Recent Progress in the Design, Characterisation and Application of LaAlO3- and LaGaO3-Based Solid Oxide Fuel Cell Electrolytes
Abstract
:1. Introduction
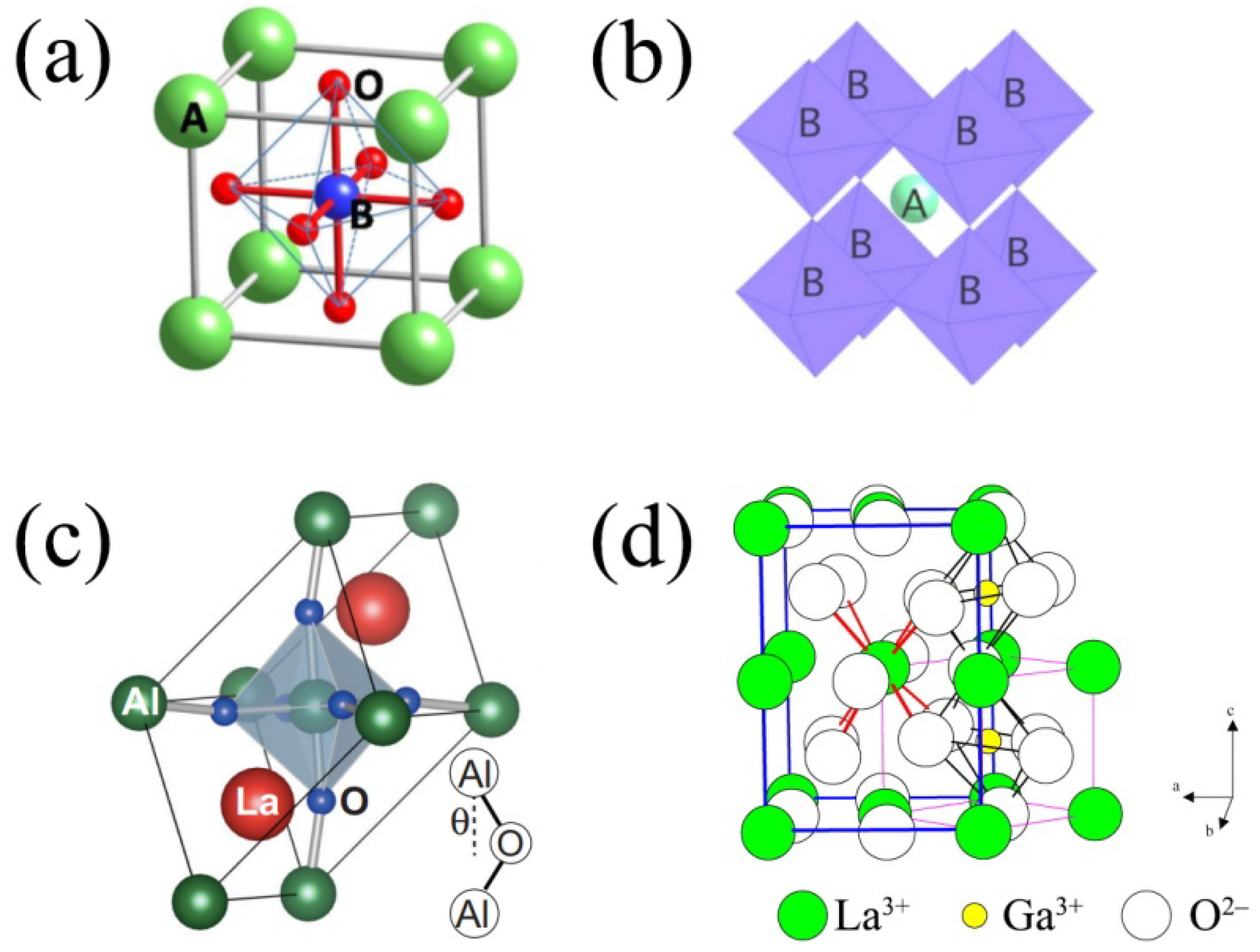
2. Electrolyte Materials Based on LaAlO3
2.1. Synthesis, Structure and Morphology
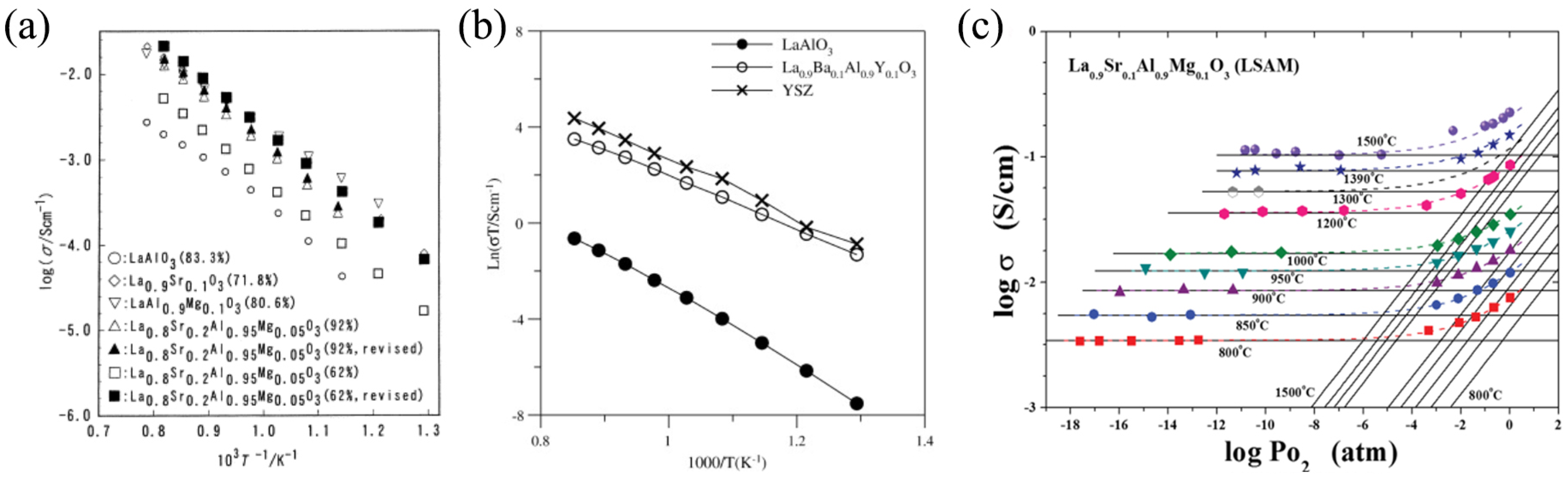
2.2. Functional Properties
2.3. Applications in SOFCs
3. Electrolyte Materials Based on Doped LaGaO3
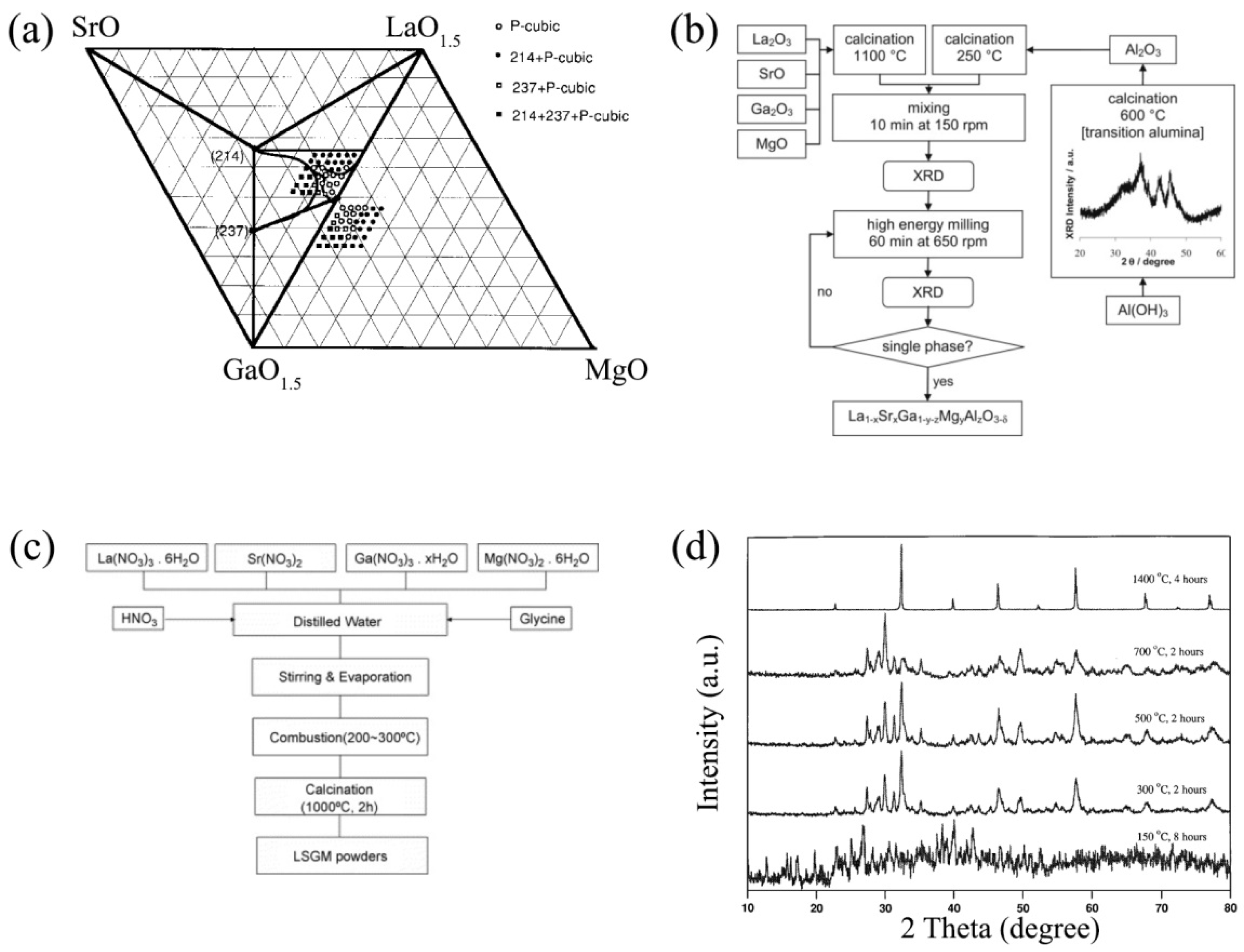
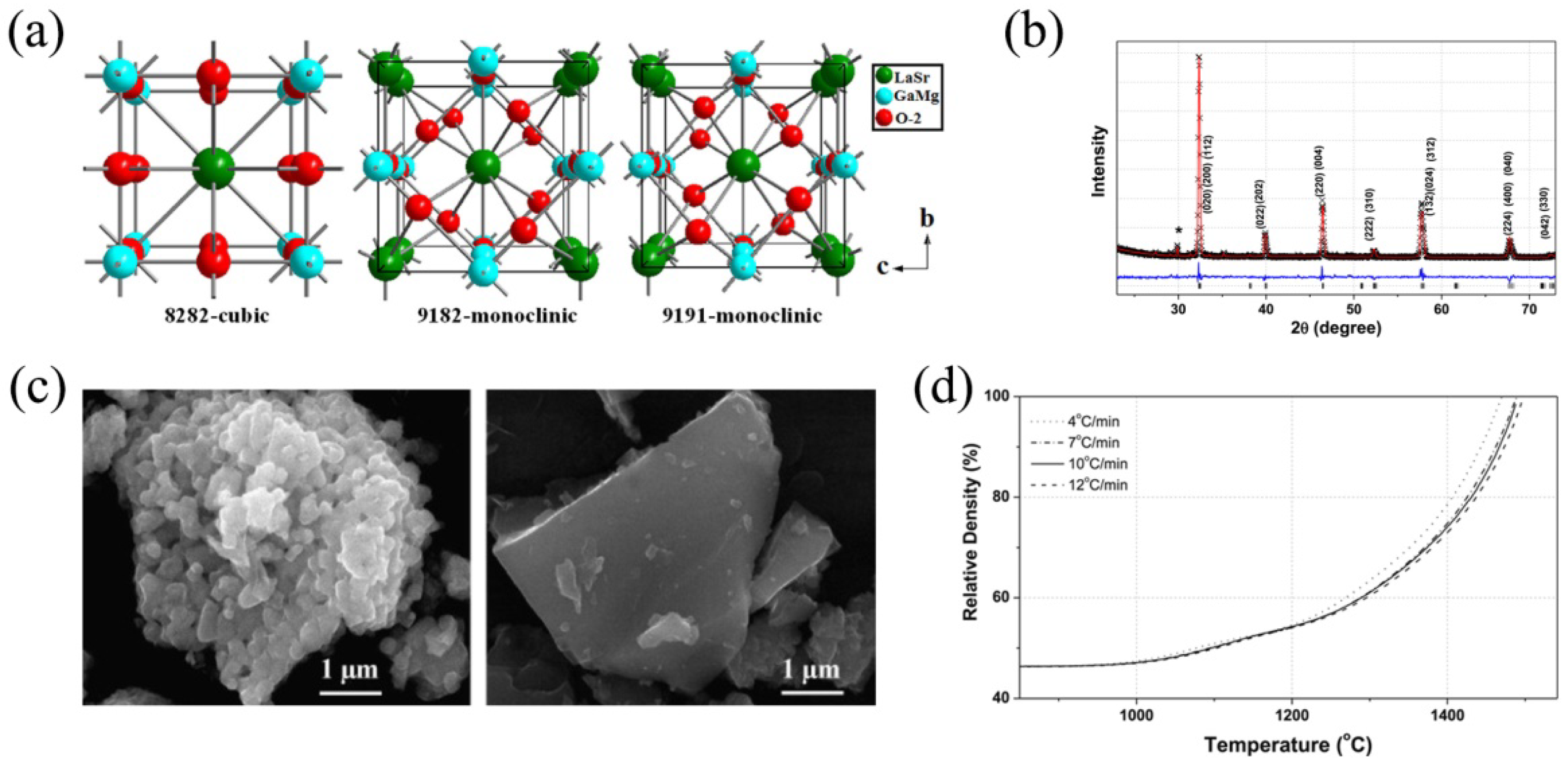
3.1. Synthesis, Structure and Morphology
3.2. Functional Properties
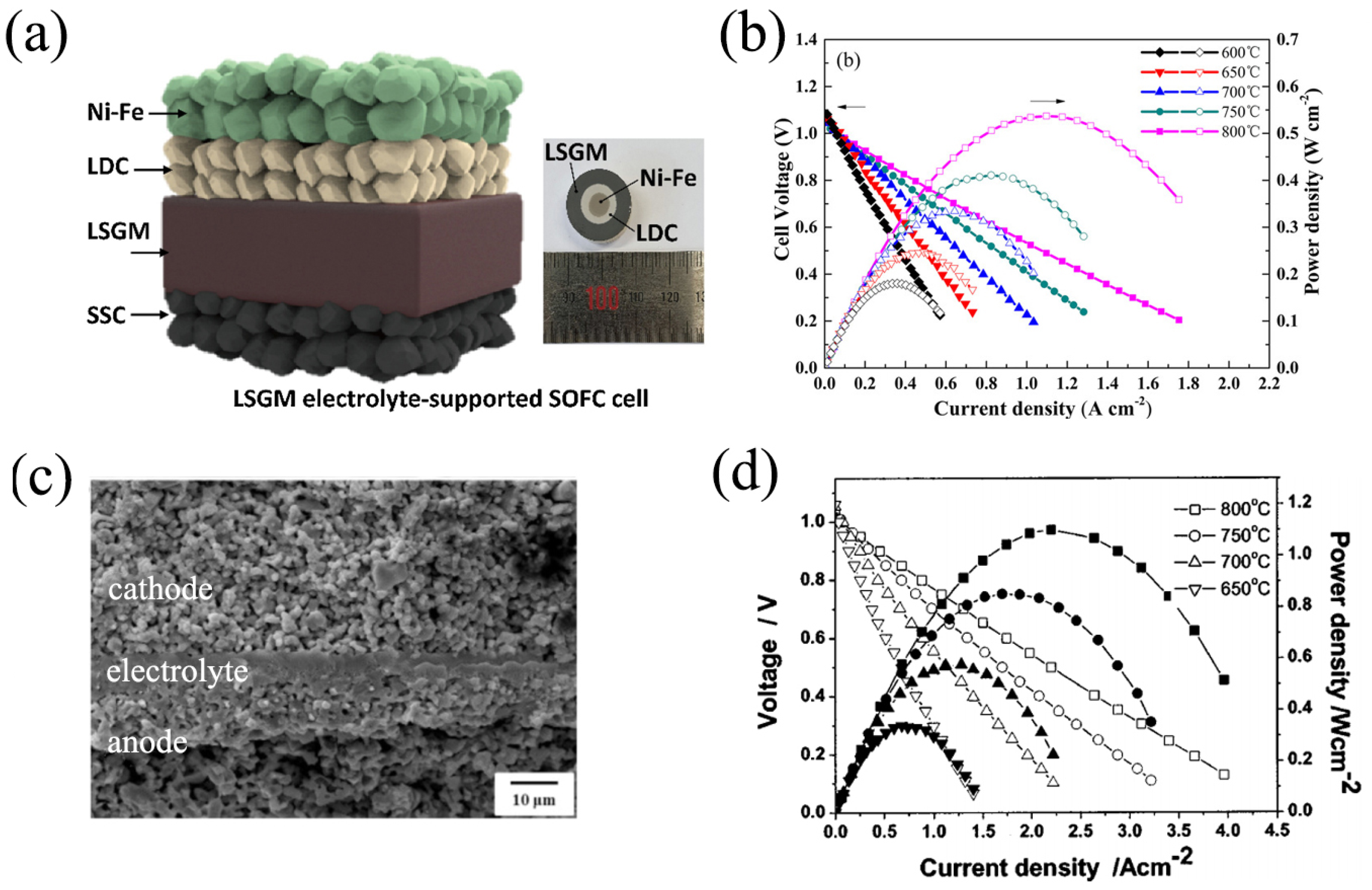
3.3. Applications in SOFCs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Holden, E.; Linnerud, K.; Rygg, B.J. A review of dominant sustainable energy narratives. Renew. Sustain. Energy Rev. 2021, 144, 110955. [Google Scholar] [CrossRef]
- Chu, W.; Calise, F.; Duić, N.; Østergaard, P.A.; Vicidomini, M.; Wang, Q. Recent advances in technology, strategy and application of sustainable energy systems. Energies 2020, 13, 5229. [Google Scholar] [CrossRef]
- Østergaard, P.A.; Duic, N.; Noorollahi, Y.; Mikulcic, H.; Kalogirou, S. Sustainable development using renewable energy technology. Renew. Energy 2020, 146, 2430–2437. [Google Scholar] [CrossRef]
- Kolosok, S.; Bilan, Y.; Vasylieva, T.; Wojciechowski, A.; Morawski, M. A scoping review of renewable energy, sustainability and the environment. Energies 2021, 14, 4490. [Google Scholar] [CrossRef]
- Erixno, O.; Rahim, N.A.; Ramadhani, F.; Adzman, N.N. Energy management of renewable energy-based combined heat and power systems: A review. Sustain. Energy Technol. Assess. 2022, 51, 101944. [Google Scholar] [CrossRef]
- Martínez, M.L.; Vázquez, G.; Pérez-Maqueo, O.; Silva, R.; Moreno-Casasola, P.; Mendoza-González, G.; López-Portillo, J.; MacGregor-Fors, I.; Heckel, G.; Hernández-Santana, J.R.; et al. A systemic view of potential environmental impacts of ocean energy production. Renew. Sustain. Energy Rev. 2021, 149, 111332. [Google Scholar] [CrossRef]
- Pradhan, S.; Chakraborty, R.; Mandal, D.K.; Barman, A.; Bose, P. Design and performance analysis of solar chimney power plant (SCPP): A review. Sustain. Energy Technol. Assess. 2021, 47, 101411. [Google Scholar] [CrossRef]
- Nazir, M.S.; Ali, N.; Bilal, M.; Iqbal, H.M.N. Potential environmental impacts of wind energy development: A global perspective. Curr. Opin. Environ. Sci. Health. 2020, 13, 85–90. [Google Scholar] [CrossRef]
- Soltani, M.; Moradi Kashkooli, F.; Souri, M.; Rafiei, B.; Jabarifar, M.; Gharali, K.; Nathwani, J.S. Environmental, economic, and social impacts of geothermal energy systems. Renew. Sustain. Energy Rev. 2021, 140, 110750. [Google Scholar] [CrossRef]
- Yuan, X.; Su, C.-W.; Umar, M.; Shao, X.; Lobont, O.-R. The race to zero emissions: Can renewable energy be the path to carbon neutrality? J. Environ. Manag. 2022, 308, 114648. [Google Scholar] [CrossRef]
- Tomkins, P.; Müller, T.E. Evaluating the carbon inventory, carbon fluxes and carbon cycles for a long-term sustainable world. Green Chem. 2019, 21, 3994–4013. [Google Scholar] [CrossRef]
- Nurdiawati, A.; Urban, F. Towards deep decarbonisation of energy-intensive industries: A review of current status, technologies and policies. Energies 2021, 14, 2408. [Google Scholar] [CrossRef]
- Bolatkhan, K.; Kossalbayev, B.D.; Zayadan, B.K.; Tomo, T.; Veziroglu, T.N.; Allakhverdiev, S.I. Hydrogen production from phototrophic microorganisms: Reality and perspectives. Int. J. Hydrogen Energy 2019, 44, 5799–5811. [Google Scholar] [CrossRef]
- Barba, F.J.; Gavahian, M.; Es, I.; Zhu, Z.; Chemat, F.; Lorenzo, J.M.; Mousavi Khaneghah, A. Solar radiation as a prospective energy source for green and economic processes in the food industry: From waste biomass valorization to dehydration, cooking, and baking. J. Clean. Prod. 2019, 220, 1121–1130. [Google Scholar] [CrossRef]
- Wijayasekera, S.C.; Hewage, K.; Siddiqui, O.; Hettiaratchi, P.; Sadiq, R. Waste-to-hydrogen technologies: A critical review of techno-economic and socio-environmental sustainability. Int. J. Hydrogen Energy 2022, 47, 5842–5870. [Google Scholar] [CrossRef]
- Testoni, R.; Bersano, A.; Segantin, S. Review of nuclear microreactors: Status, potentialities and challenges. Prog. Nucl. Energy 2021, 138, 103822. [Google Scholar] [CrossRef]
- Timmer, M.A.G.; De Blok, K.; Van Der Meer, T.H. Review on the conversion of thermoacoustic power into electricity. J. Acoustic. Soc. Am. 2018, 143, 841–857. [Google Scholar] [CrossRef] [Green Version]
- Selvan, K.V.; Hasan, M.N.; Mohamed Ali, M.S. Methodological reviews and analyses on the emerging research trends and progresses of thermoelectric generators. Int. J. Energy Res. 2019, 43, 113–140. [Google Scholar] [CrossRef] [Green Version]
- Cigolotti, V.; Genovese, M.; Fragiacomo, P. Comprehensive review on fuel cell technology for stationary applications as sustainable and efficient poly-generation energy systems. Energies 2021, 14, 4963. [Google Scholar] [CrossRef]
- Mishra, P.; Saravanan, P.; Packirisamy, G.; Jang, M.; Wang, C. A subtle review on the challenges of photocatalytic fuel cell for sustainable power production. Int. J. Hydrogen Energy 2021, 46, 22877–22906. [Google Scholar] [CrossRef]
- Minh, N.Q. Solid oxide fuel cell technology-features and applications. Solid State Ion. 2004, 174, 271–277. [Google Scholar] [CrossRef]
- Bilal Hanif, M.; Motola, M.; Qayyum, S.; Rauf, S.; Khalid, A.; Li, C.-J.; Li, C.-X. Recent advancements, doping strategies and the future perspective of perovskite-based solid oxide fuel cells for energy conversion. Chem. Engin. J. 2022, 428, 132603. [Google Scholar] [CrossRef]
- Peng, J.; Huang, J.; Wu, X.-L.; Xu, Y.-W.; Chen, H.; Li, X. Solid oxide fuel cell (SOFC) performance evaluation, fault diagnosis and health control: A review. J. Power Sources 2021, 505, 230058. [Google Scholar] [CrossRef]
- Zarabi Golkhatmi, S.; Asghar, M.I.; Lund, P.D. A review on solid oxide fuel cell durability: Latest progress, mechanisms, and study tools. Renew. Sustain. Energy Rev. 2022, 161, 112339. [Google Scholar] [CrossRef]
- Jacobson, A.J. Materials for solid oxide fuel cells. Chem. Mater. 2010, 22, 660–674. [Google Scholar] [CrossRef]
- Saadabadi, S.A.; Thallam Thattai, A.; Fan, L.; Lindeboom, R.E.F.; Spanjers, H.; Aravind, P.V. Solid oxide fuel cells fuelled with biogas: Potential and constraints. Renew. Energy 2019, 134, 194–214. [Google Scholar] [CrossRef]
- Yang, B.C.; Koo, J.; Shin, J.W.; Go, D.; Shim, J.H.; An, J. Direct alcohol-fueled low-temperature solid oxide fuel cells: A review. Energy Technol. 2019, 7, 5–19. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, G.; Dai, R.; Lv, X.; Yang, D.; Geng, S. A review of the chemical compatibility between oxide electrodes and electrolytes in solid oxide fuel cells. J. Power Sources 2021, 492, 229630. [Google Scholar] [CrossRef]
- Brandon, N.P.; Skinner, S.; Steele, B.C.H. Recent advances in materials for fuel cells. Ann. Rev. Mater. Res. 2003, 33, 182–213. [Google Scholar] [CrossRef]
- Zhou, Z.; Nadimpalli, V.K.; Pedersen, D.B.; Esposito, V. Degradation mechanisms of metal-supported solid oxide cells and countermeasures: A review. Materials 2021, 14, 3139. [Google Scholar] [CrossRef]
- Sreedhar, I.; Agarwal, B.; Goyal, P.; Agarwal, A. An overview of degradation in solid oxide fuel cells-potential clean power sources. J. Solid State Electrochem. 2020, 24, 1239–1270. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, M.; Wang, N.; Ma, C.; Wang, J.; Han, M. A short review of cathode poisoning and corrosion in solid oxide fuel cell. Int. J. Hydrogen Energy 2017, 42, 24948–24959. [Google Scholar] [CrossRef]
- Zakaria, Z.; Abu Hassan, S.H.; Shaari, N.; Yahaya, A.Z.; Boon Kar, Y. A review on recent status and challenges of yttria stabilized zirconia modification to lowering the temperature of solid oxide fuel cells operation. Int. J. Energy Res. 2020, 44, 631–650. [Google Scholar] [CrossRef]
- Hanif, M.B.; Rauf, S.; Motola, M.; Babar, Z.U.D.; Li, C.-J.; Li, C.-X. Recent progress of perovskite-based electrolyte materials for solid oxide fuel cells and performance optimizing strategies for energy storage applications. Mat. Res. Bull. 2022, 146, 111612. [Google Scholar] [CrossRef]
- Atkinson, A.; Sun, B. Residual stress and thermal cycling of planar solid oxide fuel cells. Mater. Sci. Technol. 2007, 23, 1135–1143. [Google Scholar] [CrossRef]
- Brett, D.J.L.; Atkinson, A.; Brandon, N.P.; Skinner, S.J. Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 2008, 37, 1568–1578. [Google Scholar] [CrossRef]
- Tarancón, A. Strategies for lowering solid oxide fuel cells operating temperature. Energies 2009, 2, 1130–1150. [Google Scholar] [CrossRef]
- Kilner, J.A.; Burriel, M. Materials for intermediate-temperature solid-oxide fuel cells. Ann. Rev. Mater. Res. 2014, 44, 365–393. [Google Scholar] [CrossRef]
- Yang, D.; Chen, G.; Zhang, L.; Chen, Z.; Zhang, R.; Asghar, M.I.; Lund, P.D. Low temperature ceramic fuel cells employing lithium compounds: A review. J. Power Sources 2021, 503, 230070. [Google Scholar] [CrossRef]
- Su, H.; Hu, Y.H. Progress in low-temperature solid oxide fuel cells with hydrocarbon fuels. Chem. Eng. J. 2020, 402, 126235. [Google Scholar] [CrossRef]
- Kharton, V.V.; Marques, F.M.B.; Atkinson, A. Transport properties of solid oxide electrolyte ceramics: A brief review. Solid State Ion. 2004, 174, 135–149. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–331. [Google Scholar] [CrossRef]
- Wang, F.; Lyu, Y.; Chu, D.; Jin, Z.; Zhang, G.; Wang, D. The electrolyte materials for SOFCs of low-intermediate temperature: Review. Mater. Sci. Technol. 2019, 35, 1551–1562. [Google Scholar] [CrossRef]
- Shi, H.; Su, C.; Ran, R.; Cao, J.; Shao, Z. Electrolyte materials for intermediate-temperature solid oxide fuel cells. Prog. Nat. Sci. Mater. Int. 2020, 30, 764–774. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Azad, A.T.; Petra, P.M.I.; Begum, F.; Eriksson, S.G.; Azad, A.K. Nanomaterials for solid oxide fuel cells: A review. Renew. Sustain. Energy Rev. 2018, 82, 353–368. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, B.; Su, P.-C.; He, C. Nanomaterials and technologies for low temperature solid oxide fuel cells: Recent advances, challenges and opportunities. Nano Energy 2018, 45, 148–176. [Google Scholar] [CrossRef]
- Ellingsen, L.A.W.; Hung, C.R.; Bettez, G.M.; Singh, B.; Chen, Z.; Whittingham, M.S.; Strømman, A.H. Nanotechnology for environmentally sustainable electromobility. Nat. Nanotechnol. 2016, 11, 1039–1051. [Google Scholar] [CrossRef]
- Zhigachev, A.O.; Rodaev, V.V.; Zhigacheva, D.V.; Lyskov, N.V.; Shchukina, M.A. Doping of scandia-stabilized zirconia electrolytes for intermediate-temperature solid oxide fuel cell: A review. Ceram. Int. 2021, 47, 32490–32504. [Google Scholar] [CrossRef]
- Kasyanova, A.V.; Rudenko, A.O.; Lyagaeva, Y.G.; Medvedev, D.A. Lanthanum-containing proton-conducting electrolytes with perovskite structures. Membr. Membr. Technol. 2021, 3, 73–97. [Google Scholar] [CrossRef]
- Artini, C. Crystal chemistry, stability and properties of interlanthanide perovskites: A review. J. Eur. Ceram. Soc. 2017, 37, 427–440. [Google Scholar] [CrossRef]
- Ishihara, T.; Matsuda, H.; Takita, Y. Doped LaGaO3 perovskite type oxide as a new oxide ionic conductor. J. Am. Chem. Soc. 1994, 116, 3801–3803. [Google Scholar] [CrossRef]
- Feng, M.; Goodenough, J.B.; Huang, K.; Milliken, C. Fuel cells with doped lanthanum gallate electrolyte. J. Power Sources 1996, 63, 47–51. [Google Scholar] [CrossRef]
- Fung, K.Z.; Chen, T.Y. Cathode-supported SOFC using a highly conductive lanthanum aluminate-based electrolyte. Solid State Ion. 2011, 188, 64–68. [Google Scholar] [CrossRef]
- Kaur, J.; Singh, D.; Dubey, V.; Suryanarayana, N.S.; Parganiha, Y.; Jha, P. Review of the synthesis, characterization, and properties of LaAlO3 phosphors. Res. Chem. Intermed. 2013, 40, 2737–2771. [Google Scholar] [CrossRef]
- Rizwan, M.; Gul, S.; Iqbal, T.; Mushtaq, U.; Farooq, M.H.; Farman, M.; Bibi, R.; Ijaz, M. A review on perovskite lanthanum aluminate (LaAlO3), its properties and applications. Mat. Res. Express 2019, 6, 112001. [Google Scholar] [CrossRef]
- Morales, M.; Roa, J.J.; Tartaj, J.; Segarra, M. A review of doped lanthanum gallates as electrolytes for intermediate temperature solid oxides fuel cells: From materials processing to electrical and thermo-mechanical properties. J. Eur. Ceram. Soc. 2016, 36, 1–16. [Google Scholar] [CrossRef]
- Fu, D.; Itoh, M. Ferroelectricity in silver perovskite oxides. In Ferroelectrics-Material Aspects; Lallart, M., Ed.; InTech: Houston, TX, USA, 2011; ISBN 978-953-307-332-3. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.X.; Schleife, A.; Janotti, A.; Van de Walle, C.G. Effects of La 5d and 4f states on the electronic and optical properties of LaAlO3. Phys. Rev. B 2016, 94, 205203. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.Y.; Fung, K.Z. A and B-site substitution of the solid electrolyte LaGaO3 and LaAlO3 with the alkaline-earth oxides MgO and SrO. J. Alloys Compd. 2004, 368, 106–115. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die Gesetze der Krystallochemie. Die Nat. 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Popova, V.F.; Tugova, E.A.; Zvereva, I.A.; Gusarov, V.V. Phase equilibria in the LaAlO3-LaSrAlO4 system. Glass Phys. Chem. 2004, 30, 564–567. [Google Scholar] [CrossRef]
- Egorova, A.V.; Belova, K.G.; Animitsa, I.E.; Morkhova, Y.A.; Kabanov, A.A. Effect of zinc doping on electrical properties of LaAlO3 perovskite. Chim. Technol. Acta 2021, 8, 20218103. [Google Scholar] [CrossRef]
- Azaiz, A.; Kadari, A.; Alves, N.; Faria, L.O. Influence of carbon doping on the thermoluminescence properties of LaAlO3 crystal grown by solid state reaction method. Int. J. Microstruct. Mater. Prop. 2020, 15, 156–167. [Google Scholar] [CrossRef]
- Beheshti, M.; Malekfar, R. A novel approach for the synthesis of lanthanum aluminate nanoparticles using thermal shock assisted solid-state method as a microwave absorber layer. Mater. Chem. Phys. 2021, 270, 124848. [Google Scholar] [CrossRef]
- Fabián, M.; Arias-Serrano, B.I.; Yaremchenko, A.A.; Kolev, H.; Kaňuchová, M.; Briančin, J. Ionic and electronic transport in calcium-substituted LaAlO3 perovskites prepared via mechanochemical route. J. Eur. Ceram. Soc. 2019, 39, 5298–5308. [Google Scholar] [CrossRef]
- Brylewski, T.; Bućko, M.M. Low-temperature synthesis of lanthanum monoaluminate powders using the co-precipitation–calcination technique. Ceram. Int. 2013, 39, 5667–5674. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, L.; Luo, H.; Fan, X.; Jin, L.; Liu, B.; Li, D.; Qiu, Z.; Gan, Y. Preparation of Eu3+ doped LaAlO3 phosphors by coprecipitation-molten salt synthesis. Integr. Ferroelectr. 2018, 188, 1–11. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, T.; Qi, X.W.; Qi, J.Q.; Sun, G.F.; Chen, H.H.; Zhong, R.X. Preparation and characterization of LaAlO3 via sol-gel process. Adv. Mater. Res. 2013, 624, 26–29. [Google Scholar] [CrossRef]
- Qin, G.; Huang, X.; Chen, J.; He, Z. Synthesis of Sr and Mg double-doped LaAlO3 nanopowders via EDTA-glycine combined process. Powder Technol. 2013, 235, 880–885. [Google Scholar] [CrossRef]
- Adak, A.K.; Pramanik, P. Synthesis and characterization of lanthanum aluminate powder at relatively low temperature. Mater. Lett. 1997, 30, 269–273. [Google Scholar] [CrossRef]
- Verma, O.N.; Singh, S.; Singh, V.K.; Najim, M.; Pandey, R.; Singh, P. Influence of Ba doping on the electrical behaviour of La0.9Sr0.1Al0.9Mg0.1O3−δ system for a solid electrolyte. J. Electron. Mater. 2021, 50, 1010–1021. [Google Scholar] [CrossRef]
- Rivera-Montalvo, T.; Morales-Hernandez, A.; Barrera-Angeles, A.A.; Alvarez-Romero, R.; Falcony, C.; Zarate-Medina, J. Modified Pechini’s method to prepare LaAlO3:RE thermoluminescent. Mater. Radiat. Phys. Chem. 2017, 140, 68–73. [Google Scholar] [CrossRef]
- Garcia, A.B.S.; Bispo-Jr, A.G.; Lima, S.A.M.; Pires, A.M. Effects of the Pechini’s modified synthetic route on structural and photophysical properties of Eu3+ or Tb3+-doped LaAlO3. Mat. Res. Bull. 2021, 143, 111462. [Google Scholar] [CrossRef]
- Silveira, I.S.; Ferreira, N.S.; Souza, D.N. Structural, morphological and vibrational properties of LaAlO3 nanocrystals produced by four different methods. Ceram. Int. 2021, 47, 27748–27758. [Google Scholar] [CrossRef]
- da Silva, C.A.; de Miranda, P.E.V. Synthesis of LaAlO3 based materials for potential use as methane-fueled solid oxide fuel cell anodes. Int. J. Hydrogen Energy 2015, 40, 10002–10015. [Google Scholar] [CrossRef]
- Lee, G.; Kim, I.; Yang, I.; Ha, J.-M.; Na, H.B.; Jung, J.C. Effects of the preparation method on the crystallinity and catalytic activity of LaAlO3 perovskites for oxidative coupling of methane. Appl. Surf. Sci. 2018, 429, 55–61. [Google Scholar] [CrossRef]
- Stathopoulos, V.N.; Kuznetsova, T.; Lapina, O.; Khabibulin, D.; Pandis, P.K.; Krieger, T.; Chesalov, Y.; Gulyalev, R.; Krivensov, V.; Larina, T.; et al. Evolution of bulk and surface structures in stoichiometric LaAlO3 mixed oxide prepared by using starch as template. Mater. Chem. Phys. 2018, 207, 423–434. [Google Scholar] [CrossRef]
- Lessing, P.A. Mixed-cation oxide powders via polymeric precursors. Am. Ceram. Soc. Bull. 1989, 68, 1002–1007. [Google Scholar]
- Howard, C.J.; Kennedy, B.J.; Chakoumakos, B.C. Neutron powder diffraction study of rhombohedral rare-earth aluminates and the rhombohedral to cubic phase transition. J. Phys. Condens. Matter 2000, 12, 349–365. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Dokiya, M.; Wang, S.; Tagawa, H.; Hashimoto, T. The effect of oxygen vacancy on the oxide ion mobility in LaAlO3-based oxides. Solid State Ion. 2000, 130, 229–241. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, H.J. High temperature electrical properties of Sr- and Mg-doped LaAlO3. J. Korean Cryst. Growth. Cryst. Technol. 2019, 29, 187–191. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, G.M. Electrical conductivity of Sr and Mg doped LaAlO3. Solid State Ion. 2002, 154–155, 535–540. [Google Scholar] [CrossRef]
- Chen, T.Y.; Fung, K.Z. Comparison of dissolution behavior and ionic conduction between Sr and/or Mg doped LaGaO3 and LaAlO3. J. Power Sources 2004, 132, 1–10. [Google Scholar] [CrossRef]
- Fu, Q.X.; Tietz, F.; Lersch, P.; Stöver, D. Evaluation of Sr- and Mn-substituted LaAlO3 as potential SOFC anode materials. Solid State Ion. 2006, 177, 1059–1069. [Google Scholar] [CrossRef]
- Villas-Boas, L.A.; De Souza, D.P.F. The effect of Pr co-doping on the densification and electrical properties of Sr-LaAlO3. Mater. Res. 2013, 16, 982–989. [Google Scholar] [CrossRef] [Green Version]
- Verma, O.N.; Jha, P.A.; Melkeri, A.; Singh, P. A comparative study of aqueous tape and pellet of (La0.89Ba0.01)Sr0.1Al0.9Mg0.1O3−δ electrolyte material. Phys. B Condens. Matter 2017, 521, 230–238. [Google Scholar] [CrossRef]
- Guo-Heng, Q.; Xiao-Wei, H.; Zh, H.U. Chemical compatibility and electrochemical performance between LaAlO3-based electrolyte and selected anode materials. Wuli Huaxue Xuebao/Acta Phys.-Chim. Sin. 2013, 29, 311–318. [Google Scholar] [CrossRef]
- Verma, O.N.; Jha, P.A.; Singh, P.; Jha, P.K.; Singh, P. Influence of iso-valent “Sm” double substitution on the ionic conductivity of La0.9Sr0.1Al0.9Mg0.1O3−δ ceramic system. Mater. Chem. Phys. 2020, 241, 122345. [Google Scholar] [CrossRef]
- Villas-Boas, L.A.; Goulart, C.A.; De Souza, D.P.F. Desenvolvimento microestrutural e mobilidade de ions oxigênio em perovskitas do tipo LaAlO3 dopadas com Sr, Ba e Ca. Rev. Mater. 2020, 25, e-12801. [Google Scholar] [CrossRef]
- Villas-Boas, L.A.; Goulart, C.A.; De Souza, D.P.F. Effects of Sr and Mn co-doping on microstructural evolution and electrical properties of LaAlO3. Process. Appl. Ceram. 2019, 13, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Zvonareva, I.; Fu, X.-Z.; Medvedev, D.; Shao, Z. Electrochemistry and energy conversion features of protonic ceramic cells with mixed ionic-electronic electrolytes. Energy Environ. Sci. 2022, 15, 439–465. [Google Scholar] [CrossRef]
- Tietz, F. Thermal expansion of SOFC materials. Ionics 1999, 5, 129–139. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Dokiya, M. Electrical conductivity, thermal expansion and reaction of (La, Sr)(Ga, Mg)O3 and (La, Sr)AlO3 system. Solid State Ion. 2000, 132, 217–226. [Google Scholar] [CrossRef]
- Venâncio, S.A.; de Miranda, P.E.V. Direct utilization of carbonaceous fuels in multifunctional SOFC anodes for the electrosynthesis of chemicals or the generation of electricity. Int. J. Hydrogen Energy 2017, 42, 13927–13938. [Google Scholar] [CrossRef]
- Huang, K.; Tichy, R.S.; Goodenough, J.B. Superior perovskite oxide-ion conductor; strontium- and magnesium-doped LaGaO3: I, phase relationships and electrical properties. J. Am. Ceram. Soc. 1998, 81, 2565–2575. [Google Scholar] [CrossRef]
- Chang, J.; Lee, H.-W.; Kang, S.-J.L. Low-temperature pressureless sintering of Sr- and Mg-doped lanthanum gallate ceramics by sintering atmosphere control. J. Am. Ceram. Soc. 2009, 92, 927–930. [Google Scholar] [CrossRef]
- Moure, A.; Castro, A.; Tartaj, J.; Moure, C. Single-phase ceramics with La1−xSrxGa1−yMgyO3−δ composition from precursors obtained by mechanosynthesis. J. Power Sources 2009, 188, 489–497. [Google Scholar] [CrossRef]
- Domingues, E.M.; Gonçalves, P.; Figueiredo, F.M. Synthesis of nanopowders of the aluminum-substituted lanthanum gallate solid electrolyte by mechanochemical route. Solid State Sci. 2012, 14, 820–827. [Google Scholar] [CrossRef]
- Lee, D.; Han, J.-H.; Chun, Y.; Song, R.-H.; Shin, D.R. Preparation and characterization of strontium and magnesium doped lanthanum gallates as the electrolyte for IT-SOFC. J. Power Sources 2007, 166, 35–40. [Google Scholar] [CrossRef]
- Huang, K.; Goodenough, J.B. Wet chemical synthesis of Sr- and Mg-doped LaGaO3, a perovskite-type oxide-ion conductor. J. Solid State Chem. 1998, 136, 274–283. [Google Scholar] [CrossRef]
- Colomer, M.T.; Kilner, J.A. Ni-doped lanthanum gallate perovskites: Synthesis and structural, microstructural, and electrical characterization. Solid State Ion. 2011, 182, 76–81. [Google Scholar] [CrossRef]
- Kuncewicz-Kupczyk, W.; Kobertz, D.; Miller, M.; Singheiser, L.; Hilpert, K. Vaporization of Sr- and Mg-doped lanthanum gallate and implications for solid oxide fuel cells. J. Electrochem. Soc. 2001, 148, E276–E281. [Google Scholar] [CrossRef]
- Cho, P.-S.; Park, S.-Y.; Cho, Y.H.; Kim, S.-J.; Kang, Y.C.; Mori, T.; Lee, J.-H. Preparation of LSGM powders for low temperature sintering. Solid State Ion. 2009, 180, 788–791. [Google Scholar] [CrossRef]
- Chae, N.S.; Park, K.S.; Yoon, Y.S.; Yoo, I.S.; Kim, J.S.; Yoon, H.H. Sr- and Mg-doped LaGaO3 powder synthesis by carbonate coprecipitation. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313–314, 154–157. [Google Scholar] [CrossRef]
- Shi, M.; Xu, Y.; Liu, A.; Liu, N.; Wang, C.; Majewski, P.; Aldinger, F. Synthesis and characterization of Sr- and Mg-doped lanthanum gallate electrolyte materials prepared via the Pechini method. Mater. Chem. Phys. 2009, 114, 43–46. [Google Scholar] [CrossRef]
- Cong, L.; He, T.; Ji, Y.; Guan, P.; Huang, Y.; Su, W. Synthesis and characterization of IT-electrolyte with perovskite structure La0.8Sr0.2Ga0.85Mg0.15O3−δ by glycine-nitrate combustion method. J. Alloys Compd. 2003, 348, 325–331. [Google Scholar] [CrossRef]
- Kumar, M.; Nesaraj, A.S.; Raj, I.A.; Pattabiraman, R. Synthesis and characterization of La0.9Sr0.1Ga0.8Mg0.2O3−δ electrolyte for intermediate temperature solid oxide fuel cells (IT SOFC). Ionics 2004, 10, 93–98. [Google Scholar] [CrossRef]
- Ishikawa, H.; Enoki, M.; Ishihara, T.; Akiyama, T. Combustion synthesis of doped lanthanum gallate as an electrolyte for solid oxide fuel cells. Mater. Trans. 2006, 47, 149–155. [Google Scholar] [CrossRef]
- Ha, S.B.; Cho, Y.H.; Ji, H.-I.; Lee, J.-H.; Kang, Y.C.; Lee, J.-H. Low-temperature sintering and electrical properties of strontium- and magnesium-doped lanthanum gallate with V2O5 additive. J. Power Sources 2011, 196, 2971–2978. [Google Scholar] [CrossRef]
- Ishikawa, H.; Enoki, M.; Ishihara, T.; Akiyama, T. Self-propagating high-temperature synthesis of La(Sr)Ga(Mg)O3−δ for electrolyte of solid oxide fuel cells. J. Alloys Compd. 2007, 430, 246–251. [Google Scholar] [CrossRef]
- Ishikawa, H.; Enoki, M.; Ishihara, T.; Akiyama, T. Mechanically activated self-propagating high-temperature synthesis of La0.9Sr0.1Ga0.8Mg0.2O3−δ as an electrolyte for SOFC. J. Alloys Compd. 2009, 488, 238–242. [Google Scholar] [CrossRef]
- Jung, D.S.; Koo, H.Y.; Jang, H.C.; Kim, J.H.; Cho, Y.H.; Lee, J.-H.; Kang, Y.C. Firing characteristics of La0.8Sr0.2Ga0.8Mg0.2O3−δ electrolyte powders prepared by spray pyrolysis. J. Alloys Compd. 2009, 487, 693–697. [Google Scholar] [CrossRef]
- Sammes, N.M.; Tompsett, G.A.; Phillips, R.J.; Cartner, A.M. Characterisation of doped-lanthanum gallates by X-ray diffraction and Raman spectroscopy. Solid State Ion. 1998, 111, 1–7. [Google Scholar] [CrossRef]
- Yu, J.; Liu, H.; Chen, X.; Xing, J.; Yuan, B.; Wang, M.; Ma, W. Ionic conductivity and crystal structure of LSGM with different element mole ratios. Fuel Cells 2021, 21, 149–154. [Google Scholar] [CrossRef]
- Batista, R.M.; Reis, S.L.; Muccillo, R.; Muccillo, E.N.S. Sintering evaluation of doped lanthanum gallate based on thermodilatometry. Ceram. Int. 2018, 45, 5218–5222. [Google Scholar] [CrossRef]
- Lerch, M.; Boysen, H.; Hansen, T. High-temperature neutron scattering investigation of pure and doped lanthanum gallate. J. Phys. Chem. Solids 2001, 62, 445–455. [Google Scholar] [CrossRef]
- Traina, K.; Henrist, C.; Vertruyen, B.; Cloots, R. Dense La0.9Sr0.1Ga0.8Mg0.2O2.85 electrolyte for IT-SOFC’s: Sintering study and electrochemical characterization. J. Alloys Compd. 2011, 509, 1493–1500. [Google Scholar] [CrossRef]
- Biswal, R.C.; Biswas, K. Novel way of phase stability of LSGM and its conductivity enhancement. Int. J. Hydrogen Energy 2015, 40, 509–518. [Google Scholar] [CrossRef]
- Stevenson, J.W.; Armstrong, R.; McCready, D.E.; Pederson, L.R.; Weber, W.J. Processing and electrical properties of alkaline earth-doped lanthanum gallate. J. Electrochem. Soc. 1997, 144, 3613–3620. [Google Scholar] [CrossRef]
- Hayashi, H.; Inaba, H.; Matsuyama, M.; Lan, N.G.; Dokiya, M.; Tagawa, H. Structural consideration on the ionic conductivity of perovskite-type oxides. Solid State Ion. 1999, 122, 1–15. [Google Scholar] [CrossRef]
- Datta, P.; Majewski, P.; Aldinger, F. Thermal expansion behaviour of Sr- and Mg-doped LaGaO3 solid electrolyte. J. Eur. Ceram. Soc. 2009, 29, 1463–1468. [Google Scholar] [CrossRef]
- Yu, S.; Bi, H.; Sun, J.; Zhu, L.; Yu, H.; Lu, C.; Liu, X. Effect of grain size on the electrical properties of strontium and magnesium doped lanthanum gallate electrolytes. J. Alloys Compd. 2019, 777, 244–251. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, W.J.; Wang, J.; Liu, D.; Sun, Z.H.C. Processing of perovskite La0.9Sr0.1Ga0.8Mg0.2O3−δ electrolyte by glycine-nitrate combustion method. Int. J. Hydrogen Energy 2021, 46, 31362–31369. [Google Scholar] [CrossRef]
- Liu, N.; Yuan, Y.P.; Majewski, P.; Aldinger, F. Synthesis of La0.85Sr0.15Ga0.85Mg0.15O2.85 materials for SOFC applications by acrylamide polymerization. Mat. Res. Bull. 2006, 41, 461–468. [Google Scholar] [CrossRef]
- Shi, M.; Chen, M.; Zuo, R.; Xu, Y.; Su, H.; Wang, L.; Yu, T. Synthesis and characterization of La0.85Sr0.15Ga0.8Mg0.2O2.825 by glycine combustion method and EDTA combustion method. Powder Technol. 2010, 204, 188–193. [Google Scholar] [CrossRef]
- Morales, M.; Roa, J.J.; Perez-Falcón, J.M.; Moure, A.; Tartaj, J.; Espiell, F.; Segarra, M. Correlation between electrical and mechanical properties in La1−xSrxGa1−yMgyO3−δ ceramics used as electrolytes for solid oxide fuel cells. J. Power Sources 2014, 246, 918–925. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Fung, K.-Z. Synthesis of and densification of oxygen-conducting La0.8Sr0.2Ga0.8Mg0.2O2.8 nano powder prepared from a low temperature hydrothermal urea precipitation process. J. Eur. Ceram. Soc. 2008, 28, 803–810. [Google Scholar] [CrossRef]
- Cristiani, C.; Zampori, L.; Latorrata, S.; Pelosato, R.; Dotelli, G.; Ruffo, R. Carbonate coprecipitation synthesis of Sr- and Mg-doped LaGaO3. Mater. Lett. 2009, 63, 1892–1894. [Google Scholar] [CrossRef]
- Kaleva, G.M.; Politova, E.D.; Mosunov, A.V.; Sadovskaya, N.V. Modified ion-conducting ceramics based on lanthanum gallate: Synthesis, structure, and properties. Russ. J. Phys. Chem. 2018, 92, 1138–1144. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, D.F.; Chen, L.; Xie, S.K.; Liu, X.J.; Meng, J. Improvement in the sintering and electrical properties of strontium- and magnesium-doped lanthanum gallate by MoO3 dopant. J. Alloys Compd. 2017, 710, 748–755. [Google Scholar] [CrossRef]
- Ishii, K.; Matsunaga, C.; Munakata, F.; Uchikoshi, T. Effect of A-site ion non-stoichiometry on the chemical stability and electric conductivity of strontium and magnesium-doped lanthanum gallate. J. Am. Ceram. Soc. 2019, 103, 790–799. [Google Scholar] [CrossRef]
- Wang, L.-S.; Li, C.-X.; Ma, K.; Zhang, S.-L.; Yang, G.-J.; Li, C.-J. Microstructure and electrochemical properties of La0.8Sr0.2Ga0.8Mg0.2O3 thin film deposited by vacuum cold spray for solid oxide fuel cells. ECS Trans. 2017, 78, 405–412. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Qiao, J.; Sun, W.; Sun, K.; Wang, Z. An easily controllable flash sintering process for densification of electrolyte for application in solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 45, 17824–17832. [Google Scholar] [CrossRef]
- Savioli, J.; Watson, G.W. Defect chemistry of LaGaO3 doped with divalent cations. Solid State Ion. 2022, 374, 115828. [Google Scholar] [CrossRef]
- Rupp, G.M.; Glowacki, M.; Fleig, J. Electronic and ionic conductivity of La0.95Sr0.05Ga0.95Mg0.05O3−δ (LSGM) single crystals. J. Electrochem. Soc. 2016, 163, F1189–F1197. [Google Scholar] [CrossRef]
- Mariño, C.; Basbus, J.; Larralde, A.L.; Alonso, J.A.; Fernández-Díaz, M.T.; Troncoso, L. Structural, electrical characterization and oxygen-diffusion paths in LaSrGa1−xMgxO4−δ (x = 0.0–0.2) layered perovskites: An impedance spectroscopy and neutron diffraction study. New J. Chem. 2021, 45, 10248–10256. [Google Scholar] [CrossRef]
- Li, Y.; Yi, H.; Xu, J.; Kuang, X. High oxide ion conductivity in the Bi3+ doped melilite LaSrGa3O7. J. Alloys Compd. 2018, 740, 143–147. [Google Scholar] [CrossRef]
- Baskaran, S.; Lewinsohn, C.A.; Chou, Y.-S.; Qian, M.; Stevenson, J.W.; Armstrong, T.R. Mechanical properties of alkaline earth-doped lanthanum gallate. J. Mater. Sci. 1999, 34, 3913–3922. [Google Scholar] [CrossRef]
- Shkerin, S.N.; Bronin, D.I.; Kovyazina, S.A.; Gorelov, V.P.; Kuzmin, A.V.; Martemyanova, Z.S.; Beresnev, S.M. Structure and phase transitions of (La,Sr)(Ga,Mg)O3−α solid electrolyte. Solid State Ion. 2004, 171, 129–134. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Rao, C.-R. An investigation on the microstructural and electrical properties of La0.85DxSr0.15–xGa0.8Mg0.2O2.825 (D = Ba and Ca) electrolytes in solid oxide fuel cells. Ceram. Int. 2018, 44, 19706–19717. [Google Scholar] [CrossRef]
- Nesaraj, A.S.; Kumar, M.; Arul Raj, I.; Radhakrishna, I.; Pattabiraman, R. Investigations on chemical interactions between alternate cathodes and lanthanum gallate electrolyte for intermediate temperature solid oxide fuel cell (ITSOFC). J. Iran. Chem. Soc. 2007, 4, 89–106. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Tang, Y.; Gotor, F.J.; Sayagués, M.J. Development by mechanochemistry of La0.8Sr0.2Ga0.8Mg0.2O2.8 electrolyte for SOFCs. Materials 2020, 13, 1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sydyknazar, S.; Cascos, V.; Troncoso, L.; Larralde, A.L.; Fernández-Díaz, M.T.; Alonso, J.A. Design, synthesis, structure and properties of Ba-doped derivatives of SrCo0.95Ru0.05O3−δ perovskite as cathode materials for SOFCs. Materials 2019, 12, 1957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Li, X.; Zhang, L.; Hongdongcai, H.; Xu, J.; Wang, L.; Long, W. Improved thermal expansion and electrochemical performance of La0.4Sr0.6Co0.9Sb0.1O3−δ-Ce0.8Sm0.2O1.9 composite cathode for IT-SOFCs. Solid State Sci. 2019, 91, 126–132. [Google Scholar] [CrossRef]
- Wang, S.; Jin, F.; Li, L.; Li, R.; Qu, B.; He, T. Stability, compatibility and performance improvement of SrCo0.8Fe0.1Nb0.1O3−δ perovskite as a cathode for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2017, 42, 4465–4477. [Google Scholar] [CrossRef]
- Yang, X.; Han, X.; He, T.; Du, Y. Enhanced stability of BaCoO3−δ using doping process as a cathode material for IT-SOFCs. ECS Trans. 2017, 78, 543–550. [Google Scholar] [CrossRef]
- Niu, B.; Lu, C.; Yi, W.; Luo, S.; Li, X.; Zhong, X.; Zhao, X.; Xu, B. In-situ growth of nanoparticles-decorated double perovskite electrode materials for symmetrical solid oxide cells. Appl. Catal. B Environ. 2020, 270, 118842. [Google Scholar] [CrossRef]
- Tarancón, A.; Peña-Martínez, J.; Marrero-López, D.; Morata, A.; Ruiz-Morales, J.; Núñez, P. Stability, chemical compatibility and electrochemical performance of GdBaCo2O5+x layered perovskite as a cathode for intermediate temperature solid oxide fuel cells. Solid State Ion. 2008, 179, 2372–2378. [Google Scholar] [CrossRef]
- Zhou, Q.; Qu, L.; Zhang, T.; He, Y.; Zhao, C.; Wang, M.; Wei, T.; Zhang, Y. Preparation and electrochemical properties of an La-doped Pr2Ni0.85Cu0.1Al0.05O4+δ cathode material for an IT-SOFC. J. Alloys Compd. 2020, 824, 153967. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Y.; Yuan, C.; Liu, M.; Meng, X.; Zhan, Z.; Xia, C.; Wang, S. Impregnated Nd2NiO4+δ-scandia stabilized zirconia composite cathode for intermediate-temperature solid oxide fuel cells. J. Power Sources 2014, 269, 812–817. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Medvedev, D.A.; Khasanov, A.F. Structure, stability, and thermomechanical properties of Ca-substituted Pr2NiO4+δ. Phys. Solid State 2017, 59, 694–702. [Google Scholar] [CrossRef]
- Pikalova, E.; Kolchugin, A.; Bogdanovich, N.; Medvedev, D.; Lyagaeva, J.; Vedmid’, L.; Ananyev, M.; Plaksin, S.; Farlenkov, A. Suitability of Pr2–xCaxNiO4+δ as cathode materials for electrochemical devices based on oxygen ion and proton conducting solid state electrolytes. Int. J. Hydrogen Energy 2020, 42, 4465–4477. [Google Scholar] [CrossRef]
- Skutina, L.; Filonova, E.; Medvedev, D.; Maignan, A. Undoped Sr2MMoO6 double perovskite molybdates (M = Ni, Mg, Fe) as promising anode materials for solid oxide fuel cells. Materials 2021, 14, 1715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ohara, S.; Mukai, K.; Fukui, T.; Yoshida, H.; Nishimura, M.; Inagaki, T.; Miura, K. Ni-SDC cermet anode for medium-temperature solid oxide fuel cell with lanthanum gallate electrolyte. J. Power Sources 1999, 83, 170–177. [Google Scholar] [CrossRef]
- Huang, K.; Goodenough, J.B. A solid oxide fuel cell based on Sr- and Mg-doped LaGaO3 electrolyte: The role of a rare-earth oxide buffer. J. Alloys Compd. 2000, 303–304, 454–464. [Google Scholar] [CrossRef]
- Kim, K.; Kim, B.; Son, J.; Kim, J.; Lee, H.; Lee, J.; Moon, J. Characterization of the electrode and electrolyte interfaces of LSGM-based SOFCs. Solid State Ion. 2006, 177, 2155–2158. [Google Scholar] [CrossRef]
- Eba, H.; Anzai, C.; Ootsuka, S. Observation of cation diffusion and phase formation between solid oxide layers of lanthanum gallate-based fuel cells. Mater. Trans. 2018, 59, 244–250. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Ohara, S.; Okawa, H.; Maric, R.; Fukui, T. Interactions of a La0.9Sr0.1Ga0.8Mg0.2O3−δ electrolyte with Fe2O3, Co2O3 and NiO anode materials. Solid State Ion. 2001, 139, 145–152. [Google Scholar] [CrossRef]
- Du, Y.; Sammes, N.M. Interactions and compatibilities of LSGM electrolyte and LSCM anode. ECS Proc. Vol. 2005, 2005–2007, 1127–1136. [Google Scholar] [CrossRef]
- Niu, B.; Jin, F.; Liu, J.; Zhang, Y.; Jiang, P.; Feng, T.; Xu, B.; He, T. Highly carbon– and sulfur–tolerant Sr2TiMoO6−δ double perovskite anode for solid oxide fuel cells. Int. J. Hydrogen Energy 2019, 44, 20404–20415. [Google Scholar] [CrossRef]
- Dos Santos-Gómez, L.; León-Reina, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Chemical stability and compatibility of double perovskite anode materials for SOFCs. Solid State Ion. 2013, 239, 1–7. [Google Scholar] [CrossRef]
- Filonova, E.A.; Dmitriev, A.S. Physicochemical properties of potential cathode La1-xBaxMn1-yCryO3 and anode Sr2NiMoO6 materials for solid-oxide fuel cells. Eurasian Chem.-Technol. J. 2012, 14, 139–145. [Google Scholar] [CrossRef]
- Filonova, E.A.; Gilev, A.R.; Skutina, L.S.; Vylkov, A.I.; Kuznetsov, D.K.; Shur, V.Y. Double Sr2Ni1−xMgxMoO6 perovskites (x = 0, 0.25) as perspective anode materials for LaGaO3-based solid oxide fuel cells. Solid State Ion. 2018, 314, 112–118. [Google Scholar] [CrossRef]
- Filonova, E.A.; Dmitriev, A.S.; Pikalov, P.S.; Medvedev, D.A.; Pikalova, E.Y. The structural and electrical properties of Sr2Ni0.75Mg0.25MoO6 and its compatibility with solid state electrolytes. Solid State Ion. 2014, 262, 365–369. [Google Scholar] [CrossRef]
- Takano, S.; Shin-mura, K.; Niwa, E.; Hashimoto, T.; Sasaku, K. Chemical compatibility of Sr2MgMoO6−δ with representative electrolyte materials and interlayer materials for solid oxide fuel cells. J. Ceram. Soc. Japan 2018, 126, 482–487. [Google Scholar] [CrossRef] [Green Version]
- Filonova, E.A.; Dmitriev, A.S. Crystal structure and thermal properties of Sr2ZnMoO6. Inorg. Mater. 2014, 49, 602–605. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, H.; Du, Z.; Chen, T.; Chen, N. Electrical, chemical, and electrochemical properties of double perovskite oxides Sr2Mg1−xNixMoO6−δ as anode materials for solid oxide fuel cells. J. Phys. Chem. C 2014, 118, 18853–18860. [Google Scholar] [CrossRef]
- Marrero-López, D.; Peña-Martínez, J.; Ruiz-Morales, J.C.; Gabás, M.; Núñez, P.; Aranda, M.A.G.; Ramos-Barrado, J.R. Redox behaviour, chemical compatibility and electrochemical performance of Sr2MgMoO6−δ as SOFC anode. Solid State Ion. 2010, 180, 1672–1682. [Google Scholar] [CrossRef]
- Kumar, S.; Chakraborty, A.; Kobi, S.; Gopalan, P.; Prasanna, T.R.S. Phase formation between La(Sr)Ga(Mg)O3 and Ce(La)O2 for solid oxide fuel cell applications. J. Amer. Ceram. Soc. 2022, 105, 3625–3635. [Google Scholar] [CrossRef]
- Wang, J.Q.; Zhou, D.F.; Gao, J.Q.; Sun, H.R.; Zhu, X.F.; Meng, J. Effect of A/B-Site Non-stoichiometry on the structure and properties of La0.9Sr0.1Ga0.9Mg0.1O3−δ solid electrolyte in intermediate-temperature solid oxide fuel cells. ChemElectroChem 2018, 5, 665–673. [Google Scholar] [CrossRef]
- Hwang, K.-J.; Jang, M.; Kim, M.K.; Lee, S.H.; Shin, T.H. Effective buffer layer thickness of La-doped CeO2 for high durability and performance on La0.9Sr0.1Ga0.8Mg0.2O3−δ electrolyte supported type solid oxide fuel cells. J. Eur. Ceram. Soc. 2021, 41, 2674–2681. [Google Scholar] [CrossRef]
- Zhu, T.; Troiani, H.E.; Mogni, L.V.; Han, M.; Barnett, S.A. Ni-Substituted Sr(Ti,Fe)O3 SOFC anodes: Achieving high performance via metal alloy nanoparticle exsolution. Joule 2018, 2, 478–496. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Tichy, R.S.; Goodenough, J.B.; Milliken, C. Superior perovskite oxide-ion conductor; strontium- and magnesium-doped LaGaO3: III. Performance tests of single ceramic fuel cells. J. Am. Ceram. Soc. 1998, 81, 2581–2585. [Google Scholar] [CrossRef]
- Fukui, T.; Ohara, S.; Murata, K.; Yoshida, H.; Miura, K.; Inagaki, T. Performance of intermediate temperature solid oxide fuel cells with La(Sr)Ga(Mg)O3 electrolyte film. J. Power Sources 2002, 106, 142–145. [Google Scholar] [CrossRef]
- Matsuda, M.; Ohara, O.; Murata, K.; Ohara, S.; Fukui, T.; Miyake, M. Electrophoretic fabrication and cell performance of dense Sr- and Mg-doped LaGaO3-based electrolyte films. Electrochem. Solid State Lett. 2003, 6, A140–A143. [Google Scholar] [CrossRef]
- Bozza, F.; Polini, R.; Traversa, E. High performance anode-supported intermediate temperature solid oxide fuel cells (IT-SOFCs) with La0.8Sr0.2Ga0.8Mg0.2O3−δ electrolyte films prepared by electrophoretic deposition. Electrochem. Commun. 2009, 11, 1680–1683. [Google Scholar] [CrossRef]
- Huang, K.; Wan, J.; Goodenough, J.B. Increasing power density of LSGM-based solid oxide fuel cells using new anode materials. J. Electrochem. Soc. 2001, 148, A788–A794. [Google Scholar] [CrossRef]
- Bi, Z.; Yi, B.; Wang, Z.; Dong, Y.; Wu, H.; She, Y.; Cheng, M. A high-performance anode-supported SOFC with LDC-LSGM bilayer electrolytes. Electrochem. Solid State Lett. 2004, 7, A105–A107. [Google Scholar] [CrossRef]
- Bi, Z.; Dong, Y.; Cheng, M.; Yi, B. Behavior of lanthanum-doped ceria and Sr-, Mg-doped LaGaO3 electrolytes in an anode-supported solid oxide fuel cell with a La0.6Sr0.4CoO3 cathode. J. Power Sources 2006, 161, 34–39. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Z.; Wang, H.; Ma, G.; Gao, W.; Zhou, Z. Desirable performance of intermediate-temperature solid oxide fuel cell with an anode-supported La0.9Sr0.1Ga0.8Mg0.2O3−δ electrolyte membrane. J. Power Sources 2011, 196, 3539–3543. [Google Scholar] [CrossRef]
- Ju, Y.W.; Eto, H.; Inagaki, T.; Ida, S.; Ishihara, T. Preparation of Ni–Fe bimetallic porous anode support for solid oxide fuel cells using LaGaO3 based electrolyte film with high power density. J. Power Sources 2010, 195, 6294–6300. [Google Scholar] [CrossRef]
- Lin, Y.B.; Barnett, S.A. Co-firing of anode-supported SOFCs with thin La0.9Sr0.1Ga0.8Mg0.2O3−δ electrolytes. Electrochem. Solid State Lett. 2006, 9, A285–A288. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.; Zhang, Y. Electrical and stability performance of anode-supported solid oxide fuel cells with strontium- and magnesium-doped lanthanum gallate thin electrolyte. Electrochim. Acta 2008, 53, 4420–4427. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Wang, S.-F.; Hsu, Y.-F.; Yeh, W.-Y. Solid oxide fuel cells incorporating doped lanthanum gallate films deposited by radio-frequency magnetron sputtering at various Ar/O2 ratios and annealing conditions. Surf. Coat. Technol. 2018, 344, 507–513. [Google Scholar] [CrossRef]
- Wang, Y.-P.; Gao, J.-T.; Li, J.-H.; Li, C.-J.; Li, C.-X. Preparation of bulk-like La0.8Sr0.2Ga0.8Mg0.2O3−δ coatings for porous metal-supported solid oxide fuel cells via plasma spraying at increased particle temperatures. Int. J. Hydrogen Energy 2021, 46, 32655–32664. [Google Scholar] [CrossRef]
- Wei, T.; Ji, Y.; Meng, X.; Zhang, Y. Sr2NiMoO6−δ as anode material for LaGaO3-based solid oxide fuel cell. Electrochem. Commun. 2008, 10, 1369–1372. [Google Scholar] [CrossRef]
- Gilev, A.R.; Kiselev, E.A.; Cherepanov, V.A. Performance of the lanthanum gallate based solid oxide fuel cells with the La2−xCaxNi1−yFeyO4+δ cathodes and Sr2Ni0.75Mg0.25MoO6−δ anode. Solid State Ion. 2019, 339, 115001. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Y.; Li, W.; Guan, B.; Qi, H.; Tian, H.; Zhou, L.; De Santiago, H.A.; Liu, X. Redox-stable symmetrical solid oxide fuel cells with exceptionally high performance enabled by electrode/electrolyte diffuse interface. J. Power Sources 2021, 488, 229458. [Google Scholar] [CrossRef]
- Gao, J.; Meng, X.; Luo, T.; Wu, H.; Zhan, Z. Symmetrical solid oxide fuel cells fabricated by phase inversion tape casting with impregnated SrFe0.75Mo0.25O3−δ (SFMO) electrodes. Int. J. Hydrogen Energy 2017, 42, 18499–18503. [Google Scholar] [CrossRef]
- He, W.; Wu, X.; Dong, F.; Ni, M. A novel layered perovskite electrode for symmetrical solid oxide fuel cells: PrBa(Fe0.8Sc0.2)2O5+δ. J. Power Sources 2017, 363, 16–19. [Google Scholar] [CrossRef]
- Liu, J.; Lei, Y.; Li, Y.; Gao, J.; Han, D.; Zhan, W.; Huang, F.; Wang, S. Infiltrated Sr2Fe1.5Mo0.5O6/La0.9Sr0.1Ga0.8Mg0.2O3 electrodes towards high performance symmetrical solid oxide fuel cells fabricated by an ultra-fast and time-saving procedure. Electrochem. Commun. 2017, 78, 6–10. [Google Scholar] [CrossRef]
- Lu, X.; Yang, Y.; Ding, Y.; Chen, Y.; Gu, Q.; Tian, D.; Yu, W.; Lin, B. Mo-doped Pr0.6Sr0.4Fe0.8Ni0.2O3−δ as potential electrodes for intermediate-temperature symmetrical solid oxide fuel cells. Electrochim. Acta 2017, 227, 33–40. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, K.; Liu, Y.; He, B. A novel layered perovskite as symmetric electrode for direct hydrocarbon solid oxide fuel cells. J. Power Sources 2017, 342, 313–319. [Google Scholar] [CrossRef]
- Bian, L.; Duan, C.; Wang, L.; Zhu, L.; O’Hayre, R.; Chou, K.-C. Electrochemical performance and stability of La0.5Sr0.5Fe0.9Nb0.1O3−δ symmetric electrode for solid oxide fuel cells. J. Power Sources 2018, 399, 398–405. [Google Scholar] [CrossRef]
- Niu, B.; Jin, F.; Feng, T.; Zhang, L.; Zhang, Y.; He, T. A-site deficient (La0.6Sr0.4)1−xCo0.2Fe0.6Nb0.2O3−δ symmetrical electrode materials for solid oxide fuel cells. Electrochim. Acta 2018, 270, 174–182. [Google Scholar] [CrossRef]
- Niu, B.; Jin, F.; Zhang, L.; Shen, P.; He, T. Performance of double perovskite symmetrical electrode materials Sr2TiFe1−xMoxO6−δ (x = 0.1, 0.2) for solid oxide fuel cells. Electrochim. Acta 2018, 263, 217–227. [Google Scholar] [CrossRef]
- Gou, M.; Ren, R.; Sun, W.; Xu, C.; Meng, X.; Wang, Z.; Qiao, J.; Sun, K. Nb-doped Sr2Fe1.5Mo0.5O6−δ electrode with enhanced stability and electrochemical performance for symmetrical solid oxide fuel cells. Ceram. Int. 2019, 45, 15696–15704. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Huang, W.-H. Processing improvement and performance analysis of La0.85Sr0.15Ga0.8Mg0.2O2.825 electrolyte-supported fuel cells. Ceram. Int. 2017, 43, S729–S738. [Google Scholar] [CrossRef]
- Gao, K.; Liu, X.; Wang, Z.; Xiong, Y. A Sm0.2Ce0.8O1.9 (SDC) interlayer method to prevent the elemental interdiffusion between Sm0.5Sr0.5CoO3−δ (SSC) cathode and La0.8Sr0.2Ga0.8Mg0.2O3−δ (LSGM) electrolyte. Int. J. Hydrogen Energy 2017, 42, 19170–19177. [Google Scholar] [CrossRef]
- Xu, S.; Lin, X.; Ge, B.; Ai, D.; Ma, J.; Peng, Z. Microstructure and electrical conductivity of La0.9Sr0.1Ga0.8Mg0.2O2.85-Ce0.8Gd0.2O1.9 composite electrolytes for SOFCs. Int. J. Appl. Ceram. Technol. 2019, 16, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Abubaker, O.A.; Singh, K.; Thangadurai, V. Investigating the effect of Cu-doping on the electrochemical properties of perovskite-type Ba0.5Sr0.5Fe1−xCuxO3−δ (0 ≤ x ≤ 0.20) cathodes. J. Power Sources 2020, 451, 227777. [Google Scholar] [CrossRef]
- Hong, G.; Kim, T.W.; Kwak, M.J.; Song, J.; Choi, Y.; Woo, S.-K.; Han, M.H.; Cho, C.H.; Kim, S.-D. Composite electrodes of Ti-doped SrFeO3−δ and LSGMZ electrolytes as both the anode and cathode in symmetric solid oxide fuel cells. J. Alloys Compd. 2020, 846, 156154. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Choi, S.; Shin, J.; Kim, G. A Nano-Structured SOFC Composite cathode prepared via infiltration of La0.5Ba0.25Sr0.25Co0.8Fe0.2O3−δ into La0.9Sr0.1Ga0.8Mg0.2O3−δ for extended triple-phase boundary area. J. Electrochem. Soc. 2019, 166, F805–F809. [Google Scholar] [CrossRef]
- Fujimoto, T.G.; Reis, S.L.; dos Santos Muccillo, E.N. Influence of yttria-stabilized zirconia on microstructure and electrical properties of doped lanthanum gallate. Mater. Res. 2019, 22, 20190043. [Google Scholar] [CrossRef] [Green Version]
- Malik, Y.T.; Noviyanti, A.R.; Syarif, D.G. Lowered sintering temperature on synthesis of La9.33Si6O26 (LSO)–La0.8Sr0.2Ga0.8Mg0.2O2.55 (LSGM) electrolyte composite and the electrical performance on La0.7Ca0.3MnO3 (LCM) cathode. J. Kim. Sains Apl. 2018, 21, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.P.; Zhong, H.T.; Chen, X.; Ge, B.; Ai, D.S. Preparation and property of LSGM-carbonate composite electrolyte for low temperature solid oxide fuel cell. Solid State Phenom. 2018, 281, 754–760. [Google Scholar] [CrossRef]
- Wang, S.-F.; Lu, H.-C.; Hsu, Y.-F.; Jasinski, P. High-performance anode-supported solid oxide fuel cells with co-fired Sm0.2Ce0.8O2−δ/La0.8Sr0.2Ga0.8Mg0.2O3−δ/Sm0.2Ce0.8O2−δ sandwiched electrolyte. Int. J. Hydrogen Energy 2022, 47, 5429–5438. [Google Scholar] [CrossRef]
- Noviyanti, A.R.; Malik, Y.T.; Rahayu, I.; Eddy, D.R.; Pratomo, U. Electrochemical properties of La9.33Si6O26(LSO)-La0.8Sr0.2Ga0.8Mg0.2O2.55(LSGM) electrolyte over NiO and La0.1Ca0.9MnO3(LCM) electrodes. Mat. Res. Exp. 2021, 8, 115505. [Google Scholar] [CrossRef]
- Pandey, R.; Singh, P.; Singh, A.K.; Singh, P. Polyol-mediated synthesis of La0.9Sr0.1Ga0.8Mg0.2O2.85-Ce0.85Sm0.15O1.925 composite electrolyte for IT-SOFCs. Mater. Today Proc. 2020, 49, 3071–3075. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, D.F.; Wang, Y.; Zhu, X.F.; Meng, J. Enhanced sintering of Ce0.8Nd0.2O2−δ-La0.8Sr0.2Ga0.8Mg0.2O3−δ using CoO as a sintering aid. Ceram. Int. 2017, 43, 3583–3589. [Google Scholar] [CrossRef]
- Glisenti, A.; Bedon, A.; Carollo, G.; Savaniu, C.; Irvine, J.T.S. Reversible, all-perovskite SOFCs based on La, Sr gallates. Int. J. Hydrogen Energy 2020, 45, 29155–29165. [Google Scholar] [CrossRef]
- Kwon, Y.; Kang, S.; Bae, J. Development of a PrBaMn2O5+δ-La0.8Sr0.2Ga0.85Mg0.15O3−δ composite electrode by scaffold infiltration for reversible solid oxide fuel cell applications. Int. J. Hydrogen Energy 2020, 45, 1748–1758. [Google Scholar] [CrossRef]
- Tan, Z.; Ishihara, T. Reversible operation of tubular type solid oxide fuel cells using LaGaO3 electrolyte porous layer on dense film prepared by dip-coating method. J. Electrochem. Soc. 2017, 164, F1690–F1696. [Google Scholar] [CrossRef]
- Tan, Z.; Ishihara, T. Redox stability of tubular solid oxide cell using LaGaO3 electrolyte film prepared by dip-coating. ECS Trans. 2021, 103, 1685–1693. [Google Scholar] [CrossRef]
- Tan, Z.; Song, J.T.; Takagaki, A.; Ishihara, T. Infiltration of cerium into a NiO–YSZ tubular substrate for solid oxide reversible cells using a LSGM electrolyte film. J. Mater. Chem. A 2021, 9, 1530–1540. [Google Scholar] [CrossRef]



| Sample | T (°C) | σ (S cm−1) | Ea (eV) | Ref. |
|---|---|---|---|---|
| LaAlO3 | 900 | 6 × 10−4 | 1.30 | [53] |
| LaAlO3 | 700 | 6.7 × 10−4 | 0.99 | [71] |
| LaAlO3 | 900 | 1.1 × 10−6 | 1.83 | [75] |
| LaAlO3 | 900 | 1.4 × 10−3 | 1.88 | [80] |
| LaAlO3 | 800 | 2.0 × 10−4 | 1.30 | [83] |
| La0.9Ca0.1AlO3−δ | 900 | 6.0 × 10−3 | 1.08 | [65] |
| La0.9Sr0.1AlO3−δ | 900 | 1.1 × 10−2 | 1.14 | [80] |
| La0.9Sr0.1AlO3−δ | 800 | 9.0×10−3 | 0.93 | [85] |
| La0.8Sr0.2AlO3−δ | 800 | 6.2 × 10−3 | 1.06 | [75] |
| La0.8Sr0.2AlO3−δ | 900 | 1.5 × 10−2 | 1.06 | [75] |
| La0.8Sr0.2AlO3−δ | 900 | 1.1 × 10−2 | 1.16 | [80] |
| La0.8Sr0.2AlO3−δ | 810 | 4.3 × 10−3 | 1.06 | [84] |
| La0.7Pr0.2Sr0.1AlO3−δ | 800 | 2.3 × 10−2 | 0.84 | [85] |
| LaAl0.95Zn0.05O3−δ | 700 | 8.5 × 10−4 | 1.05 | [62] |
| LaAl0.95Zn0.05O3−δ | 900 | 1.1 × 10−3 | 1.05 | [62] |
| LaAl0.9Mg0.1O3−δ | 900 | 9.6 × 10−3 | 1.05 | [80] |
| LaAl0.5Mn0.5O3−δ | 800 | 4.7(2) | 0.22 | [75] |
| LaAl0.5Mn0.5O3−δ | 900 | 5.8(2) | 0.22 | [75] |
| La0.9Sr0.1Al0.9Mg0.1O3−δ | 700 | 2.6 × 10−3 | 1.56 | [71] |
| La0.9Sr0.1Al0.9Mg0.1O3−δ | 700 | 5.3 × 10−4 | 1.38 | [88] |
| La0.9Sr0.1Al0.9Mg0.1O3−δ | 900 | 2.0 × 10−2 | 0.90 | [82] |
| La0.8Sr0.2Al0.95Mg0.05O3−δ | 900 | 1.3 × 10−2 | 1.15 | [80] |
| La0.89Sr0.1Ba0.01Al0.9Mg0.1O3−δ | 700 | 2.6 × 10−3 | 1.48 | [71] |
| La0.89Sr0.1Ba0.01Al0.9Mg0.1O3−δ tape | 700 | 6.0 × 10−4 | 0.60 | [86] |
| La0.89Sr0.1Ba0.01Al0.9Mg0.1O3−δ pellet | 700 | 4.6 × 10−2 | 0.75 | [86] |
| La0.87Sr0.1Ba0.03Al0.9Mg0.1O3−δ | 700 | 1.7 × 10−3 | 1.38 | [71] |
| La0.8Sr0.2Al0.5Mn0.5O3−δ | 800 | 8.6(3) | 0.15 | [75] |
| La0.8Sr0.2Al0.5Mn0.5O3−δ | 900 | 9.8(2) | 0.15 | [75] |
| La0.8Sr0.2Al0.7Mn0.3O3−δ | 810 | 0.75 | 0.29 | [84] |
| La0.8Sr0.2Al0.5Mn0.5O3−δ | 810 | 10 | 0.17 | [84] |
| (La0.8Sr0.2)0.94Al0.5Mn0.5O3−δ | 810 | 12 | 0.14 | [84] |
| La0.9Ba0.1Al0.9Y0.1O3−δ | 800 | 1.8 × 10−2 | 0.82 | [53] |
| La0.9Ba0.1Al0.9Y0.1O3−δ | 900 | 3.1 × 10−2 | 0.82 | [53] |
| La0.87Sr0.1Sm0.03Al0.9Mg0.1O3−δ | 700 | 1.2 × 10−3 | 1.09 | [88] |
| La0.85Sr0.1Sm0.05Al0.9Mg0.1O3−δ | 700 | 1.1 × 10−3 | 1.10 | [88] |
| Sample | Samples Obtaining Method; Annealing Temperature (°C) | T (°C) | σ (S cm−1) | Ref. |
|---|---|---|---|---|
| LaGaO3 | Solid-state route; 1500 | 950 | 0.02 | [51] |
| La0.9Sr0.1Ga0.9Mg0.1O3−δ | Solid-state route; 1500 | 950 | 0.20 | [51] |
| La0.9Sr0.1Ga0.85Mg0.15O3−δ | Solid-state route; 1500 | 950 | 0.27 | [51] |
| La0.9Sr0.1Ga0.8Mg0.2O3−δ | Solid-state route; 1500 | 950 | 0.29 | [51] |
| La0.9Sr0.1Ga0.7Mg0.3O3−δ | Solid-state route; 1500 | 950 | 0.28 | [51] |
| La0.9Sr0.1Ga0.6Mg0.4O3−δ | Solid-state route; 1500 | 950 | 0.10 | [51] |
| La0.9Sr0.1Ga0.8Mg0.2O3−δ | Glycine-combustion method; 1400 | 1000 | 0.26 | [51] |
| La0.85Sr0.15Ga0.8Mg0.2O3−δ | Glycine-combustion method; 1400 | 1000 | 0.36 | [51] |
| La0.8Sr0.2Ga0.85Mg0.15O3−δ | Glycine-combustion method; 1400 | 1000 | 0.31 | [51] |
| La0.8Sr0.2Ga0.8Mg0.2O3−δ | Glycine-combustion method; 1400 | 1000 | 0.40 | [51] |
| La0.9Sr0.1Ga0.9Mg0.1O3−δ | Solid-state route; 1470 | 800 | 0.116 | [95] |
| La0.9Sr0.1Ga0.85Mg0.15O3−δ | Solid-state route; 1470 | 800 | 0.127 | [95] |
| La0.9Sr0.1Ga0.8Mg0.2O3−δ | Solid-state route; 1470 | 800 | 0.132 | [95] |
| La0.9Sr0.1Ga0.7Mg0.3O3−δ | Solid-state route; 1470 | 800 | 0.096 | [95] |
| La0.85Sr0.15Ga0.8Mg0.2O3−δ | Solid-state route; 1470 | 800 | 0.150 | [95] |
| La0.8Sr0.2Ga0.85Mg0.15O3−δ | Solid-state route; 1470 | 800 | 0.149 | [95] |
| La0.8Sr0.2Ga0.83Mg0.17O3−δ | Solid-state route; 1470 | 800 | 0.17 | [95] |
| La0.8Sr0.2Ga0.8Mg0.2O3−δ | Solid-state route; 1470 | 800 | 0.14 | [95] |
| La0.7Sr0.3Ga0.8Mg0.2O3−δ | Solid-state route; 1470 | 800 | 0.109 | [95] |
| La0.9Sr0.1Ga0.8Mg0.2O3−δ | Self-propagating high-temperature synthesis; 1500 | 800 | 0.11 | [110] |
| La0.9Sr0.1Ga0.8Mg0.2O3−δ | Carbonate co-precipitation; 1400 | 800 | 0.045 | [104] |
| La0.9Sr0.1Ga0.9Mg0.1O3−δ | Solid-state route; 1450 | 800 | 0.071 | [114] |
| La0.9Sr0.1Ga0.8Mg0.2O3−δ | Solid-state route; 1450 | 800 | 0.1095 | [114] |
| La0.9Sr0.1Ga0.8Mg0.2O3−δ | Glycine-combustion method; 1500 | 800 | 0.092 | [122] |
| La0.9Sr0.1Ga0.8Mg0.2O3−δ | Glycine-combustion method; 1400 | 800 | 0.0395 | [123] |
| La0.85Sr0.15Ga0.85Mg0.15O3−δ | Acrylamide polymerization technique; 1432 | 800 | 0.093 | [124] |
| La0.85Sr0.15Ga0.8Mg0.2O3−δ | Mechanochemical route; 1380 | 600 | 0.016 | [97] |
| La0.85Sr0.15Ga0.8Mg0.2O3−δ | Glycine-combustion method; 1300 | 800 | 0.053 | [125] |
| La0.85Sr0.15Ga0.8Mg0.2O3−δ | EDTA-combustion method; 1300 | 800 | 0.06 | [125] |
| La0.85Sr0.15Ga0.8Mg0.2O3−δ | Glycine-combustion method; 1400 | 800 | 0.096 | [105] |
| La0.85Sr0.15Ga0.8Mg0.2O3−δ | Pechini method; 1400 | 800 | 0.135 | [126] |
| La0.8Sr0.2Ga0.8Mg0.2O3−δ | Carbonate co-precipitation; 1300 | 600 | 0.014 | [103] |
| La0.8Sr0.2Ga0.8Mg0.2O3−δ | Glycine-combustion method; 1300 | 700 | 0.022 | [109] |
| La0.8Sr0.2Ga0.8Mg0.2O3−δ | Glycine-combustion method; 1400 | 700 | 0.085 | [109] |
| La0.8Sr0.2Ga0.8Mg0.2O3−δ | Spray pyrolysis; 1400 | 500 | 0.0029 | [112] |
| La0.8Sr0.2Ga0.8Mg0.2O3−δ | Solid-state route; 1450 | 800 | 0.126 | [127] |
| La0.8Sr0.2Ga0.8Mg0.2O3−δ | Solid-state route; 1400 | 800 | 0.035 | [127] |
| La0.8Sr0.2Ga0.8Mg0.2O3−δ | Hydrothermal urea hydrolysis precipitation; 1400 | 800 | 0.056 | [127] |
| La0.8Sr0.2Ga0.8Mg0.2O3−δ | Carbonate co-precipitation; 1400 | 800 | 0.137 | [128] |
| La0.8Sr0.2Ga0.8Mg0.2O3−δ | Solid-state route; 1250 | 727 | 0.019 | [129] |
| La0.8Sr0.2Ga0.8Mg0.2O3−δ | Sol-gel technique; 1300 | 450 | 2.9 × 10−4 | [130] |
| La0.8Sr0.2Ga0.8Mg0.2O3−δ | Solid-state route; 1400 | 800 | 0.132 | [131] |
| La0.8Sr0.2Ga0.8Mg0.2O3−δ | Thin film deposited by vacuum cold spray; 200 | 750 | 0.043 | [132] |
| La0.8Sr0.2Ga0.8Mg0.2O3−δ | Step-wise current-limiting flash sintering process; 690 | 850 | 0.072 | [133] |
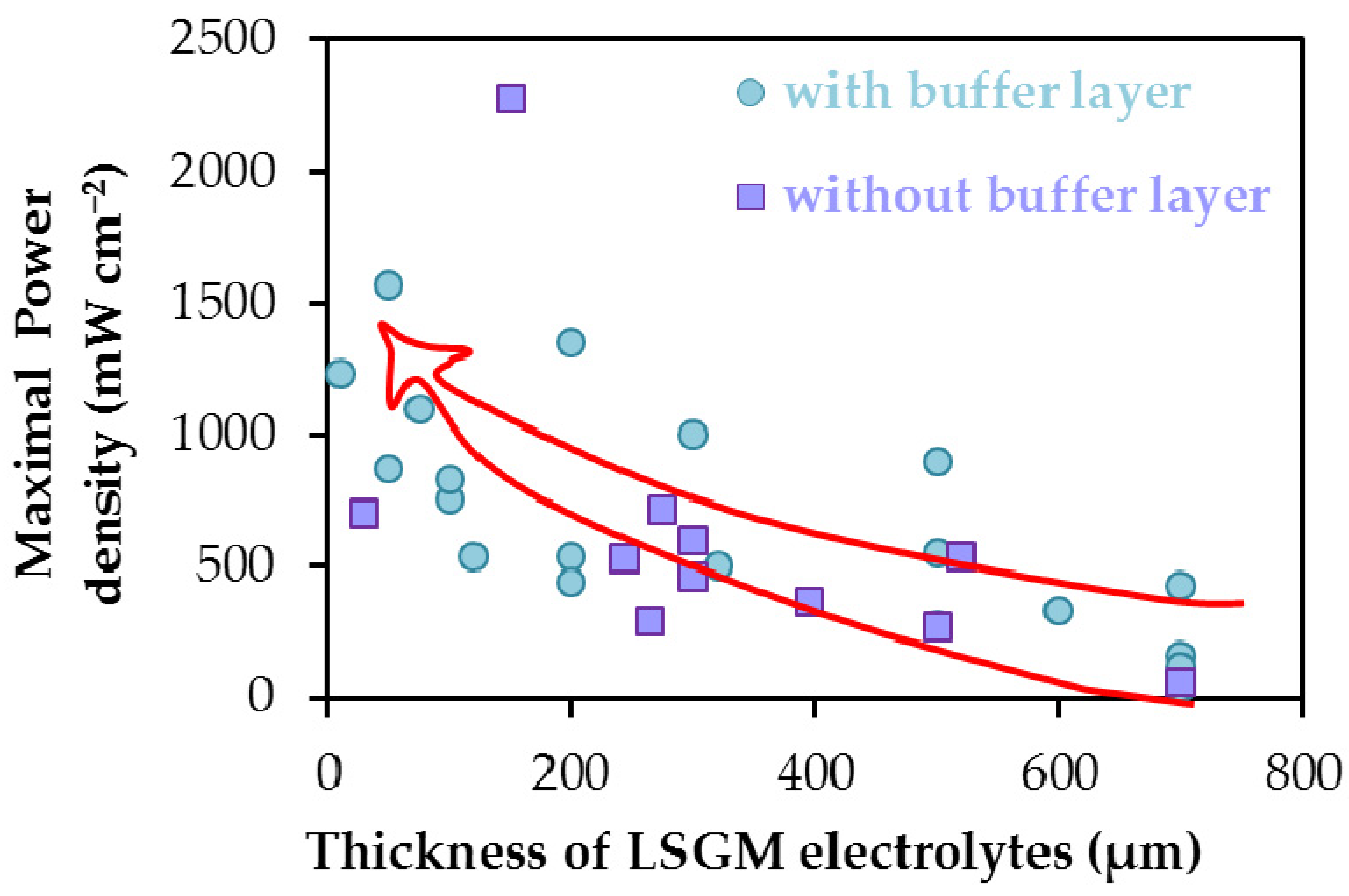
| Anode | Buffer Layer/ Electrolyte (Thickness, μm)/ Buffer Layer | Cathode | T (°C) | Power Density (mW cm−2) | Ref. |
|---|---|---|---|---|---|
| Ni-Ce0.8Sm0.2O2−δ | La0.8Sr0.2Ga0.83Mg0.17O3−δ (265) | La0.6Sr0.4O3−δ | 800 | 290 | [52] |
| Ni-La0.8Sr0.2Ga0.83Mg0.17O2.815 | La0.8Sr0.2Ga0.83Mg0.17O3−δ (395) | La0.6Sr0.4O3−δ | 800 | 363 | [52] |
| Ni-Ce0.8Sm0.2O2−δ | Ce0.8Sm0.2O2−δ/La0.9Sr0.1Ga0.8Mg0.2O3−δ (300) | La0.4Sr0.6Co0.9Sb0.1O3−δ- Ce0.8Sm0.2O2−δ | 700 | 432 | [144] |
| Ni-Ce0.8Sm0.2O2−δ | Ce0.8Sm0.2O2−δ/La0.9Sr0.1Ga0.8Mg0.2O3−δ (100) | SrCo0.8Fe0.1Nb0.1O3−δ | 800 | 756 | [145] |
| Ni-Ce0.8Sm0.2O2−δ | Ce0.8Sm0.2O2−δ/La0.9Sr0.1Ga0.8Mg0.2O3−δ (100) | SrCo0.8Fe0.1Nb0.1O3−δ– Ce0.9Gd0.1O2−δ | 800 | 829 | [145] |
| Ni-Ce0.8Sm0.2O2−δ | La0.9Sr0.1Ga0.8Mg0.2O3−δ (300) | BaCo0.7Fe0.2Ta0.1O3−δ | 800 | 460 | [146] |
| Ni-Ce0.8Sm0.2O2−δ | Ce0.8Sm0.2O2−δ/La0.9Sr0.1Ga0.8Mg0.2O3−δ (300) | Pr2Ni0.85Cu0.1Al0.05O4+δ | 700 | 392 | [149] |
| Ni-Ce0.8Sm0.2O2−δ | La0.8Sr0.2Ga0.83Mg0.17O3−δ (500) | La0.6Sr0.4O3−δ | 800 | 270 | [155,173] |
| Ni-Ce0.8Sm0.2O2−δ | Ce0.8Sm0.2O2−δ/La0.8Sr0.2Ga0.83Mg0.17O3−δ (500) | La0.6Sr0.4O3−δ | 800 | 550 | [155,173] |
| Ni-Ce0.8Sm0.2O2−δ | La0.87Sr0.13Ga0.85Mg0.15O3−δ (3.8) | La0.87Sr0.13Ga0.85Mg0.15O3−δ- La0.6Sr0.4Fe0.8Co0.2O3−δ | 750 | 1420 | [174] |
| Ni-Ce0.8Y0.2O2−δ | La0.9Sr0.1Ga0.8Mg0.2O3−δ (45) | La0.6Sr0.4O3−δ | 700 | 500 | [175] |
| Ni-Ce0.6La0.4O2−δ | La0.8Sr0.2Ga0.8Mg0.2O3−δ (30) | La0.8Sr0.2Fe0.8Co0.2O3−δ | 700 | 780 | [176] |
| Ni-Ce0.6La0.4O2−δ | Ce0.6La0.4O2−δ/La0.8Sr0.2Ga0.83Mg0.17O3−δ (500) | SrCo0.8Fe0.2O3−δ | 800 | 900 | [177] |
| Ni-Ce0.9Gd0.1O2−δ | Ce0.55La0.45O2−δ/La0.9Sr0.1Ga0.8Mg0.2O3−δ (75) | La0.9Sr0.1O3−δ- Ce0.55La0.45O2−δ | 800 | 1100 | [178] |
| Ni-Ce0.9Gd0.1O2−δ | Ce0.55La0.45O2−δ/La0.9Sr0.1Ga0.8Mg0.2O3−δ (50) | La0.6Sr0.4O3−δ | 800 | 1565 | [179] |
| Ni-Ce0.9Gd0.1O2−δ | Ce0.55La0.45O2−δ/La0.9Sr0.1Ga0.8Mg0.2O3−δ (50)/Ce0.55La0.45O2−δ | La0.6Sr0.4O3−δ | 800 | 871 | [179] |
| Ni-Ce0.8Gd0.2O2−δ | Ce0.8Gd0.2O2−δ/La0.9Sr0.1Ga0.8Mg0.2O3−δ (75) | Ba0.5Sr0.5Co0.8Fe0.2O3−δ | 700 | 760 | [180] |
| Ni-Fe | Ce0.8Sm0.2O2−δ/La0.9Sr0.1Ga0.8Mg0.2O3−δ (6) | Sm0.5Sr0.5O3−δ | 700 | 1790 | [181] |
| Ni-Ce0.6La0.4O2−δ | Ce0.6La0.4O2−δ/La0.9Sr0.1Ga0.8Mg0.2O3−δ (9)/Ce0.6La0.4O1.8 | La0.9Sr0.1Ga0.8Mg0.2O3−δ- La0.6Sr0.4Fe0.8Co0.2O3−δ | 700 | 910 | [182] |
| Ni-Ce0.8Sm0.2O2−δ | Ce0.6La0.4O2−δ/La0.9Sr0.1Ga0.8Mg0.2O3−δ (11)/Ce0.6La0.4O1.8 | La0.9Sr0.1Ga0.8Mg0.2O3−δ- La0.6Sr0.4Fe0.8Co0.2O3−δ | 800 | 1230 | [183] |
| Ni-Ce0.8Gd0.2O2−δ | Ce0.8Gd0.2O2−δ/(La0.9Sr0.1)0.97Ga0.9Mg0.1O3−δ (120) | La0.6Sr0.4Fe0.8Co0.2O3−δ | 800 | 540 | [170] |
| Ni-Ce0.8Sm0.2O2−δ | La0.9Sr0.1Ga0.8Mg0.2O3−δ (3.4) | La0.9Sr0.1Ga0.8Mg0.2O3−δ- La0.6Sr0.4Fe0.8Co0.2O3−δ | 750 | 736 | [184] |
| Ni-Ce0.8Gd0.2O2−δ | La0.8Sr0.2Ga0.8Mg0.2O3−δ (50) | La0.6Sr0.4Fe0.8Co0.2O3−δ | 700 | 831 | [185] |
| Ni-Fe | Ce0.6La0.4O2−δ/La0.9Sr0.1Ga0.8Mg0.2O3−δ (200) | Sm0.5Sr0.5O3−δ | 800 | 1350 | [171] |
| Pd-Sr2TiMoO6−δ | Ce0.8Sm0.2O2−δ/La0.9Sr0.1Ga0.8Mg0.2O3−δ (300) | NdBaCo0.67Fe0.67Cu0.67O5+δ | 850 | 1009 | [160] |
| Sr2NiMoO6−δ | La0.88Sr0.12Ga0.82Mg0.18O3−δ (700)/Ce0.8Sm0.2O2−δ | La0.7Sr0.3Fe0.9Co0.1O3−δ | 800 | 61 | [163] |
| Sr2NiMoO6−δ | La0.9Sr0.1Ga0.8Mg0.2O3−δ (300) | Ba0.5Sr0.5Co0.8Fe0.2O3−δ | 800 | 595 | [186] |
| Sr2MgMoO6−δ | Ce0.8Sm0.2O2−δ/La0.8Sr0.2Ga0.8Mg0.2O3−δ (700) | SmBaCo2O5+δ | 800 | 39 | [167] |
| Sr2MgMoO6−δ | Ce0.8Gd0.2O2−δ/La0.8Sr0.2Ga0.8Mg0.2O3−δ (600) | La0.6Sr0.4Fe0.8Co0.2O3−δ | 800 | 330 | [168] |
| Sr2Ni0.75Mg0.25MoO6−δ | La0.88Sr0.12Ga0.82Mg0.18O3−δ (700)/Ce0.8Sm0.2O2−δ | La0.7Sr0.3Fe0.9Co0.1O3−δ | 800 | 429 | [163] |
| Sr2Ni0.75Mg0.25MoO6−δ | La0.88Sr0.12Ga0.82Mg0.18O3−δ (500)/Ce0.8Sm0.2O2−δ | La2NiO4+δ | 800 | 276 | [187] |
| Sr2Ni0.75Mg0.25MoO6−δ | La0.88Sr0.12Ga0.82Mg0.18O3−δ (500)/Ce0.8Sm0.2O2−δ | La1.5Ca0.5Ni0.67Fe0.33O4+δ | 800 | 273 | [187] |
| Sr2Ni0.7Mg0.3MoO6−δ | Ce0.8Sm0.2O2−δ/La0.8Sr0.2Ga0.8Mg0.2O3−δ (700) | SmBaCo2O5+δ | 800 | 160 | [167] |
| Sr2Ni0.3Mg0.7MoO6−δ | Ce0.8Sm0.2O2−δ/La0.8Sr0.2Ga0.8Mg0.2O3−δ (700) | SmBaCo2O5+δ | 800 | 119 | [167] |
| Ba0.5Sr0.5Mo0.1Fe0.9O3−δ | La0.8Sr0.2Ga0.8Mg0.2O3−δ (150) | Ba0.5Sr0.5Mo0.1Fe0.9O3−δ | 800 | 2280 | [188] |
| SrFe0.75Mo0.25O3−δ | La0.9Sr0.1Ga0.8Mg0.2O3−δ (30) | SrFe0.75Mo0.25O3−δ | 800 | 703 | [189] |
| PrBa(Fe0.8Sc0.2)2O5+δ | La0.9Sr0.1Ga0.8Mg0.2O3−δ (275) | PrBa(Fe0.8Sc0.2)2O5+δ | 800 | 713 | [190] |
| Sr2Fe1.5Mo0.5O6−δ- La0.9Sr0.1Ga0.8Mg0.2O2.85 | La0.9Sr0.1Ga0.8Mg0.2O3−δ (10) | Sr2Fe1.5Mo0.5O6−δ- La0.9Sr0.1Ga0.8Mg0.2O3−δ | 700 | 880 | [191] |
| Pr0.6Sr0.4Fe0.8Ni0.2O3−δ | Ce0.8Gd0.2O2−δ/La0.9Sr0.1Ga0.8Mg0.2O3−δ (320)/Ce0.8Gd0.2O1.9 | Pr0.6Sr0.4Fe0.8Ni0.2O3−δ | 800 | 500 | [192] |
| PrBaMn1.5Fe0.5O5+δ | La0.8Sr0.2Ga0.8Mg0.2O3−δ (520) | PrBaMn1.5Fe0.5O5+δ | 800 | 540 | [193] |
| La0.5Sr0.5Fe0.9Nb0.1O3−δ | La0.82Sr0.18Ga0.83Mg0.17O3−δ (300) | La0.5Sr0.5Fe0.9Nb0.1O3−δ | 750 | 630 | [194] |
| La0.54Sr0.36Co0.2Fe0.6Nb0.2O3−δ | Ce0.8Sm0.2O2−δ/La0.9Sr0.1Ga0.8Mg0.2O3−δ (200)/Ce0.8Sm0.2O1.9 | La0.54Sr0.36Co0.2Fe0.6Nb0.2O3−δ | 800 | 539 | [195] |
| Sr2TiFe0.9Mo0.1O6−δ | Ce0.8Sm0.2O2−δ/La0.9Sr0.1Ga0.8Mg0.2O3−δ (200)/Ce0.8Sm0.2O1.9 | Sr2TiFe0.9Mo0.1O6−δ | 800 | 444 | [196] |
| Sr2Fe1.4Nb0.1Mo0.5O6−δ | La0.8Sr0.2Ga0.83Mg0.17O3−δ (243) | Sr2Fe1.4Nb0.1Mo0.5O6−δ | 800 | 531 | [197] |
| Sr0.95Ti0.3Fe0.63Ni0.07O3−δ | Ce0.6La0.4O2−δ/La0.8Sr0.2Ga0.83Mg0.17O3−δ (300) | La0.6SSr0.4Co0.2Fe0.8O3−δ- Gd0.1Ce0.9O2−δ | 800 | 1000 | [172] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filonova, E.; Medvedev, D. Recent Progress in the Design, Characterisation and Application of LaAlO3- and LaGaO3-Based Solid Oxide Fuel Cell Electrolytes. Nanomaterials 2022, 12, 1991. https://doi.org/10.3390/nano12121991
Filonova E, Medvedev D. Recent Progress in the Design, Characterisation and Application of LaAlO3- and LaGaO3-Based Solid Oxide Fuel Cell Electrolytes. Nanomaterials. 2022; 12(12):1991. https://doi.org/10.3390/nano12121991
Chicago/Turabian StyleFilonova, Elena, and Dmitry Medvedev. 2022. "Recent Progress in the Design, Characterisation and Application of LaAlO3- and LaGaO3-Based Solid Oxide Fuel Cell Electrolytes" Nanomaterials 12, no. 12: 1991. https://doi.org/10.3390/nano12121991
APA StyleFilonova, E., & Medvedev, D. (2022). Recent Progress in the Design, Characterisation and Application of LaAlO3- and LaGaO3-Based Solid Oxide Fuel Cell Electrolytes. Nanomaterials, 12(12), 1991. https://doi.org/10.3390/nano12121991