Nanoporous Silicon with Graphene-like Coating for Pseudocapacitor Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoporous Layer Formation
2.2. GLC Deposition
2.3. Characterization and Measurements
2.4. Calculations and Formulae
2.4.1. CV Measurements
2.4.2. GCD Measurements
2.4.3. Gravimetric Calculations
3. Results and Discussion
3.1. Sample Characterization
3.2. Electrolyte Impregnation
3.3. CV Measurements and EIS
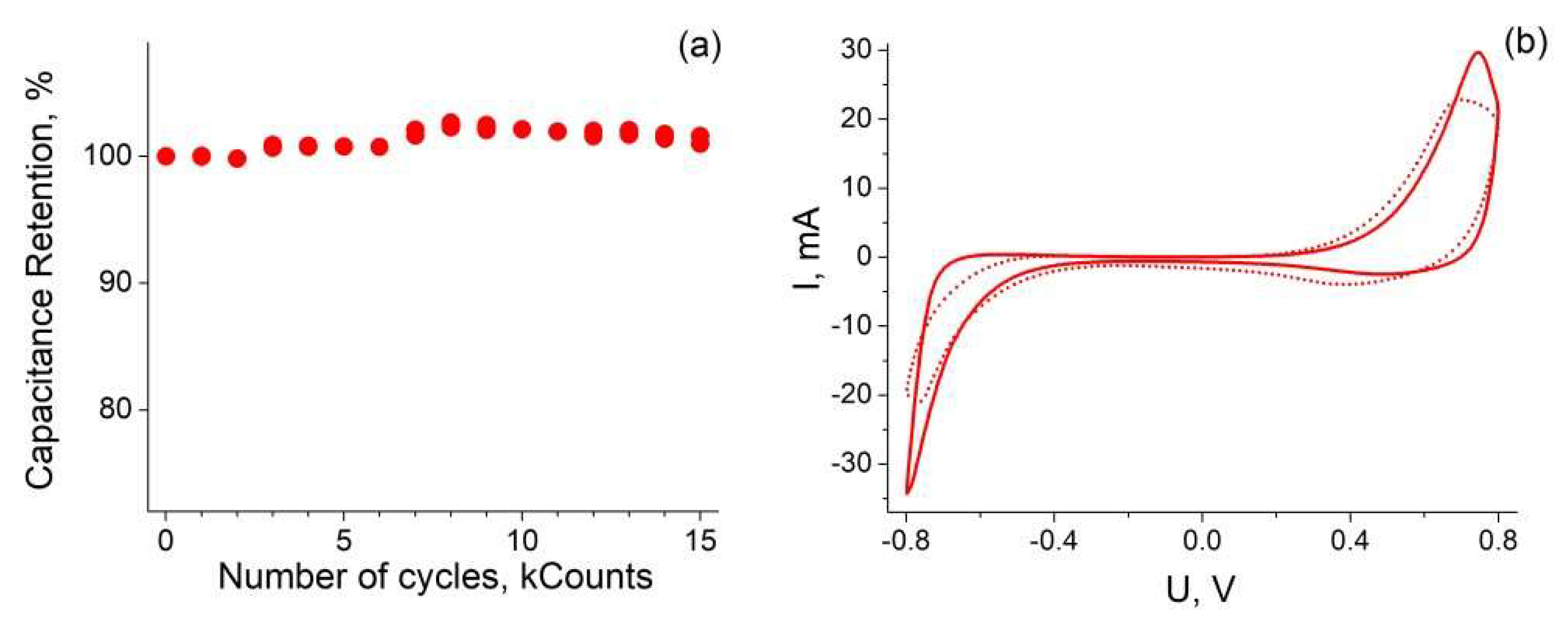
3.4. Cyclic Stability and GCD Measurements
3.5. Comparison of Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Granitzer, P.; Rumpf, K. Porous silicon—A versatile host material. Materials 2010, 3, 943–998. [Google Scholar] [CrossRef]
- Starkov, V. Production, properties, and application of porous silicon. All Materials. Encycl. Ref. Book 2009, 4, 13–21. [Google Scholar]
- RoyChaudhuri, C. A review on porous silicon based electrochemical biosensors: Beyond surface area enhancement factor. Sens. Actuators B Chem. 2015, 210, 310–323. [Google Scholar] [CrossRef]
- Lin, X.; Yang, Q.; Ding, L.; Su, B. Ultrathin silica membranes with highly ordered and perpendicular nanochannels for precise and fast molecular separation. ACS Nano 2015, 9, 11266–11277. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gaur, G.; Retterer, S.T.; Laibinis, P.E.; Weiss, S.M. Flow-through porous silicon membranes for real-time label-free biosensing. Anal. Chem. 2016, 88, 10940–10948. [Google Scholar] [CrossRef] [Green Version]
- Presting, H.; Konle, J.; Starkov, V.; Vyatkin, A.; König, U. Porous silicon for micro-sized fuel cell reformer units. Mater. Sci. Eng. B 2004, 108, 162–165. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W.; Sheldon, B.W.; Wu, J. Silicon-based nanomaterials for lithium-ion batteries: A review. Adv. Energy Mater. 2014, 4, 1300882. [Google Scholar] [CrossRef]
- Lysenko, V.; Bidault, F.; Alekseev, S.; Zaitsev, V.; Barbier, D.; Turpin, C.; Geobaldo, F.; Rivolo, P.; Garrone, E. Study of porous silicon nanostructures as hydrogen reservoirs. J. Phys. Chem. B 2005, 109, 19711–19718. [Google Scholar] [CrossRef]
- Shiraz, H.G.; Seyfollahi, R. Hybrid system for potential room temperature hydrogen storage. Vacuum 2016, 131, 115–119. [Google Scholar] [CrossRef]
- Desplobain, S.; Gautier, G.; Semai, J.; Ventura, L.; Roy, M. Investigations on porous silicon as electrode material in electrochemical capacitors. Phys. Status Solidi C 2007, 4, 2180–2184. [Google Scholar] [CrossRef]
- Ferreira, N.; Azevedo, A.; Beloto, A.; Amaral, M.; Almeida, F.; Oliveira, F.; Silva, R. Nanodiamond films growth on porous silicon substrates for electrochemical applications. Diam. Relat. Mater. 2005, 14, 441–445. [Google Scholar] [CrossRef]
- Rowlands, S.; Latham, R.; Schlindwein, W. Supercapacitor devices using porous silicon electrodes. Ionics 1999, 5, 144–149. [Google Scholar] [CrossRef]
- Grigoras, K.; Keskinen, J.; Grönberg, L.; Yli-Rantala, E.; Laakso, S.; Välimäki, H.; Kauranen, P.; Ahopelto, J.; Prunnila, M. Conformal titanium nitride in a porous silicon matrix: A nanomaterial for in-chip supercapacitors. Nano Energy 2016, 26, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Alper, J.P.; Wang, S.; Rossi, F.; Salviati, G.; Yiu, N.; Carraro, C.; Maboudian, R. Selective ultrathin carbon sheath on porous silicon nanowires: Materials for extremely high energy density planar micro-supercapacitors. Nano Lett. 2014, 14, 1843–1847. [Google Scholar] [CrossRef] [PubMed]
- Ortaboy, S.; Alper, J.P.; Rossi, F.; Bertoni, G.; Salviati, G.; Carraro, C.; Maboudian, R. MnOx-decorated carbonized porous silicon nanowire electrodes for high performance supercapacitors. Energy Environ. Sci. 2017, 10, 1505–1516. [Google Scholar] [CrossRef]
- Chatterjee, S.; Carter, R.; Oakes, L.; Erwin, W.R.; Bardhan, R.; Pint, C.L. Electrochemical and corrosion stability of nanostructured silicon by graphene coatings: Toward high power porous silicon supercapacitors. J. Phys. Chem. C 2014, 118, 10893–10902. [Google Scholar] [CrossRef]
- Grigoras, K.; Keskinen, J.; Grönberg, L.; Ahopelto, J.; Prunnila, M. Coated porous Si for high performance on-chip supercapacitors. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2014; p. 012058. [Google Scholar]
- Oakes, L.; Westover, A.; Mares, J.W.; Chatterjee, S.; Erwin, W.R.; Bardhan, R.; Weiss, S.M.; Pint, C.L. Surface engineered porous silicon for stable, high performance electrochemical supercapacitors. Sci. Rep. 2013, 3, 3020. [Google Scholar] [CrossRef] [PubMed]
- Romanitan, C.; Varasteanu, P.; Mihalache, I.; Culita, D.; Somacescu, S.; Pascu, R.; Tanasa, E.; Eremia, S.A.; Boldeiu, A.; Simion, M. High-performance solid state supercapacitors assembling graphene interconnected networks in porous silicon electrode by electrochemical methods using 2, 6-dihydroxynaphthalen. Sci. Rep. 2018, 8, 9654. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.-H.; Chang, C.-T.; Wang, C.-C.; Parwaiz, S.; Lai, C.-C.; Chen, Y.-Z.; Lu, S.-Y.; Chueh, Y.-L. Few-layer graphene sheet-passivated porous silicon toward excellent electrochemical double-layer supercapacitor electrode. Nanoscale Res. Lett. 2018, 13, 242. [Google Scholar] [CrossRef] [Green Version]
- Honarpazhouh, Y.; Astaraei, F.R.; Naderi, H.R.; Tavakoli, O. Electrochemical hydrogen storage in Pd-coated porous silicon/graphene oxide. Int. J. Hydrogen Energy 2016, 41, 12175–12182. [Google Scholar] [CrossRef]
- Westover, A.S.; Freudiger, D.; Gani, Z.S.; Share, K.; Oakes, L.; Carter, R.E.; Pint, C.L. On-chip high power porous silicon lithium ion batteries with stable capacity over 10000 cycles. Nanoscale 2015, 7, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.X. Porous silicon: Morphology and formation mechanisms. In Modern Aspects of Electrochemistry; Springer: Berlin/Heidelberg, Germany, 2006; pp. 65–133. [Google Scholar]
- Lee, J.W.; Ko, J.M.; Kim, J.-D. Hydrothermal preparation of nitrogen-doped graphene sheets via hexamethylenetetramine for application as supercapacitor electrodes. Electrochim. Acta 2012, 85, 459–466. [Google Scholar] [CrossRef]
- Münder, H.; Andrzejak, C.; Berger, M.; Klemradt, U.; Lüth, H.; Herino, R.; Ligeon, M. A detailed Raman study of porous silicon. Thin Solid Film. 1992, 221, 27–33. [Google Scholar] [CrossRef]
- Goodes, S.; Jenkins, T.; Beale, M.; Benjamin, J.; Pickering, C. The characterisation of porous silicon by Raman spectroscopy. Semicond. Sci. Technol. 1988, 3, 483. [Google Scholar] [CrossRef]
- Sui, Z.; Leong, P.P.; Herman, I.P.; Higashi, G.S.; Temkin, H. Raman analysis of light-emitting porous silicon. Appl. Phys. Lett. 1992, 60, 2086–2088. [Google Scholar] [CrossRef]
- Lenshin, A.; Kashkarov, V.; Seredin, P.; Spivak, Y.M.; Moshnikov, V. XANES and IR spectroscopy study of the electronic structure and chemical composition of porous silicon on n-and p-type substrates. Semiconductors 2011, 45, 1183–1188. [Google Scholar] [CrossRef]
- Yang, M.; Shrestha, N.K.; Schmuki, P. Toward self-ordered silica nanotubes by electrochemical anodization of Si (100). Electrochem. Solid State Lett. 2010, 13, C25. [Google Scholar] [CrossRef]
- Merazga, S.; Cheriet, A.; M’hammedi, K.; Mefoued, A.; Gabouze, N. Investigation of porous silicon thin films for electrochemical hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 9994–10002. [Google Scholar] [CrossRef]
- Starkov, V.; Gavrilin, E. Gradient-porous structure of silicon. Phys. Status Solidi C 2007, 4, 2026–2028. [Google Scholar] [CrossRef]
- Starkov, V.; Gosteva, E.; Sedlovets, D.; Kah, M. Silicon structures with variable morphology of pores as a function of depth. Methods of obtaining physical and optical properties. J. Electrochem. Soc. 2018, 165, 534–539. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Jurewicz, K.; Frackowiak, E.; Béguin, F. Towards the mechanism of electrochemical hydrogen storage in nanostructured carbon materials. Appl. Phys. A 2004, 78, 981–987. [Google Scholar] [CrossRef]
- Starkov, V.; Gosteva, E.; Sedlovets, D.; Belorus, A. Nanoporous silicon structure with graphene coating. Microporous Mesoporous Mater. 2021, 316, 110981. [Google Scholar] [CrossRef]
- Pletcher, D.; Greff, R.; Peat, R.; Peter, L.; Robinson, J. Instrumental Methods in Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Kissinger, P.T.; Heineman, W.R. Cyclic voltammetry. J. Chem. Educ. 1983, 60, 702. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Penner, R.M. Energy storage in nanomaterials–capacitive, pseudocapacitive, or battery-like? ACS Nano 2018, 12, 2081–2083. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.Y.; Shinde, D.V.; Kim, E.-K.; Lee, W.; Oh, I.-W.; Shrestha, N.K.; Lee, J.K.; Han, S.-H. Supercapacitive property of metal–organic-frameworks with different pore dimensions and morphology. Microporous Mesoporous Mater. 2013, 171, 53–57. [Google Scholar] [CrossRef]
- Zhao, J.; Burke, A.F. Electrochemical capacitors: Materials, technologies and performance. Energy Storage Mater. 2021, 36, 31–55. [Google Scholar] [CrossRef]
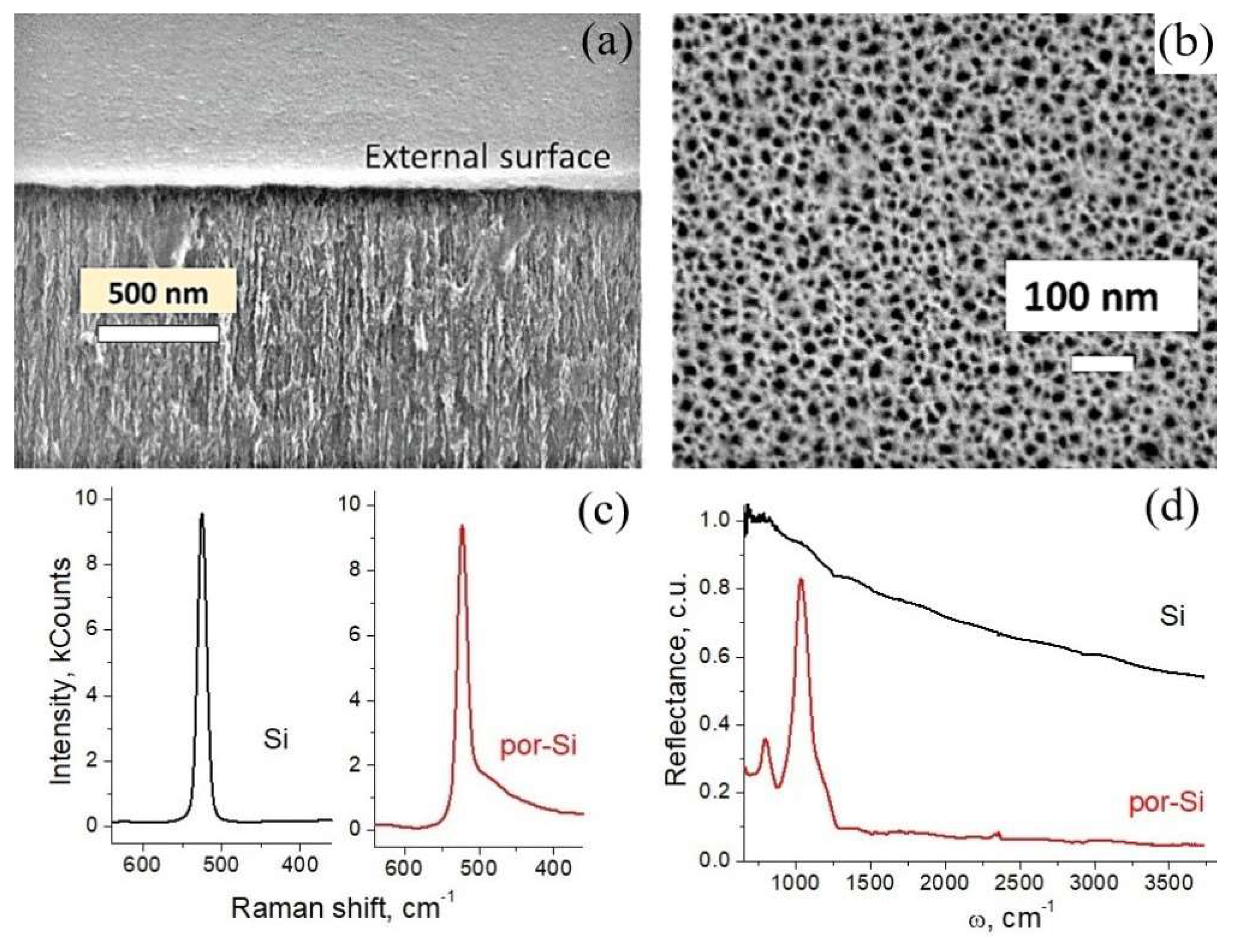


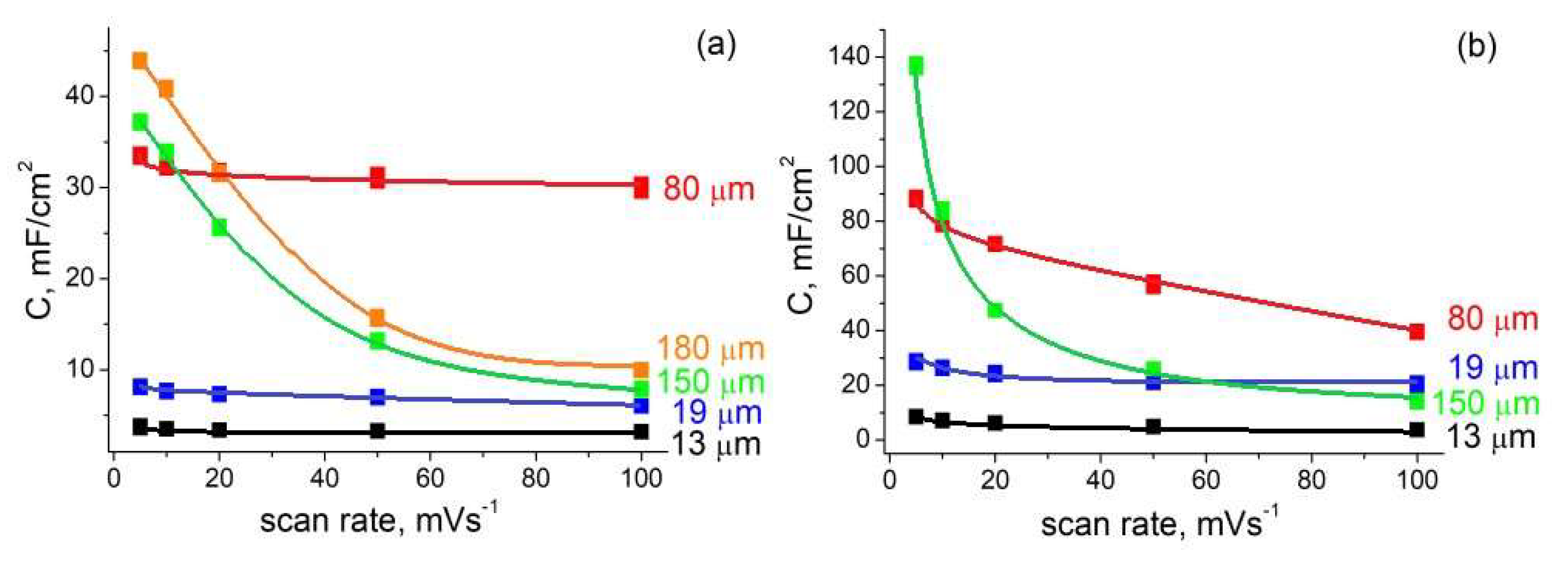
| Etching time, min | 3 | 5 | 20 | 45 | 60 |
|---|---|---|---|---|---|
| Thickness of porous layer *, μm | 13 | 19 | 80 | 150 | 180 |
| Porosity, % | 32 | 34 | 55 | 58 | 64 |
| Ref. | Capacitance Achieved | Por-Si Characteristics | Electrolyte | ||
|---|---|---|---|---|---|
| mF/cm2 | at | Depth, m | Coating | ||
| [15] | 381 | 20 A/g | ~ 80 | carbon + MnOx | 0.1 M EMIM-TSFI |
| [14] | 325 | 1 mA/cm2 | 120 | carbon | EMIM-TSFI |
| This work | 47 87 | 1 mA/cm2 5 mV/s | 80 | GLC | 3 M H2SO4 |
| [19] | 26.31 ** 15.38 ** | 0.5 A/g 100 A/g | 5 | GLC | 1 M KCl |
| [13] | 9 ** | – | 6 | TiN | 0.1 M NaCl |
| [20] | 8.16 | 1000 mV/s | – | few-layer graphene | |
| [17] | 4.38 ** | – | 6 | TiN | TEABF4/PC |
| [11] | 0.99 | 100 mV/s | – | nanocryst. diamond | 0.1 M KCl |
| [10] | 0.32 | – | 43 | Au | 20% H2SO4 |
| [12] | 0.2 | 10 mV/s | – | – | 0.25 M TEABF4/PC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedlovets, D.M.; Naumov, A.P.; Korotitsky, V.I.; Starkov, V.V. Nanoporous Silicon with Graphene-like Coating for Pseudocapacitor Application. Nanomaterials 2022, 12, 2191. https://doi.org/10.3390/nano12132191
Sedlovets DM, Naumov AP, Korotitsky VI, Starkov VV. Nanoporous Silicon with Graphene-like Coating for Pseudocapacitor Application. Nanomaterials. 2022; 12(13):2191. https://doi.org/10.3390/nano12132191
Chicago/Turabian StyleSedlovets, Daria M., Anton P. Naumov, Victor I. Korotitsky, and Vitaly V. Starkov. 2022. "Nanoporous Silicon with Graphene-like Coating for Pseudocapacitor Application" Nanomaterials 12, no. 13: 2191. https://doi.org/10.3390/nano12132191
APA StyleSedlovets, D. M., Naumov, A. P., Korotitsky, V. I., & Starkov, V. V. (2022). Nanoporous Silicon with Graphene-like Coating for Pseudocapacitor Application. Nanomaterials, 12(13), 2191. https://doi.org/10.3390/nano12132191





