Microwave-Assisted Solvothermal Synthesis of Mo-Doped TiO2 with Exceptional Textural Properties and Superior Adsorption Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
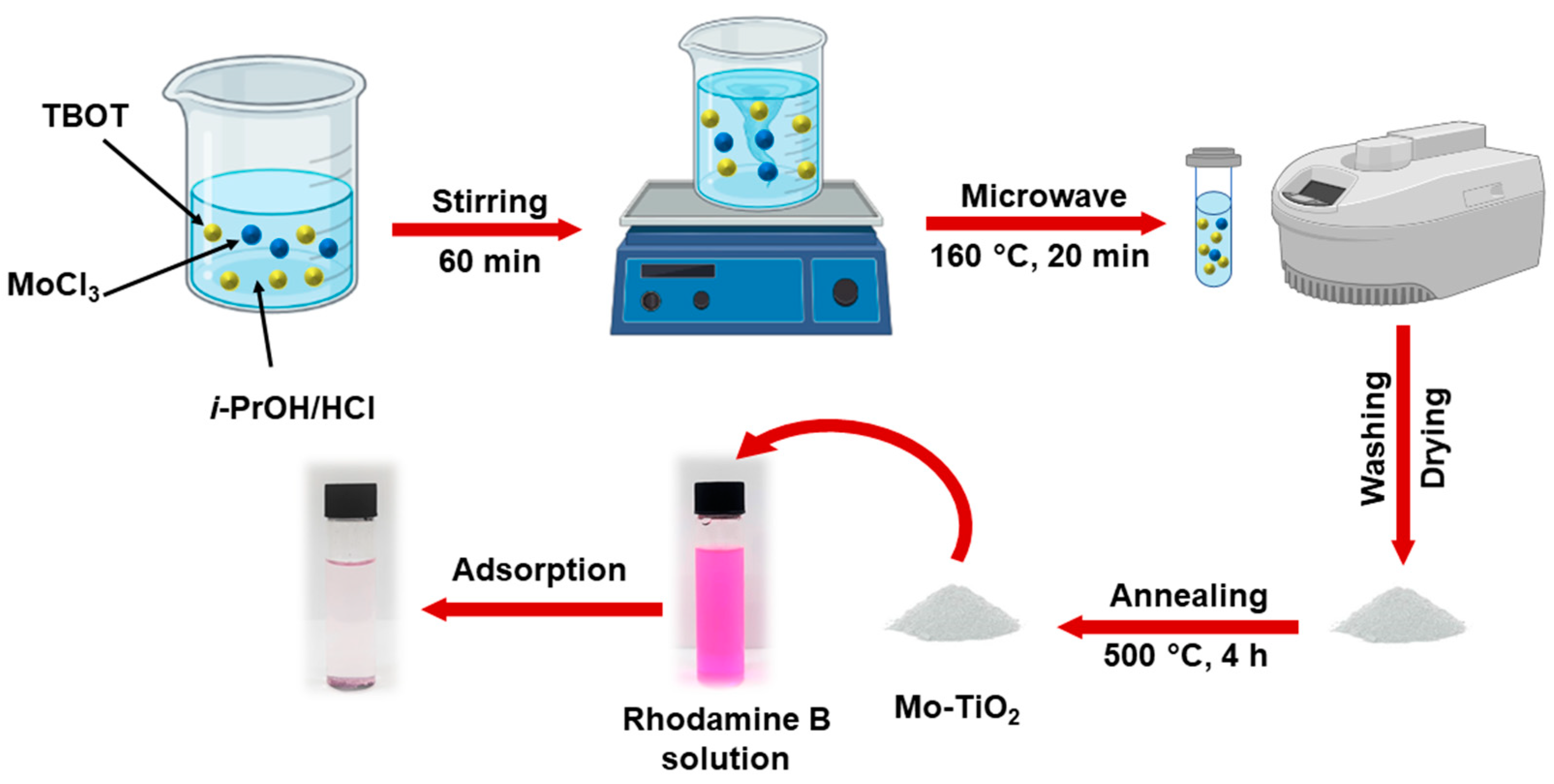
2.2. Materials Synthesis
Synthesis of Mo-Doped TiO2 (Mo-TiO2) Nanoparticles
2.3. Characterization
2.4. Adsorption Activity
3. Results and Discussion
3.1. Synthesis of TiO2 and Mo-TiO2
3.2. Morphology
3.3. Textural Properties
3.4. XRD Analysis

| Preparation Method | Metals Precursors | Reaction Conditions | Mo Ratio (%) | Specific Surface Area m2 g−1 (Pore Volume cm3 g−1) | Reference |
|---|---|---|---|---|---|
| Reverse micelle micro-emulsion sol-gel method | Titanium butoxide & ammonium heptamolybdate tetrahydrate | polyoxyethylene (20) oleyl ether (Brij O20) surfactants are dispersed in cyclohexane at 50 °C, then Ti precursor then emulsion broken by 2-propanol, dry, calcine at 500 °C, 2 h. | 0 (TiO2) 1% 5% 10% | 71 (0.091) 76 (0.112) 74 (0.141) 96 (0.137) | [42] |
| One-pot hydrothermal method | dihydroxy bis (ammonium lactato) titanium (IV) & ammonium heptamolybdate tetrahydrate | aqueous medium 200 °C, 12 h, 8 °C/min. | 0 (TiO2) 0.1% 0.3% 0.5% | 119 (0.32) 140 (0.38) 145 (0.40) 143 (0.36) | [25] |
| Sol-gel technique | Titanium butoxide & molybdenum pentachloride | Ethanol/acetylacetone/HCl sol is formed, left for 48 h, dry at 80 °C, then calcine at 450 °C | - | - | [43] |
| Hydrothermal method | TiO2 powder & ammonium heptamolybdate tetrahydrate | 10 M NaOH, 180 °C/24 h Calcination 500 °C/1 h | 0 (TiO2) 3% 5% 10% | 112 156 168 172 | [44] |
| Evaporation Induced Self Assembly | Titanium butoxide & ammonium heptamolybdate tetrahydrate | Ethanol/HNO3, rotary evaporator/17 h, calcine at 300 °C/1 h, then calcine at 400 °C | 0 (TiO2) 1% 2% 3% | 144 151 161 163 | [45] |
| Hydrothermal method | titanium tetra-isopropoxide & molybdenum pentachloride | Isopropanol/H2O, hydrothermal 150 °C/20 h, calcine 500 °C/4 h | 0 (TiO2) 0.02% 0.08% | 129 127 124 | [46] |
| Reverse micelle sol-gel synthesis | Titanium butoxide & ammonium heptamolybdate tetrahydrate | Polyoxyethylene, oleyl ether, Brij O20 surfactants, cyclohexane/H2O, then emulsion broken by 2-propanol, calcine at 500 °C/2 h | 0 (TiO2) 0.83% 3.2% 5.8% 7.7% 9.75% | 71 42 92 74 65 96 | [26] |
| Microwave-assisted solvothermal method | Titanium butoxide & molybdenum (III) chloride | Dodecylamine surfactant, isopropanol/20% HCl, 160 °C, 30 min. | 0 (TiO2) 0.9% 1.5% 2.2% 3.1% | 91.7 (0.35) 173.7 (0.62) 191.7 (0.36) 206.6 (0.33) 256.6 (0.41) | This work |
3.5. Electronic Structure
3.6. Raman Spectroscopy
3.7. Zeta-Potential Measurements
3.8. Adsorption Study
3.8.1. Adsorption Isotherms
3.8.2. Adsorption Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghasemi, Z.; Seif, A.; Ahmadi, T.S.; Zargar, B.; Rashidi, F.; Rouzbahani, G.M. Thermodynamic and kinetic studies for the adsorption of Hg(II) by nano-TiO2 from aqueous solution. Adv. Powder Technol. 2012, 23, 148–156. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzaman, M. Adsorption of phenolic compounds on low-cost adsorbents: A review. Adv. Colloid Interface Sci. 2008, 143, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.G.; Murthy, B.N.; Kumar, S.G. Photocatalytic activity of V5+, Mo6+ and Th4+ doped polycrystalline TiO2 for the degradation of chlorpyrifos under UV/solar light. J. Mol. Catal. A Chem. 2009, 308, 174–181. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Zhang, J.; Chen, H.; Anpo, M. Fe3+-TiO2 photocatalysts prepared by combining sol–gel method with hydrothermal treatment and their characterization. J. Photochem. Photobiol. A Chem. 2006, 180, 196–204. [Google Scholar] [CrossRef]
- Lu, B.; Ma, B.; Deng, X.; Wu, B.; Wu, Z.; Luo, J.; Wang, X.; Chen, G. Dual stabilized architecture of hollow Si@ TiO2@C nanospheres as anode of high-performance Li-ion battery. Chem. Eng. J. 2018, 351, 269–279. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Mariappan, V.K.; Kim, S.-J. Blue TiO2 nanosheets as a high-performance electrode material for supercapacitors. J. Colloid Interface Sci. 2019, 536, 62–70. [Google Scholar] [CrossRef]
- Galstyan, V.; Ponzoni, A.; Kholmanov, I.; Natile, M.M.; Comini, E.; Nematov, S.; Sberveglieri, G. Reduced graphene oxide–TiO2 nanotube composite: Comprehensive study for gas-sensing applications. ACS Appl. Nano Mater. 2018, 1, 7098–7105. [Google Scholar] [CrossRef]
- Zhu, Z.; Cai, H.; Sun, D.-W. Titanium dioxide (TiO2) photocatalysis technology for nonthermal inactivation of microorganisms in foods. Trends Food Sci. Technol. 2018, 75, 23–35. [Google Scholar] [CrossRef]
- Dréno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of titanium dioxide nanoparticles in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, Y.H.; Mohamed, A.T.; Sliem, M.H.; Abdullah, A.M.; Al-Qaradawi, S.Y. Enhanced photocatalytic performance of WON@porous TiO2 nanofibers towards sunlight-assisted degradation of organic contaminants. RSC Adv. 2018, 8, 32747–32755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gang, R.; Xia, Y.; Xu, L.; Zhang, L.; Ju, S.; Wang, Z.; Koppala, S. Size controlled Ag decorated TiO2 plasmonic photocatalysts for tetracycline degradation under visible light. Surf. Interfaces 2022, 31, 102018. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Javed, H.M.A.; Javed, S.; Bashir, A.; Usman, M.; Akram, A.; Ahmad, M.I.; Ali, U.; Shahid, M.; Rizwan, M.; et al. Incorporation of Zr-doped TiO2 nanoparticles in electron transport layer for efficient planar perovskite solar cells. Surf. Interfaces 2021, 25, 101299. [Google Scholar] [CrossRef]
- Jakobsson, A.-M. Measurement and Modeling of Th Sorption onto TiO2. J. Colloid Interface Sci. 1999, 220, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Fang, M.; Li, J.; Lu, Y.; Wang, X. Adsorption of Eu(III) onto TiO2: Effect of pH, concentration, ionic strength and soil fulvic acid. J. Hazard. Mater. 2009, 168, 458–465. [Google Scholar] [CrossRef]
- Fan, J.; Cai, W.; Yu, J. Adsorption of N719 Dye on Anatase TiO2 Nanoparticles and Nanosheets with Exposed (001) Facets: Equilibrium, Kinetic, and Thermodynamic Studies. Chem. Asian J. 2011, 6, 2481–2490. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Thevenet, F.; Gravejat, P.; Rousseau, A. Investigation of NO and NO2 adsorption mechanisms on TiO2 at room temperature. Appl. Catal. B Environ. 2013, 142–143, 196–204. [Google Scholar] [CrossRef]
- Pettibone, J.M.; Cwiertny, D.M.; Scherer, M.; Grassian, V.H. Adsorption of Organic Acids on TiO2 Nanoparticles: Effects of pH, Nanoparticle Size, and Nanoparticle Aggregation. Langmuir 2008, 24, 6659–6667. [Google Scholar] [CrossRef]
- Yoong, L.S.; Chong, F.K.; Dutta, B.K. Development of copper-doped TiO2 photocatalyst for hydrogen production under visible light. Energy 2009, 34, 1652–1661. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, W.-F.; Zhang, X.; Zhang, J.; Koshy, P.; Sorrell, C.C. Structural and microstructural effects of Mo3+/Mo5+ codoping on properties and photocatalytic performance of nanostructured TiO2 Thin films. J. Phys. Chem. C 2019, 123, 11781–11790. [Google Scholar] [CrossRef]
- Sreedhar, M.; Brijitta, J.; Reddy, I.N.; Cho, M.; Shim, J.; Bera, P.; Joshi, B.N.; Yoon, S.S. Dye degradation studies of Mo-doped TiO2 thin films developed by reactive sputtering. Surf. Interface Anal. 2018, 50, 171–179. [Google Scholar] [CrossRef]
- Mardare, D.; Cornei, N.; Luca, D.; Dobromir, M.; Irimiciuc, Ş.A.; Pungă, L.; Pui, A.; Adomniţei, C. Synthesis and hydrophilic properties of Mo doped TiO2 thin films. J. Appl. Phys. 2014, 115, 213501. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Nguyen, T.-T.; Akalework, N.G.; Pan, C.-J.; Rick, J.; Liao, Y.-F.; Su, W.-N.; Hwang, B.-J. Interplay between molybdenum dopant and oxygen vacancies in a TiO2 support enhances the oxygen reduction reaction. ACS Catal. 2016, 6, 6551–6559. [Google Scholar] [CrossRef]
- Liao, H.; Xie, L.; Zhang, Y.; Qiu, X.; Li, S.; Huang, Z.; Hou, H.; Ji, X. Mo-doped gray anatase TiO2: Lattice expansion for enhanced sodium storage. Electrochim. Acta 2016, 219, 227–234. [Google Scholar] [CrossRef]
- Feng, S.; Zhao, J.; Bai, Y.; Liang, X.; Wang, T.; Wang, C. Facile synthesis of Mo-doped TiO2 for selective photocatalytic CO2 reduction to methane: Promoted H2O dissociation by Mo doping. J. CO2 Util. 2020, 38, 1–9. [Google Scholar] [CrossRef]
- Esposito, S.; Ditaranto, N.; Dell’Agli, G.; Nasi, R.; Rivolo, P.; Bonelli, B. Effective Inclusion of Sizable Amounts of Mo within TiO2 Nanoparticles Can Be Obtained by Reverse Micelle Sol–Gel Synthesis. ACS Omega 2021, 6, 5379–5388. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Deng, Q.; Ng, D.H.L.; Zhao, H. Microwave-Assisted Fabrication of Nanoparticulate TiO2 Microspheres for Synergistic Photocatalytic Removal of Cr(VI) and Methyl Orange. ACS Appl. Mater. Interfaces 2014, 6, 3008–3015. [Google Scholar] [CrossRef]
- Kumar, A.; Kuang, Y.; Liang, Z.; Sun, X. Microwave chemistry, recent advancements, and eco-friendly microwave-assisted synthesis of nanoarchitectures and their applications: A review. Mater. Today Nano 2020, 11, 100076. [Google Scholar] [CrossRef]
- Cui, L.; Hui, K.N.; Hui, K.S.; Lee, S.K.; Zhou, W.; Wan, Z.P.; Thuc, C.-N.H. Facile microwave-assisted hydrothermal synthesis of TiO2 nanotubes. Mater. Lett. 2012, 75, 175–178. [Google Scholar] [CrossRef]
- Ding, K.; Miao, Z.; Liu, Z.; Zhang, Z.; Han, B.; An, G.; Miao, S.; Xie, Y. Facile Synthesis of High Quality TiO2 Nanocrystals in Ionic Liquid via a Microwave-Assisted Process. J. Am. Chem. Soc. 2007, 129, 6362–6363. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Zhang, D.; Gao, Y.; Shao, X.; Ding, G.; Li, S.; Zhao, S. One-pot microwave-assisted combustion synthesis of graphene oxide–TiO2 hybrids for photodegradation of methyl orange. J. Alloys Compd. 2013, 551, 382–388. [Google Scholar] [CrossRef]
- Esquivel, K.; Nava, R.; Zamudio-Méndez, A.; González, M.V.; Jaime-Acuña, O.E.; Escobar-Alarcón, L.; Peralta-Hernández, J.M.; Pawelec, B.; Fierro, J.L.G. Microwave-assisted synthesis of (S)Fe/TiO2 systems: Effects of synthesis conditions and dopant concentration on photoactivity. Appl. Catal. B Environ. 2013, 140–141, 213–224. [Google Scholar] [CrossRef]
- Suwarnkar, M.B.; Dhabbe, R.S.; Kadam, A.N.; Garadkar, K.M. Enhanced photocatalytic activity of Ag doped TiO2 nanoparticles synthesized by a microwave assisted method. Ceram. Int. 2014, 40, 5489–5496. [Google Scholar] [CrossRef]
- Calleja, G.; Serrano, D.P.; Sanz, R.; Pizarro, P.; García, A. Study on the synthesis of high-surface-area mesoporous TiO2 in the presence of nonionic surfactants. Ind. Eng. Chem. Res. 2004, 43, 2485–2492. [Google Scholar] [CrossRef]
- Kluson, P.; Kacer, P.; Cajthaml, T.; Kalaji, M. Preparation of titania mesoporous materials using a surfactant-mediated sol–gel method. J. Mater. Chem. 2001, 11, 644–651. [Google Scholar] [CrossRef]
- Lee, D.-U.; Jang, S.-R.; Vittal, R.; Lee, J.; Kim, K.-J. CTAB facilitated spherical rutile TiO2 particles and their advantage in a dye-sensitized solar cell. Sol. Energy 2008, 82, 1042–1048. [Google Scholar] [CrossRef]
- Tian, G.; Fu, H.; Jing, L.; Xin, B.; Pan, K. Preparation and Characterization of Stable Biphase TiO2 Photocatalyst with High Crystallinity, Large Surface Area, and Enhanced Photoactivity. J. Phys. Chem. C 2008, 112, 3083–3089. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-Y.; Guo, R.-T.; Pan, W.-G.; Tang, J.-Y.; Zhou, W.-G.; Liu, X.-Y.; Qin, H.; Jia, P.-Y. One-dimension TiO2 nanostructures with enhanced activity for CO2 photocatalytic reduction. Appl. Surf. Sci. 2019, 464, 534–543. [Google Scholar] [CrossRef]
- Bregani, F.; Casale, C.; Depero, L.; Natali-Sora, I.; Robba, D.; Sangaletti, L.; Toledo, G. Temperature effects on the size of anatase crystallites in Mo-TiO2 and W-TiO2 powders. Sens. Actuat. B Chem. 1996, 31, 25–28. [Google Scholar] [CrossRef]
- Nasi, R.; Esposito, S.; Freyria, F.S.; Armandi, M.; Gadhi, T.A.; Hernandez, S.; Rivolo, P.; Ditaranto, N.; Bonelli, B. Application of Reverse Micelle Sol–Gel Synthesis for Bulk Doping and Heteroatoms Surface Enrichment in Mo-Doped TiO2 Nanoparticles. Materials 2019, 12, 937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Bai, L.; Sun, H.; Jiang, Q.; Lian, J. Structure and photocatalytic property of Mo-doped TiO2 nanoparticles. Powder Technol. 2013, 244, 9–15. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Liu, H.; Zhu, Z. Multilayered Mo-doped TiO2 nanofibers and enhanced photocatalytic activity. Mater. Manuf. Process 2012, 27, 631–635. [Google Scholar] [CrossRef]
- Avilés-García, O.; Espino-Valencia, J.; Romero, R.; Rico-Cerda, J.L.; Arroyo-Albiter, M.; Natividad, R. W and Mo doped TiO2: Synthesis, characterization and photocatalytic activity. Fuel 2017, 198, 31–41. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.-F.; Bahmanrokh, G.; Kumar, V.; Ho, N.; Koshy, P.; Sorrell, C.C. Synthesis of V- and Mo-doped/codoped TiO2 powders for photocatalytic degradation of methylene blue. Nano-Struct. Nano-Objects 2020, 24, 100557. [Google Scholar] [CrossRef]
- Luo, S.-Y.; Yan, B.-X.; Shen, J. Enhancement of photoelectric and photocatalytic activities: Mo doped TiO2 thin films deposited by sputtering. Thin Solid Film. 2012, 522, 361–365. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Yang, H.G.; Cheng, H.-M.; Lu, G.Q. Titania-based photocatalysts—crystal growth, doping and heterostructuring. J. Mater. Chem. 2010, 20, 831–843. [Google Scholar] [CrossRef]
- Štengl, V.; Bakardjieva, S. Molybdenum-Doped Anatase and Its Extraordinary Photocatalytic Activity in the Degradation of Orange II in the UV and vis Regions. J. Phys. Chem. C 2010, 114, 19308–19317. [Google Scholar] [CrossRef]
- Khan, H.; Berk, D. Characterization and mechanistic study of Mo+6 and V+5 codoped TiO2 as a photocatalyst. J. Photochem. Photobiol. A Chem. 2014, 294, 96–109. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Hedhili, M.N.; Ahmed, E.; Shi, L.; Wang, P. Microwave-Assisted Self-Doping of TiO2 Photonic Crystals for Efficient Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2014, 6, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, W.-F.; Koshy, P.; Sorrell, C.C. Enhanced photocatalytic performance of nanostructured TiO2 thin films through combined effects of polymer conjugation and Mo-doping. J. Mater. Sci. 2019, 54, 5266–5279. [Google Scholar] [CrossRef]
- Niu, X.; Yan, W.; Shao, C.; Zhao, H.; Yang, J. Hydrothermal synthesis of Mo-C co-doped TiO2 and coupled with fluorine-doped tin oxide (FTO) for high-efficiency photodegradation of methylene blue and tetracycline: Effect of donor-acceptor passivated co-doping. Appl. Surf. Sci. 2019, 466, 882–892. [Google Scholar] [CrossRef]
- Wang, D.-H.; Jia, L.; Wu, X.-L.; Lu, L.-Q.; Xu, A.-W. One-step hydrothermal synthesis of N-doped TiO2/C nanocomposites with high visible light photocatalytic activity. Nanoscale 2012, 4, 576–584. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.; Zhang, J.; Pan, C. Raman Spectroscopy: A New Approach to Measure the Percentage of Anatase TiO2 Exposed (001) Facets. J. Phys. Chem. C 2012, 116, 7515–7519. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Feng, Z.; Chen, J.; Li, C. UV Raman Spectroscopic Study on TiO2. I. Phase Transformation at the Surface and in the Bulk. J. Phys. Chem. B 2006, 110, 927–935. [Google Scholar] [CrossRef]
- Hu, H.; Wachs, I.E.; Bare, S.R. Surface Structures of Supported Molybdenum Oxide Catalysts: Characterization by Raman and Mo L3-Edge XANES. J. Phys. Chem. 1995, 99, 10897–10910. [Google Scholar] [CrossRef]
- Stampfl, S.R.; Chen, Y.; Dumesic, J.; Niu, C.; Hill Jr, C. Interactions of molybdenum oxide with various oxide supports: Calcination of mechanical mixtures. J. Catal. 1987, 105, 445–454. [Google Scholar] [CrossRef]
- Shimada, T.; Yamazaki, H.; Mimura, M.; Inui, Y.; Guengerich, F.P. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: Studies with liver microsomes of 30 Japanese and 30 Caucasians. J. Pharmacol. Exp. Ther. 1994, 270, 414. [Google Scholar]
- Alakhras, F.; Alhajri, E.; Haounati, R.; Ouachtak, H.; Addi, A.A.; Saleh, T.A. A comparative study of photocatalytic degradation of Rhodamine B using natural-based zeolite composites. Surf. Interfaces 2020, 20, 100611. [Google Scholar] [CrossRef]
- Li, Z.; Meng, X.; Zhang, Z. Equilibrium and kinetic modelling of adsorption of Rhodamine B on MoS2. Mater. Res. Bull. 2019, 111, 238–244. [Google Scholar] [CrossRef]
- Ding, L.; Zou, B.; Gao, W.; Liu, Q.; Wang, Z.; Guo, Y.; Wang, X.; Liu, Y. Adsorption of Rhodamine-B from aqueous solution using treated rice husk-based activated carbon. Colloids Surf. A Physicochem. Eng. Asp. 2014, 446, 1–7. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Y.; Zhang, C.; Huang, X.; Ma, K.; Zhang, Y. Preparation of graphene oxide/4A molecular sieve composite and evaluation of adsorption performance for Rhodamine B. Sep. Purif. Technol. 2022, 286, 120400. [Google Scholar] [CrossRef]
- Cheng, Z.-L.; Li, Y.-X.; Liu, Z. Study on adsorption of rhodamine B onto Beta zeolites by tuning SiO2/Al2O3 ratio. Ecotoxicol. Environ. Saf. 2018, 148, 585–592. [Google Scholar] [CrossRef]
- Khan, T.A.; Dahiya, S.; Ali, I. Use of kaolinite as adsorbent: Equilibrium, dynamics and thermodynamic studies on the adsorption of Rhodamine B from aqueous solution. Appl. Clay Sci. 2012, 69, 58–66. [Google Scholar] [CrossRef]
- Mittal, H.; Mishra, S.B. Gum ghatti and Fe3O4 magnetic nanoparticles based nanocomposites for the effective adsorption of rhodamine B. Carbohydr. Polym. 2014, 101, 1255–1264. [Google Scholar] [CrossRef]
- Cui, W.; Kang, X.; Zhang, X.; Cui, X. Gel-like ZnO/Zr-MOF(bpy) nanocomposite for highly efficient adsorption of Rhodamine B dye from aqueous solution. J. Phys. Chem. Solids 2019, 134, 165–175. [Google Scholar] [CrossRef]










| Sample | Atomic Ratio of Mo from EDX | Atomic Ratio of Mo from XPS | L (Crystallite Size, nm) | ε (Lattice Strain) | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Radius (nm) |
|---|---|---|---|---|---|---|---|
| TiO2 | - | - | 13.71 | 2.53 × 10−2 | 91.7 | 0.35 | 7.6 |
| TiO2-Mo-0.9 | 0.93 | 1.23 | 10.74 | 3.23 × 10−2 | 173.7 | 0.62 | 7.2 |
| TiO2-Mo-1.5 | 1.45 | 1.92 | 9.94 | 3.49 × 10−2 | 191.7 | 0.36 | 3.7 |
| TiO2-Mo-2.2 | 2.23 | 2.75 | 8.96 | 3.87 × 10−2 | 206.6 | 0.33 | 3.2 |
| TiO2-Mo-3.1 | 3.09 | 4.46 | 7.93 | 4.37 × 10−2 | 256.5 | 0.41 | 3.2 |
| Isotherm | Parameter | Value |
|---|---|---|
| Langmuir | qmax | 69.01 |
| K (L mg−1) | 0.288 | |
| R2 | 0.981 | |
| Freundlich | n | 2.09 |
| Kf (L g−1) | 17.273 | |
| R2 | 0.943 | |
| Temkin | B | 15.054 |
| AT (L g−1) | 1.053 | |
| bT (J mol−1) | 162.374 | |
| R2 | 0.971 | |
| Dubinin–Radushkevich | qs (mg g−1) | 45.403 |
| R2 | 0.881 |
| Co (ppm) | Pseudo-First Order Model | Pseudo-Second Order Model | ||||
|---|---|---|---|---|---|---|
| k1 (min−1) | qe (mg g−1) | R2 | k2 (g mg−1 min−1) | qe (mg g−1) | R2 | |
| 15 | 0.0568 | 1.468 | 0.8399 | 0.0916 | 14.138 | 0.9999 |
| 20 | 0.0474 | 1.807 | 0.8455 | 0.0542 | 18.615 | 0.9999 |
| 25 | 0.0212 | 2.515 | 0.7726 | 0.0126 | 24.073 | 0.9998 |
| 30 | 0.0541 | 3.611 | 0.8272 | 0.0084 | 27.115 | 0.9997 |
| 40 | 0.0579 | 3.863 | 0.9581 | 0.0074 | 37.779 | 0.9998 |
| 50 | 0.0641 | 3.846 | 0.9797 | 0.0071 | 45.167 | 0.9994 |
| 60 | 0.0630 | 3.472 | 0.9603 | 0.0109 | 50.505 | 0.9999 |
| 70 | 0.0630 | 5.884 | 0.9455 | 0.0136 | 57.208 | 0.9999 |
| Adsorbent | RhB Concentration, mg L−1 | Temperature (K) | Rate Constant, k2 × 10−3 (g mg−1 min−1) | Reference |
|---|---|---|---|---|
| MoS2 mesoporous microspheres prepared using CH4N2S | 10 15 20 | 293 | 3.0 3.0 1.0 | [61] |
| treated rice husk-based activated carbon | 100 | 293 | 1.68 | [62] |
| graphene oxide/4A molecular sieve composite | 10 | 298 | 23.0 | [63] |
| Beta zeolites (SiO2/Al2O3 = 18.4) | 20 | 295 | 61.0 | [64] |
| Kaolinite | 20 40 60 | 303 | 4.69 2.30 1.26 | [65] |
| Gum ghatti/Fe3O4 nanocomposite | 50 | 298 | 67.0 | [66] |
| ZnO/Zr-MOF(bpy) nanocomposite | 10 30 50 | 298 | 2.69 1.06 0.334 | [67] |
| TiO2-Mo-3.1 | 15 20 40 50 60 | 293 | 91.6 54.2 8.40 7.40 7.10 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, Y.H.; Abu Hatab, A.S.; Mohamed, A.T.; Al-Kuwari, M.S.; Aljaber, A.S.; Al-Qaradawi, S.Y. Microwave-Assisted Solvothermal Synthesis of Mo-Doped TiO2 with Exceptional Textural Properties and Superior Adsorption Kinetics. Nanomaterials 2022, 12, 2051. https://doi.org/10.3390/nano12122051
Ahmad YH, Abu Hatab AS, Mohamed AT, Al-Kuwari MS, Aljaber AS, Al-Qaradawi SY. Microwave-Assisted Solvothermal Synthesis of Mo-Doped TiO2 with Exceptional Textural Properties and Superior Adsorption Kinetics. Nanomaterials. 2022; 12(12):2051. https://doi.org/10.3390/nano12122051
Chicago/Turabian StyleAhmad, Yahia H., Aymen S. Abu Hatab, Assem T. Mohamed, Mohammed S. Al-Kuwari, Amina S. Aljaber, and Siham Y. Al-Qaradawi. 2022. "Microwave-Assisted Solvothermal Synthesis of Mo-Doped TiO2 with Exceptional Textural Properties and Superior Adsorption Kinetics" Nanomaterials 12, no. 12: 2051. https://doi.org/10.3390/nano12122051
APA StyleAhmad, Y. H., Abu Hatab, A. S., Mohamed, A. T., Al-Kuwari, M. S., Aljaber, A. S., & Al-Qaradawi, S. Y. (2022). Microwave-Assisted Solvothermal Synthesis of Mo-Doped TiO2 with Exceptional Textural Properties and Superior Adsorption Kinetics. Nanomaterials, 12(12), 2051. https://doi.org/10.3390/nano12122051







