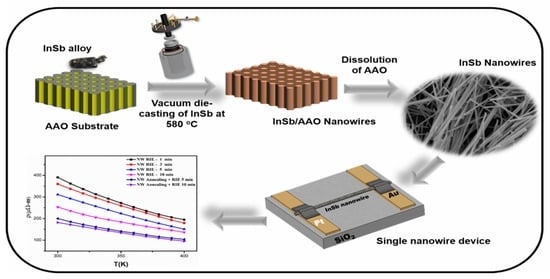
Tailoring InSb Nanowires for High Thermoelectric Performance Using AAO Template-Assisted Die Casting Process
Abstract
:1. Introduction
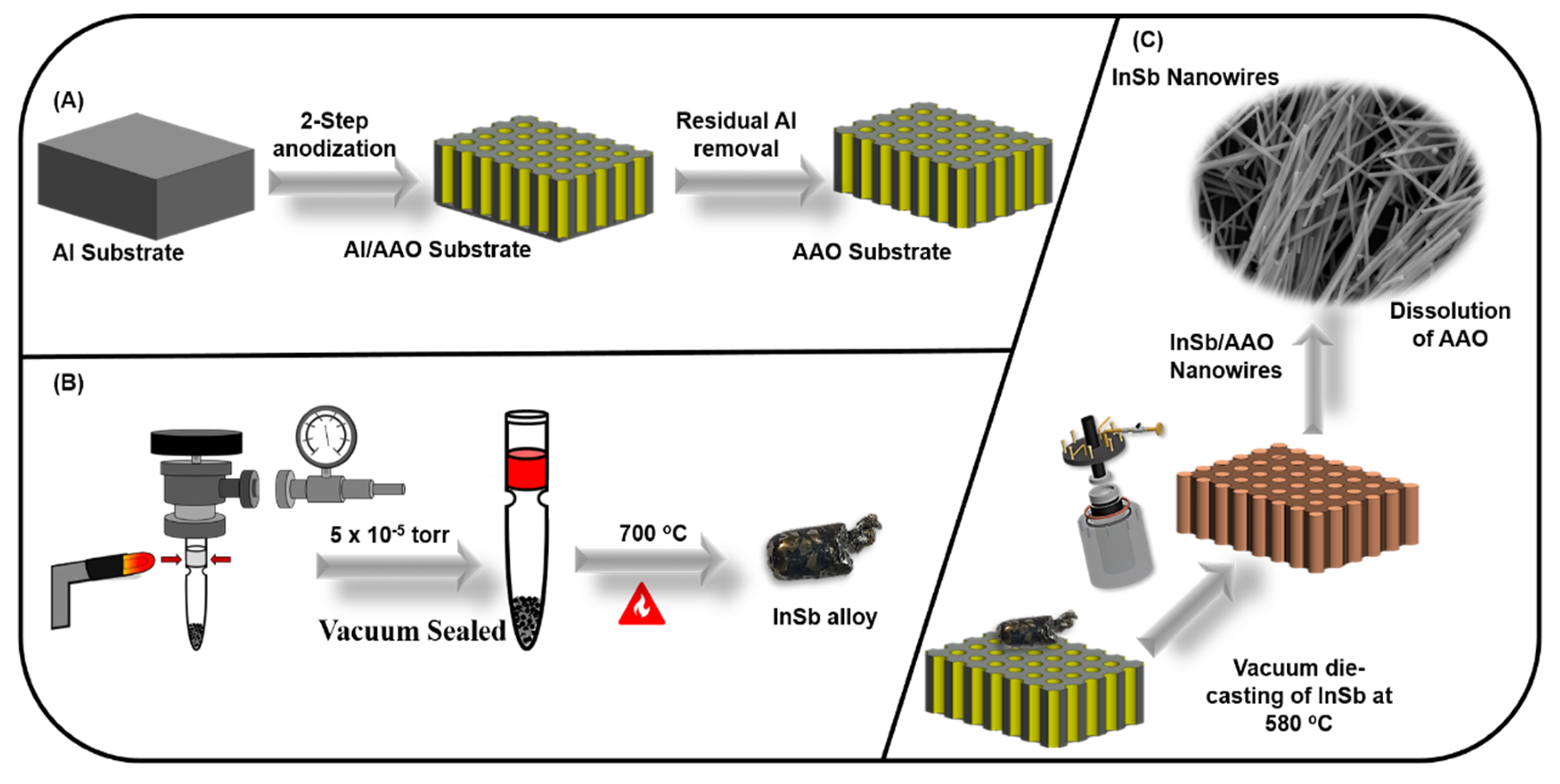
2. Experimental Procedure
2.1. Characterization
3. Results and Discussion
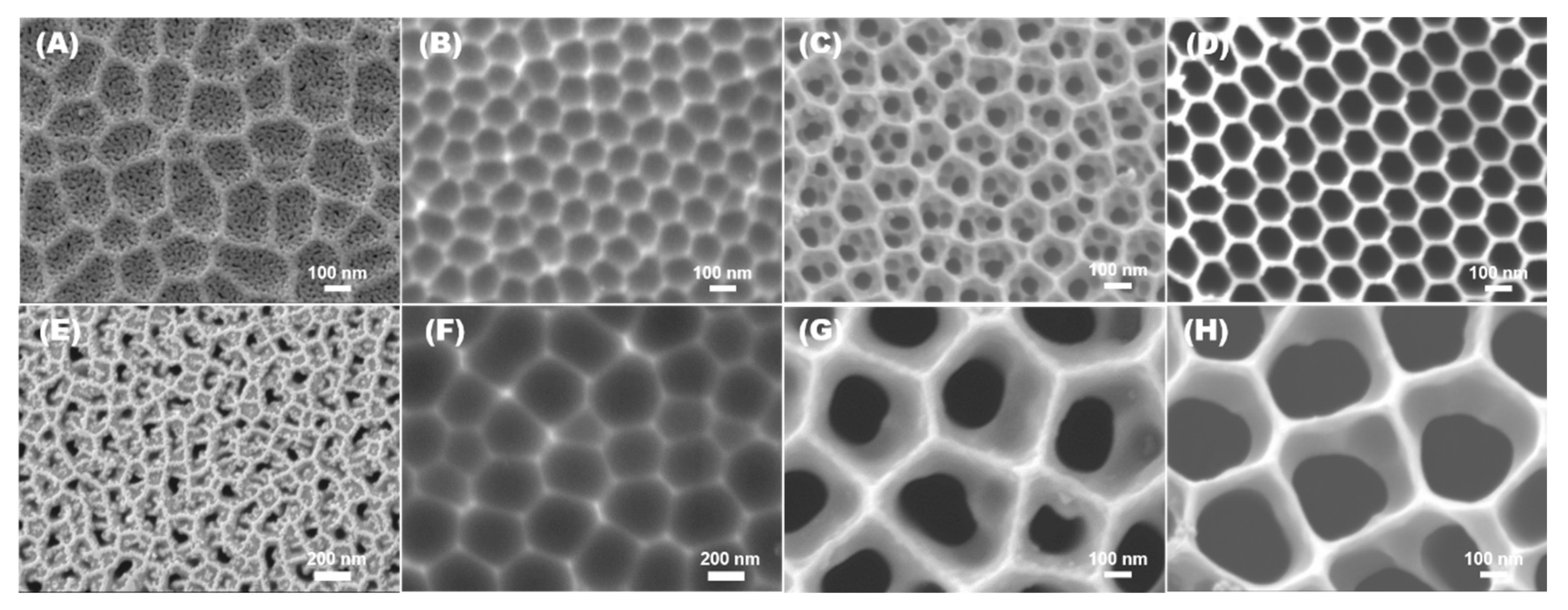
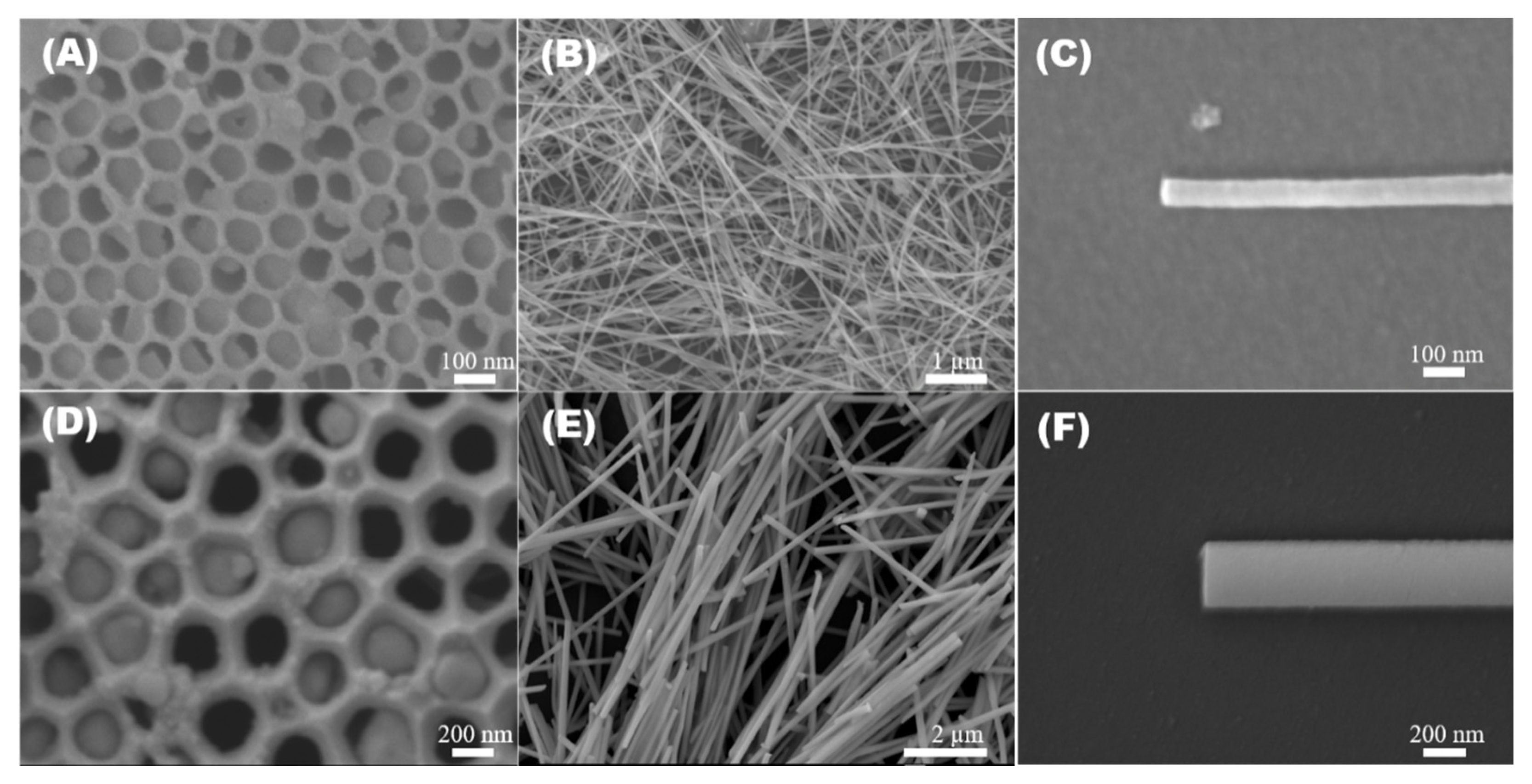
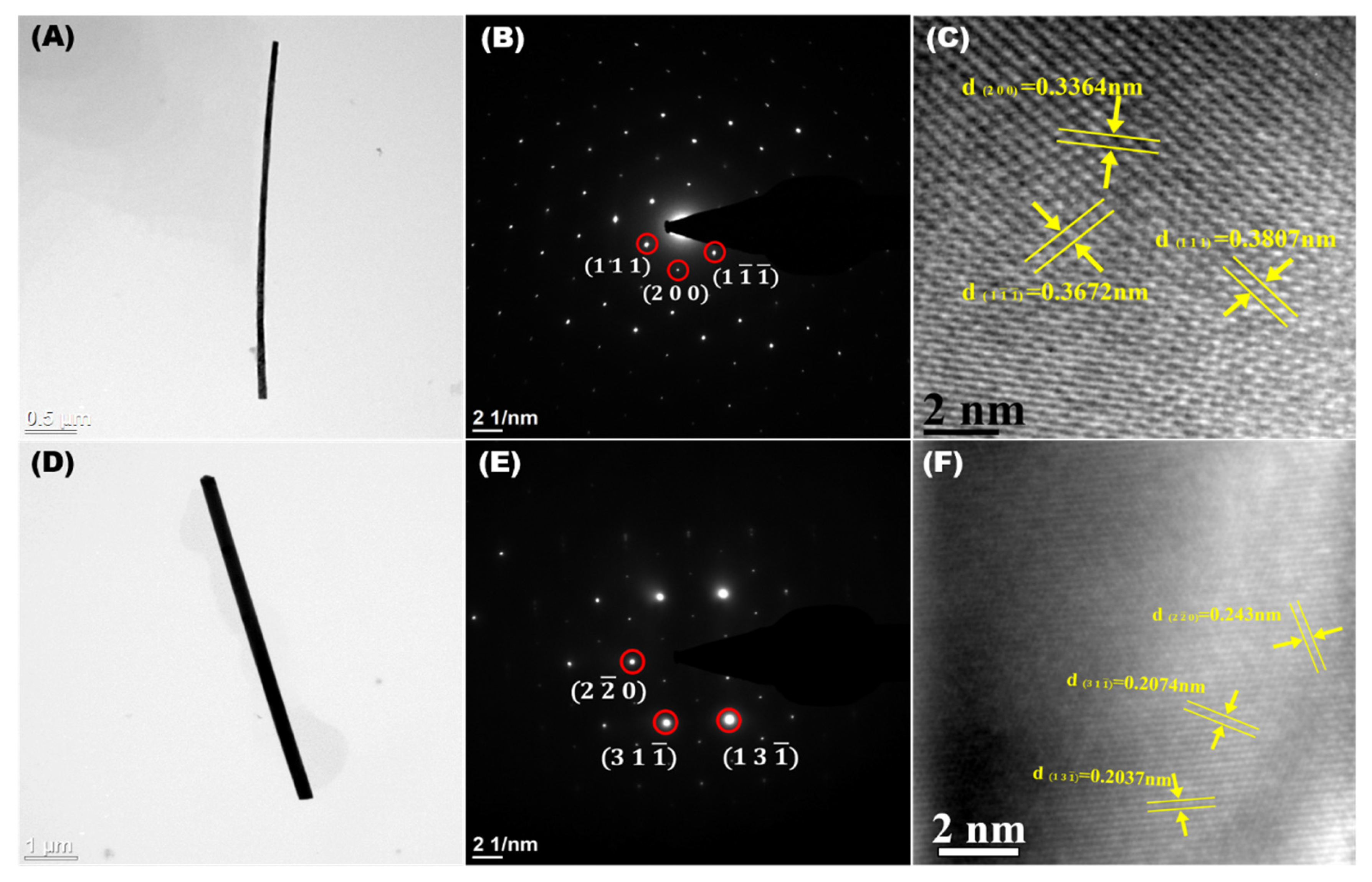
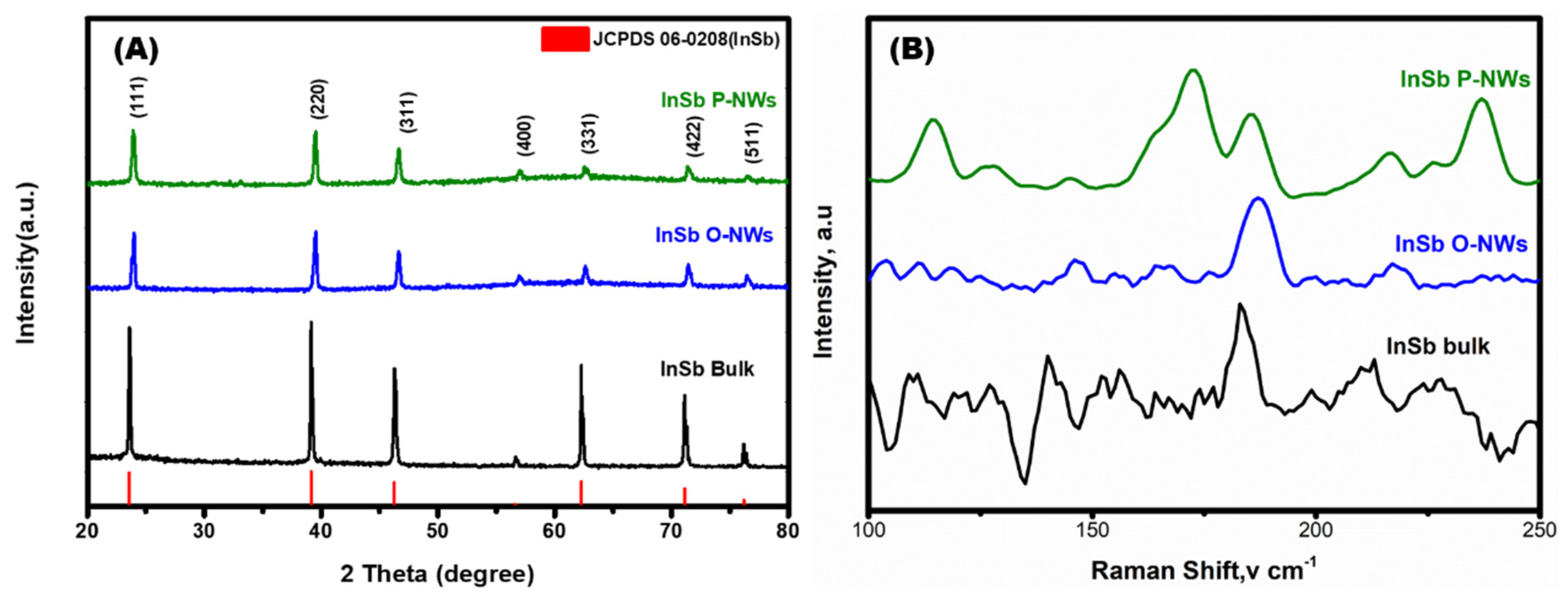
3.1. Morphological and Microstructural Analysis
3.2. Thermoelectrical Property of InSb NWs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, X.-L.; Zou, J.; Chen, Z.-G. Advanced Thermoelectric Design: From Materials and Structures to Devices. Chem. Rev. 2020, 120, 7399–7515. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Lee, J.; Lee, W.; Li, D. Thermoelectrics of Nanowires. Chem. Rev. 2019, 119, 9260–9302. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wang, S.; Wang, X.; Suwardi, A.; Chua, M.H.; Soo, X.Y.D.; Xu, J. Bottom-Up Engineering Strategies for High-Performance Thermoelectric Materials. Nano-Micro Lett. 2021, 13, 119. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.G.; Hana, G.; Yanga, L.; Cheng, L.; Zou, J. Nanostructured Thermoelectric Materials: Current Research and Future Challenge. Prog. Nat. Sci. Mater. Int. 2012, 22, 535–549. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.; Liu, Z.; Ren, Z. Size Effect in Thermoelectric Materials. npj Quantum Mater. 2016, 1, 16028. [Google Scholar] [CrossRef]
- Liu, S.; Li, G.; Lan, M.; Piao, Y.; Zhang, Y.; Wang, Q. Study on the Thermoelectric Properties of Porous Bi-Te Films Deposited Using Thermal Evaporation on AAO Template. Curr. Appl. Phys. 2020, 20, 400–405. [Google Scholar] [CrossRef]
- Prete, D.; Dimaggio, E.; Demontis, V.; Zannier, V.; Rodriguez-Douton, M.J.; Guazzelli, L.; Beltram, F.; Sorba, L.; Pennelli, G.; Rossella, F. Electrostatic Control of the Thermoelectric Figure of Merit in Ion-Gated Nanotransistors. Adv. Funct. Mater. 2021, 31, 2104175. [Google Scholar] [CrossRef]
- Roslyak, O.; Piryatinski, A. Thermoelectric Properties of Semiconductor Nanowire Networks articles you may be interested in. J. Appl. Phys. 2016, 119, 125107. [Google Scholar] [CrossRef] [Green Version]
- Ju, H.; Park, D.; Kim, J. Organic Acid-Assisted Chemical Transformation of SnSe Bulk Crystals into Se Nanowires and the Influence on Thermoelectric Behaviors. J. Alloy. Compd. 2018, 732, 436–442. [Google Scholar] [CrossRef]
- Domínguez-Adame, F.; Martín-González, M.; Sánchez, D.; Cantarero, A. Nanowires: A Route to Efficient Thermoelectric Devices. Phys. E Low-Dimensional Syst. Nanostructures 2019, 113, 213–225. [Google Scholar]
- Hsin, C.-L.; Huang, C.-W.; Wu, M.-H.; Cheng, S.-Y.; Pan, R.-C. Synthesis and Thermoelectric Properties of Indium Telluride Nanowires. Mater. Res. Bull. 2019, 112, 61–65. [Google Scholar] [CrossRef]
- Demontis, V.; Zannier, V.; Sorba, L.; Rossella, F. Surface Nano-Patterning for the Bottom-up Growth of Iii-v Semiconductor Nanowire Ordered Arrays. Nanomaterials 2021, 11, 2079. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, P.; i Morral, A.F. Semiconductor Nanowires: To Grow or Not to Grow? Mater. Today Nano 2020, 9, 100058. [Google Scholar] [CrossRef]
- Pak, A.; Ivashutenko, A.; Zakharova, A.; Vassilyeva, Y. Cubic SiC Nanowire Synthesis by DC Arc Discharge under Ambient Air Conditions. Surf. Coat. Technol. 2020, 387, 125554. [Google Scholar] [CrossRef]
- Nair, N.; Sankapal, B.R. Cationic-Exchange Approach for Conversion of Two Dimensional CdS to Two Dimensional Ag2S Nanowires with an Intermediate Core-Shell Nanostructure towards Supercapacitor Application. New J. Chem. 2016, 40, 10144–10152. [Google Scholar] [CrossRef]
- Ma, Z.; Chai, S.; Feng, Q.; Li, L.; Li, X.; Huang, L.; Liu, D.; Sun, J.; Jiang, R.; Zhai, T.; et al. Chemical Vapor Deposition Growth of High Crystallinity Sb2Se3 Nanowire with Strong Anisotropy for Near-Infrared Photodetectors. Small 2019, 15, e1805307. [Google Scholar] [CrossRef]
- Bertero, E.; Manzano, C.V.; Bürki, G.; Philippe, L. Stainless Steel-like FeCrNi Nanostructures via Electrodeposition into AAO Templates Using a Mixed-Solvent Cr(III)-Based Electrolyte. Mater. Des. 2020, 190, 108559. [Google Scholar] [CrossRef]
- Shkurmanov, A.; Sturm, C.; Franke, H.; Lenzner, J.; Grundmann, M. Low-Temperature PLD-Growth of Ultrathin ZnO Nanowires by Using ZnxAl1−xO and ZnxGa1−xO Seed Layers. Nanoscale Res. Lett. 2017, 12, 134. [Google Scholar] [CrossRef] [Green Version]
- Sztaberek, L.; Mabey, H.; Beatrez, W.; Lore, C.; Santulli, A.C.; Koenigsmann, C. Sol-Gel Synthesis of Ruthenium Oxide Nanowires to Enhance Methanol Oxidation in Supported Platinum Nanoparticle Catalysts. ACS Omega 2019, 4, 14226–14233. [Google Scholar] [CrossRef] [Green Version]
- Zettler, J.K.; Corfdir, P.; Hauswald, C.; Luna, E.; Jahn, U.; Flissikowski, T.; Schmidt, E.; Ronning, C.; Trampert, A.; Geelhaar, L.; et al. Observation of Dielectrically Confined Excitons in Ultrathin GaN Nanowires up to Room Temperature. Nano Lett. 2016, 16, 973–980. [Google Scholar] [CrossRef]
- Chen, S.H.; Chen, C.C.; Luo, Z.P.; Chao, C.G. Fabrication and Characterization of Eutectic Bismuth-Tin (Bi-Sn) Nanowires. Mater. Lett. 2009, 63, 1165–1168. [Google Scholar] [CrossRef]
- Chen, S.H.; Wang, C.Y.; Chen, Y.C.; Hun, C.W.; Chen, S.F.; Yang, S.M. Fabrication of Pure Aluminum Nanowires by Using Injection Molding Process in Ambient Air. Mater. Lett. 2015, 148, 30–33. [Google Scholar] [CrossRef]
- Qu, X.; Dai, J.; Tian, J.; Huang, X.; Liu, Z.; Shen, Z.; Wang, P. Syntheses of Nd2O3 Nanowires through Sol-Gel Process Assisted with Porous Anodic Aluminum Oxide (AAO) Template. J. Alloy. Compd. 2009, 469, 332–335. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, P.; Qu, Y.; Jiang, Y.; Huang, J.; Yan, W.; Liu, G. AFM Characterization and Electrochemical Property of Ag Nanowires by Modified AAO Template Method. J. Alloys Compd. 2008, 466, 479–482. [Google Scholar] [CrossRef]
- Chen, S.H.; Chen, C.C.; Chao, C.G. Novel Morphology and Solidification Behavior of Eutectic Bismuth-Tin (Bi-Sn) Nanowires. J. Alloys Compd. 2009, 481, 270–273. [Google Scholar] [CrossRef]
- Nazarkina, Y.; Gavrilov, S.; Terryn, H.; Petrova, M.; Ustarroz, J. Investigation of the Ordering of Porous Anodic Alumina Formed by Anodization of Aluminum in Selenic Acid. J. Electrochem. Soc. 2015, 162, E166–E172. [Google Scholar] [CrossRef]
- Chen, Y.H.; Shen, Y.M.; Wang, S.C.; Huang, J.L. Fabrication of One-Dimensional ZnO Nanotube and Nanowire Arrays with an Anodic Alumina Oxide Template via Electrochemical Deposition. Thin Solid Film. 2014, 570, 303–309. [Google Scholar] [CrossRef]
- Lekwongderm, P.; Chumkaew, R.; Thainoi, S.; Kiravittaya, S.; Tandaechanurat, A.; Nuntawong, N.; Sopitpan, S.; Yordsri, V.; Thanachayanont, C.; Kanjanachuchai, S.; et al. Study on Raman Spectroscopy of InSb Nano-Stripes Grown on GaSb Substrate by Molecular Beam Epitaxy and Their Raman Peak Shift with Magnetic Field. J. Cryst. Growth 2019, 512, 198–202. [Google Scholar] [CrossRef]
- Oviroh, P.O.; Akbarzadeh, R.; Pan, D.; Coetzee, R.A.M.; Jen, T.C. New Development of Atomic Layer Deposition: Processes, Methods and Applications. Sci. Technol. Adv. Mater. 2019, 20, 465–496. [Google Scholar] [CrossRef] [Green Version]
- Vivekanandan, A.K.; Azher, K.; Chang, S.F.; Chen, S.H. Size-Controllable Zinc Oxide Nanowires Fabricated via the Combination of Die-Casting and Oxidation Process. J. Alloy. Compd. 2021, 861, 157964. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, J.; Jiang, Q.; He, D.; He, J.; Luo, Y.; Zhang, D.; Zhou, Z.; Ren, Y.; Xin, J. New Insight into InSb-Based Thermoelectric Materials: From a Divorced Eutectic Design to a Remarkably High Thermoelectric Performance. J. Mater. Chem. A 2017, 5, 5163–5170. [Google Scholar] [CrossRef]
- Wang, K.; Qin, P.; Ge, Z.H.; Feng, J. Synthesis and Thermoelectric Properties of InSb Alloys by Solid Reaction. Mater. Lett. 2017, 209, 373–375. [Google Scholar] [CrossRef]
- Wang, Y.; Chi, J.; Banerjee, K.; Grützmacher, D.; Schäpers, T.; Lu, J.G. Field Effect Transistor Based on Single Crystalline InSb Nanowire. J. Mater. Chem. 2011, 21, 2459–2462. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-H.; Wu, J.-M.; Lin, S.-J.; Chang, W.-C. High Sensitivity of Middle-Wavelength Infrared Photodetectors Based on an Individual InSb Nanowire. Nanoscale Res. Lett. 2013, 8, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.-H.; Wu, J.-M.; Lin, S.-J. Room Temperature-Synthesized Vertically Aligned InSb Nanowires: Electrical Transport and Field Emission Characteristics. Nanoscale Res. Lett. 2013, 81, 69. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.; Bai, X.; Tang, Z.; Zhao, S.; Chen, Y.; Zhou, L.; Peng, Y.; Chen, X.; Xu, X. Fabrication of InSb Crystal via Horizontal Bridgman Method and Investigation on Its Thermoelectric Properties. Mater. Res. Bull. 2021, 142, 111411. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered Metal Nanohole Arrays Made by a Two-Step Replication of Honeycomb Structures of Anodic Alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef]
- Dedi; Lee, P.C.; Wei, P.C.; Chen, Y.Y. Thermoelectric Characteristics of a Single-Crystalline Topological Insulator Bi2Se3 Nanowire. Nanomaterials 2021, 11, 819. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivekanandan, A.K.; Lee, C.-W.; Wu, R.-Z.; Tsai, W.-H.; Chen, S.-H.; Chen, Y.-Y.; Lin, C.-T. Tailoring InSb Nanowires for High Thermoelectric Performance Using AAO Template-Assisted Die Casting Process. Nanomaterials 2022, 12, 2032. https://doi.org/10.3390/nano12122032
Vivekanandan AK, Lee C-W, Wu R-Z, Tsai W-H, Chen S-H, Chen Y-Y, Lin C-T. Tailoring InSb Nanowires for High Thermoelectric Performance Using AAO Template-Assisted Die Casting Process. Nanomaterials. 2022; 12(12):2032. https://doi.org/10.3390/nano12122032
Chicago/Turabian StyleVivekanandan, Alangadu Kothandan, Chen-Wei Lee, Rui-Zhe Wu, Wei-Han Tsai, Shih-Hsun Chen, Yang-Yuan Chen, and Chia-Ting Lin. 2022. "Tailoring InSb Nanowires for High Thermoelectric Performance Using AAO Template-Assisted Die Casting Process" Nanomaterials 12, no. 12: 2032. https://doi.org/10.3390/nano12122032