In Situ Decoration of ZnSnO3 Nanosheets on the Surface of Hollow Zn2SnO4 Octahedrons for Enhanced Solar Energy Application
Abstract
1. Introduction
2. Materials and Methods
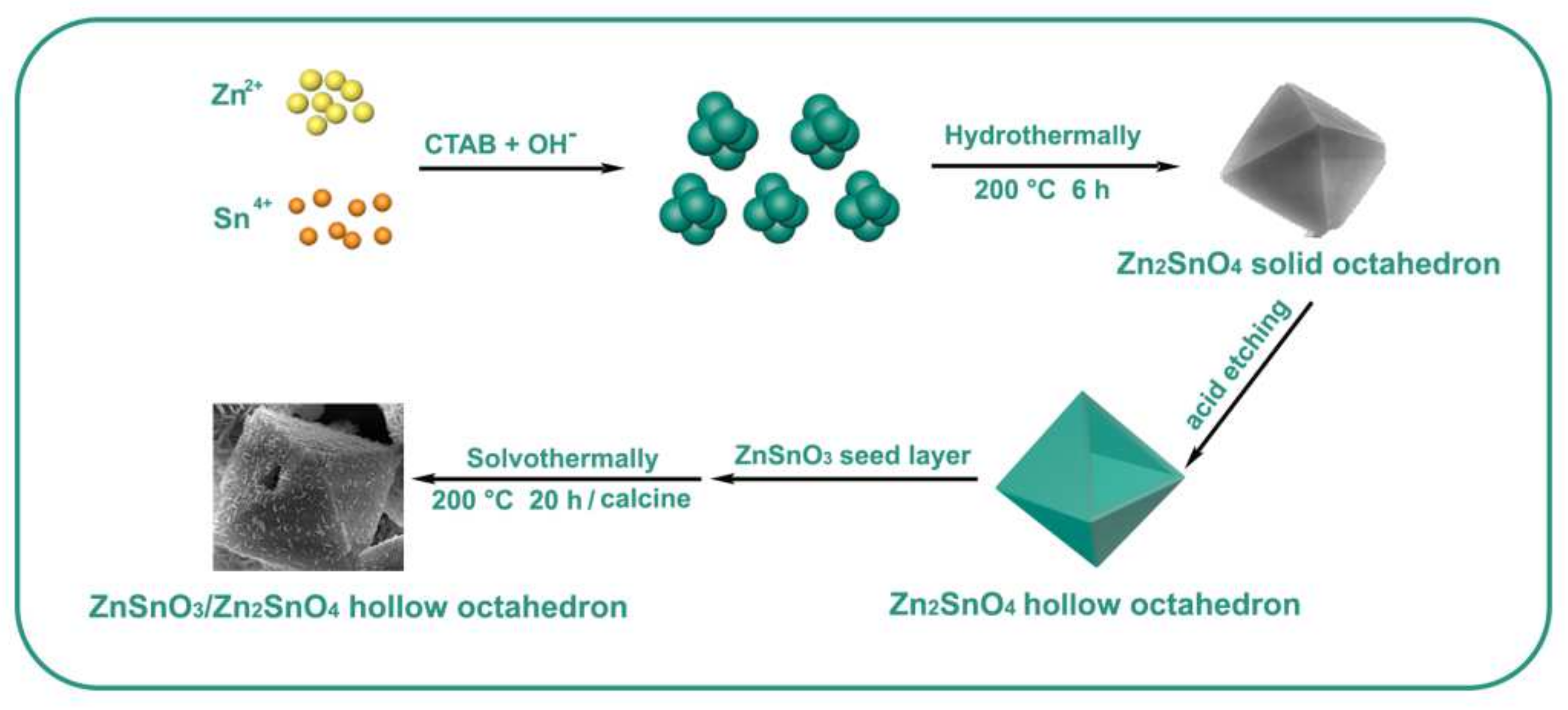
2.1. Materials Synthesis
2.2. Materials Characterization
2.3. Preparation of the Electrode
2.4. Photovoltaic Characterization
2.5. Photocatalytic Measurements
3. Results and Discussion
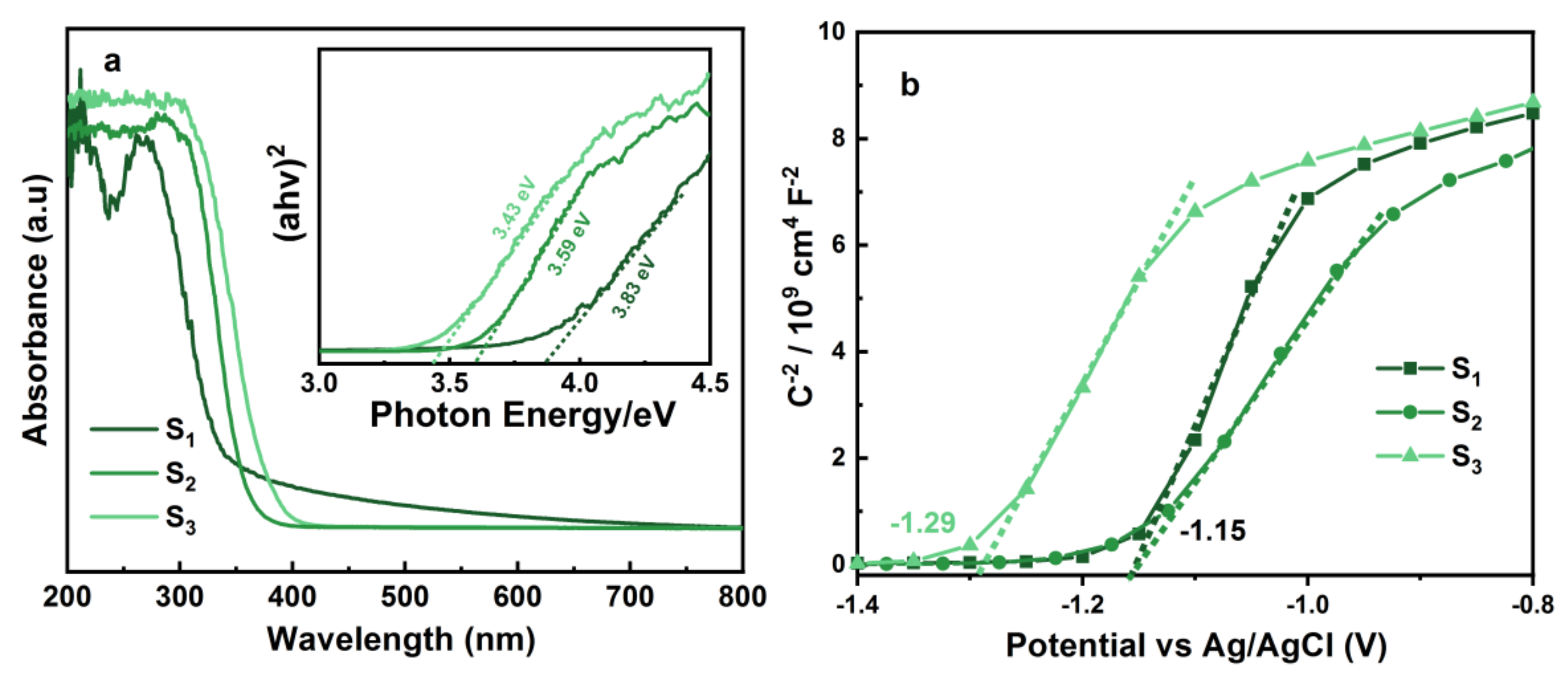
3.1. Structure Characterization
3.2. Photovoltaic Characteristics of Zn2SnO4 and ZnSnO3/Zn2SnO4
3.2.1. Photovoltaic Characteristics of Zn2SnO4
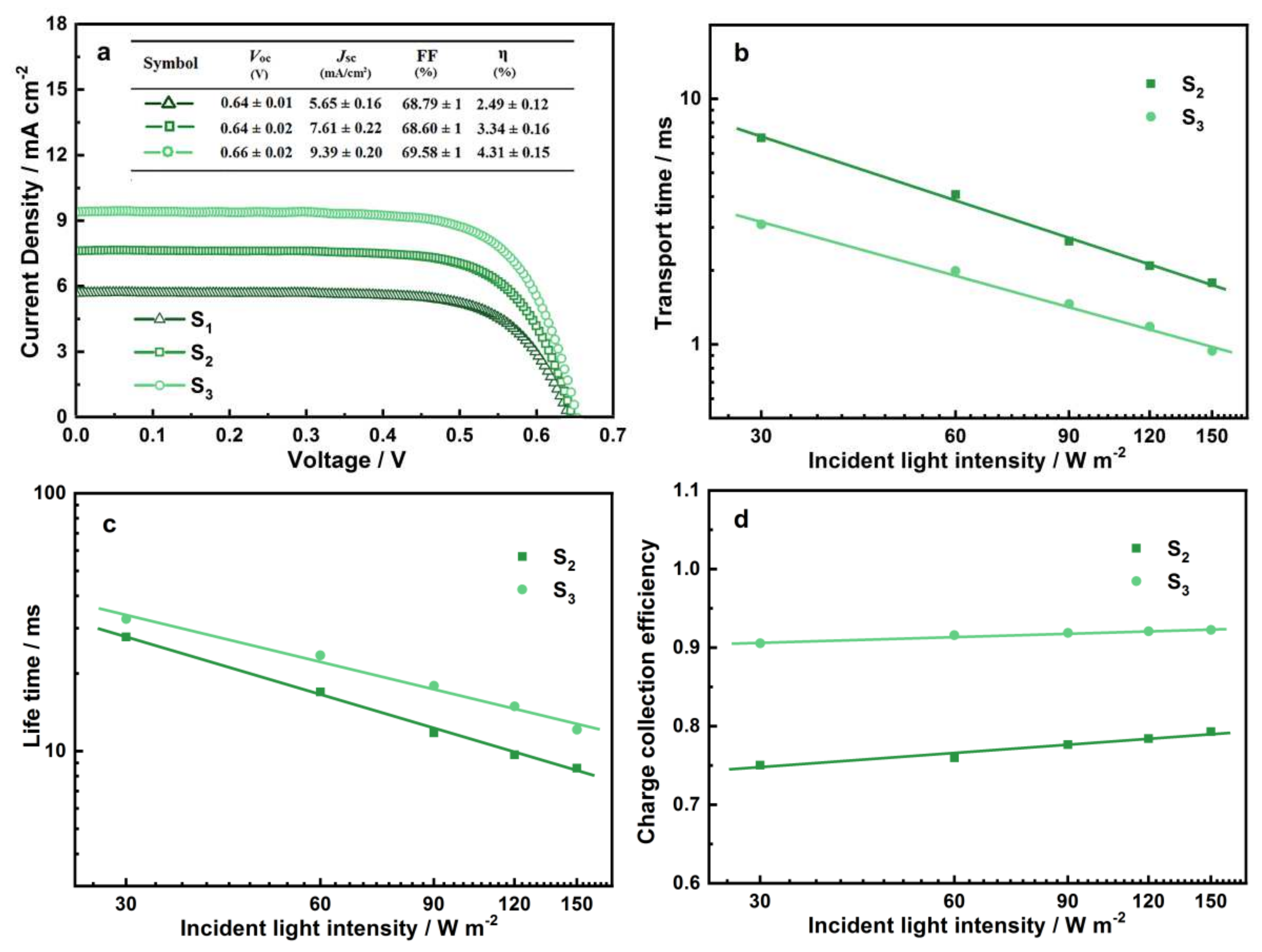
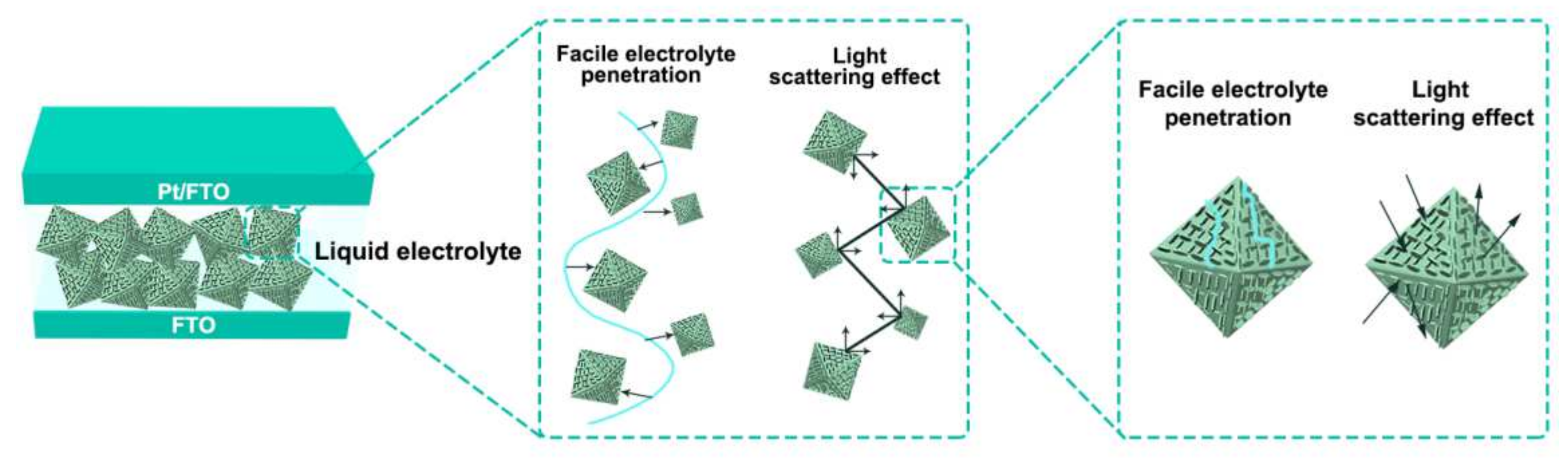
3.2.2. Photovoltaic Characteristics of ZnSnO3/Zn2SnO4
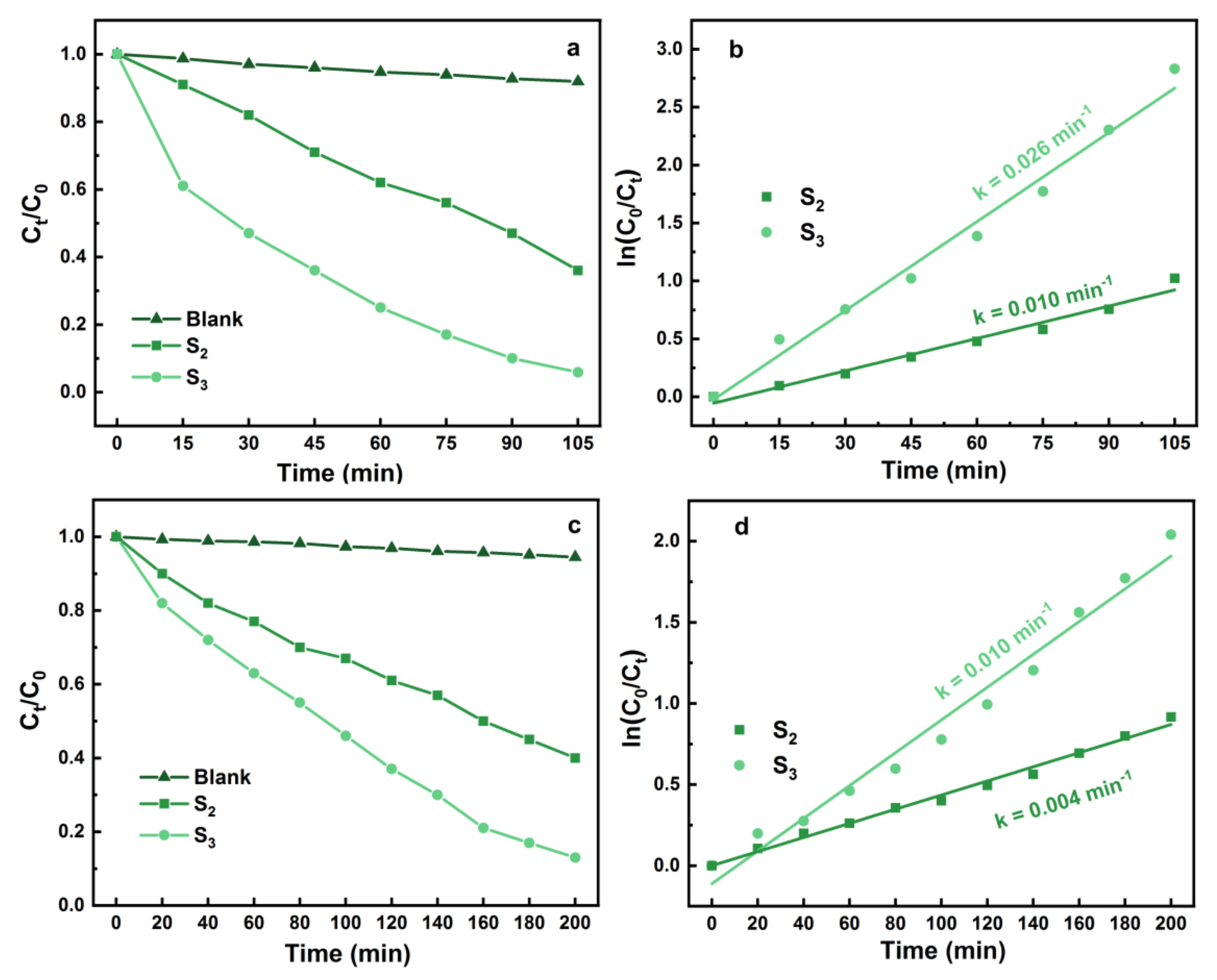
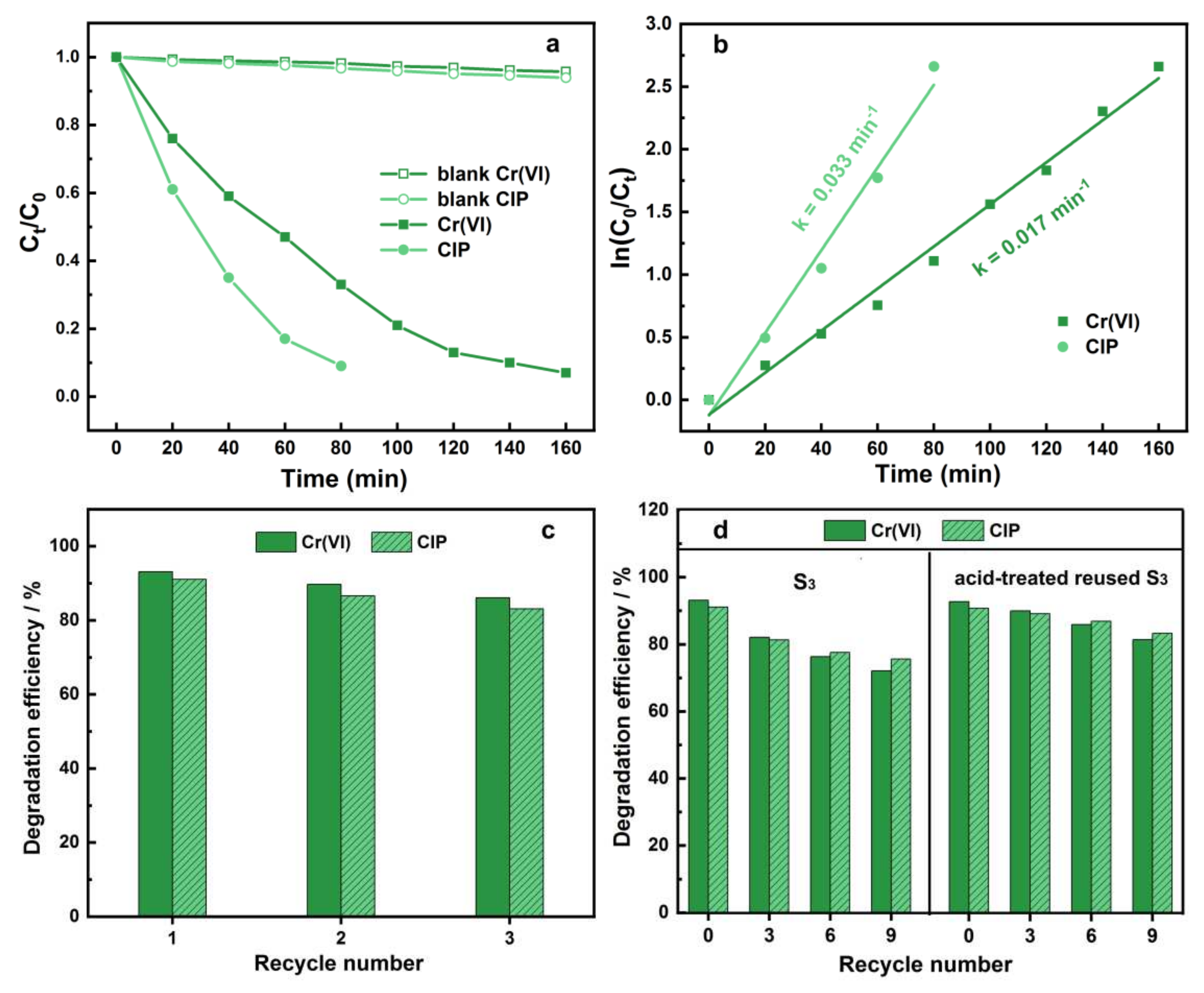
3.3. Photocatalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hussain, I.; Sahoo, S.; Mohapatra, D.; Ahmad, M.; Iqbal, S.; Javed, M.S.; Gu, S.; Qin, N.; Lamiel, C.; Zhang, K. Recent progress in trimetallic/ternary-metal oxides nanostructures: Misinterpretation/misconception of electrochemical data and devices. Appl. Mater. Today 2022, 26, 101297. [Google Scholar] [CrossRef]
- Liu, L.; Li, M.; Chu, L.; Jiang, B.; Lin, R.; Zhu, X.; Cao, G. Layered ternary metal oxides: Performance degradation mechanisms as cathodes, and design strategies for high-performance batteries. Prog. Mater. Sci. 2020, 111, 100655. [Google Scholar] [CrossRef]
- He, H.; Liao, A.; Guo, W.; Luo, W.; Zhou, Y.; Zou, Z. State-of-the-art progress in the use of ternary metal oxides as photoelectrode materials for water splitting and organic synthesis. Nano Today 2019, 28, 100763. [Google Scholar] [CrossRef]
- Dou, J.; Li, X.; Li, Y.; Chen, Y.; Wei, M. Fabrication of Zn2SnO4 microspheres with controllable shell numbers for highly efficient dye-sensitized solar cells. Sol. Energy 2019, 181, 424–429. [Google Scholar] [CrossRef]
- Hirano, T.; Takemoto, M. Photovoltaic properties of dye sensitized solar cells employing amorphous ZnSnO3 as semiconducting porous film material. Trans. Mat. Res. Soc. Jpn. 2016, 41, 93–96. [Google Scholar] [CrossRef][Green Version]
- Yu, S.; Jia, X.; Yang, J.; Wang, S.; Li, Y.; Song, H. Highly sensitive and low detection limit of ethanol gas sensor based on CeO2 nanodot-decorated ZnSnO3 hollow microspheres. Ceram. Int. 2022, 48, 14865–14875. [Google Scholar] [CrossRef]
- Dong, S.; Jin, X.; Wei, J.; Wu, H. Electrospun ZnSnO3/ZnO composite nanofibers and its ethanol-sensitive properties. Metals 2022, 12, 196. [Google Scholar] [CrossRef]
- Song, J.; Lu, X.; Tian, Q.; Cui, L.; Chen, J.; Sui, Z. Dual-stable engineering enables high-performance Zn2SnO4-based lithium-ion battery anode. J. Alloy. Compd. 2022, 910, 164924. [Google Scholar] [CrossRef]
- Tan, H.; Cho, H.; Wu, J. Binder-free ZnO@ZnSnO3 quantum dots core-shell nanorod array anodes for lithium-ion batteries. J. Power Sources 2018, 388, 11–18. [Google Scholar] [CrossRef]
- Jia, T.; An, J.; Yu, D.; Li, J.; Fu, F.; Wang, K.; Wang, W. Continuously improved photocatalytic performance of Zn2SnO4/SnO2/Cu2O composites by structural modulation and band alignment modification. Nanomaterials 2019, 9, 1390. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N.; Polat, I.; Yasar, A.; Yüksel, Ö.F. Silver doped zinc stannate (Ag-ZnSnO3) for the photocatalytic degradation of caffeine under UV irradiation. Water 2021, 13, 1290. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, Y.; Bi, D.; Liu, Q.; Yang, C.; Zhang, J. Continuously improved gas-sensing performance of Zn2SnO4 porous octahedrons by structure evolution and further ZnSnO3 nanosheets decoration. J. Alloy. Compd. 2022, 901, 163744. [Google Scholar] [CrossRef]
- Du, L.; Zhang, H.; Zhu, M.; Zhang, M. Construction of flower-like ZnSnO3/Zn2SnO4 hybrids for enhanced phenylamine sensing performance. Inorg. Chem. Front. 2019, 6, 2311–2317. [Google Scholar] [CrossRef]
- Habibi, M.H.; Mardani, M. Synthesis and characterization of bi-component ZnSnO3/Zn2SnO4 (perovskite/spinel) nano-composites for photocatalytic degradation of Intracron Blue: Structural, opto-electronic and morphology study. J. Mol. Liq. 2017, 238, 397–401. [Google Scholar] [CrossRef]
- Manikandan, M.; Mukilraj, T.; Venkateswaran, C.; Moorthy, B.S. Exploration of photoanode characteristics of a mixed ferroelectric ZnSnO3 and semiconducting Zn2SnO4 phase for photovoltaic applications. J. Mater. Sci. Mater. Electron. 2018, 29, 15106–15111. [Google Scholar] [CrossRef]
- Wu, W.; Feng, H.; Chen, H.; Kuang, D.; Su, C. Recent advances in hierarchical three-dimensional titanium dioxide nanotree arrays for high-performance solar cells. J. Mater. Chem. A 2017, 5, 12699–12717. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Mao, W.; Zou, Z. Nanowire-based hierarchical tin oxide/zinc stannate hollow microspheres: Enhanced solar energy utilization efficiency for dye-sensitized solar cells and photocatalytic degradation of dyes. J. Power Sources 2015, 274, 575–581. [Google Scholar] [CrossRef]
- Jafri, N.N.M.; Jaafar, J.; Aziz, F.; Salleh, W.N.W.; Yusof, N.; Othman, M.H.D.; Rahman, M.A.; Ismail, A.F.; Rahman, R.A.; Khongnakorn, W. Development of free-standing titanium dioxide hollow nanofibers photocatalyst with enhanced recyclability. Membranes 2022, 12, 342. [Google Scholar] [CrossRef]
- Lee, K.-H.; Han, S.-H.; Chuquer, A.; Yang, H.-Y.; Kim, J.; Pham, X.-H.; Yun, W.-J.; Jun, B.-H.; Rho, W.-Y. Effect of Au nanoparticles and scattering layer in dye-sensitized solar cells based on freestanding TiO2 nanotube arrays. Nanomaterials 2021, 11, 328. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, C.; Wang, C.; Cheng, P.; Xu, L.; Lv, L.; Zhang, H. Design of SnO2@Air@TiO2 hierarchical urchin-like double-hollow nanospheres for high performance dye-sensitized solar cells. Sol. Energy 2019, 189, 412–420. [Google Scholar] [CrossRef]
- Sun, X.; Yan, X.; Su, H.; Sun, L.; Zhao, L.; Shi, J.; Wang, Z.; Niu, J.; Qian, H.; Duan, E. Non-Stacked γ-Fe2O3/C@TiO2 double-layer hollow nanoparticles for enhanced photocatalytic applications under visible light. Nanomaterials 2022, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.S.; Cho, J.S.; Wang, X.L.; Jin, E.M.; Jeong, S.M.; Kang, D.W. Improving of the photovoltaic characteristics of dye-sensitized solar cells using a photoelectrode with electrospun porous TiO2 nanofibers. Nanomaterials 2019, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Niu, X.; Li, W.; An, J.; Liu, Y.; Chen, Y.; Wang, P.; Yang, X.; Feng, Q. Microwave-assisted synthesis of high-energy faceted TiO2 nanocrystals derived from exfoliated porous metatitanic acid nanosheets with improved photocatalytic and photovoltaic performance. Materials 2019, 12, 3614. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.A.; Kang, H.C.; Yoo, K.; Asiam, F.K.; Lee, J.-J.; Shim, J.W. Cosensitization of metal-based dyes for high-performance dye-sensitized photovoltaics under ambient lighting conditions. Dye. Pigment. 2021, 194, 109624. [Google Scholar] [CrossRef]
- Dong, S.; Cui, L.; Zhang, W.; Xia, L.; Zhou, S.; Russell, C.K.; Fan, M.; Feng, J.; Sun, J. Double-shelled ZnSnO3 hollow cubes for efficient photocatalytic degradation of antibiotic wastewater. Chem. Eng. J. 2020, 384, 123279. [Google Scholar] [CrossRef]
- Peng, J.; Lin, H.; Lee, C.; Tseng, C.; Suryanarayanan, V.; Vittal, R.; Ho, K. Hierarchically assembled microspheres consisting of nanosheets of highly exposed (001)-facets TiO2 for dye-sensitized solar cells. RSC Adv. 2016, 6, 14178–14191. [Google Scholar] [CrossRef]
- Tan, B.; Toman, E.; Li, Y.; Wu, Y. Zinc stannate (Zn2SnO4) dye-sensitized solar cells. J. Am. Chem. Soc. 2007, 129, 4162–4163. [Google Scholar] [CrossRef]
- Zhang, Q.; Dandeneau, C.S.; Zhou, X.; Cao, G. ZnO Nanostructures for Dye-Sensitized Solar Cells. Adv. Mater. 2009, 21, 4087–4108. [Google Scholar] [CrossRef]
- Sengupta, D.; Das, P.; Mondal, B.; Mukherjee, K. Effects of doping, morphology and film-thickness of photo-anode materials for dye sensitized solar cell application—A review. Renew. Sust. Energ. Rev. 2016, 60, 356–376. [Google Scholar] [CrossRef]
- Li, L.B.; Wang, Y.F.; Rao, H.S.; Wu, W.Q.; Li, K.N.; Su, C.Y.; Kuang, D.B. Hierarchical macroporous Zn2SnO4-ZnO nanorod composite photoelectrodes for efficient CdS/CdSe quantum dot co-sensitized solar cells. ACS Appl. Mater. Interfaces 2013, 5, 11865–11871. [Google Scholar] [CrossRef]
- Jafarzadeh, M.; Sipaut, S.C.; Dayou, J.; Mansa, F.R. Recent progresses in solar cells: Insight into hollow micro/nano–structures. Renew. Sust. Energ. Rev. 2016, 64, 543–568. [Google Scholar] [CrossRef]
- Wei, X.; He, X.; Wu, P.; Gong, F.; Wang, D.; Wang, S.; Lu, S.; Zhang, J.; Xiang, S.; Kai, T.; et al. Recent advances in the design of semiconductor hollow microspheres for enhanced photocatalytic water splitting. Int. J. Hydrogen Energ. 2021, 46, 27974–27996. [Google Scholar] [CrossRef]
- Yashwantrao, G.; Saha, S. Perspective on the rational design strategies of quinoxaline derived organic sensitizers for dye-sensitized solar cells (DSSC). Dye. Pigment. 2022, 199, 110093. [Google Scholar] [CrossRef]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P.D. Nanowire dye-sensitized solar cells. Nat. Mater. 2005, 4, 455–459. [Google Scholar] [CrossRef]
- Sasidharan, S.; Jagadeesh, A.; Pradhan, S.C.; Nair, B.N.; Mohamed, A.A.; Unni, K.N.N.; Soman, S.; Hareesh, U.N. ZnO hierarchical structures as sacrificial inclusions for enhanced performance under full sun and indoor light in bifacial dye sensitized solar cells. Sol. Energy 2021, 226, 214–224. [Google Scholar] [CrossRef]
- Merazga, A.; Al-Zahrani, J.; Al-Baradi, A.; Omer, B.; Badawi, A.; Al-Omairy, S. Optical band-gap of reduced graphene oxide/TiO2 composite and performance of associated dye-sensitized solar cells. Mat. Sci. Eng. B 2020, 259, 114581. [Google Scholar] [CrossRef]
- Akman, E.; Akin, S.; Ozturk, T.; Gulveren, B.; Sonmezoglu, S. Europium and terbium lanthanide ions co-doping in TiO2 photoanode to synchronously improve light-harvesting and open-circuit voltage for high-efficiency dye-sensitized solar cells. Sol. Energy 2020, 202, 227–237. [Google Scholar] [CrossRef]
- Khan, M.W.; Zuo, X.; Yang, Q.; Tang, H.; Rehman, K.M.U.; Wu, M.; Li, G. Functionalized multi-walled carbon nanotubes embedded with nanoflakes boost the short-circuit current of Ru(II) based dye-sensitized solar cells. Dye. Pigment. 2020, 181, 108573. [Google Scholar] [CrossRef]
- Devadiga, D.; Selvakumar, M.; Shetty, P.; Santosh, M.S. Recent progress in dye sensitized solar cell materials and photo-supercapacitors: A review. J. Power Sources 2021, 493, 229698. [Google Scholar] [CrossRef]
- Agrawal, A.; Siddiqui, S.A.; Soni, A.; Sharma, G.D. Advancements, frontiers and analysis of metal oxide semiconductor, dye, electrolyte and counter electrode of dye sensitized solar cell. Sol. Energy 2022, 233, 378–407. [Google Scholar] [CrossRef]
- Dou, J.; Lia, Y.; Wu, J.; Chang, Y.J.; Wei, M. Improving the photovoltaic performance of Zn2SnO4 solar cells by doping Sr2+/Ba2+ ions: Efficient electron injection and transfer. Sol. Energy 2018, 165, 122–130. [Google Scholar] [CrossRef]
- Ursu, D.; Banică, R.; Vajda, M.; Baneasa, C.B.; Miclau, M. Investigation of silver nanowires in Zn2SnO4 spheres for enhanced dye-sensitized solar cells performance. J. Alloy. Compd. 2022, 902, 163890. [Google Scholar] [CrossRef]
- Li, B.; Guo, E.; Wang, C.; Yin, L. Novel Au inlaid Zn2SnO4/SnO2 hollow rounded cubes for dye-sensitized solar cells with enhanced photoelectric conversion performance. J. Mater. Chem. A 2016, 4, 466–477. [Google Scholar] [CrossRef]
- Karimi, V.; Asemi, M.; Ghanaatshoar, M. Improving photovoltaic properties of ZTO-based DSSCs using surface modifcation of Zn2SnO4 nanoparticles prepared by co-precipitation method. Mat. Sci. Semicon. Proc. 2021, 127, 105664. [Google Scholar] [CrossRef]
- Siwatcha, S.; Kundua, V.S.; Kumarb, A.; Kumara, S.; Chauhana, N.; Kumaria, M. Effect of novel ZnO/Zn2SnO4 photoanode on the performance of dye sensitized solar cell. Optik 2019, 194, 163117. [Google Scholar] [CrossRef]
- Saeed, M.A.; Yoo, K.; Kang, H.C.; Shim, J.W.; Lee, J.-J. Recent developments in dye-sensitized photovoltaic cells under ambient illumination. Dye. Pigment. 2021, 194, 109626. [Google Scholar] [CrossRef]
- Lei, D.; Xue, J.; Bi, Q.; Tang, C.; Zhang, L. 3D/2D direct Z-scheme photocatalyst Zn2SnO4/CdS for simultaneous removal of Cr(VI) and organic pollutant. Appl. Surf. Sci. 2020, 517, 146030. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Hu, S.; Jiang, W.; Liu, Y.; Liu, J. A novel 3D Z-scheme heterojunction photocatalyst: Ag6Si2O7 anchored on flower-like Bi2WO6 and its excellent photocatalytic performance for the degradation of toxic pharmaceutical antibiotics. Inorg. Chem. Front. 2020, 7, 529–541. [Google Scholar] [CrossRef]
- Li, Z.; Bi, D.; Zhao, Y.; Liu, R.; Ye, J.; Zhou, Y. In situ growth of zinc oxide nanoribbons within the interstices of a zinc stannate nanoplates network on compacted woven metal wires and their enhanced solar energy application. Electrochim. Acta 2018, 262, 124–134. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Liu, K.; Sun, R.; Yang, C.; Liu, X. In Situ Decoration of ZnSnO3 Nanosheets on the Surface of Hollow Zn2SnO4 Octahedrons for Enhanced Solar Energy Application. Nanomaterials 2022, 12, 2124. https://doi.org/10.3390/nano12122124
Li Z, Liu K, Sun R, Yang C, Liu X. In Situ Decoration of ZnSnO3 Nanosheets on the Surface of Hollow Zn2SnO4 Octahedrons for Enhanced Solar Energy Application. Nanomaterials. 2022; 12(12):2124. https://doi.org/10.3390/nano12122124
Chicago/Turabian StyleLi, Zhengdao, Kecheng Liu, Ruixue Sun, Chuanyun Yang, and Xiaodi Liu. 2022. "In Situ Decoration of ZnSnO3 Nanosheets on the Surface of Hollow Zn2SnO4 Octahedrons for Enhanced Solar Energy Application" Nanomaterials 12, no. 12: 2124. https://doi.org/10.3390/nano12122124
APA StyleLi, Z., Liu, K., Sun, R., Yang, C., & Liu, X. (2022). In Situ Decoration of ZnSnO3 Nanosheets on the Surface of Hollow Zn2SnO4 Octahedrons for Enhanced Solar Energy Application. Nanomaterials, 12(12), 2124. https://doi.org/10.3390/nano12122124




