Reversible Diels–Alder Addition to Fullerenes: A Study of Dimethylanthracene with H2@C60
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kroto, H. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Murata, M.; Murata, Y.; Komatsu, K. Surgery of fullerenes. Chem. Commun. 2008, 6083–6094. [Google Scholar] [CrossRef]
- Vougioukalakis, G.C.; Roubelakis, M.M.; Orfanopoulos, M. Open-cage fullerenes: Towards the construction of nanosized molecular containers. Chem. Soc. Rev. 2010, 39, 817–844. [Google Scholar] [CrossRef]
- Shi, L.; Gan, L. Open-cage fullerenes as tailor-made container for a single water molecule. J. Phys. Org. Chem. 2013, 26, 766–772. [Google Scholar] [CrossRef]
- Chen, C.P.; Lin, Y.W.; Horng, J.C.; Chuang, S.C. Open-Cage Fullerenes as n-Type Materials in Organic Photovoltaics: Relevance of Frontier Energy Levels, Carrier Mobility and Morphology of Different Sizable Open-Cage Fullerenes with Power Conversion Efficiency in Devices. Adv. Energy Mater. 2011, 1, 776–780. [Google Scholar] [CrossRef]
- Murata, M.; Morinaka, Y.; Murata, Y.; Yoshikawa, O.; Sagawa, T.; Yoshikawa, S. Modification of the σ-framework of [60] fullerene for bulk-heterojunction solar cells. Chem. Commun. 2011, 47, 7335–7337. [Google Scholar] [CrossRef][Green Version]
- Castro, E.; Artigas, A.; Pla-Quintana, A.; Roglans, A.; Liu, F.; Perez, F.; Lledó, A.; Zhu, X.-Y.; Echegoyen, L. Enhanced open-circuit voltage in perovskite solar cells with open-cage [60] fullerene derivatives as electron-transporting materials. Materials 2019, 12, 1314. [Google Scholar] [CrossRef]
- Komatsu, K.; Murata, M.; Murata, Y. Encapsulation of Molecular Hydrogen in Fullerene C60 by Organic Synthesis. Science 2005, 307, 238–240. [Google Scholar] [CrossRef]
- Kurotobi, K.; Murata, Y. A Single Molecule of Water Encapsulated in Fullerene C60. Science 2011, 333, 613–616. [Google Scholar] [CrossRef]
- Aoyagi, S.; Nishibori, E.; Sawa, H.; Sugimoto, K.; Takata, M.; Miyata, Y.; Kitaura, R.; Shinohara, H.; Okada, H.; Sakai, T. A layered ionic crystal of polar Li@C60 superatoms. Nat. Chem. 2010, 2, 678–683. [Google Scholar] [CrossRef]
- Nikawa, H.; Araki, Y.; Slanina, Z.; Tsuchiya, T.; Akasaka, T.; Wada, T.; Ito, O.; Dinse, K.-P.; Ata, M.; Kato, T. The effect of atomic nitrogen on the C60 cage. Chem. Commun. 2010, 46, 631–633. [Google Scholar] [CrossRef]
- Liu, G.; Gimenez-Lopez, M.d.C.; Jevric, M.; Khlobystov, A.N.; Briggs, G.A.D.; Porfyrakis, K. Alignment of N@C60 Derivatives in a Liquid Crystal Matrix. J. Phys. Chem. B 2013, 117, 5925–5931. [Google Scholar] [CrossRef]
- Krachmalnicoff, A.; Bounds, R.; Mamone, S.; Alom, S.; Concistrè, M.; Meier, B.; Kouřil, K.; Light, M.E.; Johnson, M.R.; Rols, S. The dipolar endofullerene hf@C60. Nat. Chem. 2016, 8, 953–957. [Google Scholar] [CrossRef]
- Levitt, M.H. Spectroscopy of light-molecule endofullerenes. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20120429. [Google Scholar] [CrossRef]
- Horsewill, A.; Goh, K.; Rols, S.; Ollivier, J.; Johnson, M.; Levitt, M.; Carravetta, M.; Mamone, S.; Murata, Y.; Chen, J.-C. Potential energy and dipole moment surfaces for HF@C60: Prediction of spectral and electric response properties. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20110627. [Google Scholar] [CrossRef]
- Horsewill, A.; Panesar, K.; Rols, S.; Johnson, M.; Murata, Y.; Komatsu, K.; Mamone, S.; Danquigny, A.; Cuda, F.; Maltsev, S. Quantum translator-rotator: Inelastic neutron scattering of dihydrogen molecules trapped inside anisotropic fullerene cages. Phys. Rev. Lett. 2009, 102, 013001. [Google Scholar] [CrossRef]
- Beduz, C.; Carravetta, M.; Chen, J.Y.-C.; Concistrè, M.; Denning, M.; Frunzi, M.; Horsewill, A.J.; Johannessen, O.G.; Lawler, R.; Lei, X. Quantum rotation of ortho and para-water encapsulated in a fullerene cage. Proc. Natl. Acad. Sci. USA 2012, 109, 12894–12898. [Google Scholar] [CrossRef]
- Mamone, S.; Ge, M.; Hüvonen, D.; Nagel, U.; Danquigny, A.; Cuda, F.; Grossel, M.; Murata, Y.; Komatsu, K.; Levitt, M. Rotor in a cage: Infrared spectroscopy of an endohedral hydrogen-fullerene complex. J. Chem. Phys. 2009, 130, 081103. [Google Scholar] [CrossRef]
- Ge, M.; Nagel, U.; Hüvonen, D.; Rõõm, T.; Mamone, S.; Levitt, M.; Carravetta, M.; Murata, Y.; Komatsu, K.; Lei, X. Infrared spectroscopy of endohedral HD and D2 in C60. J. Chem. Phys. 2011, 135, 114511. [Google Scholar] [CrossRef]
- Rõõm, T.; Peedu, L.; Ge, M.; Hüvonen, D.; Nagel, U.; Ye, S.; Xu, M.; Bačić, Z.; Mamone, S.; Levitt, M. Infrared spectroscopy of small-molecule endofullerenes. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20110631. [Google Scholar]
- Vidal, S.; Izquierdo, M.; Alom, S.; Garcia-Borràs, M.; Filippone, S.; Osuna, S.; Solà, M.; Whitby, R.J.; Martín, N. Effect of incarcerated HF on the exohedral chemical reactivity of HF@C60. Chem. Commun. 2017, 53, 10993–10996. [Google Scholar] [CrossRef]
- Mamone, S.; Concistrè, M.; Carignani, E.; Meier, B.; Krachmalnicoff, A.; Johannessen, O.G.; Lei, X.; Li, Y.; Denning, M.; Carravetta, M. Nuclear spin conversion of water inside fullerene cages detected by low-temperature nuclear magnetic resonance. J. Chem. Phys. 2014, 140, 194306. [Google Scholar] [CrossRef]
- Frunzi, M.; Jockusch, S.; Chen, J.Y.-C.; Calderon, R.M.K.; Lei, X.; Murata, Y.; Komatsu, K.; Guldi, D.M.; Lawler, R.G.; Turro, N.J. A Photochemical On–Off Switch for Tuning the Equilibrium Mixture of H2 Nuclear Spin Isomers as a Function of Temperature. J. Am. Chem. Soc. 2011, 133, 14232–14235. [Google Scholar] [CrossRef]
- Li, Y.; Lei, X.; Jockusch, S.; Chen, J.Y.-C.; Frunzi, M.; Johnson, J.A.; Lawler, R.G.; Murata, Y.; Murata, M.; Komatsu, K. A magnetic switch for spin-catalyzed interconversion of nuclear spin isomers. J. Am. Chem. Soc. 2010, 132, 4042–4043. [Google Scholar] [CrossRef]
- Sato, S.; Maeda, Y.; Guo, J.-D.; Yamada, M.; Mizorogi, N.; Nagase, S.; Akasaka, T. Mechanistic Study of the Diels–Alder Reaction of Paramagnetic Endohedral Metallofullerene: Reaction of La@C82 with 1,2,3,4,5-Pentamethylcyclopentadiene. J. Am. Chem. Soc. 2013, 135, 5582–5587. [Google Scholar] [CrossRef]
- Cioslowski, J.; Fleischmann, E.D. Endohedral complexes: Atoms and ions inside the C60 cage. J. Chem. Phys. 1991, 94, 3730–3734. [Google Scholar] [CrossRef]
- Garcia-Borràs, M.; Osuna, S.; Luis, J.M.; Swart, M.; Solà, M. A Complete Guide on the Influence of Metal Clusters in the Diels–Alder Regioselectivity of Ih-C80 Endohedral Metallofullerenes. Chem.—A Eur. J. 2013, 19, 14931–14940. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, X.; Ehara, M. Regioselectivity of Sc2C2@C3v(8)-C82: Role of the Sumanene-Type Hexagon in Diels–Alder Reaction. J. Org. Chem. 2016, 81, 8169–8174. [Google Scholar] [CrossRef]
- Lamparth, I.; Maichle–Mössmer, C.; Hirsch, A. Reversible Template-Directed Activation of Equatorial Double Bonds of the Fullerene Framework: Regioselective Direct Synthesis, Crystal Structure, and Aromatic Properties of Th-C66(COOEt)12. Angew. Chem. Int. Ed. Engl. 1995, 34, 1607–1609. [Google Scholar] [CrossRef]
- Hirsch, A.; Vostrowsky, O. C60 Hexakisadducts with an Octahedral Addition Pattern − A New Structure Motif in Organic Chemistry. Eur. J. Org. Chem. 2001, 2001, 829–848. [Google Scholar] [CrossRef]
- Lamparth, I.; Herzog, A.; Hirsch, A. Synthesis of [60] fullerene derivatives with an octahedral addition pattern. Tetrahedron 1996, 52, 5065–5075. [Google Scholar] [CrossRef]
- Wang, G.-W.; Saunders, M.; Cross, R.J. Reversible Diels−Alder Addition to Fullerenes: A Study of Equilibria Using 3He NMR Spectroscopy. J. Am. Chem. Soc. 2001, 123, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Buehl, M.; Thiel, W.; Jiao, H.; Schleyer, P.v.R.; Saunders, M.; Anet, F.A. Helium and lithium NMR chemical shifts of endohedral fullerene compounds: An ab initio study. J. Am. Chem. Soc. 1994, 116, 6005–6006. [Google Scholar] [CrossRef]
- Elser, V.; Haddon, R. Icosahedral C60: An aromatic molecule with a vanishingly small ring current magnetic susceptibility. Nature 1987, 325, 792–794. [Google Scholar] [CrossRef]
- Pasquarello, A.; Schlüter, M.; Haddon, R. Ring Currents in Icosahedral C60. Science 1992, 257, 1660–1661. [Google Scholar] [CrossRef]
- Saunders, M.; Jiménez-Vázquez, H.A.; Cross, R.J.; Mroczkowski, S.; Freedberg, D.I.; Anet, F.A. Probing the interior of fullerenes by 3He NMR spectroscopy of endohedral 3He@ C60 and 3He@ C70. Nature 1994, 367, 256–258. [Google Scholar] [CrossRef]
- Smith, A.B., III; Strongin, R.M.; Brard, L.; Romanow, W.J.; Saunders, M.; Jimenez-Vazquez, H.A.; Cross, R.J. Synthesis and 3He NMR studies of C60 and C70 epoxide, cyclopropane, and annulene derivatives containing endohedral helium. J. Am. Chem. Soc. 1994, 116, 10831–10832. [Google Scholar] [CrossRef]
- Saunders, M.; Cross, R.J.; Jiménez-Vázquez, H.A.; Shimshi, R.; Khong, A. Noble gas atoms inside fullerenes. Science 1996, 271, 1693–1697. [Google Scholar] [CrossRef]
- Saunders, M.; Jimenez-Vazquez, H.A.; Bangerter, B.W.; Cross, R.J.; Mroczkowski, S.; Freedberg, D.I.; Anet, F.A. 3He NMR: A powerful new tool for following fullerene chemistry. J. Am. Chem. Soc. 1994, 116, 3621–3622. [Google Scholar] [CrossRef]
- Saunders, M.; Jimenez-Vazquez, H.A.; Cross, R.; Billups, W.; Gesenberg, C.; Gonzalez, A.; Luo, W.; Haddon, R.; Diederich, F.; Herrmann, A. Analysis of isomers of the higher fullerenes by 3He NMR spectroscopy. J. Am. Chem. Soc. 1995, 117, 9305–9308. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, P.; Cai, W.; Bao, L.; Chen, M.; Xie, Y.; Zhao, X.; Lu, X. Stabilization of Giant Fullerenes C2(41)-C90, D3(85)-C92, C1(132)-C94, C2(157)-C96, and C1(175)-C98 by Encapsulation of a Large La2C2 Cluster: The Importance of Cluster–Cage Matching. J. Am. Chem. Soc. 2017, 139, 4724–4728. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Wang, S.; Lu, X.; Tan, Y.; Huang, J.; Liu, F.; Li, Q.; Xie, S.; Yang, S. Entrapping a Group-VB Transition Metal, Vanadium, within an Endohedral Metallofullerene: VxSc3–xN@Ih-C80 (x = 1, 2). J. Am. Chem. Soc. 2016, 138, 207–214. [Google Scholar] [CrossRef]
- Frunzi, M.; Xu, H.; Cross, R.J.; Saunders, M. NMR Temperature-Jump Method for Measuring Reaction Rates: Reaction of Dimethylanthracene with H2@C60. J. Phys. Chem. A 2009, 113, 4996–4999. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, K. Classic Carbon Nanostructures. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20110636. [Google Scholar] [CrossRef][Green Version]
- Krachmalnicoff, A.; Levitt, M.H.; Whitby, R.J. An optimised scalable synthesis of H2O@C60 and a new synthesis of H2@C60. Chem. Commun. 2014, 50, 13037–13040. [Google Scholar] [CrossRef]
- Cross, R.; Khong, A.; Saunders, M. Using Cyanide To Put Noble Gases inside C60. J. Org. Chem. 2003, 68, 8281–8283. [Google Scholar] [CrossRef]
- Rubin, Y.; Jarrosson, T.; Wang, G.W.; Bartberger, M.D.; Houk, K.; Schick, G.; Saunders, M.; Cross, R.J. Insertion of helium and molecular hydrogen through the orifice of an open fullerene. Angew. Chem. Int. Ed. 2001, 40, 1543–1546. [Google Scholar] [CrossRef]
- Yao, J.; Xiao, Z.; Zhang, J.; Yang, X.; Gan, L.; Zhang, W.-X. Switched role of fullerene in the Diels–Alder reaction: Facile addition of dienophiles to the conjugated fullerene diene moiety. Chem. Commun. 2008. [Google Scholar] [CrossRef]
- Kadish, K.M.; Ruoff, R.S. Fullerenes: Chemistry, Physics, and Technology; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Filippone, S.; Maroto, E.E.; Martín-Domenech, Á.; Suarez, M.; Martín, N. An efficient approach to chiral fullerene derivatives by catalytic enantioselective 1,3-dipolar cycloadditions. Nat. Chem. 2009, 1, 578–582. [Google Scholar] [CrossRef]
- Maroto, E.E.; de Cózar, A.; Filippone, S.; Martín-Domenech, Á.; Suarez, M.; Cossío, F.P.; Martín, N. Hierarchical Selectivity in Fullerenes: Site-, Regio-, Diastereo-, and Enantiocontrol of the 1,3-Dipolar Cycloaddition to C70. Angew. Chem. Int. Ed. 2011, 50, 6060–6064. [Google Scholar] [CrossRef] [PubMed]
- Maroto, E.E.; Filippone, S.; Martín-Domenech, A.; Suarez, M.; Martín, N. Switching the stereoselectivity:(Fullero) pyrrolidines “a la carte”. J. Am. Chem. Soc. 2012, 134, 12936–12938. [Google Scholar] [CrossRef] [PubMed]

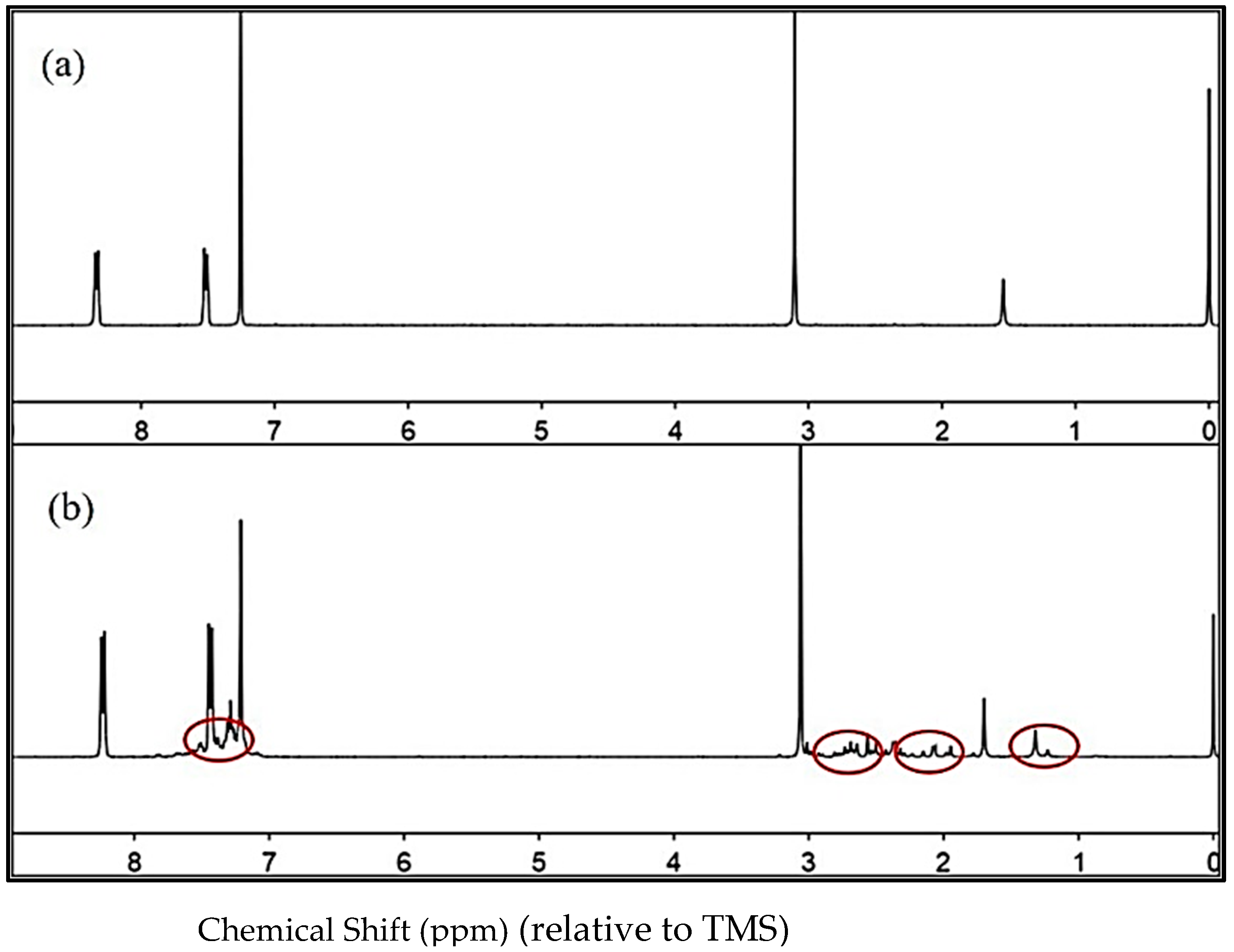
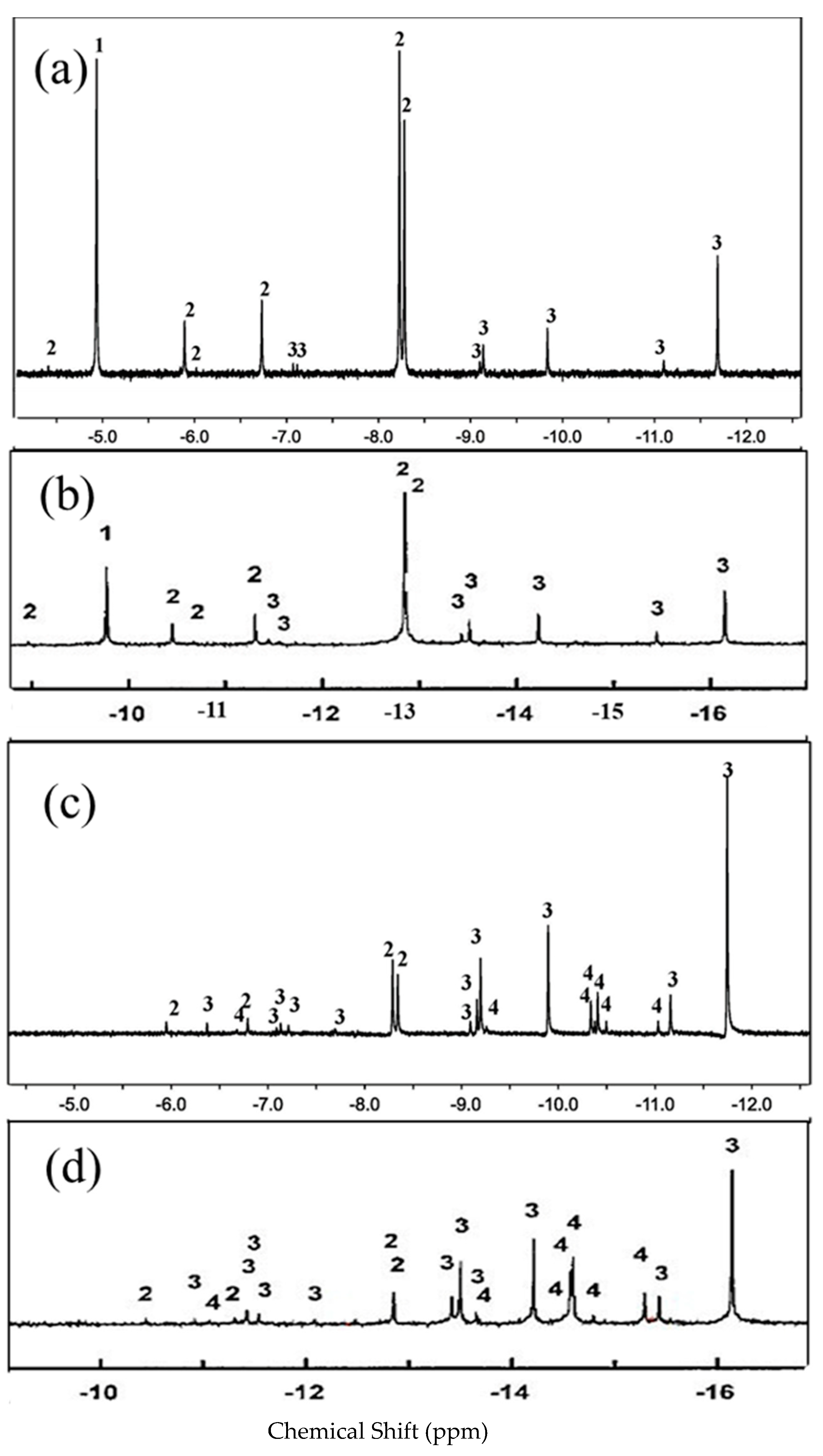
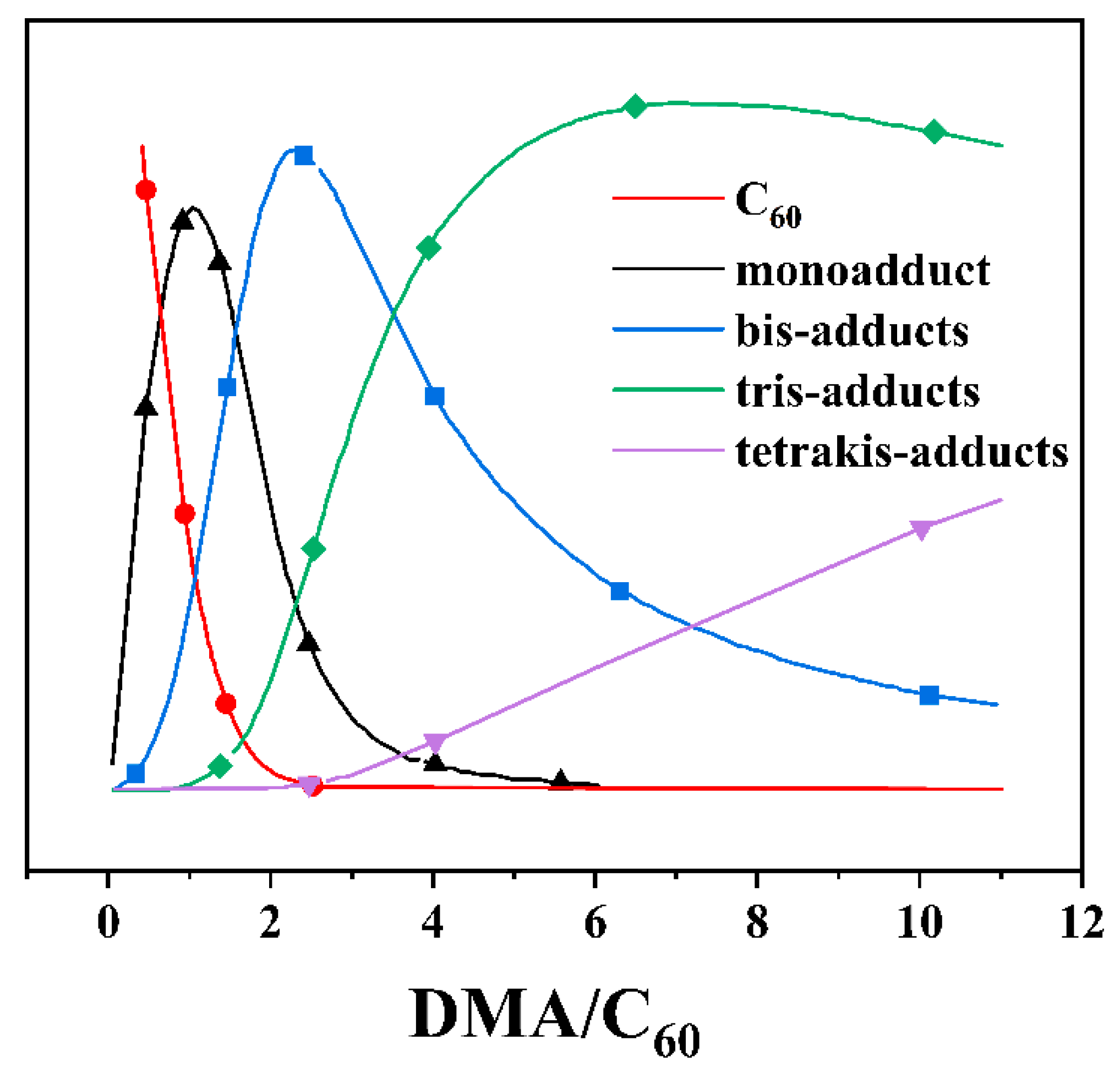
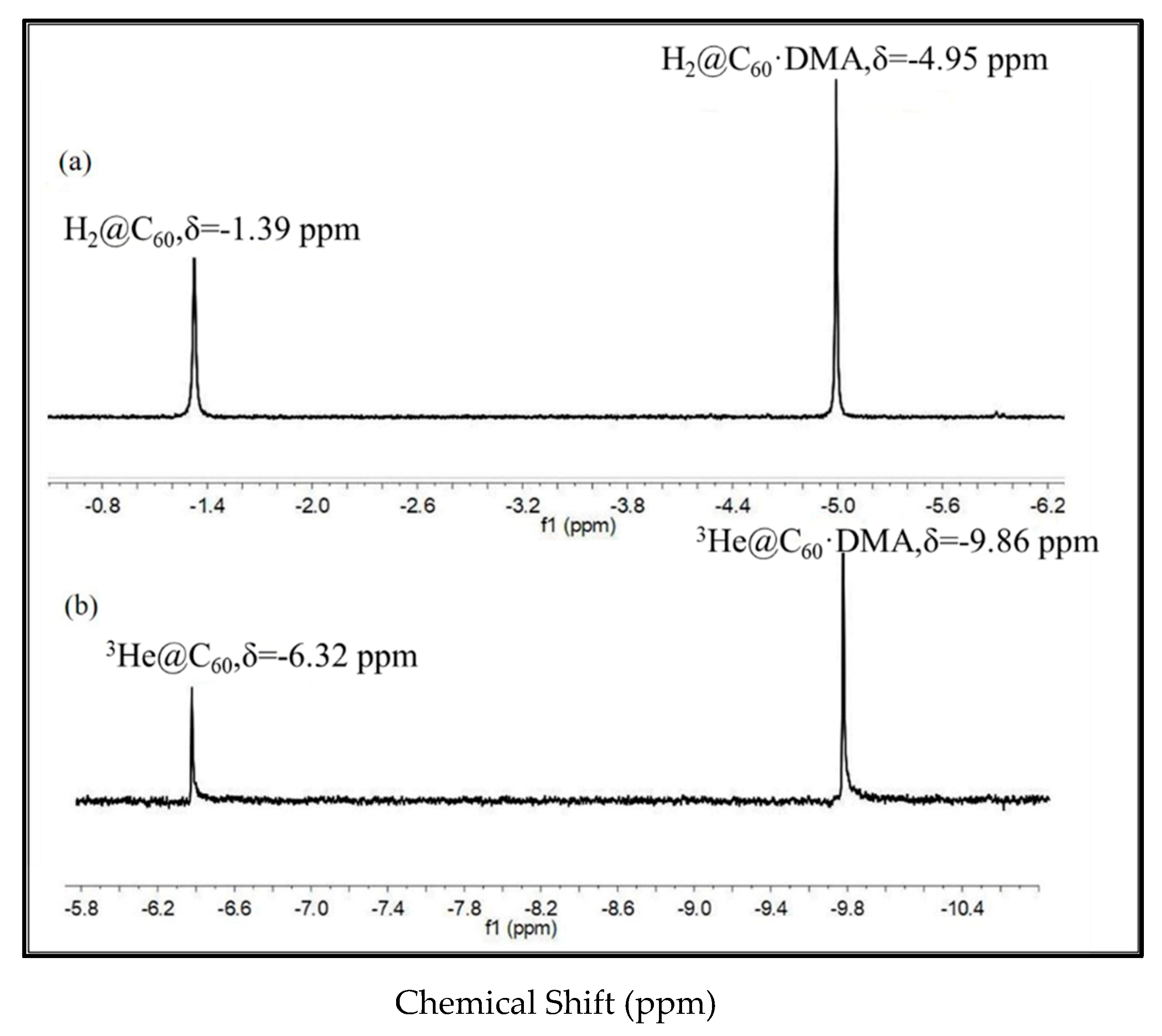
| δ a (ppm) | f b | δ a (ppm) | f b | ||||
|---|---|---|---|---|---|---|---|
| Mono | Tetrakis | ||||||
| 1 | −4.954 | 1.000 | 1 | −6.667 | 0.008 | ||
| Bis | 2 | −8.092 | 0.009 | ||||
| 1 | −4.591 | 0.012 | 3 | −9.397 | 0.031 | ||
| 2 | −5.896 | 0.073 | 4 5 | −10.041 −9.531 | 0.047 0.031 | ||
| 3 | −5.941 | 0.008 | 6 | −10.321 | 0.282 | ||
| 4 | −6.779 | 0.092 | 7 | −10.391 | 0.354 | ||
| 5 | −8.276 | 0.435 | 8 | −10.482 | 0.042 | ||
| 6 | −8.329 | 0.380 | 9 | −11.013 | 0.166 | ||
| Tris | 10 | −11.145 | 0.030 | ||||
| 1 | −6.073 | 0.005 | |||||
| 2 | −7.076 | 0.008 | |||||
| 3 | −7.120 | 0.024 | |||||
| 4 | −7.198 | 0.042 | |||||
| 5 | −8.618 | 0.053 | |||||
| 6 | −9.142 | 0.009 | |||||
| 7 | −9.182 | 0.162 | |||||
| 8 | −9.249 | 0.018 | |||||
| 9 | −9.881 | 0.217 | |||||
| 10 | −11.142 | 0.054 | |||||
| 11 | −11.733 | 0.408 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subhani, M.; Zhou, J.; Sui, Y.; Zou, H.; Frunzi, M.; Cross, J.; Saunders, M.; Shuai, C.; Liang, W.; Xu, H. Reversible Diels–Alder Addition to Fullerenes: A Study of Dimethylanthracene with H2@C60. Nanomaterials 2022, 12, 1667. https://doi.org/10.3390/nano12101667
Subhani M, Zhou J, Sui Y, Zou H, Frunzi M, Cross J, Saunders M, Shuai C, Liang W, Xu H. Reversible Diels–Alder Addition to Fullerenes: A Study of Dimethylanthracene with H2@C60. Nanomaterials. 2022; 12(10):1667. https://doi.org/10.3390/nano12101667
Chicago/Turabian StyleSubhani, Mahboob, Jinrong Zhou, Yuguang Sui, Huijing Zou, Michael Frunzi, James Cross, Martin Saunders, Cijun Shuai, Wenjie Liang, and Hai Xu. 2022. "Reversible Diels–Alder Addition to Fullerenes: A Study of Dimethylanthracene with H2@C60" Nanomaterials 12, no. 10: 1667. https://doi.org/10.3390/nano12101667
APA StyleSubhani, M., Zhou, J., Sui, Y., Zou, H., Frunzi, M., Cross, J., Saunders, M., Shuai, C., Liang, W., & Xu, H. (2022). Reversible Diels–Alder Addition to Fullerenes: A Study of Dimethylanthracene with H2@C60. Nanomaterials, 12(10), 1667. https://doi.org/10.3390/nano12101667







