpl. 2019, 28, 349–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbiah, R.; Guldberg, R.E. Materials science and design principles of growth factor delivery systems in tissue engineering and regenerative medicine. Adv. Healthc. Mater. 2019, 8, e1801000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferenz, K.B.; Gast, R.E.; Rose, K.; Finger, I.E.; Hasche, A.; Krieglstein, J. Nerve growth factor and brain-derived neurotrophic factor but not granulocyte colony-stimulating factor, nimodipine and dizocilpine, require ATP for neuroprotective activity after oxygen–glucose deprivation of primary neurons. Brain Res. 2012, 1448, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Levi-Montalcini, R. Growth control of nerve cells by a protein factor and its antiserum: Discovery of this factor may provide new leads to understanding of some neurogenetic processes. Science 1964, 143, 105–110. [Google Scholar] [CrossRef]
- Ivanova, L.; Karelson, M.; Dobchev, D.A. Identification of natural compounds against neurodegenerative diseases using in silico techniques. Molecules 2018, 23, 1847. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Hudalla, G.A. Using self-assembling peptides to integrate biomolecules into functional supramolecular biomaterials. Molecules 2019, 24, 1450. [Google Scholar] [CrossRef] [Green Version]
- Prakash, A.; Medhi, B.; Chopra, K. Granulocyte colony stimulating factor (GCSF) improves memory and neurobehavior in an amyloid-β induced experimental model of Alzheimer’s disease. Pharm. Biochem Behav. 2013, 110, 46–57. [Google Scholar] [CrossRef]
- Lucini, C.; D’Angelo, L.; Cacialli, P.; Palladino, A.; De Girolamo, P. BDNF, brain, and regeneration: Insights from zebrafish. Int. J. Mol. Sci. 2018, 19, 3155. [Google Scholar] [CrossRef] [Green Version]
- Numakawa, T.; Odaka, H.; Adachi, N. Actions of brain-derived neurotrophin factor in the neurogenesis and neuronal function, and its involvement in the pathophysiology of brain diseases. Int. J. Mol. Sci. 2018, 19, 3650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Runeberg-Roos, P.; Piccinini, E.; Penttinen, A.M.; Mätlik, K.; Heikkinen, H.; Kuure, S.; Bespalov, M.M.; Peränen, J.; Garea-Rodríguez, E.; Fuchs, E.; et al. Developing therapeutically more efficient neurturin variants for treatment of Parkinson’s disease. Neurobiol. Dis. 2016, 96, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, J.L.; Murray, S.S.; Xiao, J. Brain-derived neurotrophin factor in central nervous system nyelination: A New mechanism to promote myelin plasticity and repair. Int. J. Mol. Sci. 2018, 19, 4131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, A.M.; Toulouse, A. Neurotrophic factors for the treatment of Parkinson’s disease. Cytokine Growth Factor Rev. 2011, 22, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aron, L.; Klein, R. Repairing the parkinsonian brain with neurotrophic factors. Trends Neurosci. 2011, 34, 88–100. [Google Scholar] [CrossRef]
- Paul, G.; Zachrisson, O.; Varrone, A.; Almqvist, P.; Jerling, M.; Lind, G.; Rehncrona, S.; Linderoth, B.; Bjartmarz, H.; Shafer, L.L.; et al. Safety and tolerability of intracerebroventricular PDGF-BB in Parkinson’s disease patients. J. Clin. Investig. 2015, 125, 1339–1346. [Google Scholar] [CrossRef] [Green Version]
- Dharmadana, D.; Adamcik, J.; Ryan, T.M.; Appiah Danso, S.; Chong, C.J.H.; Conn, C.E.; Reynolds, N.P.; Mezzenga, R.; Valéry, C. Peptide substance P self-assembles into semi-flexible nanotubes that can be manipulated for nanotechnology. Nanoscale 2020, 12, 22680–22687. [Google Scholar] [CrossRef]
- Schäbitz, W.R.; Krüger, C.; Pitzer, C.; Weber, D.; Laage, R.; Gassler, N.; Aronowski, J.; Mier, W.; Kirsch, F.; Dittgen, T.; et al. A neuroprotective function for the hematopoietic protein granulocyte-macrophage colony stimulating factor (GM-CSF). J. Cereb. Blood Flow Metab. 2008, 28, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, V.E.; Locatelli, V.; Rizzi, L. Neurotrophic and neuroregenerative effects of GH/IGF1. Int. J. Mol. Sci. 2017, 18, 2441. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, K.; Das, G.; Gupta, V.; Mondal, P.; Barman, S.; Khan, J.; Ghosh, S. Discovery of neuroregenerative peptoid from amphibian neuropeptide that inhibits amyloid-β toxicity and crosses blood-brain barrier. ACS Chem. Neurosci. 2019, 10, 1355–1368. [Google Scholar] [CrossRef]
- Chermenina, M.; Schouten, P.; Nevalainen, N.; Johansson, F.; Orädd, G.; Strömberg, I. GDNF is important for striatal organization and maintenance of dopamine neurons grown in the presence of the striatum. Neuroscience 2014, 270, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Grondin, R.; Littrell, O.M.; Zhang, Z.; Ai, Y.; Huettl, P.; Pomerleau, F.; Quintero, J.E.; Andersen, A.H.; Stenslik, M.J.; Bradley, L.H.; et al. GDNF revisited: A novel mammalian cell-derived variant form of GDNF increases dopamine turnover and improves brain biodistribution. Neuropharmacology 2019, 147, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, C.F.; Andressoo, J.O. Biology of GDNF and its receptors—Relevance for disorders of the central nervous system. Neurobiol. Dis. 2017, 97, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.C.; O’Bryan, L.M.; Mitchell, P.J.; Leung, D.; Ghanem, M.; Wilson, J.M.; Hanson, J.C.; Sossick, S.; Cooper, J.; Huang, L.; et al. Increased brain bio-distribution and chemical stability and decreased immunogenicity of an engineered variant of GDNF. Exp. Neurol. 2015, 267, 165–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, K.J.; Tsai, Y.C.; Shen, C.K. G-CSF rescues the memory impairment of animal models of Alzheimer’s disease. J. Exp. Med. 2007, 204, 1273–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Ramos, J.; Song, S.; Sava, V.; Catlow, B.; Lin, X.; Mori, T.; Cao, C.; Arendash, G.W. Granulocyte colony stimulating factor decreases brain amyloid burden and reverses cognitive impairment in Alzheimer’s mice. Neuroscience 2009, 163, 55–72. [Google Scholar] [CrossRef] [Green Version]
- Schneider, A.; Krüger, C.; Steigleder, T.; Weber, D.; Pitzer, C.; Laage, R.; Aronowski, J.; Maurer, M.H.; Gassler, N.; Mier, W.; et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J. Clin. Investig. 2005, 115, 2083–2098. [Google Scholar] [CrossRef] [Green Version]
- Nutt, J.G.; Burchiel, K.J.; Comella, C.L.; Jankovic, J.; Lang, A.E.; Laws, E.R.; Lozano, A.M.; Penn, R.D.; Simpson, R.K.; Stacy, M.; et al. Implanted intracerebroventricular. Glial cell line-derived neurotrophic factor. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 2003, 60, 69–73. [Google Scholar] [CrossRef]
- Gill, S.S.; Patel, N.K.; Hotton, G.R.; O’Sullivan, K.; McCarter, R.; Bunnage, M.; Brooks, D.J.; Svendsen, C.N.; Heywood, P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 2003, 9, 589–595. [Google Scholar] [CrossRef]
- Whone, A.; Luz, M.; Boca, M.; Woolley, M.; Mooney, L.; Dharia, S.; Broadfoot, J.; Cronin, D.; Schroers, C.; Barua, N.U.; et al. Randomized trial of intermittent intraputamenal glial cell linederived neurotrophic factor in Parkinson’s disease. Brain 2019, 142, 512–525. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishna, S.; Muddashetty, R.S. Emerging role of microRNAs in dementia. J. Mol. Biol. 2019, 431, 1743–1762. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ge, X.; Chen, Y.; Lv, N.; Liu, Z.; Yuan, W. Advances with RNA interference in Alzheimer’s disease research. Drug Des. Devel. Ther. 2013, 7, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, V.M.; Gouvion, C.M.; Davidson, B.L.; Paulson, H.L. Targeting Alzheimer’s disease genes with RNA interference: An efficient strategy for silencing mutant alleles. Nucleic Acids Res. 2004, 32, 661–668. [Google Scholar] [CrossRef] [Green Version]
- Hegarty, S.V.; Lee, D.J.; O’Keeffe, G.W.; Sullivan, A.M. Effects of intracerebral neurotrophic factor application on motor symptoms in Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism Relat. Disord. 2017, 38, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Yan, Z.; He, R.; Pang, Z.; Guo, L.; Qian, Y.; Jiang, X.; Fang, L. Brain targeting and toxicity study of odorranalectin-conjugated nanoparticles following intranasal administration. Drug Deliv. 2011, 18, 555–561. [Google Scholar] [CrossRef]
- Li, X.; Su, J.; Kamal, Z.; Guo, P.; Wu, X.; Lu, L.; Wu, H.; Qiu, M. Odorranalectin modified PEG-PLGA/PEG-PBLG curcumin-loaded nanoparticle for intranasal administration. Drug Dev. Ind. Pharm. 2020, 46, 899–909. [Google Scholar] [CrossRef]
- Ahirrao, M.; Shrotriya, S. In vitro and in vivo evaluation of cubosomal in situ nasal gel containing resveratrol for brain targeting. Drug Dev. Ind. Pharm. 2017, 43, 1686–1693. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; Zhang, Q.; Yan, X.; Guo, L.; Gao, X.; Qiu, M.; Jiang, X.; Lai, R.; Chen, H. A novel small Odorranalectin-bearing cubosomes: Preparation, brain delivery and pharmacodynamic study on amyloid-β₂₅₋₃₅-treated rats following intranasal administration. Eur. J. Pharm. Biopharm. 2012, 80, 368–378. [Google Scholar] [CrossRef]
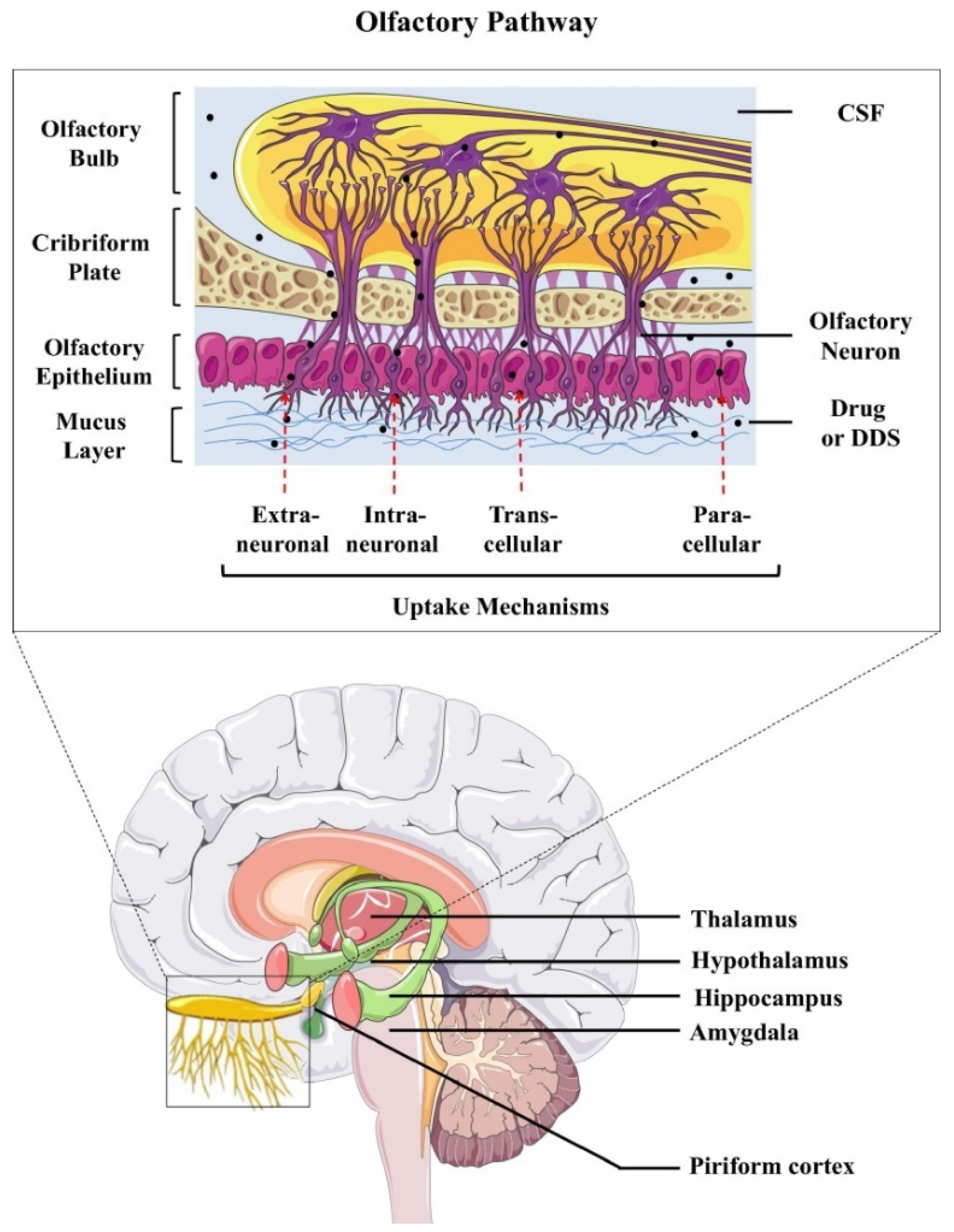
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Samaridou, E.; Alonso, M.J. Nose-to-brain peptide delivery—The potential of nanotechnology. Bioorg. Med. Chem. 2018, 26, 2888–2905. [Google Scholar] [CrossRef]
- Sajja, R.K.; Cudic, P.; Cucullo, L. In vitro characterization of odorranalectin for peptide-based drug delivery across the blood-brain barrier. BMC Neurosci. 2019, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, M.; Zhang, J.; Maincent, P.; Xia, X.; Wu, W. Updated progress of nanocarrier-based intranasal drug delivery systems for treatment of brain diseases. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 433–467. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; Wolak, D.J.; Pizzo, M.E.; Thorne, R.G. Rapid transport within cerebral perivascular spaces underlies widespread tracer distribution in the brain after intranasal administration. J. Cereb. Blood Flow Metab. 2015, 35, 371–381. [Google Scholar] [CrossRef]
- Alcalá-Barraza, S.R.; Lee, M.S.; Hanson, L.R.; McDonald, A.A.; Frey, W.H.; McLoon, L.K. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J. Drug Target. 2010, 18, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Kirik, D.; Cederfjäll, E.; Halliday, G.; Petersén, Å. Gene therapy for Parkinson’s disease: Disease modification by GDNF family of ligands. Neurobiol. Dis. 2017, 97 Pt B, 179–188. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Yang, K.J.; Park, S.N.; Kim, D.K.; Kim, J.D. The effect of dexamethasone/cell-penetrating peptide nanoparticles on gene delivery for inner ear therapy. Int. J. Nanomed. 2016, 11, 6123–6134. [Google Scholar] [CrossRef] [Green Version]
- Bartus, R.T.; Baumann, T.L.; Siffert, J.; Herzog, C.D.; Alterman, R.; Boulis, N.; Turner, D.A.; Stacy, M.; Lang, A.E.; Lozano, A.M.; et al. Safety/feasibility of targeting the substantia nigra with AAV2-neurturin in Parkinson patients. Neurology 2013, 80, 1698–1701. [Google Scholar] [CrossRef] [Green Version]
- Marks, W.J., Jr.; Ostrem, J.L.; Verhagen, L.; Starr, P.A.; Larson, P.S.; Bakay, R.A.; Taylor, R.; Cahn-Weiner, D.A.; Stoessl, A.J.; Olanow, C.W.; et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: An open-label, phase I trial. Lancet Neurol. 2008, 7, 400–408. [Google Scholar] [CrossRef]
- Marks, W.J., Jr.; Bartus, R.T.; Siffert, J.; Davis, C.S.; Lozano, A.; Boulis, N.; Vitek, J.; Stacy, M.; Turner, D.; Verhagen, L.; et al. Gene delivery of AAV2-neurturin for Parkinson’s disease: A double-blind, randomised, controlled trial. Lancet Neurol. 2010, 9, 1164–1172. [Google Scholar] [CrossRef]
- Richardson, R.T.; Wise, A.K.; Thompson, B.C.; Flynn, B.O.; Atkinson, P.J.; Fretwell, N.J.; Fallon, J.B.; Wallace, G.G.; Shepherd, R.K.; Clark, G.M.; et al. Polypyrrole-coated electrodes for the delivery of charge and neurotrophins to cochlear neurons. Biomaterials 2009, 30, 2614–2624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikkawa, Y.S.; Nakagawa, T.; Ying, L.; Tabata, Y.; Tsubouchi, H.; Ido, A.; Ito, J. Growth factor-eluting cochlear implant electrode: Impact on residual auditory function, insertional trauma, and fibrosis. J. Transl. Med. 2014, 12, 280. [Google Scholar] [CrossRef]
- Chikar, J.A.; Hendricks, J.L.; Richardson-Burns, S.M.; Raphael, Y.; Pfingst, B.E.; Martin, D.C. The use of a dual PEDOT and RGD-functionalized alginate hydrogel coating to provide sustained drug delivery and improved cochlear implant function. Biomaterials 2012, 33, 1982–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, T.; Nakagawa, T.; Kita, T.; Iguchi, F.; Kim, T.S.; Tamura, T.; Iwai, K.; Tabata, Y.; Ito, J. Novel strategy for treatment of inner ears using a biodegradable gel. Laryngoscope 2005, 115, 2016–2020. [Google Scholar] [CrossRef] [PubMed]
- Madduri, S.; Gander, B. Growth factor delivery systems and repair strategies for damaged peripheral nerves. J. Control. Release 2012, 161, 274–282. [Google Scholar] [CrossRef]
- Wang, Y.; Wise, A.K.; Tan, J.; Maina, J.W.; Shepherd, R.K.; Caruso, F. Mesoporous silica supraparticles for sustained inner-ear drug delivery. Small 2014, 10, 4244–4248. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Edin, F.; Hayashi, H.; Gudjonsson, O.; Danckwardt-Lillieström, N.; Engqvist, H.; Rask-Andersen, H.; Xia, W. Guided growth of auditory neurons: Bioactive particles towards gapless neural-electrode interface. Biomaterials 2017, 122, 1–9. [Google Scholar] [CrossRef]
- Roy, S.; Glueckert, R.; Johnston, A.H.; Perrier, T.; Bitsche, M.; Newman, T.A.; Saulnier, P.; Schrott-Fischer, A. Strategies for drug delivery to the human inner ear by multifunctional nanoparticles. Nanomedicine 2012, 7, 55–63. [Google Scholar] [CrossRef]
- Mitra, S.; Behbahani, H.; Eriksdotter, M. Innovative therapy for Alzheimer’s disease with focus on biodelivery of NGF. Front. Neurosci. 2019, 13, 38. [Google Scholar] [CrossRef] [Green Version]
- Xi, Y.; Chen, Y.; Jin, Y.; Han, G.; Song, M.; Song, T.; Shi, Y.; Tao, L.; Huang, Z.; Zhou, J.; et al. Versatile nanomaterials for Alzheimer’s disease: Pathogenesis inspired disease-modifying therapy. J. Control Release. 2022, 345, 38. [Google Scholar] [CrossRef]
- Akel, H.; Ismail, R.; Csóka, I. Progress and perspectives of brain-targeting lipid-based nanosystems via the nasal route in Alzheimer’s disease. Eur. J. Pharm. Biopharm. 2020, 148, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Babazadeh, A.; Mohammadi Vahed, F.; Jafari, S.M. Nanocarrier-mediated brain delivery of bioactives for treatment/prevention of neurodegenerative diseases. J. Control. Release 2020, 321, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Sonvico, F.; Clementino, A.; Buttini, F.; Colombo, G.; Pescina, S.; Stanisçuaski Guterres, S.; Raffin Pohlmann, A.; Nicoli, S. Surface-modified nanocarriers for nose-to-brain delivery: From bioadhesion to targeting. Pharmaceutics 2018, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Jao, D.; Xue, Y.; Medina, J.; Hu, X. Protein-based drug-delivery materials. Materials 2017, 10, 517. [Google Scholar] [CrossRef]
- Goldsmith, M.; Abramovitz, L.; Peer, D. Precision nanomedicine in neurodegenerative diseases. ACS Nano 2014, 8, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Burnouf, P.A.; Burnouf, T.; Agrahari, V. Nanoformulation properties, characterization, and behavior in complex biological matrices: Challenges and opportunities for brain-targeted drug delivery applications and enhanced translational potential. Adv. Drug Deliv. Rev. 2019, 148, 146–180. [Google Scholar] [CrossRef]
- Teixeira, M.I.; Lopes, C.M.; Amaral, M.H.; Costa, P.C. Current insights on lipid nanocarrier-assisted drug delivery in the treatment of neurodegenerative diseases. Eur. J. Pharm. Biopharm. 2020, 149, 192–217. [Google Scholar] [CrossRef]
- Harilal, S.; Jose, J.; Parambi, D.G.T.; Kumar, R.; Mathew, G.E.; Uddin, M.S.; Kim, H.; Mathew, B. Advancements in nanotherapeutics for Alzheimer’s disease: Current perspectives. J. Pharm. Pharmacol. 2019, 71, 1370–1383. [Google Scholar] [CrossRef] [Green Version]
- Zorkina, Y.; Abramova, O.; Ushakova, V.; Morozova, A.; Zubkov, E.; Valikhov, M.; Melnikov, P.; Majouga, A.; Chekhonin, V. Nano carrier drug delivery systems for the treatment of neuropsychiatric disorders: Advantages and limitations. Molecules 2020, 25, 5294. [Google Scholar] [CrossRef]
- Yokel, R.A. Nanoparticle brain delivery: A guide to verification methods. Nanomedicine 2020, 15, 409–432. [Google Scholar] [CrossRef] [Green Version]
- Wen, M.M.; El-Salamouni, N.S.; El-Refaie, W.M.; Hazzah, H.A.; Ali, M.M.; Tosi, G.; Farid, R.M.; Blanco-Prieto, M.J.; Billa, N.; Hanafy, A.S. Nanotechnology-based drug delivery systems for Alzheimer’s disease management: Technical, industrial, and clinical challenges. J. Control. Release 2017, 245, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Sahni, J.K.; Doggui, S.; Ali, J.; Baboota, S.; Dao, L.; Ramassamy, C. Neurotherapeutic applications of nanoparticles in Alzheimer’s disease. J. Control. Release 2011, 152, 208–231. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, D.; Le Droumaguet, B.; Nicolas, J.; Hashemi, S.H.; Wu, L.P.; Moghimi, S.M.; Couvreur, P.; Andrieux, K. Nanotechnologies for Alzheimer’s disease: Diagnosis, therapy, and safety issues. Nanomedicine 2011, 7, 521–540. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of nanotechnology in drug delivery to the central nervous system. Biomed Pharm. 2019, 111, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.C.; Santos, A.O. Nanosystems in nose-to-brain drug delivery: A review of non-clinical brain targeting studies. J. Control. Release 2018, 270, 89–100. [Google Scholar] [CrossRef]
- Géral, C.; Angelova, A.; Angelov, B.; Nicolas, V.; Lesieur, S. Chapter 11: Multicompartment lipid nanocarriers for targeting of cells expressing brain receptors. In Self-Assembled Supramolecular Architectures: Lyotropic Liquid Crystals; Garti, N., Mezzenga, R., Somasundaran, P., Eds.; John Wiley & Sons, Inc.: New Jersey, NJ, USA, 2012; pp. 319–355. [Google Scholar] [CrossRef]
- Liaw, K.; Zhang, Z.; Kannan, S. Neuronanotechnology for brain regeneration. Adv. Drug Deliv. Rev. 2019, 148, 3–18. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C. Nanomaterial-based bone regeneration. Nanoscale 2017, 9, 4862–4874. [Google Scholar] [CrossRef]
- Bu, M.; Tang, J.; Wei, Y.; Sun, Y.; Wang, X.; Wu, L.; Liu, H. Enhanced bioavailability of nerve growth factor with phytantriol lipid-based crystalline nanoparticles in cochlea. Int. J. Nanomed. 2015, 10, 6879–6889. [Google Scholar] [CrossRef] [Green Version]
- Guccione, C.; Oufir, M.; Piazzini, V.; Eigenmann, D.E.; Jähne, E.A.; Zabela, V.; Faleschini, M.T.; Bergonzi, M.C.; Smiesko, M.; Hamburger, M.; et al. Andrographolide-loaded nanoparticles for brain delivery: Formulation, characterisation and in vitro permeability using hCMEC/D3 cell line. Eur. J. Pharm. Biopharm. 2017, 119, 253–263. [Google Scholar] [CrossRef]
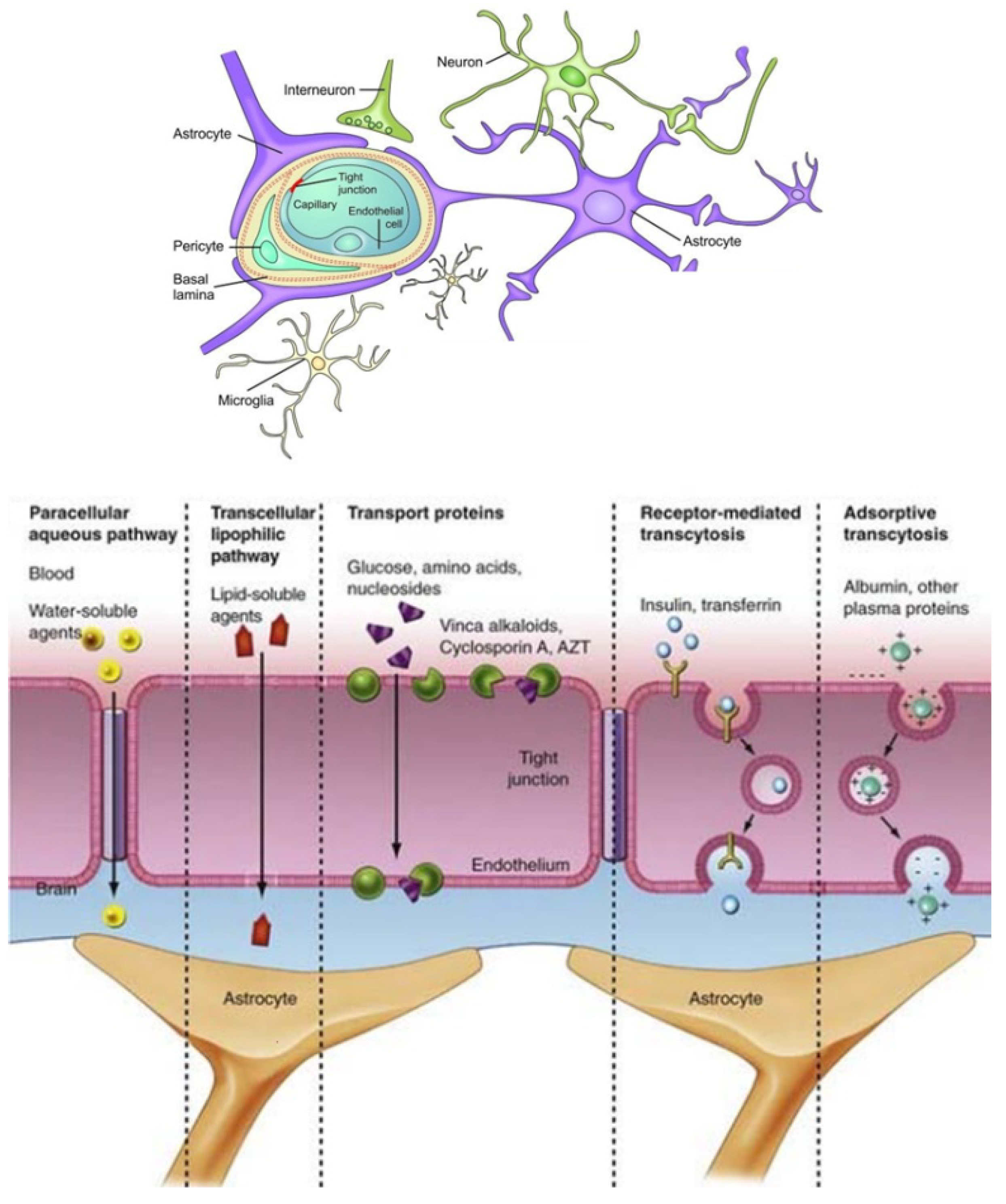
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.T.; Li, W.; Meng, G.; Wang, P.; Liao, W. Strategies for transporting nanoparticles across the blood-brain barrier. Biomater. Sci. 2016, 4, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Ajazuddin Tripathi, D.K.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Mourtas, S.; Hammarlund-Udenaes, M.; Alexander, A. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease. J. Control. Release 2017, 260, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, A.R.; Lee, S.S.; Bhattacharya, M.; Nam, J.S.; Chakraborty, C. Advances in nanocarriers enabled brain targeted drug delivery across blood brain barrier. Int. J. Pharm. 2019, 559, 360–372. [Google Scholar] [CrossRef]
- Kim, J.; Ahn, S.I.; Kim, Y. Nanotherapeutics engineered to cross the blood-brain barrier for advanced drug delivery to the central nervous system. J. Ind. Eng. Chem. 2019, 73, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Wairkar, S.; Sridhar, V.; Gaud, R. Pramipexole dihydrochloride loaded chitosan nanoparticles for nose to brain delivery: Development, characterization and in vivo anti-Parkinson activity. Int. J. Biol. Macromol. 2018, 109, 27–35. [Google Scholar] [CrossRef]
- Eskinazi-Budge, A.; Manickavasagam, D.; Czech, T.; Novak, K.; Kunzler, J.; Oyewumi, M.O. Preparation of emulsifying wax/glyceryl monooleate nanoparticles and evaluation as a delivery system for repurposing simvastatin in bone regeneration. Drug Dev. Ind. Pharm. 2018, 44, 1583–1590. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Novel piperine-loaded Tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in Alzheimer’s disease: Pharmaceutical, biological, and toxicological studies. Int. J. Nanomed. 2015, 10, 5459–5473. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Song, H.; Xiong, S.; Tian, T.; Liu, T.; Sun, Y. Chitosan-stablized bovine serum albumin nanoparticles having ability to control the release of NELL-1 protein. Int. J. Biol. Macromol. 2018, 109, 672–680. [Google Scholar] [CrossRef]
- Chatterjee, B.; Gorain, B.; Mohananaidu, K.; Sengupta, P.; Mandal, U.K.; Choudhury, H. Targeted drug delivery to the brain via intranasal nanoemulsion: Available proof of concept and existing challenges. Int. J. Pharm. 2019, 565, 258–268. [Google Scholar] [CrossRef]
- Oshiro, J.A.; Sato, M.R.; Scardueli, C.R.; Lopes de Oliveira, G.J.P.; Abucafy, M.P.; Chorilli, M. Bioactive molecule-loaded drug delivery systems to optimize bone tissue repair. Curr. Protein Pept. Sci. 2017, 18, 850–863. [Google Scholar] [CrossRef]
- Chung, E.P.; Cotter, J.D.; Prakapenka, A.V.; Cook, R.L.; DiPerna, D.M.; Sirianni, R.W. Targeting small molecule delivery to the brain and spinal cord via intranasal administration of Rabies Virus Glycoprotein (RVG29)-modified PLGA nanoparticles. Pharmaceutics 2020, 12, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piazzini, V.; Landucci, E.; D’Ambrosio, M.; Tiozzo Fasiolo, L.; Cinci, L.; Colombo, G.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Luceri, C.; Bergonzi, M.C. Chitosan coated human serum albumin nanoparticles: A promising strategy for nose-to-brain drug delivery. Int. J. Biol. Macromol. 2019, 129, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Yang, L.; Liu, Y.; Zhou, X.; Sun, J.; Liu, J. Sialic acid (SA)-modified selenium nanoparticles coated with a high blood-brain barrier permeability peptide-B6 peptide for potential use in Alzheimer’s disease. Acta Biomater. 2015, 25, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Barnabas, W. Drug targeting strategies into the brain for treating neurological diseases. J. Neurosci. Methods 2019, 311, 133–146. [Google Scholar] [CrossRef]
- Yu, S.; Xu, X.; Feng, J.; Liu, M.; Hu, K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int. J. Pharm. 2019, 560, 282–293. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, C.; Guo, Q.; Wan, X.; Shao, X.; Liu, Q.; Zhang, Q. Dual-functional nanoparticles for precise drug delivery to Alzheimer’s disease lesions: Targeting mechanisms, pharmacodynamics and safety. Int. J. Pharm. 2017, 525, 237–248. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Negut, I.; Grumezescu, V.; Grumezescu, A.M.; Teleanu, R.I. Nanomaterials for drug delivery to the central nervous system. Nanomaterials 2019, 9, 371. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Wei, M.; Zhang, N.; Li, H.; Tan, X.; Zhang, Y.; Zheng, W. Enhanced permeability of blood-brain barrier and targeting function of brain via borneol-modified chemically solid lipid nanoparticle. Int. J. Nanomed. 2018, 13, 1869–1879. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Yang, F.; Liao, Y.; Li, L.; Zhang, L. Cholesterol-PEG comodified poly (N-butyl) cyanoacrylate nanoparticles for brain delivery: In vitro and in vivo evaluations. Drug Deliv. 2017, 24, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Gajbhiye, K.R.; Pawar, A.; Mahadik, K.R.; Gajbhiye, V. PEGylated nanocarriers: A promising tool for targeted delivery to the brain. Colloids Surf. B Biointerfaces 2020, 187, 110770. [Google Scholar] [CrossRef]
- Azhari, H.; Strauss, M.; Hook, S.; Boyd, B.J.; Rizwan, S.B. Stabilising cubosomes with Tween 80 as a step towards targeting lipid nanocarriers to the blood-brain barrier. Eur. J. Pharm. Biopharm. 2016, 104, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, M.; Re, F.; Canovi, M.; Beeg, M.; Gregori, M.; Sesana, S.; Sonnino, S.; Brogioli, D.; Musicanti, C.; Gasco, P.; et al. Lipid-based nanoparticles with high binding affinity for amyloid-beta1-42 peptide. Biomaterials 2010, 31, 6519–6529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wan, X.; Zheng, X.; Shao, X.; Liu, Q.; Zhang, Q.; Qian, Y. Dual-functional nanoparticles targeting amyloid plaques in the brains of Alzheimer’s disease mice. Biomaterials 2014, 35, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Naki, T. Chitosan-based nanocarriers for nose to brain delivery. Appl. Sci. 2019, 9, 2219. [Google Scholar] [CrossRef] [Green Version]
- Ramalho, M.J.; Andrade, S.; Loureiro, J.A.; do Carmo Pereira, M. Nanotechnology to improve the Alzheimer’s disease therapy with natural compounds. Drug Deliv. Transl. Res. 2020, 10, 380–402. [Google Scholar] [CrossRef]
- Prabhath, A.; Vernekar, V.N.; Sanchez, E.; Laurencin, C.T. Growth factor delivery strategies for rotator cuff repair and regeneration. Int. J. Pharm. 2018, 544, 358–371. [Google Scholar] [CrossRef]
- Bayer, E.A.; Gottardi, R.; Fedorchak, M.V.; Little, S.R. The scope and sequence of growth factor delivery for vascularized bone tissue regeneration. J. Control. Release 2015, 219, 129–140. [Google Scholar] [CrossRef]
- Van Rijt, S.; Habibovic, P. Enhancing regenerative approaches with nanoparticles. J. R. Soc. Interface 2017, 14, 20170093. [Google Scholar] [CrossRef]
- Prades, R.; Guerrero, S.; Araya, E.; Molina, C.; Salas, E.; Zurita, E.; Selva, J.; Egea, G.; López-Iglesias, C.; Teixidó, M.; et al. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials 2012, 33, 7194–7205. [Google Scholar] [CrossRef]
- Songjiang, Z.; Lixiang, W. Amyloid-beta associated with chitosan nano-carrier has favorable immunogenicity and permeates the BBB. AAPS PharmSciTech 2009, 10, 900–905. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zheng, X.; Wan, X.; Shao, X.; Liu, Q.; Zhang, Z.; Zhang, Q. The potential use of H102 peptide-loaded dual-functional nanoparticles in the treatment of Alzheimer’s disease. J. Control. Release 2014, 192, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Zensi, A.; Begley, D.; Pontikis, C.; Legros, C.; Mihoreanu, L.; Wagner, S.; Büchel, C.; von Briesen, H.; Kreuter, J. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones. J. Control. Release 2009, 137, 78–86. [Google Scholar] [CrossRef] [PubMed]
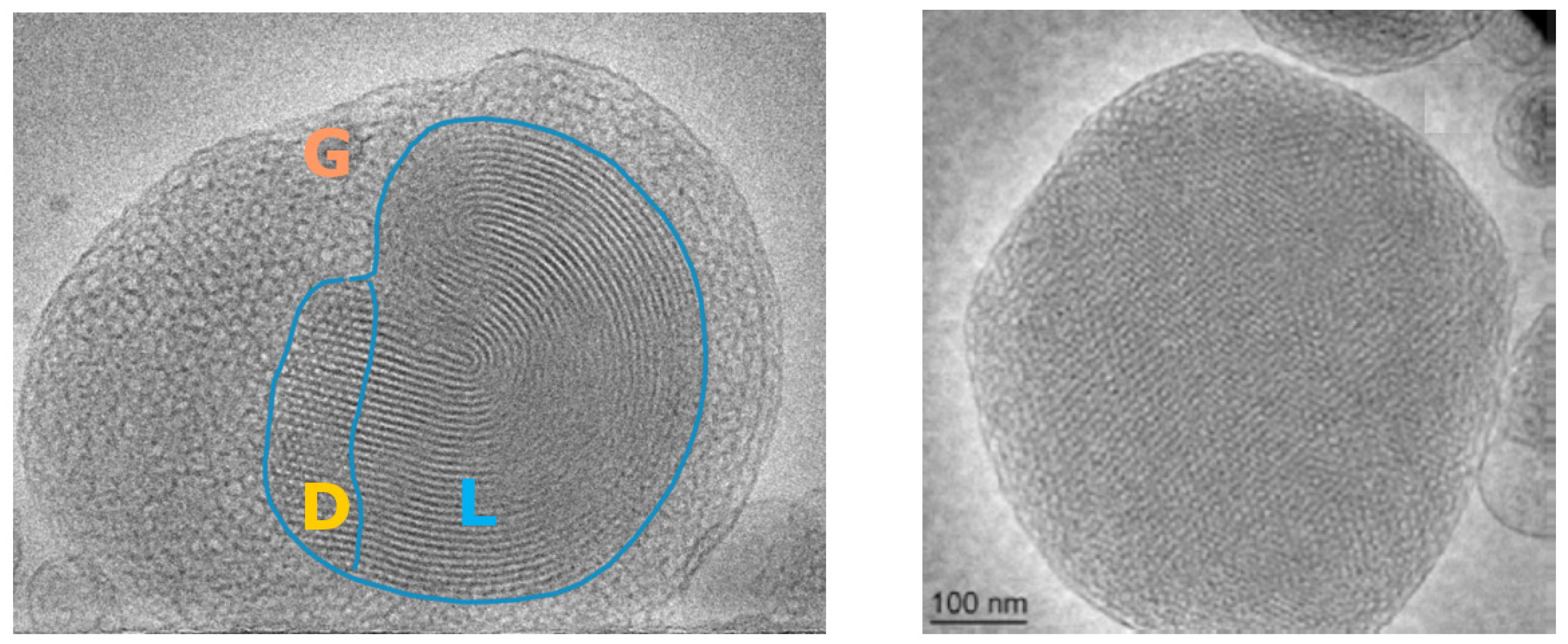
- Angelov, B.; Angelova, A.; Filippov, S.K.; Drechsler, M.; Štěpánek, P.; Lesieur, S. Multicompartment lipid cubic nanoparticles with high protein upload: Millisecond dynamics of formation. ACS Nano 2014, 8, 5216–5226. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wu, B.; Zhang, Q.; Chen, J.; Zhu, J.; Zhang, W.; Rong, Z.; Chen, H.; Jiang, X. Brain delivery of vasoactive intestinal peptide enhanced with the nanoparticles conjugated with wheat germ agglutinin following intranasal administration. J. Control. Release 2007, 121, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.; Feng, C.; Shao, X.; Liu, Q.; Zhang, Q.; Pang, Z.; Jiang, X. Intranasal nanoparticles of basic fibroblast growth factor for brain delivery to treat Alzheimer’s disease. Int. J. Pharm. 2014, 461, 192–202. [Google Scholar] [CrossRef]
- Park, T.E.; Singh, B.; Li, H.; Lee, J.Y.; Kang, S.K.; Choi, Y.J.; Cho, C.S. Enhanced BBB permeability of osmotically active poly(mannitol-co-PEI) modified with rabies virus glycoprotein via selective stimulation of caveolar endocytosis for RNAi therapeutics in Alzheimer’s disease. Biomaterials 2015, 38, 61–71. [Google Scholar] [CrossRef]
- Liu, Y.; An, S.; Li, J.; Kuang, Y.; He, X.; Guo, Y.; Ma, H.; Zhang, Y.; Ji, B.; Jiang, C. Brain-targeted co-delivery of therapeutic gene and peptide by multifunctional nanoparticles in Alzheimer’s disease mice. Biomaterials 2016, 80, 33–45. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, D.; Singh, J. GLUT-1: An effective target to deliver brain-derived neurotrophic factor gene across the blood brain barrier. ACS Chem. Neurosci. 2020, 11, 1620–1633. [Google Scholar] [CrossRef]
- Gandhi, M.; Bhatt, P.; Chauhan, G.; Gupta, S.; Misra, A.; Mashru, R. IGF-II-conjugated nanocarrier for brain-targeted delivery of p11 gene for depression. AAPS PharmSciTech 2019, 20, 50. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, X.; Guo, Q.; Yang, P.; Pang, X.; Qian, K.; Lu, W.; Zhang, Q.; Jiang, X. Systemic delivery of BACE1 siRNA through neuron-targeted nanocomplexes for treatment of Alzheimer’s disease. J. Control. Release 2018, 279, 220–233. [Google Scholar] [CrossRef]
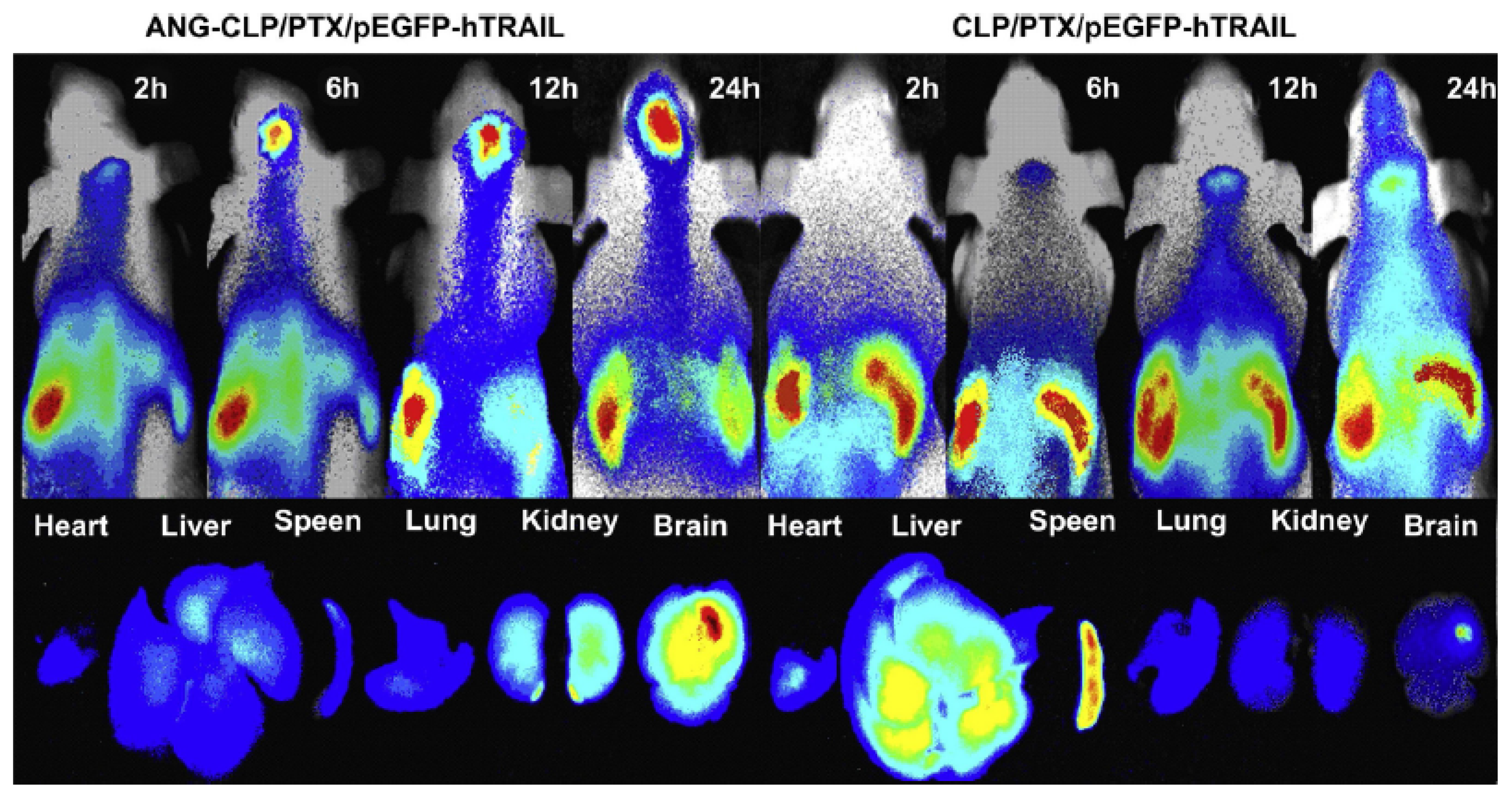
- Sun, X.; Pang, Z.; Ye, H.; Qiu, B.; Guo, L.; Li, J.; Ren, J.; Qian, Y.; Zhang, Q.; Chen, J. Co-delivery of pEGFP-hTRAIL and paclitaxel to brain glioma mediated by an angiopep-conjugated liposome. Biomaterials 2012, 33, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Tarus, D.; Hamard, L.; Caraguel, F.; Wion, D.; Szarpak-Jankowska, A.; van der Sanden, B.; Auzély-Velty, R. Design of hyaluronic acid hydrogels to promote neurite outgrowth in three dimensions. ACS Appl. Mater. Interfaces 2016, 8, 25051–25059. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.; Boto, C.; Saraiva, C.M.; Bernardino, L.; Ferreira, L. Nanomedicine approaches to modulate neural stem cells in brain repair. Trends Biotechnol. 2016, 34, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Entekhabi, E.; Haghbin Nazarpak, M.; Moztarzadeh, F.; Sadeghi, A. Design and manufacture of neural tissue engineering scaffolds using hyaluronic acid and polycaprolactone nanofibers with controlled porosity. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Chu, H.; Edalat, F.; Cha, J.M.; Sant, S.; Kashyap, A.; Ahari, A.F.; Kwon, C.H.; Nichol, J.W.; Manoucheri, S.; et al. Development of functional biomaterials with micro- and nanoscale technologies for tissue engineering and drug delivery applications. J. Tissue Eng. Regen. Med. 2014, 8, 1–14. [Google Scholar] [CrossRef]
- Kuihua, Z.; Chunyang, W.; Cunyi, F.; Xiumei, M. Aligned SF/P(LLA-CL)-blended nanofibers encapsulating nerve growth factor for peripheral nerve regeneration. J. Biomed. Mater. Res. A 2014, 102, 2680–2691. [Google Scholar] [CrossRef]
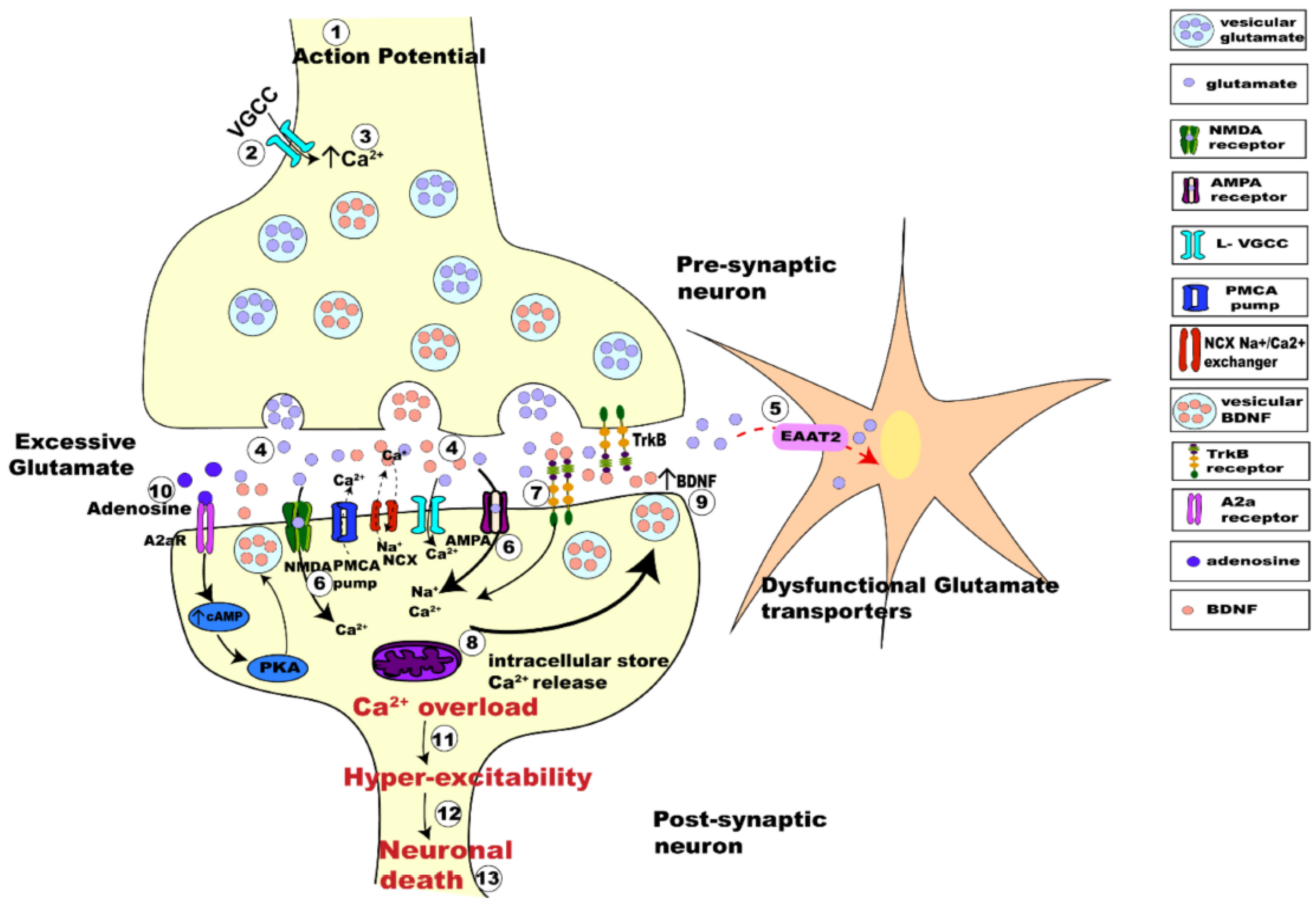
- Pradhan, J.; Noakes, P.G.; Bellingham, M.C. The role of altered BDNF/TrkB signaling in Amyotrophic Lateral Sclerosis. Front. Cell Neurosci. 2019, 13, 368. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Drechsler, M.; Nicolas, V.; Bizien, T.; Gorshkova, Y.E.; Deng, Y.; Angelova, A. Liquid crystalline lipid nanoparticles for combined delivery of curcumin, fish oil and BDNF: In vitro neuroprotective potential in a cellular model of tunicamycin-induced endoplasmic reticulum stress. Smart Mater. Med. 2022, 3, 274–288. [Google Scholar] [CrossRef]
- Guo, W.; Nagappan, G.; Lu, B. Differential effects of transient and sustained activation of BDNF-TrkB signaling. Dev. Neurobiol. 2018, 78, 647–659. [Google Scholar] [CrossRef]
- Minuzzi, L.G.; Seelaender, M.; Silva, B.S.D.A.; Cunha, E.d.B.B.; Deus, M.D.C.; Vasconcellos, F.T.F.; Marqueze, L.F.B.; Gadotti, A.C.; Baena, C.P.; Pereira, T.; et al. COVID-19 outcome relates with circulating BDNF, according to patient adiposity and age. Front. Nutr. 2021, 8, 784429. [Google Scholar] [CrossRef]
- Motaghinejad, M.; Gholami, M. Possible neurological and mental outcomes of COVID-19 infection: A hypothetical role of ACE-2\Mas\BDNF signaling pathway. Int. J. Prev. Med. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Rivellini, E.; Lavitrano, M.; Combi, R. Can SARS-CoV-2 Infection exacerbate Alzheimer’s disease? An overview of shared risk factors and pathogenetic mechanisms. J. Pers. Med. 2021, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.S.; Ho, C.S.H.; Tam, W.W.S.; Kua, E.H.; Ho, R.C.M. Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with Alzheimer’s disease (AD): A systematic review and meta-analysis. Int. J. Mol. Sci. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, N.M.; Ritzel, R.; Mancini, N.S.; Jiang, Y.; Yi, X.; Manickam, D.S.; Banks, W.A.; Kabanov, A.V.; McCullough, L.D.; Verma, R. Nano-particle delivery of brain derived neurotrophic factor after focal cerebral ischemia reduces tissue injury and enhances behavioral recovery. Pharmacol. Biochem. Behav. 2016, 150–151, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Fay, J.M.; Poon, C.D.; Vinod, N.; Zhao, Y.; Bullock, K.; Qin, S.; Manickam, D.S.; Yi, X.; Banks, W.A.; et al. Nanoformulation of brain-derived neurotrophic factor with target receptor-triggered-release in the central nervous system. Adv. Funct. Mater. 2018, 28, 1703982. [Google Scholar] [CrossRef]
- Limongi, T.; Rocchi, A.; Cesca, F.; Tan, H.; Miele, E.; Giugni, A.; Orlando, M.; Perrone Donnorso, M.; Perozziello, G.; Benfenati, F.; et al. Delivery of brain-derived neurotrophic factor by 3D biocompatible polymeric scaffolds for neural tissue engineering and neuronal regeneration. Mol. Neurobiol. 2018, 55, 8788–8798. [Google Scholar] [CrossRef]
- Hassannejad, Z.; Zadegan, S.A.; Vaccaro, A.R.; Rahimi-Movaghar, V.; Sabzevari, O. Biofunctionalized peptide-based hydrogel as an injectable scaffold for BDNF delivery can improve regeneration after spinal cord injury. Injury 2019, 50, 278–285. [Google Scholar] [CrossRef]
- Cook, D.J.; Nguyen, C.; Chun, H.N.L.; Llorente, I.; Chiu, A.S.; Machnicki, M.; Zarembinski, T.I.; Carmichael, S.T. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J. Cereb. Blood Flow. Metab. 2017, 37, 1030–1045. [Google Scholar] [CrossRef]
- Obermeyer, J.M.; Tuladhar, A.; Payne, S.L.; Ho, E.; Morshead, C.M.; Shoichet, M.S. Local delivery of brain-derived neurotrophic factor enables behavioral recovery and tissue repair in stroke-injured rats. Tissue Eng. Part A 2019, 25, 1175–1187. [Google Scholar] [CrossRef]
- Kandalam, S.; Sindji, L.; Delcroix, G.J.; Violet, F.; Garric, X.; André, E.M.; Schiller, P.C.; Venier-Julienne, M.C.; des Rieux, A.; Guicheux, J.; et al. Pharmacologically active microcarriers delivering BDNF within a hydrogel: Novel strategy for human bone marrow-derived stem cells neural/neuronal differentiation guidance and therapeutic secretome enhancement. Acta Biomater. 2017, 49, 167–180. [Google Scholar] [CrossRef]
- Xing, Y.; Wen, C.Y.; Li, S.T.; Xia, Z.X. Non-viral liposome-mediated transfer of brain-derived neurotrophic factor across the blood-brain barrier. Neural Regen. Res. 2016, 11, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Low, W.C.; Rujitanaroj, P.O.; Wang, F.; Wang, J.; Chew, S.Y. Nanofiber-mediated release of retinoic acid and brain-derived neurotrophic factor for enhanced neuronal differentiation of neural progenitor cells. Drug Deliv. Transl. Res. 2015, 5, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.; Schulze, J.; Warwas, D.P.; Ehlert, N.; Lenarz, T.; Warnecke, A.; Behrens, P. Long-term delivery of brain-derived neurotrophic factor (BDNF) from nanoporous silica nanoparticles improves the survival of spiral ganglion neurons in vitro. PLoS ONE 2018, 13, e0194778. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chau, Y. Polymeric nanoparticles decorated with BDNF-derived peptide for neuron-targeted delivery of PTEN inhibitor. Eur. J. Pharm. Sci. 2018, 124, 37–45. [Google Scholar] [CrossRef]
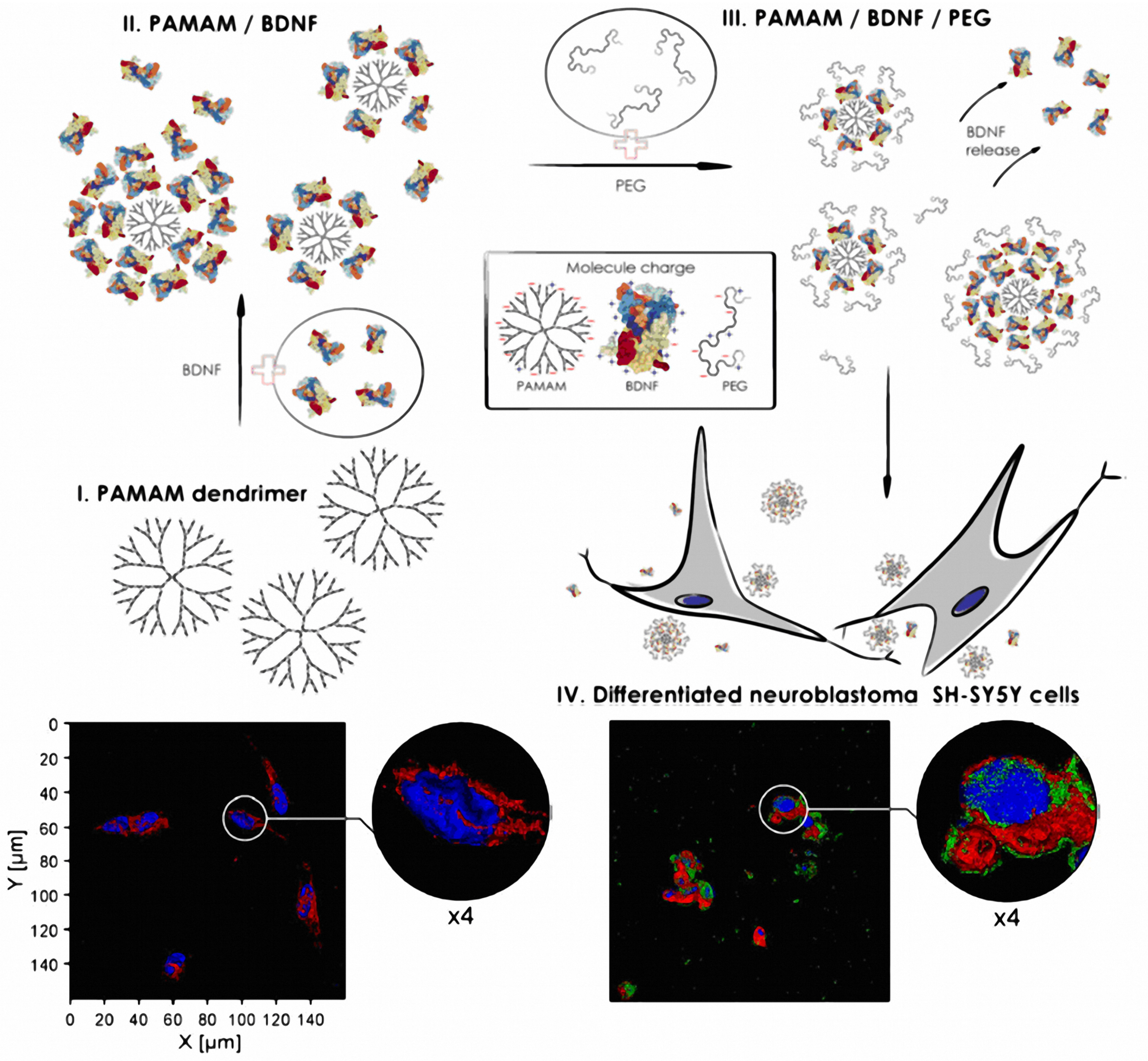
- Dąbkowska, M.; Łuczkowska, K.; Rogińska, D.; Sobuś, A.; Wasilewska, M.; Ulańczyk, Z.; Machaliński, B. Novel design of (PEG-ylated)PAMAM-based nanoparticles for sustained delivery of BDNF to neurotoxin-injured differentiated neuroblastoma cells. J. Nanobiotechnol. 2020, 18, 120. [Google Scholar] [CrossRef]
- Lu, J.; Yan, X.; Sun, X.; Shen, X.; Yin, H.; Wang, C.; Liu, Y.; Lu, C.; Fu, H.; Yang, S.; et al. Synergistic effects of dual-presenting VEGF- and BDNF-mimetic peptide epitopes from self-assembling peptide hydrogels on peripheral nerve regeneration. Nanoscale 2019, 11, 19943–19958. [Google Scholar] [CrossRef]
- Edelbrock, A.N.; Àlvarez, Z.; Simkin, D.; Fyrner, T.; Chin, S.M.; Sato, K.; Kiskinis, E.; Stupp, S.I. Supramolecular nanostructure activates TrkB receptor signaling of neuronal cells by mimicking brain-derived neurotrophic factor. Nano Lett. 2018, 18, 6237–6247. [Google Scholar] [CrossRef]
- Lopes, C.D.F.; Gonçalves, N.P.; Gomes, C.P.; Saraiva, M.J.; Pêgo, A.P. BDNF gene delivery mediated by neuron-targeted nanoparticles is neuroprotective in peripheral nerve injury. Biomaterials 2017, 121, 83–96. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Yuan, J.; Chen, X.; Xu, J.; Li, Y.; Dong, P. Functional regeneration of the transected recurrent laryngeal nerve using a collagen scaffold loaded with laminin and laminin-binding BDNF and GDNF. Sci. Rep. 2016, 6, 32292. [Google Scholar] [CrossRef] [Green Version]
- Ravina, K.; Briggs, D.I.; Kislal, S.; Warraich, Z.; Nguyen, T.; Lam, R.K.; Zarembinski, T.I.; Shamloo, M. Intracerebral delivery of brain-derived neurotrophic factor using HyStem®-C hydrogel implants improves functional recovery and reduces neuroinflammation in a rat model of ischemic stroke. Int. J. Mol. Sci. 2018, 19, 3782. [Google Scholar] [CrossRef] [Green Version]
- Gransee, H.M.; Zhan, W.Z.; Sieck, G.C.; Mantilla, C.B. Localized delivery of brain-derived neurotrophic factor-expressing mesenchymal stem cells enhances functional recovery following cervical spinal cord injury. J. Neurotrauma 2015, 32, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Sandner, B.; Schackel, T.; Nicholson, L.; Chtarto, A.; Tenenbaum, L.; Puttagunta, R.; Müller, R.; Weidner, N.; Blesch, A. Regulated viral BDNF delivery in combination with Schwann cells promotes axonal regeneration through capillary alginate hydrogels after spinal cord injury. Acta Biomater. 2017, 60, 167–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schendzielorz, P.; Scherzed, A.; Rak, K.; Völker, J.; Hagen, R.; Mlynski, R.; Frölich, K.; Radeloff, A. A hydrogel coating for cochlear implant arrays with encapsulated adipose-derived stem cells allows brain-derived neurotrophic factor delivery. Acta Otolaryngol. 2014, 134, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Drechsler, M.; Garamus, V.M.; Angelov, B. Liquid crystalline nanostructures as PEGylated reservoirs of omega-3 polyunsaturated fatty acids: Structural insights toward delivery formulations against neurodegenerative disorders. ACS Omega 2018, 3, 3235–3247. [Google Scholar] [CrossRef] [Green Version]
- Guerzoni, L.P.B.; Nicolas, V.; Angelova, A. In vitro modulation of TrkB receptor signaling upon sequential delivery of curcumin-DHA loaded carriers towards promoting neuronal survival. Pharm. Res. 2017, 34, 492–505. [Google Scholar] [CrossRef]
- Fujino, T.; Yamada, T.; Asada, T.; Tsuboi, Y.; Wakana, C.; Mawatari, S.; Kono, S. Efficacy and blood plasmalogen changes by oral administration of plasmalogen in patients with mild Alzheimer’s disease and mild cognitive impairment: A multicenter, randomized, double-blind, placebo-controlled trial. eBiomedicine 2017, 17, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Angelova, A. Coronavirus-induced host cubic membranes and lipid-related antiviral therapies: A focus on bioactive plasmalogens. Front. Cell Dev. Biol. 2021, 9, 630242. [Google Scholar] [CrossRef]
- Hossain, M.S.; Mawatari, S.; Fujino, T. Plasmalogens, the vinyl ether-linked glycerophospholipids, enhance learning and memory by regulating brain-derived neurotrophic factor. Front. Cell Dev. Biol. 2022, 10, 828282. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B.; Drechsler, M.; Bizien, T.; Gorshkova, Y.E.; Deng, Y. Plasmalogen-based liquid crystalline multiphase structures involving docosapentaenoyl derivatives inspired by biological cubic membranes. Front. Cell Dev. Biol. 2021, 9, 617984. [Google Scholar] [CrossRef]
- Park, H.; Oh, J.; Shim, G.; Cho, B.; Chang, Y.; Kim, S.; Baek, S.; Kim, H.; Shin, J.; Cho, H.; et al. In vivo neuronal gene editing via CRISPR–Cas9 amphiphilic nanocomplexes alleviates deficits in mouse models of Alzheimer’s disease. Nat Neurosci. 2019, 22, 524–528. [Google Scholar] [CrossRef]
- Kolli, N.; Lu, M.; Maiti, P.; Rossignol, J.; Dunbar, G.L. Application of the gene editing tool, CRISPR-Cas9, for treating neurodegenerative diseases. Neurochem. Int. 2018, 112, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Han, Y.; Yang, C.; Lu, S.; Du, J.; Li, H.; Lin, J. CRISPR-Cas9-mediated gene therapy in neurological disorders. Mol. Neurobiol. 2022, 59, 968–982. [Google Scholar] [CrossRef] [PubMed]
- Rozhin, A.; Batasheva, S.; Kruychkova, M.; Cherednichenko, Y.; Rozhina, E.; Fakhrullin, R. Biogenic silver nanoparticles: Synthesis and application as antibacterial and antifungal Agents. Micromachines 2021, 12, 1480. [Google Scholar] [CrossRef] [PubMed]

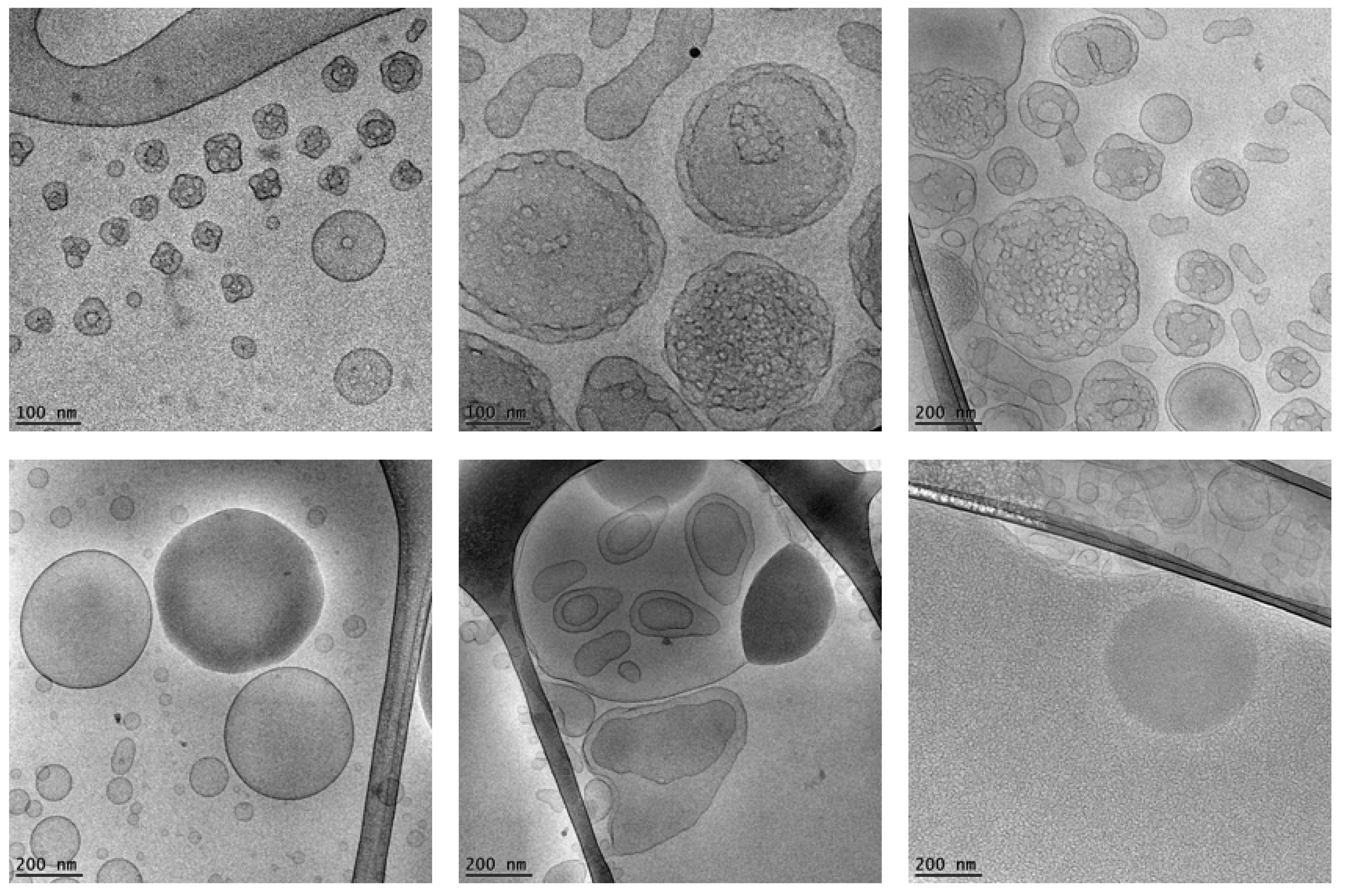
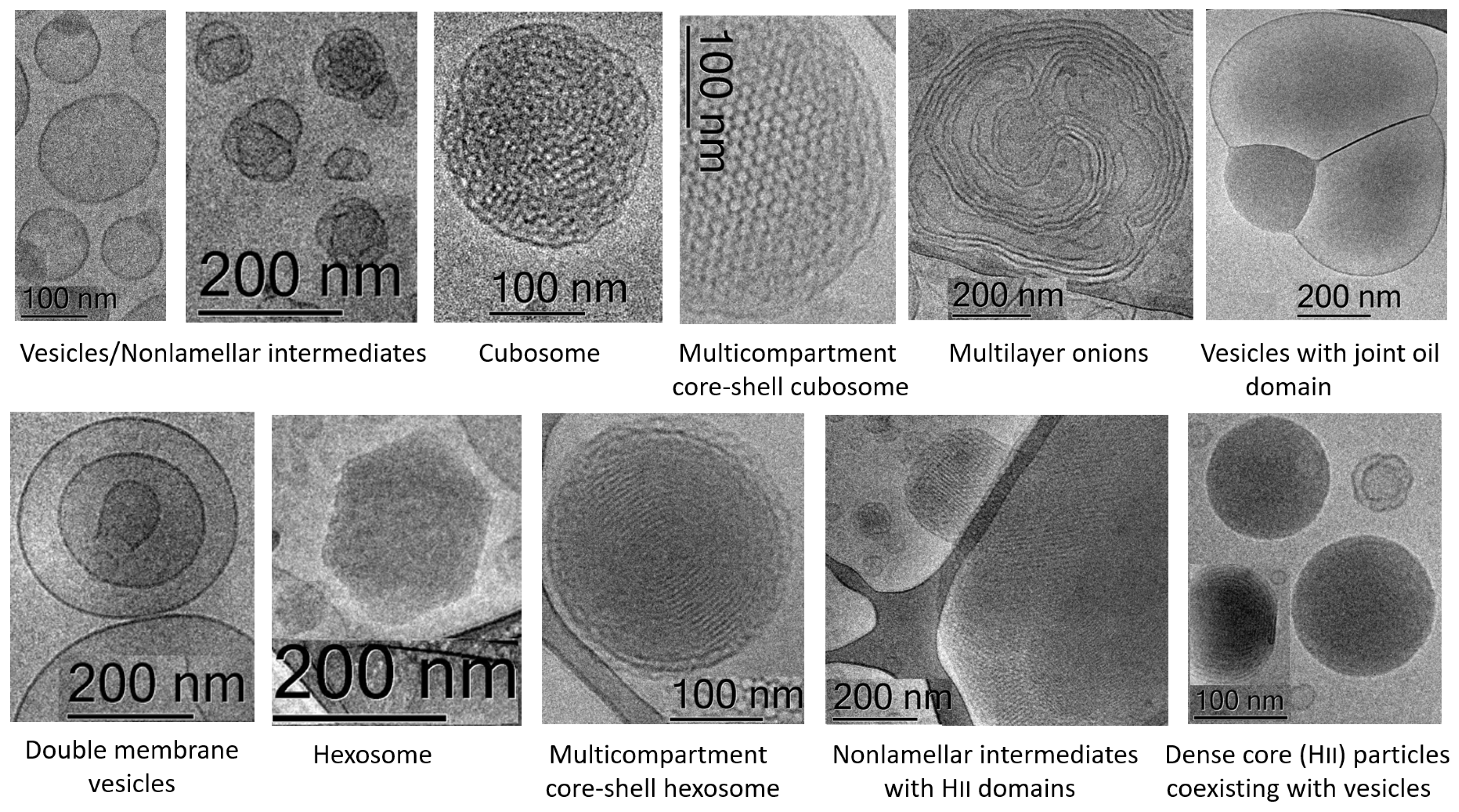
| Nanoformulation | Disease Indications | Administration Route/Model | Outcomes |
|---|---|---|---|
| Lipid-based nanoparticles | |||
| Liposomes conjugated with polyethylene glycol (PEG) and transferrin (Tf) as carriers for encapsulated BDNF gene, modified with a glial fibrillary acidic protein promoter (pGFAP) [Tf-pGFAP-BDNF-PEG] or a cytomegalovirus promoter (pCMV) [Tf-pCMV-BDNF-PEG] | Brain injury (degeneration ischemia, and inflammation) | In vivo tail-vein injection | Tf-pGFAP-BDNF-PEG and Tf-pCMV-BDNF-PEG carriers are able to cross the BBB. Predominant expression of BDNF in the cerebral cortex. The Tf-pGFAP-BDNF-PEG group is promoting more significantly the BDNF expression in the cerebral cortex than the Tf-pCMV-BDNF-PEG group [172]. |
| Polymeric-based nanoparticles and hydrogels | |||
| PEG-PGA nanoparticle polyion complexes with BDNF | Ischemic stroke | In vivo subcutaneous injection in mice | Reduced tissue injury. Behavioral improvements [165]. |
| BDNF mixed in poly(ethylene glycol)-b-poly(l-glutamic acid) (PEG-PLE) copolymer solution | Neurologic diseases | In vivo Intranasal | Protection of BDNF in the circulation. Better distribution than the native protein. Improved BDNF delivery efficiency [166]. |
| BDNF-loaded micropillarred poly-ε-caprolactone (MP-PCL) or flat PCL (F-PCL) scaffolds | Neuronal lesion | In vitro primary neuronal cultures | Sustained release of BDNF up to 21 days. Increased neuronal survival and synaptic density. Suitable for neural tissue engineering and prosthetics [167]. |
| BDNF in self-assembled IKVAV PA hydrogel | Traumatic spinal cord injuries (TSCI) | In vivo Injection, Spinal cord injury induced using clip compression at T7-T8 vertebral segment | Sustained release of BDNF. Axonal preservation. Astrogliosis decreased at 6 weeks post-injury without inflammation. Locomotor functional recovery failed [168]. |
| BDNF encapsulated in hyaluronic acid hydrogel | Stroke | In vivo Stroke models in mouse (strains C57Bl/6, DBA) and non-human primate (chronic stroke) | Distribution of BDNF-loaded hydrogel from the stroke cavity into the peri-infarct tissue up to 3 weeks compared to 1 week for direct BDNF injection in a mouse model. Recovery of motor function. Migration of immature neurons into the peri-infarct cortex and long-term survival. Released BDNF sufficient for functional recovery from stroke in a non-human primate [169]. |
| BDNF dispersed in a hydrogel, consisting of hyaluronan and methylcellulose, with embedded poly(lactic-co-glycolic acid) nanoparticles | Stroke | In vivo stroke lesions; Stroke-injured rat | Unchanged lesion volume compared to a vehicle group. Synaptophysin expression in homotopic contralesional hemisphere. Better plasticity. [170]. |
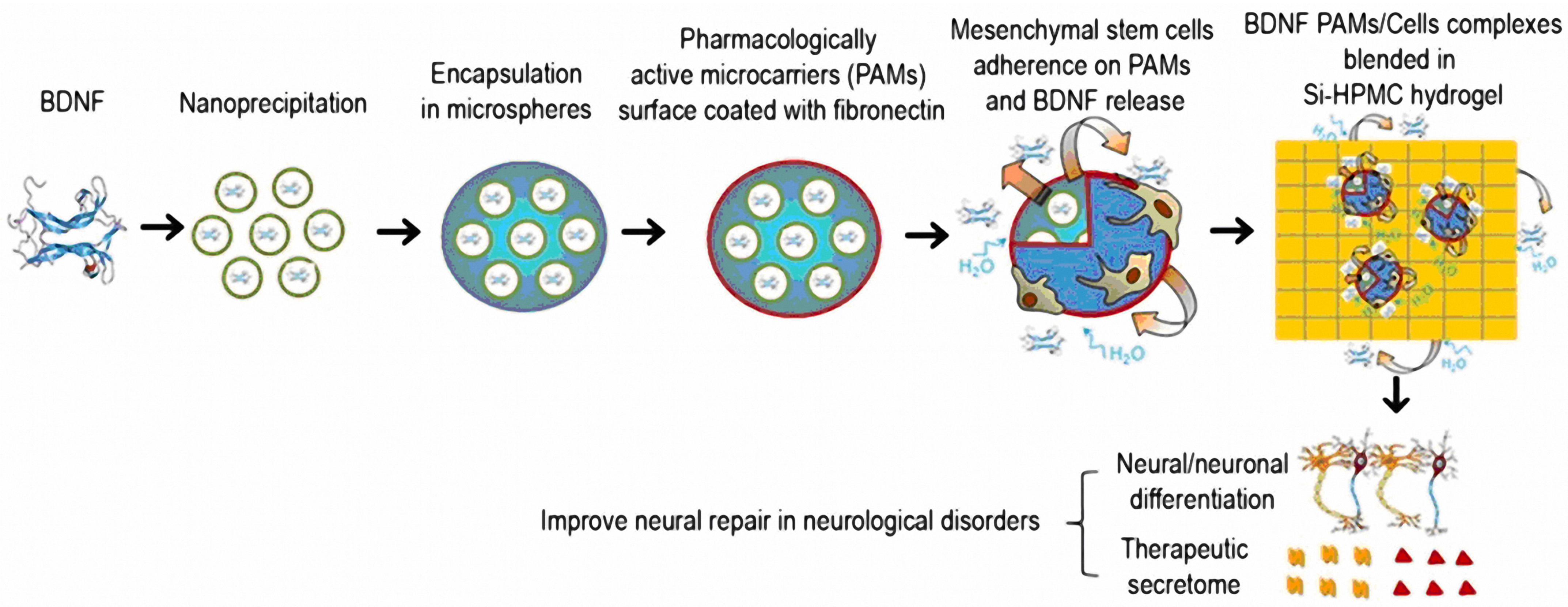
| Fibronectin-coated pharmacologically active microcarriers (PAMs) modified with silanized- hydroxypropyl methylcellulose (Si-HPMC) hydrogel for BDNF delivery | Neurological disorders | Human marrow-isolated adult multilineage-inducible (MIAMI) stem cells | The PAMs Si-HPMC hydrogel facilitated the expression of neuronal differentiation markers in MIAMI cells. Improved secretion of growth factors (e.g., b-NGF, HGF, SCF, LIF, SDF-1α, VEGF-A & D) and chemokines (MIP-1α & β, RANTES, IL-8) [171]. |
| PEGylated PAMAM-based nanoparticles | Neurodegenerative diseases | In vitro SH-SY5Y cells | Increased BDNF expression and release for the PEGylated PAMAM nanoparticle group versus the PAMAM-based nanoparticles [176]. |
| BDNF-mimetic peptide nanofiber scaffolds | |||
| Self-assemble nanofiber hydrogel including a BDNF mimetic peptide | Peripheral nerve injury | In vivo Rat model | Nerve regeneration and functional recovery observed in a rat model after implantation of nanofiber hydrogels [177]. |
| Nanofibers involving a BDNF mimetic peptide | CNS injuries and diseases | Primary cortical neurons | Neuronal survival and increased functional maturation [178]. |
| Silica nanoparticles | |||
| BDNF-loaded porous silica nanoparticles (NPSNPs) | Degeneration of SGNs, inner ear disease | In vitro NIH3T3 fibroblats, SGNs | Sustained BDNF release from amino-modified nanoparticles over 80 days. Cytocompatibility of the NPSNPs with the fibroblasts. Higher survival rate of SGNs in cell cultures as compared to unloaded control NPSNPs [174]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Rakotoarisoa, M.; Angelov, B.; Deng, Y.; Angelova, A.
Self-Assembled Nanoscale Materials for
Wu Y, Rakotoarisoa M, Angelov B, Deng Y, Angelova A.
Self-Assembled Nanoscale Materials for
Wu, Yu, Miora Rakotoarisoa, Borislav Angelov, Yuru Deng, and Angelina Angelova.
2022. "Self-Assembled Nanoscale Materials for
Wu, Y., Rakotoarisoa, M., Angelov, B., Deng, Y., & Angelova, A.
(2022). Self-Assembled Nanoscale Materials for








