Emerging Trends in the Remediation of Persistent Organic Pollutants Using Nanomaterials and Related Processes: A Review
Abstract
1. Introduction
2. POPs, Source, and Fate
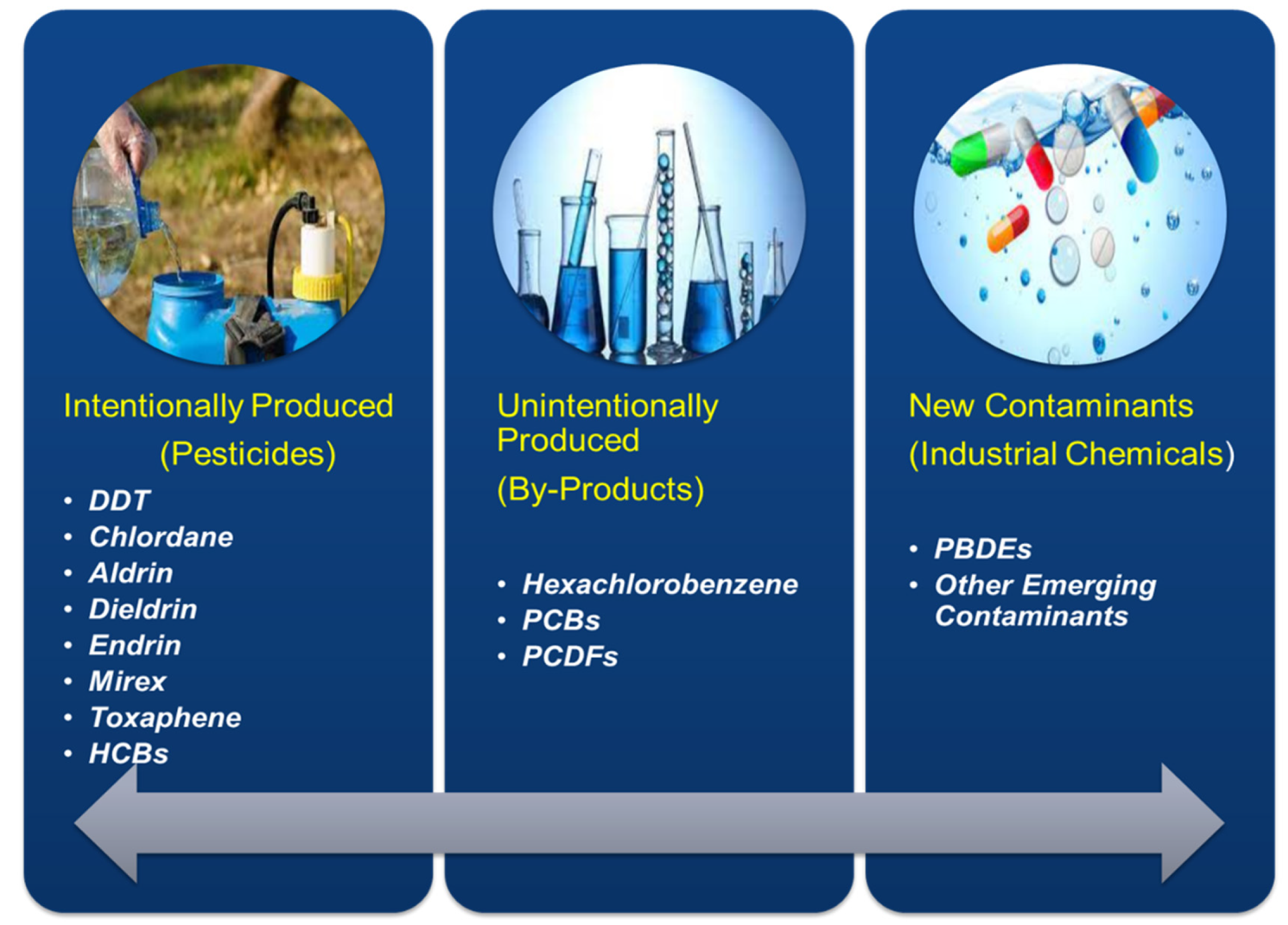
Various Categories of POPs
- (i)
- most hazardous chemical [restricted for production and utilization];
- (ii)
- medium level chemicals [confined to use during the production];
- (ii)
- unintentional discharge of chemical;
- (iv)
- use of chemicals under investigation.
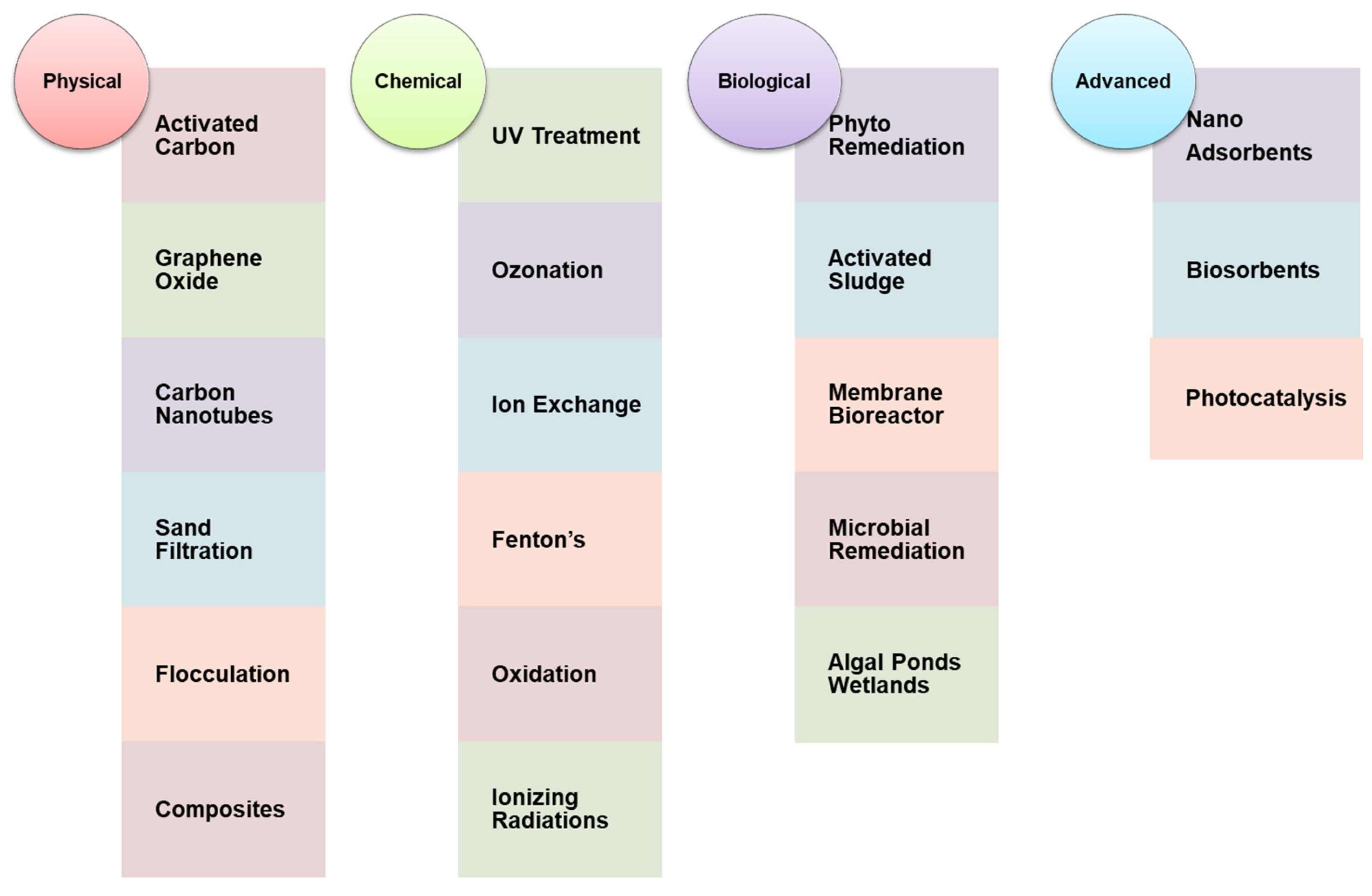
3. Conventional Treatment Technology
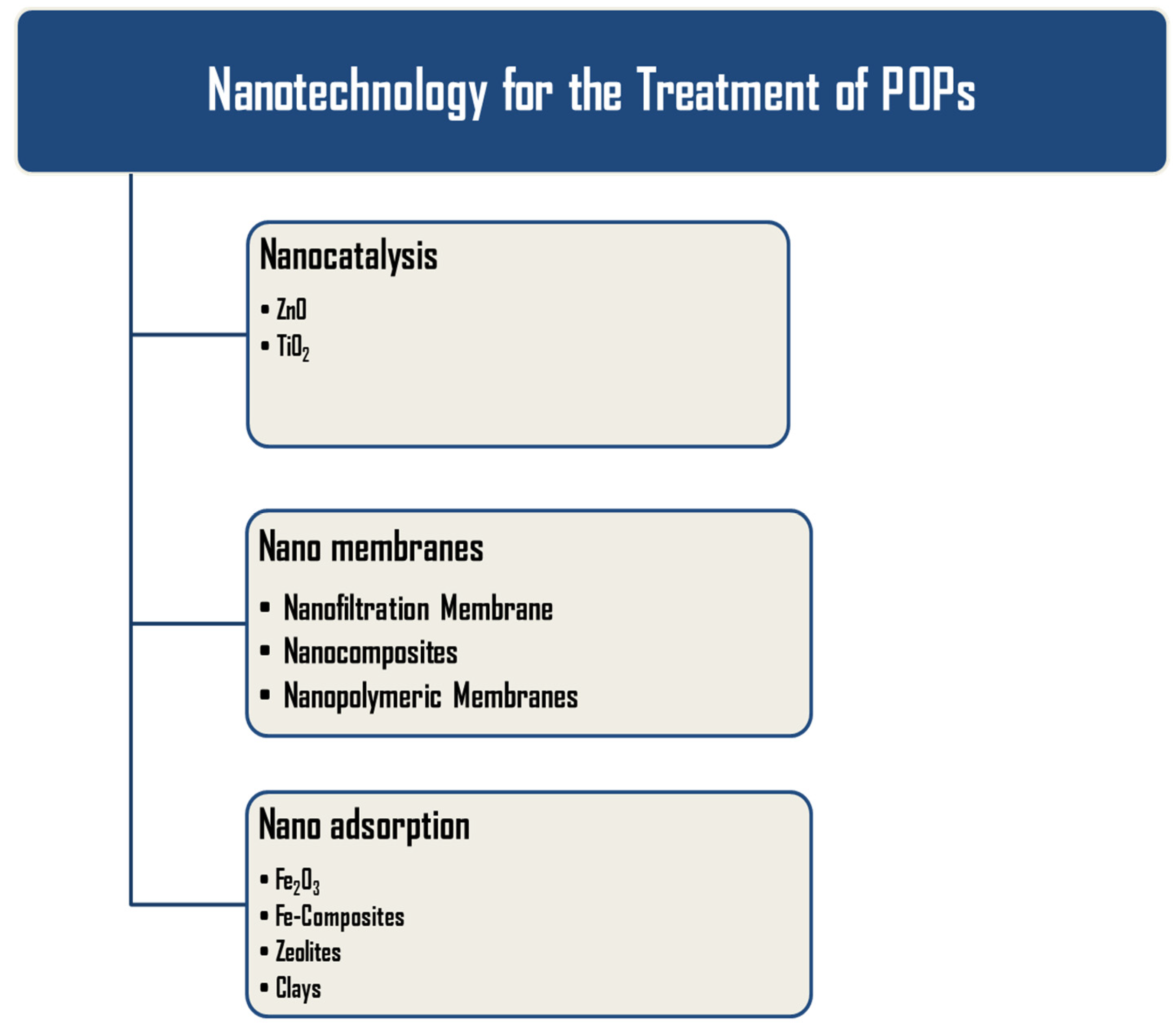
4. Nanotechnology for Environment Remediation
5. Advanced Nanotechnological Approaches for Removal of POPs
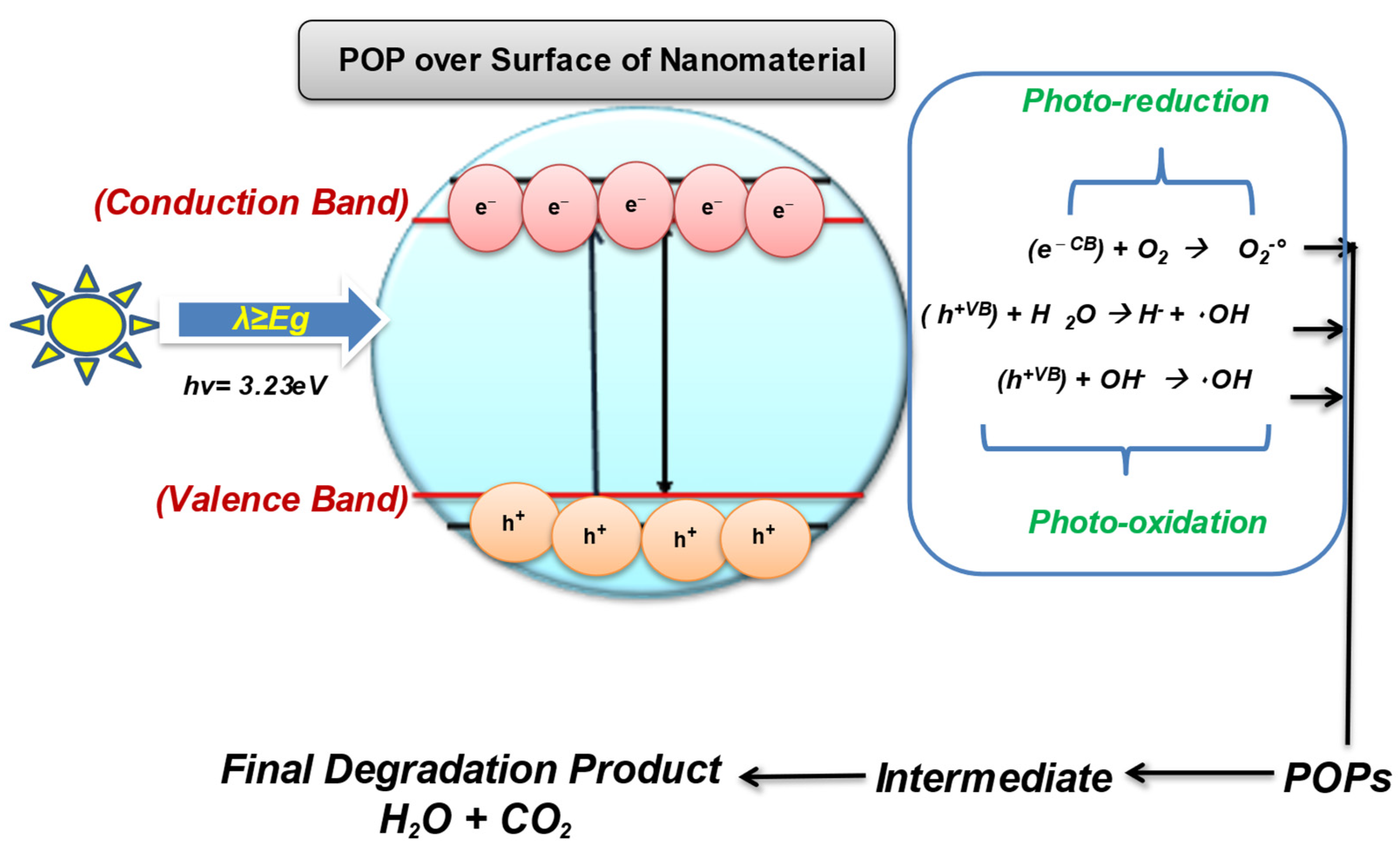
5.1. Nanocatalysis
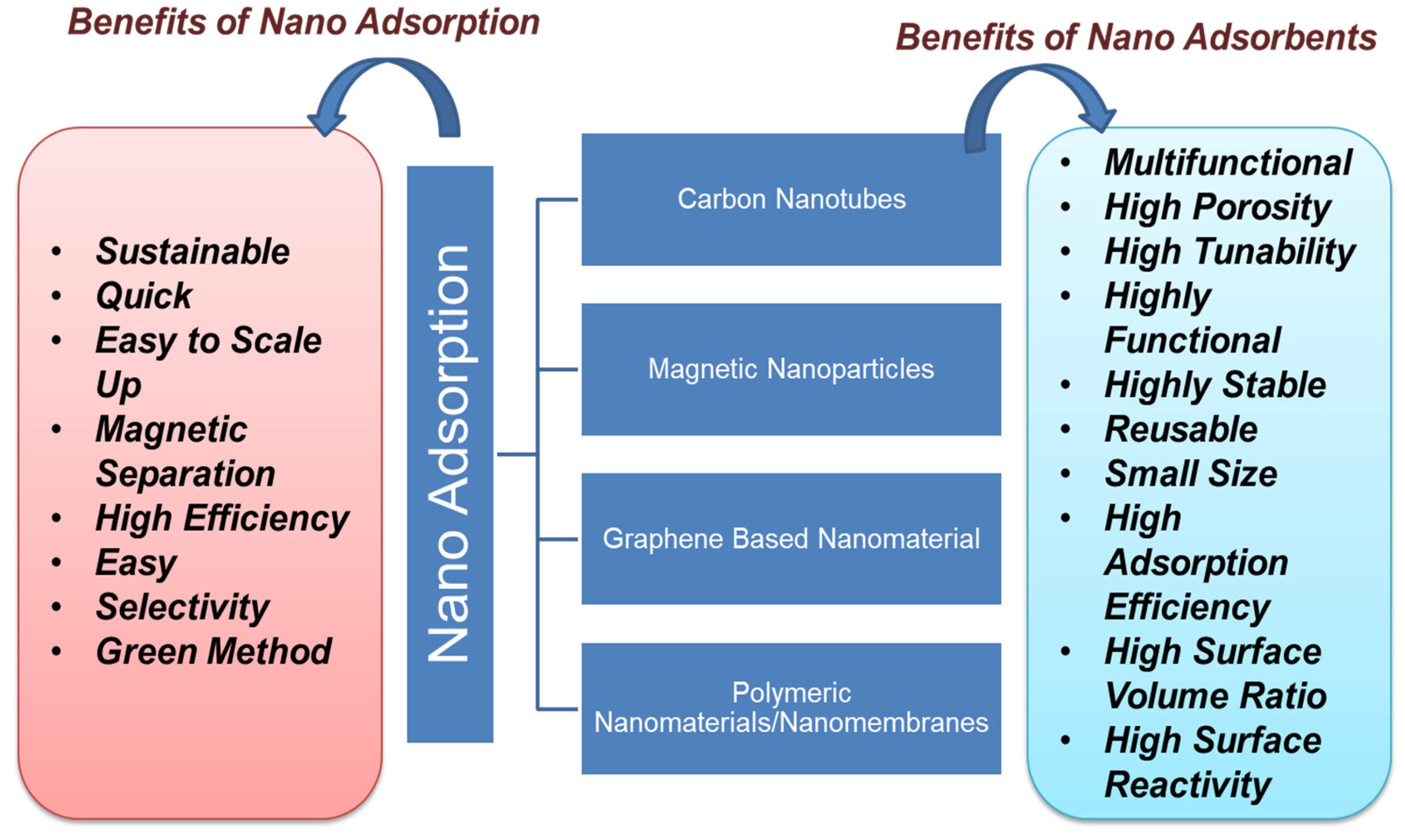
5.2. Nanoadsorption
5.3. Nanofiltration
5.4. Nanobiotechnology and Hybrid Technologies
6. Case Studies from Different Countries
7. Future Prospects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AgNP | Silver Nanoparticles |
| EDs | Endocrine disruptors |
| EPA | Environment Protection Agency |
| FC | Food Chain |
| HCB | Hexachlorobenzene |
| MF | Microfiltration |
| NF | Nanofiltration |
| OCs | Organochlorines |
| OP | Organophosphorus |
| PAHs | Polyaromatic hydrocarbons |
| PCBs | Polychlorinated biphenyls |
| PCBzs | Polychlorinated Benzenes |
| PCDD | Polychlorinated dibenzo-p-dioxins |
| PCDF | Polychlorinated dibenzofurans |
| PCP | Personal Care Products |
| PDBE | Polybrominated diphenyl ethers |
| PF | Perfluorinated compounds |
| POPs | Persistent Organic Pollutants |
| RO | Reverse osmosis |
| ROS | Reactive Oxygen Species |
| SVR | Surface Volume Ratio |
| UF | Ultrafiltration |
| VOCs | Volatile Organic Compounds |
| ZnO | Zinc Oxide |
References
- Alharbi, O.M.; Khattab, R.A.; Ali, I. Health and environmental effects of persistent organic pollutants. J. Mol. Liq. 2018, 263, 442–453. [Google Scholar] [CrossRef]
- El-Shahawi, M.S.; Hamza, A.; Bashammakh, A.S.; Al-Saggaf, W.T. An overview on the accumulation, distribution, transformations, toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta 2010, 80, 1587–1597. [Google Scholar] [CrossRef]
- Fernández, P.; Grimalt, J.O. On the global distribution of persistent organic pollutants. CHIMIA Int. J. Chem. 2003, 57, 514–521. [Google Scholar] [CrossRef]
- Stokke, O.S.; Thommessen, O.B. Stockholm Convention Persistent Organic Pollutants (Stockholm Convention: POPs). In Yearbook of International Cooperation on Environment and Development 2001-02; Routledge: London, UK, 2013; pp. 122–123. [Google Scholar]
- Alam, J.; Yadav, V.K.; Yadav, K.K.; Cabral-Pinto, M.M.; Tavker, N.; Choudhary, N.; Shukla, A.K.; Ali, F.A.A.; Alhoshan, M.; Hamid, A.A. Recent advances in methods for the recovery of carbon nanominerals and polyaromatic hydrocarbons from coal fly ash and their emerging applications. Crystals 2021, 11, 88. [Google Scholar] [CrossRef]
- Kelly, B.C.; Ikonomou, M.G.; Blair, J.D.; Morin, A.E.; Gobas, F.A. Food web specific biomagnification of persistent organic pollutants. Science 2007, 317, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, T.; Fréour, T.; Ploteau, S.; Le Bizec, B.; Antignac, J.P.; Cano-Sancho, G. Associations between human internal chemical exposure to Persistent Organic Pollutants (POPs) and In Vitro Fertilization (IVF) outcomes: Systematic review and evidence map of human epidemiological evidence. Reprod. Toxicol. 2021, 105, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Adithya, S.; Jayaraman, R.S.; Krishnan, A.; Malolan, R.; Gopinath, K.P.; Arun, J.; Govarthanan, M. A critical review on the formation, fate and degradation of the persistent organic pollutant hexachlorocyclohexane in water systems and waste streams. Chemosphere 2021, 271, 129866. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J. A review of the accumulation and distribution of persistent organic pollutants in the environment. Int. J. Biosci. Biochem. Bioinform. 2013, 3, 657. [Google Scholar] [CrossRef][Green Version]
- Lallas, P.L. The Stockholm Convention on persistent organic pollutants. Am. J. Int. Law 2001, 95, 692–708. [Google Scholar] [CrossRef]
- Xie, L.; Du, T.; Wang, J.; Ma, Y.; Ni, Y.; Liu, Z.; Wang, J. Recent advances on heterojunction-based photocatalysts for the degradation of persistent organic pollutants. Chem. Eng. J. 2021, 426, 130617. [Google Scholar] [CrossRef]
- Kanzari, F.; Syakti, A.D.; Asia, L.; Malleret, L.; Piram, A.; Mille, G.; Doumenq, P. Distributions and sources of persistent organic pollutants (aliphatic hydrocarbons, PAHs, PCBs and pesticides) in surface sediments of an industrialized urban river (Huveaune), France. Sci. Total Environ. 2014, 478, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Venier, M.; Salamova, A.; Hites, R.A. How to distinguish urban vs. agricultural sources of persistent organic pollutants? Curr. Opin. Environ. Sci. Health 2019, 8, 23–28. [Google Scholar] [CrossRef]
- Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Hong, H.Z. Persistent organic pollutants in food: Contamination sources, health effects and detection methods. Int. J. Environ. Res. Public Health 2019, 16, 4361. [Google Scholar] [CrossRef] [PubMed]
- Eljarrat, E.; Barcelo, D. Priority lists for persistent organic pollutants and emerging contaminants based on their relative toxic potency in environmental samples. TrAC Trends Anal. Chem. 2003, 1, 655–665. [Google Scholar] [CrossRef]
- Göktaş, R.K.; MacLeod, M. Remoteness from sources of persistent organic pollutants in the multi-media global environment. Environ. Pollut. 2016, 217, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Adeola, F.O. Boon or bane? The environmental and health impacts of persistent organic pollutants (POPs). Hum. Ecol. Rev. 2004, 11, 27–35. [Google Scholar]
- Abelsohn, A.; Gibson, B.L.; Sanborn, M.D.; Weir, E. Identifying and managing adverse environmental health effects: Persistent organic pollutants. CMAJ 2002, 166, 1549–1554. [Google Scholar]
- Qing Li, Q.; Loganath, A.; Seng Chong, Y.; Tan, J.; Philip Obbard, J. Persistent organic pollutants and adverse health effects in humans. J. Toxicol. Environ. Health Part A 2006, 69, 1987–2005. [Google Scholar] [CrossRef]
- Gaur, N.; Narasimhulu, K.; PydiSetty, Y. Recent advances in the bio-remediation of persistent organic pollutants and its effect on environment. J. Clean. Prod. 2018, 198, 1602–1631. [Google Scholar] [CrossRef]
- Sun, B.; Li, Q.; Zheng, M.; Su, G.; Lin, S.; Wu, M.; Meng, B. Recent advances in the removal of persistent organic pollutants (POPs) using multifunctional materials: A review. Environ. Pollut. 2020, 265, 114908. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Smith, S.M.; Wantala, K.; Kajitvichyanukul, P. Photocatalytic remediation of persistent organic pollutants (POPs): A review. Arab. J. Chem. 2020, 13, 8309–8337. [Google Scholar] [CrossRef]
- Karthigadevi, G.; Manikandan, S.; Karmegam, N.; Subbaiya, R.; Chozhavendhan, S.; Ravindran, B.; Awasthi, M.K. Chemico-nanotreatment methods for the removal of persistent organic pollutants and xenobiotics in water—A review. Bioresour. Technol. 2021, 324, 124678. [Google Scholar] [CrossRef] [PubMed]
- Ebrahiem, E.E.; Al-Maghrabi, M.N.; Mobarki, A.R. Removal of organic pollutants from industrial wastewater by applying photo-Fenton oxidation technology. Arab. J. Chem. 2017, 10, S1674–S1679. [Google Scholar] [CrossRef]
- Thirunavukkarasu, A.; Nithya, R.; Sivashankar, R. A review on the role of nanomaterials in the removal of organic pollutants from wastewater. Rev. Environ. Sci. Bio/Technol. 2020, 19, 751–778. [Google Scholar] [CrossRef]
- Kang, J.W. Removing environmental organic pollutants with bioremediation and phytoremediation. Biotechnol. Lett. 2014, 36, 1129–1139. [Google Scholar] [CrossRef]
- Tijani, J.O.; Fatoba, O.O.; Madzivire, G.; Petrik, L.F. A review of combined advanced oxidation technologies for the removal of organic pollutants from water. Water Air Soil Pollut. 2014, 225, 2102. [Google Scholar] [CrossRef]
- Puzyn, T.; Mostrag, A. (Eds.) Organic Pollutants Ten Years after the Stockholm Convention: Environmental and Analytical Update; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Seow, T.W.; Lim, C.K.; Nor, M.H.M.; Mubarak, M.F.M.; Lam, C.Y.; Yahya, A.; Ibrahim, Z. Review on wastewater treatment technologies. Int. J. Appl. Environ. Sci. 2016, 11, 111–126. [Google Scholar]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Bora, T.; Dutta, J. Applications of nanotechnology in wastewater treatment—A review. J. Nanosci. Nanotechnol. 2014, 14, 613–626. [Google Scholar] [CrossRef]
- Cheriyamundath, S.; Vavilala, S.L. Nanotechnology-based wastewater treatment. Water Environ. J. 2021, 35, 123–132. [Google Scholar] [CrossRef]
- Singh, S.P.; Batra, R.D. Nanotechnology in wastewater treatment: A review. In Novel Applications in Polymers and Waste Management, 1st ed.; Apple Academic Press: New York, NY, USA, 2018; pp. 173–182. [Google Scholar] [CrossRef]
- Khan, N.A.; Khan, S.U.; Ahmed, S.; Farooqi, I.H.; Dhingra, A.; Hussain, A.; Changani, F. Applications of nanotechnology in water and wastewater treatment: A review. Asian J. Water Environ. Pollut. 2019, 16, 81–86. [Google Scholar] [CrossRef]
- Kunduru, K.R.; Nazarkovsky, M.; Farah, S.; Pawar, R.P.; Basu, A.; Domb, A.J. Nanotechnology for water purification: Applications of nanotechnology methods in wastewater treatment. In Water Purification; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 33–74. [Google Scholar] [CrossRef]
- Khan, S.H. Green nanotechnology for the environment and sustainable development. In Green Materials for Wastewater Treatment; Springer: Cham, Switzerland, 2020; pp. 13–46. [Google Scholar]
- Zhi, D.; Lin, Y.; Jiang, L.; Zhou, Y.; Huang, A.; Yang, J.; Luo, L. Remediation of persistent organic pollutants in aqueous systems by electrochemical activation of persulfates: A review. J. Environ. Manag. 2020, 260, 110125. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Bahadur, N.; Dumée, L.F. Photo-catalytic membrane reactors for the remediation of persistent organic pollutants–A review. Sep. Purif. Technol. 2020, 230, 115878. [Google Scholar] [CrossRef]
- Pi, Y.; Li, X.; Xia, Q.; Wu, J.; Li, Y.; Xiao, J.; Li, Z. Adsorptive and photocatalytic removal of Persistent Organic Pollutants (POPs) in water by metal-organic frameworks (MOFs). Chem. Eng. J. 2018, 337, 351–371. [Google Scholar] [CrossRef]
- Lu, F.; Astruc, D. Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coord. Chem. Rev. 2020, 408, 213180. [Google Scholar] [CrossRef]
- Zhang, S.; Li, B.; Wang, X.; Zhao, G.; Hu, B.; Lu, Z.; Wang, X. Recent developments of two-dimensional graphene-based composites in visible-light photocatalysis for eliminating persistent organic pollutants from wastewater. Chem. Eng. J. 2020, 390, 124642. [Google Scholar] [CrossRef]
- Fei, L.; Bilal, M.; Qamar, S.A.; Imran, H.M.; Riasat, A.; Jahangeer, M.; Iqbal, H.M. Nano-remediation technologies for the sustainable mitigation of persistent organic pollutants. Environ. Res. 2022, 211, 113060. [Google Scholar] [CrossRef]
- Das, S.; Chakraborty, J.; Chatterjee, S.; Kumar, H. Prospects of biosynthesized nanomaterials for the remediation of organic and inorganic environmental contaminants. Environ. Sci. Nano 2018, 5, 2784–2808. [Google Scholar] [CrossRef]
- Taghizade Firozjaee, T.; Mehrdadi, N.; Baghdadi, M.; Nabi Bidhendi, G.R. Application of nanotechnology in pesticides removal from aqueous solutions-a review. Int. J. Nanosci. Nanotechnol. 2018, 14, 43–56. [Google Scholar]
- Zhang, W.; Zhang, D.; Liang, Y. Nanotechnology in remediation of water contaminated by poly-and perfluoroalkyl substances: A review. Environ. Pollut. 2019, 247, 266–276. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Akhdhar, A.; Hamza, M.F. Recent advances in greenly synthesized nanoengineered materials for water/wastewater remediation: An overview. Nanotechnol. Environ. Eng. 2021, 6, 9. [Google Scholar] [CrossRef]
- Trojanowicz, M. Removal of persistent organic pollutants (POPs) from waters and wastewaters by the use of ionizing radiation. Sci. Total Environ. 2020, 718, 134425. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H.; Pathak, B. Zinc oxide based photocatalytic degradation of persistent pesticides: A comprehensive review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100290. [Google Scholar] [CrossRef]
- Badmus, K.O.; Tijani, J.O.; Massima, E.; Petrik, L. Treatment of persistent organic pollutants in wastewater using hydrodynamic cavitation in synergy with advanced oxidation process. Environ. Sci. Pollut. Res. 2018, 25, 7299–7314. [Google Scholar] [CrossRef]
- Bhavya, G.; Belorkar, S.A.; Mythili, R.; Geetha, N.; Shetty, H.S.; Udikeri, S.S.; Jogaiah, S. Remediation of emerging environmental pollutants: A review based on advances in the uses of eco-friendly biofabricated nanomaterials. Chemosphere 2021, 275, 129975. [Google Scholar] [CrossRef]
- Jagirani, M.S.; Soylak, M. A review: Recent advances in solid phase microextraction of toxic pollutants using nanotechnology scenario. Microchem. J. 2020, 159, 105436. [Google Scholar] [CrossRef]
- Manikandan, S.; Subbaiya, R.; Saravanan, M.; Ponraj, M.; Selvam, M.; Pugazhendhi, A. A critical review of advanced nanotechnology and hybrid membrane-based water recycling, reuse, and wastewater treatment processes. Chemosphere 2022, 289, 132867. [Google Scholar] [CrossRef]
- Bhat, S.A.; Sher, F.; Hameed, M.; Bashir, O.; Kumar, R.; Vo, D.V.N.; Lima, E.C. Sustainable nanotechnology-based wastewater treatment strategies: Achievements, challenges and future perspectives. Chemosphere 2022, 288, 132606. [Google Scholar] [CrossRef]
- Desireddy, S.; Sabumon, P.C. Emerging Contaminants Removal from Wastewater by Nanotechnological Methods. In New Trends in Emerging Environmental Contaminants; Springer: Singapore, 2022; pp. 261–285. [Google Scholar]
- Daramola, I.O.; Adebayo, M.A. Recent Developments in the Application of Advanced Oxidative Processes for Remediation of Persistent Organic Pollutants from Water. Persistent Org. Pollut. (POPs) Monit. Impact Treat. 2022, 27, 139. [Google Scholar]
- Al Ja’farawy, M.S.; Purwanto, A.; Widiyandari, H. Carbon quantum dots supported zinc oxide (ZnO/CQDs) efficient photocatalyst for organic pollutant degradation–A systematic review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100681. [Google Scholar]
- Ganie, A.S.; Bano, S.; Khan, N.; Sultana, S.; Rehman, Z.; Rahman, M.M.; Khan, M.Z. Nanoremediation technologies for sustainable remediation of contaminated environments: Recent advances and challenges. Chemosphere 2021, 275, 130065. [Google Scholar] [CrossRef] [PubMed]
- Rane, N.R.; Tapase, S.; Kanojia, A.; Watharkar, A.; Salama, E.S.; Jang, M.; Jeon, B.H. Molecular insights into plant–microbe interactions for sustainable remediation of contaminated environment. Bioresour. Technol. 2022, 344, 126246. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, H.A.; Rafatullah, M.; Ali, K.A.; Umar, M.F.; Khan, M.A.; Jeon, B.H. Photocatalytic activity of graphene oxide/zinc oxide nanocomposite derived from rice husk for the degradation of phenanthrene under ultraviolet-visible light. J. Water Process Eng. 2022, 47, 102714. [Google Scholar] [CrossRef]
- Dhingra, R.; Naidu, S.; Upreti, G.; Sawhney, R. Sustainable nanotechnology: Through green methods and life-cycle thinking. Sustainability 2010, 2, 3323–3338. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D. Nanomaterials: Green synthesis for water applications. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O., Martínez, L., Kharisov, B., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–21. [Google Scholar] [CrossRef]
- Khan, S.H.; Pathak, B.; Fulekar, M.H. A study on the influence of metal (Fe, Bi, and Ag) doping on structural, optical, and antimicrobial activity of ZnO nanostructures. Adv. Compos. Hybrid Mater. 2020, 3, 551–569. [Google Scholar] [CrossRef]
- Kong, X.P.; Zhang, B.H.; Wang, J. Multiple roles of mesoporous silica in safe pesticide application by nanotechnology: A review. J. Agric. Food Chem. 2021, 69, 6735–6754. [Google Scholar] [CrossRef]
- Sarno, M.; Casa, M.; Cirillo, C.; Ciambelli, P. Complete removal of persistent pesticide using reduced graphene oxide-silver nanocomposite. Chem. Eng. Trans. 2017, 60, 151–156. [Google Scholar]
- Abdennouri, M.; Baâlala, M.; Galadi, A.; El Makhfouk, M.; Bensitel, M.; Nohair, K.; Barka, N. Photocatalytic degradation of pesticides by titanium dioxide and titanium pillared purified clays. Arab. J. Chem. 2016, 9, S313–S318. [Google Scholar] [CrossRef]
- Navarro, S.; Fenoll, J.; Vela, N.; Ruiz, E.; Navarro, G. Photocatalytic degradation of eight pesticides in leaching water by use of ZnO under natural sunlight. J. Hazard. Mater. 2009, 172, 1303–1310. [Google Scholar] [CrossRef]
- Luna-Sanguino, G.; Ruíz-Delgado, A.; Tolosana-Moranchel, A.; Pascual, L.; Malato, S.; Bahamonde, A.; Faraldos, M. Solar photocatalytic degradation of pesticides over TiO2-rGO nanocomposites at pilot plant scale. Sci. Total Environ. 2020, 737, 140286. [Google Scholar] [CrossRef]
- Veerakumar, P.; Sangili, A.; Saranya, K.; Pandikumar, A.; Lin, K.C. Palladium and silver nanoparticles embedded on zinc oxide nanostars for photocatalytic degradation of pesticides and herbicides. Chem. Eng. J. 2021, 410, 128434. [Google Scholar] [CrossRef]
- Rui, Z.E.N.G.; Jingguo, W.A.N.G.; Jianyu, C.U.I.; Lin, H.U.; Kangguo, M.U. Photocatalytic degradation of pesticide residues with RE3+-doped nano-TiO2. J. Rare Earths 2010, 28, 353–356. [Google Scholar]
- Ahamad, T.; Naushad, M.; Al-Saeedi, S.I.; Almotairi, S.; Alshehri, S.M. Fabrication of MoS2/ZnS embedded in N/S doped carbon for the photocatalytic degradation of pesticide. Mater. Lett. 2020, 263, 127271. [Google Scholar] [CrossRef]
- Tabasum, A.; Alghuthaymi, M.; Qazi, U.Y.; Shahid, I.; Abbas, Q.; Javaid, R.; Zahid, M. UV-accelerated photocatalytic degradation of pesticide over magnetite and cobalt ferrite decorated graphene oxide composite. Plants 2020, 10, 6. [Google Scholar] [CrossRef]
- Iqbal, A.; Haq, A.U.; Cerrón-Calle, G.A.; Naqvi, S.A.R.; Westerhoff, P.; Garcia-Segura, S. Green synthesis of flower-shaped copper oxide and nickel oxide nanoparticles via Capparis decidua leaf extract for synergic adsorption-photocatalytic degradation of pesticides. Catalysts 2021, 11, 806. [Google Scholar] [CrossRef]
- Khan, S.H.; Pathak, B.; Fulekar, M.H. Synthesis, characterization and photocatalytic degradation of chlorpyrifos by novel Fe: ZnO nanocomposite material. Nanotechnol. Environ. Eng. 2018, 3, 13. [Google Scholar] [CrossRef]
- Singh, M.; Rano, S.; Roy, S.; Mukherjee, P.; Dalui, S.; Gupta, G.K.; Mondal, M.K. Characterization of organophosphate pesticide sorption of potato peel biochar as low cost adsorbent for chlorpyrifos removal. Chemosphere 2022, 297, 134112. [Google Scholar] [CrossRef] [PubMed]
- Bandala, E.R.; Gelover, S.; Leal, M.T.; Arancibia-Bulnes, C.; Jimenez, A.; Estrada, C.A. Solar photocatalytic degradation of Aldrin. Catal. Today 2002, 76, 189–199. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Ismail, A.; Mostafa, M.K.; Mahmoud, M.S.; Ali, W.; Shawky, A.M. Isotherm and kinetic studies for heptachlor removal from aqueous solution using Fe/Cu nanoparticles, artificial intelligence, and regression analysis. Sep. Sci. Technol. 2020, 55, 684–696. [Google Scholar] [CrossRef]
- Nagappa, D.; Manu, B. Nano-scale Iron Oxide as Heterogeneous Fenton Catalyst for Organic Pollution Degradation and Heavy Metal Remediation in Water Sample of Byramangala Lake, Karnataka. Asian J. Water Environ. Pollut. 2019, 16, 25–33. [Google Scholar] [CrossRef]
- Mukherjee, R.; Sinha, A.; Lama, Y.; Kumar, V. Utilization of zerovalent iron (zvi) particles produced from steel industry waste for in-situ remediation of groundwater contaminated with organo-chlorine pesticide heptachlor. Int. J. Environ. Res. 2015, 9, 19–26. [Google Scholar]
- Bhuvaneswari, R.; Nagarajan, V.; Chandiramouli, R. Chemisorption of Heptachlor and Mirex molecules on beta arsenene nanotubes–A first-principles analysis. Appl. Surf. Sci. 2021, 537, 147835. [Google Scholar] [CrossRef]
- Wan, J.; Ma, Y.; Zhang, S.; Long, T.; Lin, Y.; Li, Q.; Li, C. Anionic/nonionic surfactant enhanced microscale Cu/Fe bimetal degrading chlordane and mirex in water and soil slurry. J. Ecol. Rural Environ. 2014, 30, 754–760. [Google Scholar]
- Momić, T.; Pašti, T.L.; Bogdanović, U.; Vodnik, V.; Mraković, A.; Rakočević, Z.; Vasić, V. Adsorption of organophosphate pesticide dimethoate on gold nanospheres and nanorods. J. Nanomater. 2016, 2016, 8910271. [Google Scholar] [CrossRef]
- Sarno, M.; Cirillo, C.; Ciambelli, P. Dehalogenation and contemporaneous removal of halocarbon using Graphene/Ni nanoparticles. Chem. Eng. Trans. 2016, 47, 169–174. [Google Scholar]
- Abbas, T.; Wadhawan, T.; Khan, A.; McEvoy, J.; Khan, E. Virgin (Fe0) and microbially regenerated (Fe2+) iron turning waste for treating chlorinated pesticides in water. J. Hazard. Mater. 2020, 398, 122980. [Google Scholar] [CrossRef]
- Jia, M.; Su, G.; Zheng, M.; Zhang, B.; Lin, S. Development of self-assembled 3D FexOy micro/nano materials for application in hexachlorobenzene degradation. J. Nanosci. Nanotechnol. 2011, 11, 2100–2106. [Google Scholar] [CrossRef]
- Su, G.; Liu, Y.; Huang, L.; Lu, H.; Liu, S.; Li, L.; Zheng, M. Synthesis of hierarchical Mg-doped Fe3O4 micro/nano materials for the decomposition of hexachlorobenzene. Chemosphere 2014, 99, 216–223. [Google Scholar] [CrossRef]
- Wiltschka, K.; Neumann, L.; Werheid, M.; Bunge, M.; Duering, R.A.; Mackenzie, K.; Böhm, L. Hydrodechlorination of hexachlorobenzene in a miniaturized nano-Pd (0) reaction system combined with the simultaneous extraction of all dechlorination products. Appl. Catal. B Environ. 2020, 275, 119100. [Google Scholar] [CrossRef]
- Chen, W.F.; Pan, L.; Chen, L.F.; Wang, Q.; Yan, C.C. Dechlorination of hexachlorobenzene by nano zero-valent iron/activated carbon composite: Iron loading, kinetics and pathway. RSC Adv. 2014, 4, 46689–46696. [Google Scholar] [CrossRef]
- Yang, J.; Jia, M. Novel Co–Fe–O micro/nanostructures manipulated by urea and their applications in degradation of hexachlorobenzene. Mater. Res. Innov. 2018, 22, 159–167. [Google Scholar] [CrossRef]
- Garbou, A.M.; Liu, M.; Zou, S.; Yestrebsky, C.L. Degradation kinetics of hexachlorobenzene over zero-valent magnesium/graphite in protic solvent system and modeling of degradation pathways using density functional theory. Chemosphere 2019, 222, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.H.; Hsu, C.Y.; Su, Y.F. Reduction of hexachlorobenzene by nanoscale zero-valent iron: Kinetics, pH effect, and degradation mechanism. Sep. Purif. Technol. 2011, 76, 268–274. [Google Scholar] [CrossRef]
- Fan, G.; Cang, L.; Qin, W.; Zhou, C.; Gomes, H.I.; Zhou, D. Surfactants-enhanced electrokinetic transport of xanthan gum stabilized nanoPd/Fe for the remediation of PCBs contaminated soils. Sep. Purif. Technol. 2013, 114, 64–72. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.; Yang, K.; Zhu, L.; White, J.C.; Lin, D. Synergistic remediation of PCB-contaminated soil with nanoparticulate zero-valent iron and alfalfa: Targeted changes in the root metabolite-dependent microbial community. Environ. Sci. Nano 2021, 8, 986–999. [Google Scholar] [CrossRef]
- Liu, J.; Chen, T.; Qi, Z.; Yan, J.; Buekens, A.; Li, X. Thermal desorption of PCBs from contaminated soil using nano zerovalent iron. Environ. Sci. Pollut. Res. 2014, 21, 12739–12746. [Google Scholar] [CrossRef]
- Hennebel, T.; Fitts, J.; Van Nevel, S.; Boon, N. Pd nanocatalysts for PCB removal. In Nanotechnology for Water and Wastewater Treatment; Lens, P.L.N., Virkutyte, J., Jegatheesan, V., Kim, S.-H., Al-Abed, S., Eds.; IWA Publishing: London, UK, 2013; Volume 12, pp. 209–227. [Google Scholar] [CrossRef][Green Version]
- Tunçal, T.; Uslu, O. Industrial sludge remediation with photonic treatment using Ti–Ag nano-composite thin films: Persistent organic pollutant removal from sludge matrix. J. Environ. Manag. 2015, 149, 37–45. [Google Scholar] [CrossRef]
- Wu, T.; Liao, X.; Zou, Y.; Liu, Y.; Yang, K.; White, J.C.; Lin, D. Fe-based nanomaterial transformation to amorphous Fe: Enhanced alfalfa rhizoremediation of PCBs-contaminated soil. J. Hazard. Mater. 2022, 425, 127973. [Google Scholar] [CrossRef]
- Chen, B.; Meng, G.; Zhou, F.; Huang, Q.; Zhu, C.; Hu, X.; Kong, M. Ordered arrays of Au-nanobowls loaded with Ag-nanoparticles as effective SERS substrates for rapid detection of PCBs. Nanotechnology 2014, 25, 145605. [Google Scholar] [CrossRef]
- Abdelbasir, S.M.; Shalan, A.E. An overview of nanomaterials for industrial wastewater treatment. Korean. J. Chem. Eng. 2019, 36, 1209–1225. [Google Scholar]
- Dhaka, S.; Kumar, R.; Deep, A.; Kurade, M.B.; Ji, S.W.; Jeon, B.H. Metal–organic frameworks (MOFs) for the removal of emerging contaminants from aquatic environments. Coord. Chem. Rev. 2019, 380, 330–352. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Liu, F.; Yan, H.; Li, J.; Zhang, X. Reduction of Gibbs free energy and enhancement of Methanosaeta by bicarbonate to promote anaerobic syntrophic butyrate oxidation. Bioresour. Technol. 2018, 267, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Yadav, V.K.; Gacem, A.; Ali, I.H.; Dave, D.; Khan, S.H.; Jeon, B.H. Recent and Emerging Trends in Remediation of Methylene Blue Dye from Wastewater by Using Zinc Oxide Nanoparticles. Water 2022, 14, 1749. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Ali, D.; Yadav, V.K.; Dhinagaran, G.; Venkatachalam, K.; Narayanan, V. New construction of Fe3O4/rGO/ZnSnO3 nanocomposites enhanced photoelectro chemical properties. Opt. Mater. 2020, 109, 110353. [Google Scholar] [CrossRef]
- Waghmode, T.R.; Kurade, M.B.; Sapkal, R.T.; Bhosale, C.H.; Jeon, B.H.; Govindwar, S.P. Sequential photocatalysis and biological treatment for the enhanced degradation of the persistent azo dye methyl red. J. Hazard. Mater. 2019, 371, 115–122. [Google Scholar] [CrossRef]
- Khan, S.H.; Yadav, V.K. Advanced oxidation processes for wastewater remediation: An overview. In Removal of Emerging Contaminants Through Microbial Processes, Springer: Singapore, 2021; pp. 71–93. [CrossRef]
- Doll, T.E.; Frimmel, F.H. Removal of selected persistent organic pollutants by heterogeneous photocatalysis in water. Catal. Today 2005, 101, 195–202. [Google Scholar] [CrossRef]
- Li, K.; Yan, L.; Zeng, Z.; Luo, S.; Luo, X.; Liu, X.; Guo, Y. Fabrication of H3PW12O40-doped carbon nitride nanotubes by one-step hydrothermal treatment strategy and their efficient visible-light photocatalytic activity toward representative aqueous persistent organic pollutants degradation. Appl. Catal. B Environ. 2014, 156, 141–152. [Google Scholar] [CrossRef]
- Vaya, D.; Surolia, P.K. Semiconductor based photocatalytic degradation of pesticides: An overview. Environ. Technol. Innov. 2020, 20, 101128. [Google Scholar] [CrossRef]
- Devipriya, S.; Yesodharan, S. Photocatalytic degradation of pesticide contaminants in water. Sol. Energy Mater. Sol. Cells 2005, 86, 309–348. [Google Scholar] [CrossRef]
- Hadei, M.; Mesdaghinia, A.; Nabizadeh, R.; Mahvi, A.H.; Rabbani, S.; Naddafi, K. A comprehensive systematic review of photocatalytic degradation of pesticides using nano TiO2. Environ. Sci. Pollut. Res. 2021, 28, 13055–13071. [Google Scholar] [CrossRef]
- Kurade, M.B.; Ha, Y.H.; Xiong, J.Q.; Govindwar, S.P.; Jang, M.; Jeon, B.H. Phytoremediation as a green biotechnology tool for emerging environmental pollution: A step forward towards sustainable rehabilitation of the environment. Chem. Eng. J. 2021, 415, 129040. [Google Scholar] [CrossRef]
- Valizadeh, S.; Lee, S.S.; Baek, K.; Choi, Y.J.; Jeon, B.H.; Rhee, G.H.; Park, Y.K. Bioremediation strategies with biochar for polychlorinated biphenyls (PCBs)-contaminated soils: A review. Environ. Res. 2021, 200, 111757. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Cho, I.H.; Park, J. The effect of operational parameters on the photocatalytic degradation of pesticide. J. Environ. Sci. Health Part B 2004, 39, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Bano, K.; Kaushal, S.; Singh, P.P. A review on photocatalytic degradation of hazardous pesticides using heterojunctions. Polyhedron 2021, 209, 115465. [Google Scholar] [CrossRef]
- Lwin, H.M.; Zhan, W.; Song, S.; Jia, F.; Zhou, J. Visible-light photocatalytic degradation pathway of tetracycline hydrochloride with cubic structured ZnO/SnO2 heterojunction nanocatalyst. Chem. Phys. Lett. 2019, 736, 136806. [Google Scholar] [CrossRef]
- Amir, M.; Kurtan, U.; Baykal, A.; Sözeri, H. MnFe2O4@PANI@Ag heterogeneous nanocatalyst for degradation of industrial aqueous organic pollutants. J. Mater. Sci. Technol. 2016, 32, 134–141. [Google Scholar] [CrossRef]
- Chen, L.; Maqbool, T.; Hou, C.; Fu, W.; Zhang, X. Mechanistic study of oxidative removal of bisphenol A by pristine nanocatalyst Mn3O4/peroxymonosulfate. Sep. Purif. Technol. 2022, 281, 119882. [Google Scholar] [CrossRef]
- Tara, N.; Siddiqui, S.I.; Rathi, G.; Chaudhry, S.A.; Asiri, A.M. Nano-engineered adsorbent for the removal of dyes from water: A review. Curr. Anal. Chem. 2020, 16, 14–40. [Google Scholar] [CrossRef]
- Yadav, V.K.; Ali, D.; Khan, S.H.; Gnanamoorthy, G.; Choudhary, N.; Yadav, K.K.; Manhrdas, S. Synthesis and characterization of amorphous iron oxide nanoparticles by the sonochemical method and their application for the remediation of heavy metals from wastewater. Nanomaterials 2020, 10, 1551. [Google Scholar] [CrossRef]
- Yadav, V.K.; Choudhary, N.; Khan, S.H.; Malik, P.; Inwati, G.K.; Suriyaprabha, R.; Ravi, R.K. Synthesis and Characterisation of Nano-Biosorbents and Their Applications for Waste Water Treatment. In Handbook of Research on Emerging Developments and Environmental Impacts of Ecological Chemistry; IGI Global: Hershey, PA, USA, 2020; pp. 252–290. [Google Scholar]
- Harja, M.; Ciobanu, G. Eco-friendly nano-adsorbents for pollutant removal from wastewaters. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O., Martínez, L., Kharisov, B., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–22. [Google Scholar] [CrossRef]
- Khan, S.H.; Alaie, S.A. Green nanomaterials for environmental applications. In Green Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 365–396. [Google Scholar]
- Ghosh, S.; Malloum, A.; Bornman, C.; Othmani, A.; Osagie, C.; Esfahani, Z.; Dehghani, M.H. Novel green adsorbents for removal of aniline from industrial effluents: A review. J. Mol. Liq. 2022, 345, 118167. [Google Scholar] [CrossRef]
- Sadegh, H.; Ali, G.A. Potential applications of nanomaterials in wastewater treatment: Nanoadsorbents performance. In Research Anthology on Synthesis, Characterization, and Applications of Nanomaterials; IGI Global: Hershey, PA, USA, 2021; pp. 1230–1240. [Google Scholar]
- Kurniawan, T.A.; Sillanpää, M.E.; Sillanpää, M. Nanoadsorbents for remediation of aquatic environment: Local and practical solutions for global water pollution problems. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1233–1295. [Google Scholar] [CrossRef]
- Subbaiah, M.P.; Kalimuthu, P.; Jung, J.; Jeon, B.H. Recent advances in effective capture of inorganic mercury from aqueous solutions through sulfurized 2D-material-based adsorbents. J. Mater. Chem. A 2021, 9, 18086–18101. [Google Scholar] [CrossRef]
- Gul, A.; Khaligh, N.G.; Julkapli, N.M. Surface modification of carbon-based nanoadsorbents for the advanced wastewater treatment. J. Mol. Struct. 2021, 1235, 130148. [Google Scholar] [CrossRef]
- Hussain, N.; Bilal, M.; Iqbal, H.M. Carbon-based nanomaterials with multipurpose attributes for water treatment: Greening the 21st-century nanostructure materials deployment. Biomater. Polym. Horiz. 2022, 1, 48–58. [Google Scholar] [CrossRef]
- Hu, X.; You, S.; Li, F.; Liu, Y. Recent advances in antimony removal using carbon-based nanomaterials: A review. Front. Environ. Sci. Eng. 2022, 16, 48. [Google Scholar] [CrossRef]
- Ali, I.; Alharbi, O.M.; Alothman, Z.A.; Alwarthan, A. Facile and eco-friendly synthesis of functionalized iron nanoparticles for cyanazine removal in water. Colloids Surf. B Biointerfaces 2018, 171, 606–613. [Google Scholar] [CrossRef]
- Mahdavi, V.; Taghadosi, F.; Dashtestani, F.; Bahadorikhalili, S.; Farimani, M.M.; Ma’mani, L.; Khaneghah, A.M. Aminoguanidine modified magnetic graphene oxide as a robust nanoadsorbent for efficient removal and extraction of chlorpyrifos residue from water. J. Environ. Chem. Eng. 2021, 9, 106117. [Google Scholar] [CrossRef]
- Izanloo, M.; Esrafili, A.; Jafari, A.J.; Farzadkia, M.; Behbahani, M.; Sobhi, H.R. Application of a novel bi-functional nanoadsorbent for the simultaneous removal of inorganic and organic compounds: Equilibrium, kinetic and thermodynamic studies. J. Mol. Liq. 2019, 273, 543–550. [Google Scholar] [CrossRef]
- Mohammadi, F.; Esrafili, A.; Kermani, M.; Behbahani, M. Application of modified magnetic nanoparticles with amine groups as an efficient solid sorbent for simultaneous removal of 2, 4-Dichlorophenoxyacetic acid and 2-methyl-4-chlorophenoxyacetic acid from aqueous solution: Optimization and modeling. J. Iran. Chem. Soc. 2018, 15, 421–429. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Kamalian, S.; Shayeghi, M.; Yousefi, M.; Heidarinejad, Z.; Agarwal, S.; Gupta, V.K. High-performance removal of diazinon pesticide from water using multi-walled carbon nanotubes. Microchem. J. 2019, 145, 486–491. [Google Scholar] [CrossRef]
- Kalhor, M.M.; Rafati, A.A.; Rafati, L.; Rafati, A.A. Synthesis, characterization and adsorption studies of amino-functionalized silica nano hollow sphere as an efficient adsorbent for removal of imidacloprid pesticide. J. Mol. Liq. 2018, 266, 453–459. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Padhiari, S.; Biswal, S.K.; Panda, B.B.; Hota, G. Fe3O4 nanoparticles functionalized GO/g-C3N4 nanocomposite: An efficient magnetic nano adsorbent for adsorptive removal of organic pollutants. Mater. Chem. Phys. 2020, 244, 122710. [Google Scholar] [CrossRef]
- Nikzad, S.; Amooey, A.A.; Alinejad-Mir, A. Adsorption of diazinon from aqueous solutions by magnetic guar gum-montmorillonite. Chem. Data Collect. 2019, 20, 100187. [Google Scholar] [CrossRef]
- Peralta, M.E.; Mártire, D.O.; Moreno, M.S.; Parolo, M.E.; Carlos, L. Versatile nano adsorbents based on magnetic mesostructured silica nanoparticles with tailored surface properties for organic pollutants removal. J. Environ. Chem. Eng. 2021, 9, 104841. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Mustereţ, C.P.; Teodosiu, C. Removal of Persistent Organic Pollutants from Textile Wastewater By Membrane Processes. Environ. Eng. Manag. J. 2007, 6, 175–187. [Google Scholar] [CrossRef]
- Luis, P.; Saquib, M.; Vinckier, C.; Van der Bruggen, B. Effect of membrane filtration on ozonation efficiency for removal of atrazine from surface water. Ind. Eng. Chem. Res. 2011, 50, 8686–8692. [Google Scholar] [CrossRef]
- Fujii, S.; Polprasert, C.; Tanaka, S.; Hong Lien, N.P.; Qiu, Y. New POPs in the water environment: Distribution, bioaccumulation and treatment of perfluorinated compounds–a review paper. J. Water Supply Res. Technol. AQUA 2007, 56, 313–326. [Google Scholar] [CrossRef]
- Tibi, F.; Charfi, A.; Cho, J.; Kim, J. Fabrication of polymeric membranes for membrane distillation process and application for wastewater treatment: Critical review. Process Saf. Environ. Prot. 2020, 141, 190–201. [Google Scholar] [CrossRef]
- Sarkar, S.; Chakraborty, S. Nanocomposite polymeric membrane a new trend of water and wastewater treatment: A short review. Groundw. Sustain. Dev. 2021, 12, 100533. [Google Scholar] [CrossRef]
- Vázquez-Núñez, E.; Molina-Guerrero, C.E.; Peña-Castro, J.M.; Fernández-Luqueño, F.; de la Rosa-Álvarez, M. Use of nanotechnology for the bioremediation of contaminants: A review. Processes 2020, 8, 826. [Google Scholar] [CrossRef]
- Ng, L.Y.; Chua, H.S.; Ng, C.Y. Incorporation of graphene oxide-based nanocomposite in the polymeric membrane for water and wastewater treatment: A review on recent development. J. Environ. Chem. Eng. 2021, 9, 105994. [Google Scholar] [CrossRef]
- Lau, W.J.; Ismail, A.F. Polymeric nanofiltration membranes for textile dye wastewater treatment: Preparation, performance evaluation, transport modelling, and fouling control—A review. Desalination 2009, 245, 321–348. [Google Scholar] [CrossRef]
- Oatley-Radcliffe, D.L.; Walters, M.; Ainscough, T.J.; Williams, P.M.; Mohammad, A.W.; Hilal, N. Nanofiltration membranes and processes: A review of research trends over the past decade. J. Water Process Eng. 2017, 19, 164–171. [Google Scholar] [CrossRef]
- Mulyanti, R.; Susanto, H. Wastewater treatment by nanofiltration membranes. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 142, p. 012017. [Google Scholar]
- Yoon, Y.; Lueptow, R.M. Removal of organic contaminants by RO and NF membranes. J. Membr. Sci. 2005, 261, 76–86. [Google Scholar] [CrossRef]
- Agenson, K.O.; Oh, J.I.; Urase, T. Retention of a wide variety of organic pollutants by different nanofiltration/reverse osmosis membranes: Controlling parameters of process. J. Membr. Sci. 2003, 225, 91–103. [Google Scholar] [CrossRef]
- Paul, M.; Jons, S.D. Chemistry and fabrication of polymeric nanofiltration membranes: A review. Polymer 2016, 103, 417–456. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef]
- Molinari, R.; Mungari, M.; Drioli, E.; Di Paola, A.; Loddo, V.; Palmisano, L.; Schiavello, M. Study on a photocatalytic membrane reactor for water purification. Catal. Today 2000, 55, 71–78. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Argurio, P.; Fontananova, E.; Molinari, R.; Drioli, E. Photocatalytic membranes in photocatalytic membrane reactors. Processes 2018, 6, 162. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, L.; Luo, J.; Jaffrin, M.Y.; Tang, B. Membrane fouling in photocatalytic membrane reactors (PMRs) for water and wastewater treatment: A critical review. Chem. Eng. J. 2016, 302, 446–458. [Google Scholar] [CrossRef]
- Marchetti, P.; Jimenez Solomon, M.F.; Szekely, G.; Livingston, A.G. Molecular separation with organic solvent nanofiltration: A critical review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef] [PubMed]
- Hilal, N.; Al-Zoubi, H.; Darwish, N.A.; Mohamma, A.W.; Arabi, M.A. A comprehensive review of nanofiltration membranes: Treatment, pretreatment, modelling, and atomic force microscopy. Desalination 2004, 170, 281–308. [Google Scholar] [CrossRef]
- Tul Muntha, S.; Kausar, A.; Siddiq, M. Advances in polymeric nanofiltration membrane: A review. Polym. Plast. Technol. Eng. 2017, 56, 841–856. [Google Scholar] [CrossRef]
- Shon, H.K.; Phuntsho, S.; Chaudhary, D.S.; Vigneswaran, S.; Cho, J. Nanofiltration for water and wastewater treatment–a mini review. Drink. Water Eng. Sci. 2013, 6, 47–53. [Google Scholar] [CrossRef]
- Karimi, H.; Rahimpour, A.; Kebria, M.R.Z. Pesticides removal from water using modified piperazine-based nanofiltration (NF) membranes. Desalination Water Treat. 2016, 57, 24844–24854. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.; Li, Y.; Jing, L.; Wang, J.; Li, X. Ag NPs supported on the magnetic Al-MOF/PDA as nanocatalyst for the removal of organic pollutants in water. J. Alloys Compd. 2020, 828, 154340. [Google Scholar] [CrossRef]
- Yadav, V.K.; Khan, S.H.; Choudhary, N.; Tirth, V.; Kumar, P.; Ravi, R.K.; Godha, M. Nanobioremediation: A sustainable approach towards the degradation of sodium dodecyl sulfate in the environment and simulated conditions. J. Basic Microbiol. 2022, 62, 348–360. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Show, P.L. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef]
| S. No | Chemical | Category |
|---|---|---|
| As per the 2001 Amendment (The Dirty Dozen) | ||
| 1 | PCB | Industrial waste/byproduct |
| 2 | PCD | Byproduct |
| 3 | PCDF | Byproduct |
| 4 | Chlordane | Pesticide |
| 5 | Mirex | Pesticide |
| 6 | Endrin | Pesticide |
| 7 | Aldrin | Pesticide |
| 8 | Dieldrin | Pesticide |
| 9 | HCB | Pesticide |
| 10 | Heptachlor | Pesticide |
| 11 | Toxaphene | Pesticide |
| 12 | DDT | Pesticide |
| As per the 2009 Amendment | ||
| 13 | Lindane | Pesticide |
| 14 | Chlordecone | Pesticide |
| 15 | Pentachloro benzene | Pesticide and Byproduct |
| 16 | Alpha-HCH | Pesticide and Byproduct |
| 17 | Beta-HCH | Pesticide and Byproduct |
| 18 | PFO and constituents PFOSF | Industrial |
| 19 | Hexabromobiphenyl | Industrial |
| 20 | Hexa-BDE and Hepta-BDE | Industrial |
| 21 | Tetra-BDE and Penta-BDE | Industrial |
| As per the 2011 Amendment | ||
| 22 | Endosulfan | Pesticide |
| Method | Technique | Advantage | Disadvantage |
|---|---|---|---|
| Chemical | Oxidation Reduction Hydrolysis Catalysis Photo-Fenton Ozonation Coagulation | Effective, rapid, and destructive use of hazardous chemicals | High cost, complex processes, toxic by-products, and end-products, sometimes the formation of even more toxic products than the parent pollutant |
| Physical | Adsorption Settling Membrane filtration Air stripping | Fast but comparatively less effective | The formation of by-products, cannot break the organic pollutant, high operational costs, low removal efficiency |
| Thermal | Combustion | Rapid and destructive, no by-product formed | High cost, complex process, not suggested for the recalcitrant compound |
| Biological | Microbial degradation Rhizoremediation Phytoremediation | Destructive, environmentally friendly, low energy requirement | Comparatively slow, high cost, less effective, toxic by-products/end-products, cost-benefit ratio is low |
| Nano-based | Photocatalysis Nanoadsorption Hybrid nanoremediation Nanofiltration | Superfast, high removal capacity, highly selective, highly efficient, excellent porosity, charge-based repulsion, comparatively less pressure, better selectivity | High cost, high operational cost and management, nanotoxicological concerns, membrane blocking, difficult to scale up |
| S.NO | Technique | Nanomaterial Used | Reference |
|---|---|---|---|
| 1. | Sulfate-radical-based advanced oxidation processes (SR-AOPs) for refractory organic contaminants | SR-AOPs using heterogeneous catalysis | [37] |
| 2. | Hybrid photocatalytic membrane reactors for removal of POPs | Photocatalysis and membrane filtration | [38] |
| 3. | Adsorptive and photocatalytic removal of POPs | Metal–organic frameworks (MOFs) | [39] |
| 4. | Nanocatalysts and other nanomaterials for remediation of POPs | Oxidation, adsorption | [40] |
| 5. | Photodegradation of POPs by GR-based composites | Catalysis and reduced graphene for photodegradation | [41] |
| 6. | Nanoremediation for removal of POPs | Different nanomaterials, i.e., nanoscale zero-valent iron (nZVI), CNT, silica (SiO2), magnetic and metallic nanoparticles, graphene oxide, covalent organic frameworks (COFs), and MOFs | [42] |
| 7. | Biogenic nanomaterials for the remediation of organic and inorganic pollutants | NMs, NPs, nanomembranes, and nanopowders for detection as well as for the removal of toxic metals and organic compounds | [43] |
| 8. | Nanotechnology for pesticide removal from aqueous solutions | Nanomaterials, nanocomposites | [44] |
| 9. | Removal of POPs using various multifunctional materials | Thermal, electrochemical, and photocatalytic remediation processes | [21] |
| 10. | Remediation of water contaminated by poly- and perfluoroalkyl substances | Modified CNTs, modified nano-iron oxides, metal-based nanophotocatalysts | [45] |
| 11. | Green synthesized nanoengineered materials for water/wastewater remediation | Green nanomaterials for POP removal | [46] |
| 12. | Removal of persistent organic pollutants (POPs) from waters and wastewaters | Ionizing radiation, advanced oxidation and reduction processes (AO/RPs) | [47] |
| 13. | Zinc oxide-based photocatalytic degradation of persistent pesticides | Photocatalytic degradation using ZnO | [48] |
| 14. | Nanocatalysts and other nanomaterials for remediation of organic pollutants | Oxidation, adsorption, degrading organic pollutants for water remediation. | [40] |
| 15. | Treatment of persistent organic pollutants in wastewater | Synergistic efficiency hydrodynamic cavitation with the advanced oxidation process | [49] |
| 16. | Biofabricated nanoparticles for mitigating the environmental pollutants | Removal of pollutants via adsorption, immobilization, and reduction mechanisms | [50] |
| 17. | Solid-phase microextraction of toxic pollutants using nanotechnology | Carbon-based materials, metal and metal-oxide nanomaterials | [51] |
| 18. | Advanced nanotechnology and hybrid membrane-based treatment | Ag, Fe, Zn, Ti metal nanoparticles and carbon nanotubes | [52] |
| 19. | Sustainable nanotechnology-based wastewater treatment | Graphene-based nanoparticles, their oxides (GO) and reduced graphene oxide (rGO), single-walled carbon nanotubes, multiple walled carbon nanotubes, covalent organic frameworks, metal, and metal-oxide-based nanoparticles | [53] |
| 20. | Emerging contaminants removal from wastewater | Nanoscale materials such as nanosorbents, nanofilters, and nanocatalysts in the degradation of emerging contaminants | [54] |
| 21. | Advanced oxidative processes for remediation of persistent organic pollutants from water | AOPs, such as sulfate radical, ionizing radiation, heterogeneous photocatalysis, electrohydraulic discharge system, ozonation, and Fenton processes | [55] |
| 22. | Photocatalyst for organic-pollutant degradation | Carbon quantum-dot-supported zinc oxide (ZnO/CQDs) | [56] |
| Contaminant | Nanomaterials | References |
|---|---|---|
| Organic Pollutant | Ag/ZnO, ZnO-Bi, ZnO | [62] |
| Mesoporous silica | [63] | |
| Graphene oxide-Ag NP | [64] | |
| TiO2 | [65] | |
| ZnO | [66] | |
| TiO2-rGO | [67] | |
| Palladium and AgNp-embedded-zinc oxide nanostars | [68] | |
| RE3+-doped nano-TiO2 | [69] | |
| MoS2/ZnS embedded in N/S doped carbon | [70] | |
| Magnetite and cobalt ferrite-decorated graphene oxide composite. | [71] | |
| CuO and NiO nanoparticles | [72] | |
| Chlorpyrifos | ZnO/ZnO-Bi/ZnO-Ag/ZnO-Fe | [73] |
| Potato-peel biochar | [74] | |
| Aldrin | TiO2 | [75] |
| Heptachlor | Fe/Cu nanoparticles | [76] |
| Fe2O3 | [77] | |
| NZVI | [78] | |
| Beta arsenene nanotubes | [79] | |
| Mirex | Beta arsenene nanotubes | [79] |
| Cu/Fe bimetal | [80] | |
| Dimethoate | Gold Nanospheres and Nanorods | [81] |
| Chlordane | Graphene/Ni nanocomposite | [82] |
| Cu/Fe bimetal | [80] | |
| Endrin | Virgin (Fe0) and microbially regenerated (Fe2+) iron | [83] |
| HCBs | Magnetic micro/nano FexOy-CeO2 composite | [84] |
| Mg-doped Fe3O4 | [85] | |
| Nano Pd (0) | [86] | |
| Nano zero-valent iron/activated carbon composite | [87] | |
| Co-Fe-O | [88] | |
| Zero-valent magnesium/graphite | [89] | |
| nZVI | [90] | |
| PCBs | Nano Pd/Fe | [91] |
| Zero Valent iron (ZVI) | [92] | |
| ZVI | [93] | |
| Pd nanocatalyst | [94] | |
| Ti-Ag Nanocomposite | [95] | |
| Fe2O3 | [96] | |
| Au-Ag NP and Pd-Fe Bimetallic NP | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boulkhessaim, S.; Gacem, A.; Khan, S.H.; Amari, A.; Yadav, V.K.; Harharah, H.N.; Elkhaleefa, A.M.; Yadav, K.K.; Rather, S.-u.; Ahn, H.-J.; et al. Emerging Trends in the Remediation of Persistent Organic Pollutants Using Nanomaterials and Related Processes: A Review. Nanomaterials 2022, 12, 2148. https://doi.org/10.3390/nano12132148
Boulkhessaim S, Gacem A, Khan SH, Amari A, Yadav VK, Harharah HN, Elkhaleefa AM, Yadav KK, Rather S-u, Ahn H-J, et al. Emerging Trends in the Remediation of Persistent Organic Pollutants Using Nanomaterials and Related Processes: A Review. Nanomaterials. 2022; 12(13):2148. https://doi.org/10.3390/nano12132148
Chicago/Turabian StyleBoulkhessaim, Salim, Amel Gacem, Samreen Heena Khan, Abdelfattah Amari, Virendra Kumar Yadav, Hamed N. Harharah, Abubakr M. Elkhaleefa, Krishna Kumar Yadav, Sami-ullah Rather, Hyun-Jo Ahn, and et al. 2022. "Emerging Trends in the Remediation of Persistent Organic Pollutants Using Nanomaterials and Related Processes: A Review" Nanomaterials 12, no. 13: 2148. https://doi.org/10.3390/nano12132148
APA StyleBoulkhessaim, S., Gacem, A., Khan, S. H., Amari, A., Yadav, V. K., Harharah, H. N., Elkhaleefa, A. M., Yadav, K. K., Rather, S.-u., Ahn, H.-J., & Jeon, B.-H. (2022). Emerging Trends in the Remediation of Persistent Organic Pollutants Using Nanomaterials and Related Processes: A Review. Nanomaterials, 12(13), 2148. https://doi.org/10.3390/nano12132148










