Enhanced Photocatalytic Activity of Zn-Al Layered Double Hydroxides for Methyl Violet and Peat Water Photooxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Zn-Al LDHs
2.3. Characterization of Zn-Al LDHs
2.4. Photocatalytic Activity and Adsorption Study of Zn-Al LDHs
3. Results
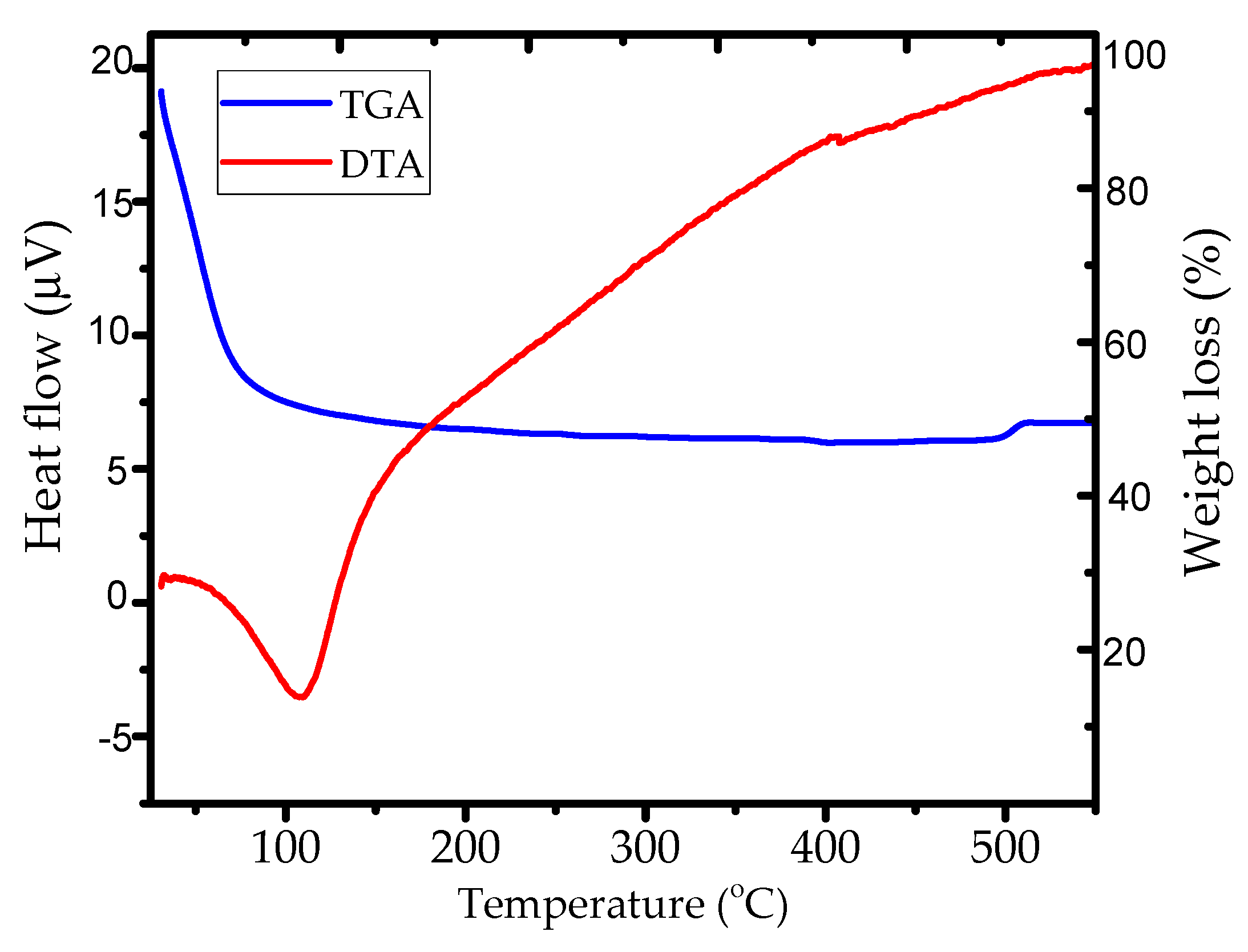
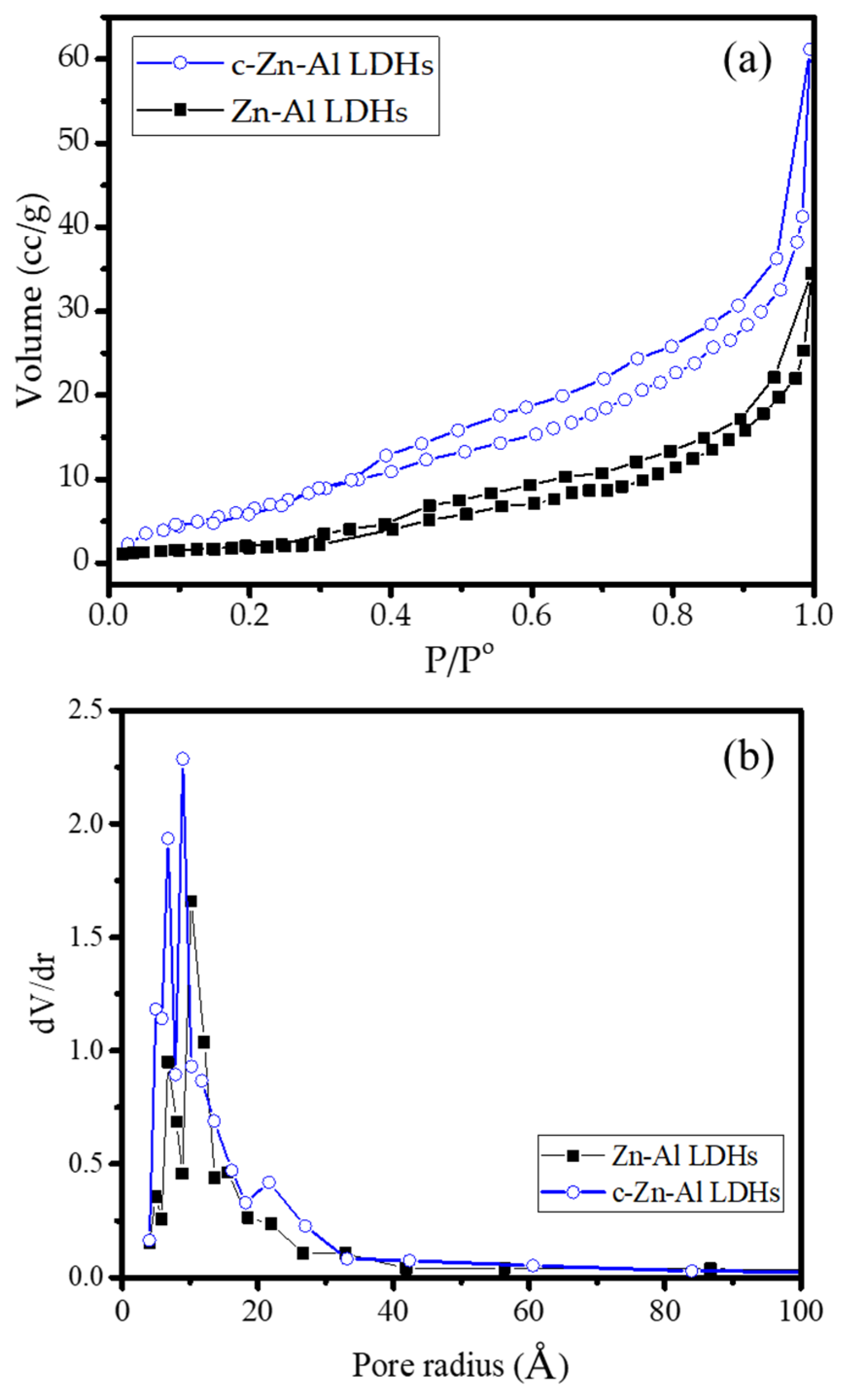
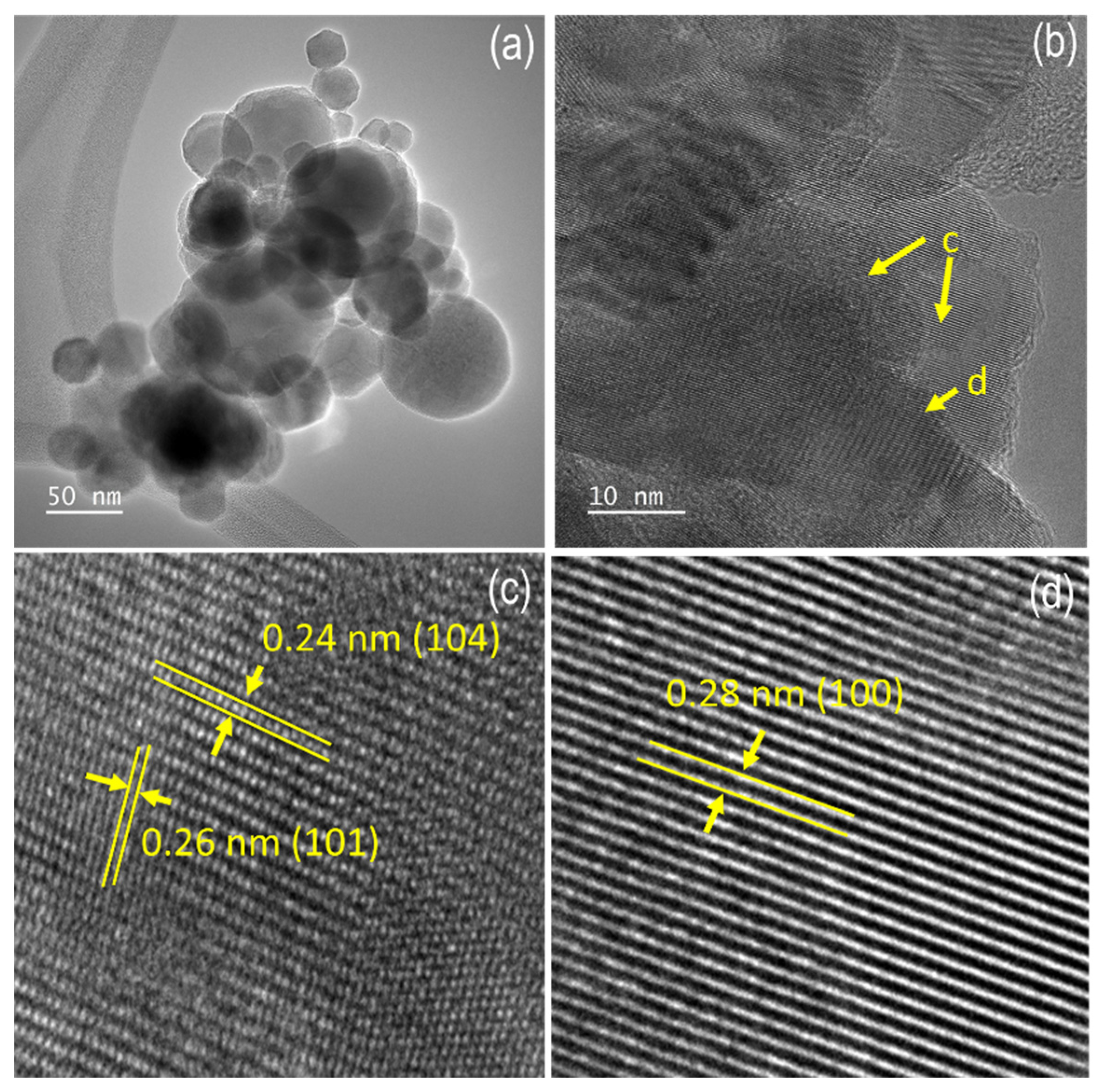
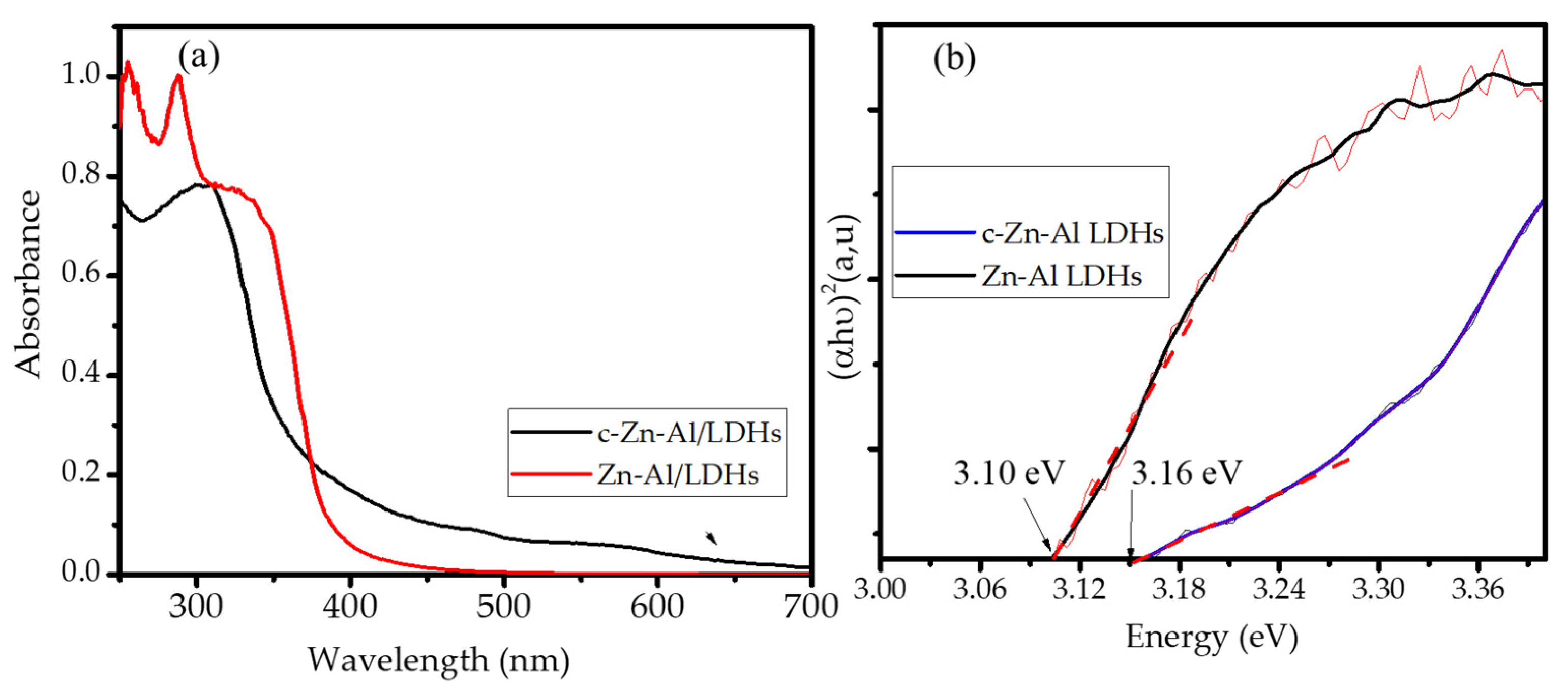
3.1. Materials Characterization
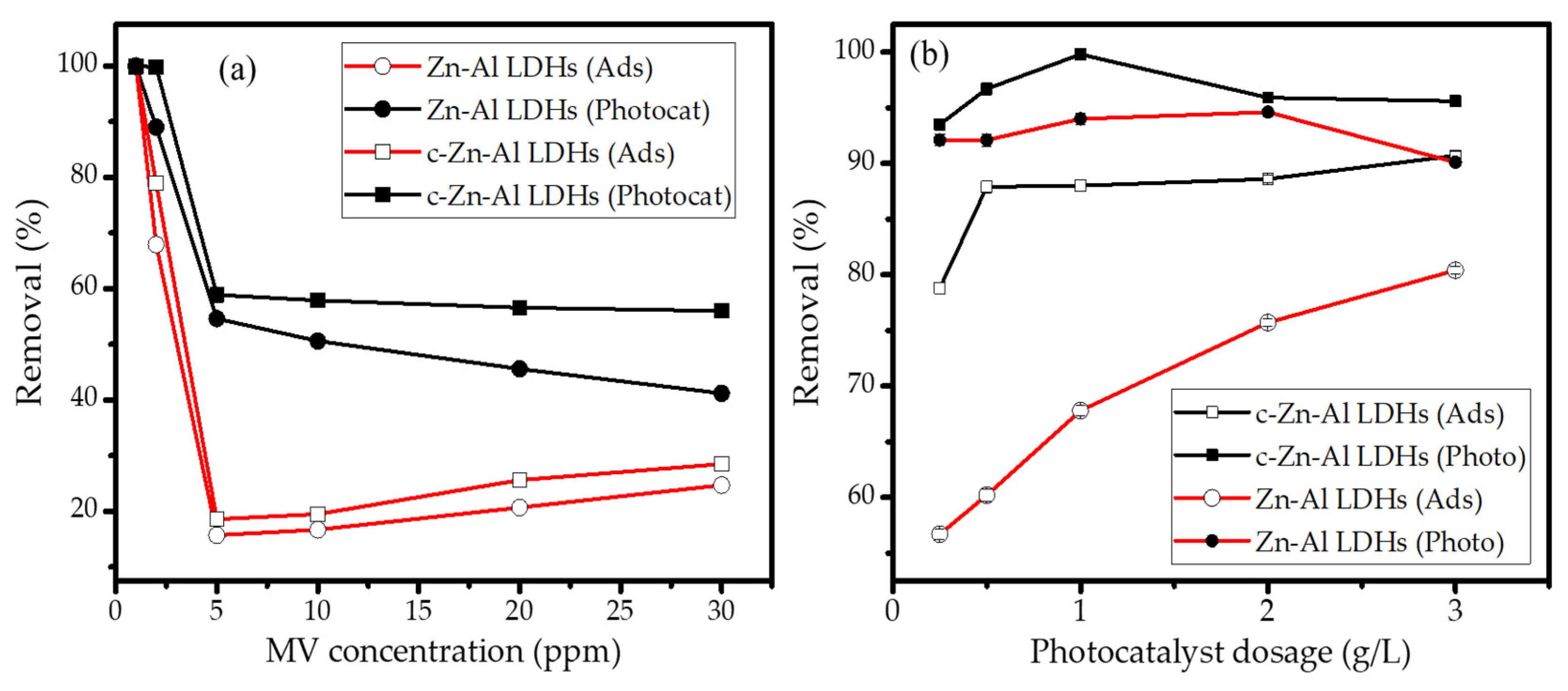
3.2. Adsorption and Photocatalytic Studies
3.3. Photocatalyst Reusability
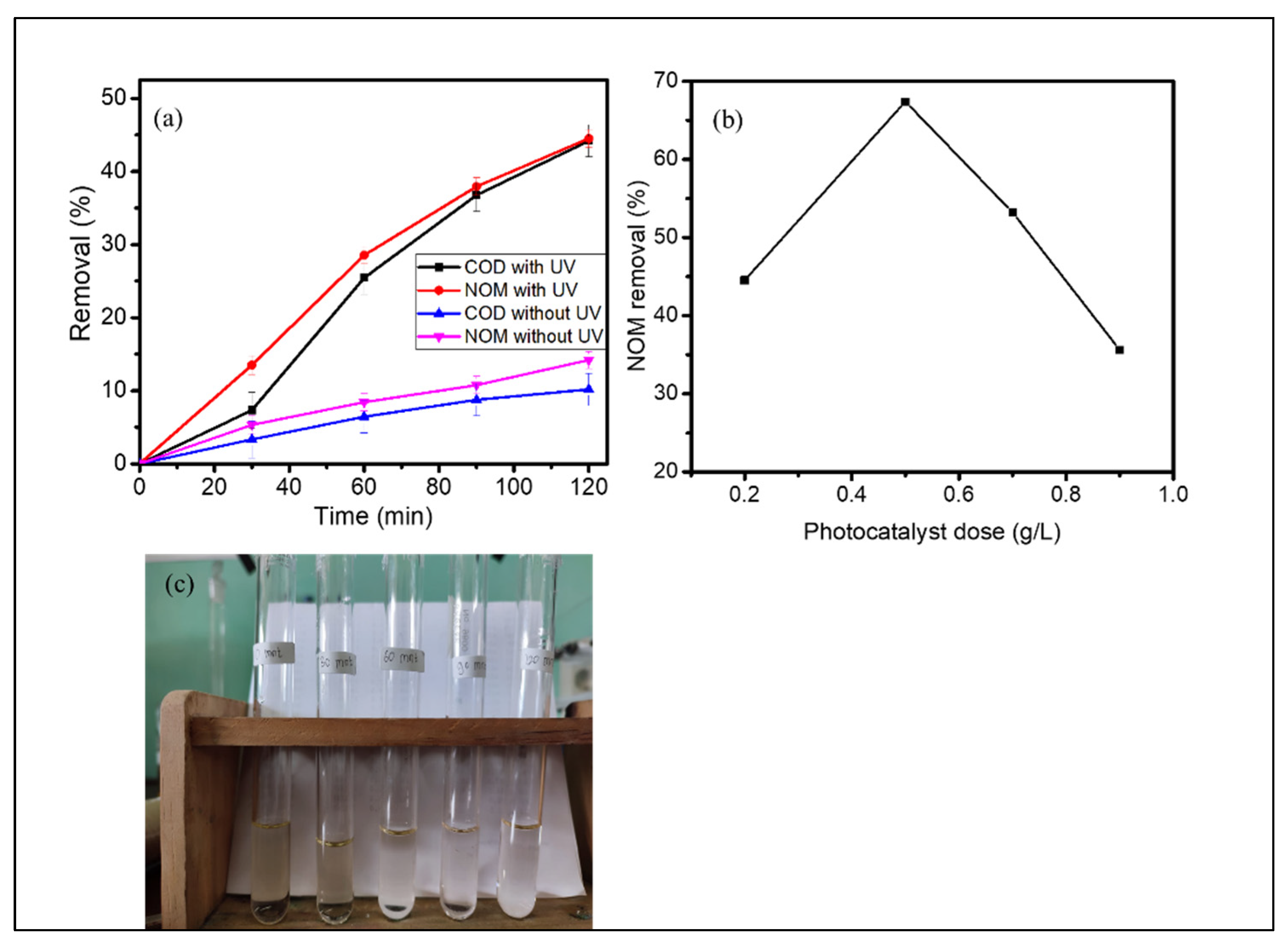
3.4. Photocatalytic Treatment of Peat Water
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hassaan, M.A.; El Nemr, A.; Madkour, F.F. Advanced oxidation processes of Mordant Violet 40 dye in freshwater and seawater. Egypt. J. Aquat. Res. 2017, 43, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hooijer, A.; Page, S.; Jauhiainen, J.; Lee, W.A.; Lu, X.X.; Idris, A.; Anshari, G. Subsidence and carbon loss in drained tropical peatlands. Biogeosciences 2012, 9, 1053–1071. [Google Scholar] [CrossRef] [Green Version]
- Elma, M.; Pratiwi, A.E.; Rahma, A.; Rampun, E.L.A.; Mahmud, M.; Abdi, C.; Rosadi, R.; Yanto, D.H.Y.; Bilad, M.R. Combination of coagulation, adsorption, and ultrafiltration processes for organic matter removal from peat water. Sustainability 2022, 14, 370. [Google Scholar] [CrossRef]
- Lin, C.H.; Chu, H.L.; Hwang, W.S.; Wang, M.C.; Ko, H.H. Synthesis and optical properties of Mg-Al layered double hydroxides precursor powders. AIP Adv. 2017, 7, 125005. [Google Scholar] [CrossRef] [Green Version]
- Amor, F.; Diouri, A.; Ellouzi, I.; Ouanji, F.; Kacimi, M. High efficient photocatalytic activity of Zn-Al-Ti layered double hydroxides nanocomposite. MATEC Web Conf. 2018, 149, 2017–2019. [Google Scholar] [CrossRef]
- Zhang, Z.; Hua, Z.; Lang, J.; Song, Y.; Zhang, Q.; Han, Q.; Fan, H.; Gao, M.; Li, X.; Yang, J. Eco-friendly nanostructured Zn-Al layered double hydroxide photocatalysts with enhanced photocatalytic activity. CrystEngComm 2019, 21, 4607–4619. [Google Scholar] [CrossRef]
- Starukh, G. Photocatalytically Enhanced Cationic Dye Removal with Zn-Al Layered Double Hydroxides. Nanoscale Res. Lett. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Li, A.; Deng, H.; Ye, C.; Jiang, Y. Fabrication and chatacterization of novel ZnAl-Layered Double Hydroxide for the superadsorption of organic contaminants from wastewater. ACS Omega 2020, 5, 15152–15161. [Google Scholar] [CrossRef]
- Omdeo, K.G.; Ajay, V.R.; Krishnan, K.; Abitha, V.K.; Nikesh, S.; Sabu, T.; Satyendra, M. Surface modification of synthesized Layered Double Hydroxide [LDH] for methylene blue dye removal in textile industry via photocatalytic activity under visible light. J. Nano Res. 2017, 46, 135–147. [Google Scholar] [CrossRef]
- EL Mersly, L.; El Mouchtari, E.M.; Moujahid, E.M.; Forano, C.; El Haddad, M.; Briche, S.; Alaoui Tahiri, A.; Rafqah, S. ZnCr-LDHs with dual adsorption and photocatalysis capability for the removal of acid orange 7 dye in aqueous solution. J. Sci. Adv. Mater. Devices 2021, 6, 118–126. [Google Scholar] [CrossRef]
- Abderrazek, K.; Najoua, F.S.; Srasra, E. Synthesis and characterization of [Zn-Al] LDH: Study of the effect of calcination on the photocatalytic activity. Appl. Clay Sci. 2016, 119, 229–235. [Google Scholar] [CrossRef]
- Elhalil, A.; Farnane, M.; Machrouhi, A.; Mahjoubi, F.Z.; Elmoubarki, R.; Tounsadi, H.; Abdennouri, M.; Barka, N. Effects of molar ratio and calcination temperature on the adsorption performance of Zn/Al layered double hydroxide nanoparticles in the removal of pharmaceutical pollutants. J. Sci. Adv. Mater. Devices 2018, 3, 188–195. [Google Scholar] [CrossRef]
- Świetlik, J.; Sikorska, E. Characterization of natural organic matter fractions by high pressure size-exclusion chromatography, specific UV absorbance and total luminescence spectroscopy. Polish J. Environ. Stud. 2006, 15, 145–153. [Google Scholar]
- Liao, L.; Zhao, N.; Xia, Z. Hydrothermal synthesis of Mg–Al layered double hydroxides (LDHs) from natural brucite and Al(OH)3. Mater. Res. Bull. 2012, 47, 3897–3901. [Google Scholar] [CrossRef]
- Mészáros, S.; Halász, J.; Kónya, Z.; Sipos, P.; Pálinkó, I. Reconstruction of calcined MgAl- and NiMgAl-layered double hydroxides during glycerol dehydration and their recycling characteristics. Appl. Clay Sci. 2013, 80–81, 245–248. [Google Scholar] [CrossRef]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva, C. Multi-phase semicrystalline microstructures drive exciton dissociation in neat plastic semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef] [Green Version]
- Nocchetti, M.; Pica, M.; Ridolfi, B.; Donnadio, A.; Boccalon, E.; Zampini, G.; Pietrella, D.; Casciola, M. AgCl-ZnAl Layered Double Hydroxides as Catalysts with Enhanced Photodegradation and Antibacterial Activities. Inorganics 2019, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- George, G.; Saravanakumar, M.P. Facile synthesis of carbon-coated layered double hydroxide and its comparative characterisation with Zn–Al LDH: Application on crystal violet and malachite green dye adsorption—isotherm, kinetics and Box-Behnken design. Environ. Sci. Pollut. Res. 2018, 25, 30236–30254. [Google Scholar] [CrossRef]
- Li, H.; Wen, J.; Yu, R.; Meng, J.; Wang, C.; Wang, C.; Sun, S. Facile synthesis of a nanocomposite based on graphene and ZnAl layered double hydroxides as a portable shelf of a luminescent sensor for DNA detection. RSC Adv. 2015, 5, 9341–9347. [Google Scholar] [CrossRef]
- Abdel-Khalek, A.A.; Mahmoud, S.A.; Zaki, A.H. Visible light assisted photocatalytic degradation of crystal violet, bromophenol blue and eosin Y dyes using AgBr-ZnO nanocomposite. Environ. Nanotechnol. Monit. Manag. 2018, 9, 164–173. [Google Scholar] [CrossRef]
- Marotti, R.E.; Giorgi, P.; Machado, G.; Dalchiele, E.A. Crystallite size dependence of band gap energy for electrodeposited ZnO grown at different temperatures. Sol. Energy Mater. Sol. Cells 2006, 90, 2356–2361. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, V.; Tanwar, A. Structural, morphological, optical and photocatalytic properties of Ag-doped ZnO nanoparticles. J. Mater. Sci. Mater. Electron. 2016, 27, 2166–2173. [Google Scholar] [CrossRef]
- Kamarulzaman, N.; Kasim, M.F.; Chayed, N.F. Elucidation of the highest valence band and lowest conduction band shifts using XPS for ZnO and Zn0.99Cu0.01O band gap changes. Results Phys. 2016, 6, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, F.; Hassaballa, S.; Shaban, M.; Ahmed, A.M. Highly Efficient Photocatalyst Fabricated from the ChemicalRecycling of Iron Waste and Natural Zeolite for SuperDye Degradation. Nanomater 2022, 12, 235. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Ahmaruzzaman, M.; Devi, T.B.; Nath, J. Photodegradation of methyl violet 6B and methylene blue using tin-oxide nanoparticles (synthesized via a green route). J. Photochem. Photobiol. A Chem. 2016, 325, 116–124. [Google Scholar] [CrossRef]
- Chen, C.C.; Fan, H.J.; Jang, C.Y.; Jan, J.L.; De Lin, H.; Lu, C.S. Photooxidative N-de-methylation of crystal violet dye in aqueous nano-TiO2 dispersions under visible light irradiation. J. Photochem. Photobiol. A Chem. 2006, 184, 147–154. [Google Scholar] [CrossRef]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar] [CrossRef]
- Khezrianjoo, S.; Revanasiddappa, H. Langmuir-Hinshelwood Kinetic Expression for the Photocatalytic Degradation of Metanil Yellow Aqueous Solutions by ZnO Catalyst. Chem. Sci. J. 2012, 2012, 85. [Google Scholar]
- Fatimah, I.; Purwiandono, G.; Hidayat, A.; Sagadevan, S.; Kamari, A. Mechanistic insight into the adsorption and photocatalytic activity of a magnetically separable γ-Fe2O3/Montmorillonite nanocomposite for rhodamine B removal. Chem. Phys. Lett. 2022, 792, 139410. [Google Scholar] [CrossRef]
- Salim, N.A.A.; Puteh, M.H.; Khamidun, M.H.; Fulazzaky, M.A.; Abdullah, N.H.; Yusoff, A.R.M.; Zaini, M.A.A.; Ahmad, N.; Lazim, Z.M.; Nuid, M. Interpretation of isotherm models for adsorption of ammonium onto granular activated carbon. Biointerface Res. Appl. Chem. 2021, 11, 9227–9241. [Google Scholar] [CrossRef]
- Soliman, N.K.; Moustafa, A.F.; El-Mageed, H.R.A.; Abdel-Gawad, O.F.; Elkady, E.T.; Ahmed, S.A.; Mohamed, H.S. Experimentally and theoretically approaches for disperse red 60 dye adsorption on novel quaternary nanocomposites. Sci. Rep. 2021, 11, 10000. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, I. Composite of TiO2-montmorillonite from indonesia and its photocatalytic properties in methylene blue and E.coli reduction. J. Mater. Environ. Sci. 2012, 3, 983–992. [Google Scholar]
- Fatimah, I.; Shukla, P.; Kooli, F. Combined photocatalytic and Fenton oxidation of methyl orange dye using iron exchanged titanium pillared montmorillonite. J. Appl. Sci. 2009, 9, 3715–3722. [Google Scholar] [CrossRef] [Green Version]
- Punithavathy, I.K.; Richard, J.P.; Jeyakumar, S.J.; Jothibas, M.; Praveen, P. Photodegradation of methyl violet dye using ZnO nanorods. J. Mater. Sci. Mater. Electron. 2017, 28, 2494–2501. [Google Scholar] [CrossRef]
- Yang, S.; Xu, Y.; Huang, Y.; Zhou, G.; Yang, Z.; Yang, Y.; Wang, G. Photocatalytic Degradation of Methyl Violet with TiSiW12O40/TiO2. Int. J. Photoenergy 2013, 2013, 191340. [Google Scholar] [CrossRef] [Green Version]
- Purnawan, C.; Wahyuningsih, S.; Nawakusuma, V. Methyl violet degradation using photocatalytic and photoelectrocatalytic processes over graphite/PbTiO3 composite. Bull. Chem. React. Eng. Catal. 2018, 13, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Rao, C.; Lu, L.; Zhang, S.; Muddassir, M.; Liu, J. Efficient photocatalytic degradation of methyl violet using two new 3D MOFs directed by different carboxylate spacers. CrystEngComm 2021, 23, 741–747. [Google Scholar] [CrossRef]
- Jeyasubramanian, K.; Hikku, G.S.; Sharma, R.K. Photo-catalytic degradation of methyl violet dye using zinc oxide nano particles prepared by a novel precipitation method and its anti-bacterial activities. J. Water Process Eng. 2015, 8, 35–44. [Google Scholar] [CrossRef]
- Bencherif, S.D.; Gallardo, J.J.; Carrillo-berdugo, I.; Bahmani, A.; Navas, J. Synthesis, characterization and photocatalytic performance of calcined zncr-layered double hydroxides. Nanomaterials 2021, 11, 3051. [Google Scholar] [CrossRef]
- Franco, P.; Sacco, O.; De Marco, I.; Vaiano, V. Zinc Oxide Nanoparticles Obtained by Supercritical Antisolvent Precipitation for the Photocatalytic Degradation of Crystal Violet Dye. Catalysts 2019, 9, 346. [Google Scholar] [CrossRef] [Green Version]
- Ameen, S.; Akhtar, M.S.; Nazim, M.; Shin, H.S. Rapid photocatalytic degradation of crystal violet dye over ZnO flower nanomaterials. Mater. Lett. 2013, 96, 228–232. [Google Scholar] [CrossRef]
- Xu, S.M.; Pan, T.; Dou, Y.B.; Yan, H.; Zhang, S.T.; Ning, F.Y.; Shi, W.Y.; Wei, M. Theoretical and Experimental Study on MIIMIII-Layered Double Hydroxides as Efficient Photocatalysts toward Oxygen Evolution from Water. J. Phys. Chem. C 2015, 119, 18823–18834. [Google Scholar] [CrossRef]
- Wang, J.; Lei, X.; Huang, C.; Xue, L.; Cheng, W.; Wu, Q. Fabrication of a novel MoO3/Zn–Al LDHs composite photocatalyst for efficient degradation of tetracycline under visible light irradiation. J. Phys. Chem. Solids 2021, 148, 109698. [Google Scholar] [CrossRef]
- Fatimah, I.; Sahroni, I.; Muraza, O.; Doong, R.A. One-pot biosynthesis of SnO2 quantum dots mediated by Clitoria ternatea flower extract for photocatalytic degradation of rhodamine B. J. Environ. Chem. Eng. 2020, 8, 103879. [Google Scholar] [CrossRef]
- Andayani, W.; Bagyo, A.N.M. TiO2 beads for photocatalytic degradation of humic acid in peat water. Indones. J. Chem. 2011, 11, 253–257. [Google Scholar] [CrossRef]
- Gusfiyesi; Alif, A.; Aziz, H.; Arief, S.; Munaf, E. Degradation of humic acid as peat water degradation model by TiO2 thin layer photocatalytic reactor. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 918–930. [Google Scholar]
- Fu, J.; Ji, M.; Jin, L. Photocatalytic oxidation of natural organic matter-fulvic acid in water. Chem. Bull./Huaxue Tongbao 2005, 68, 871–875. [Google Scholar]
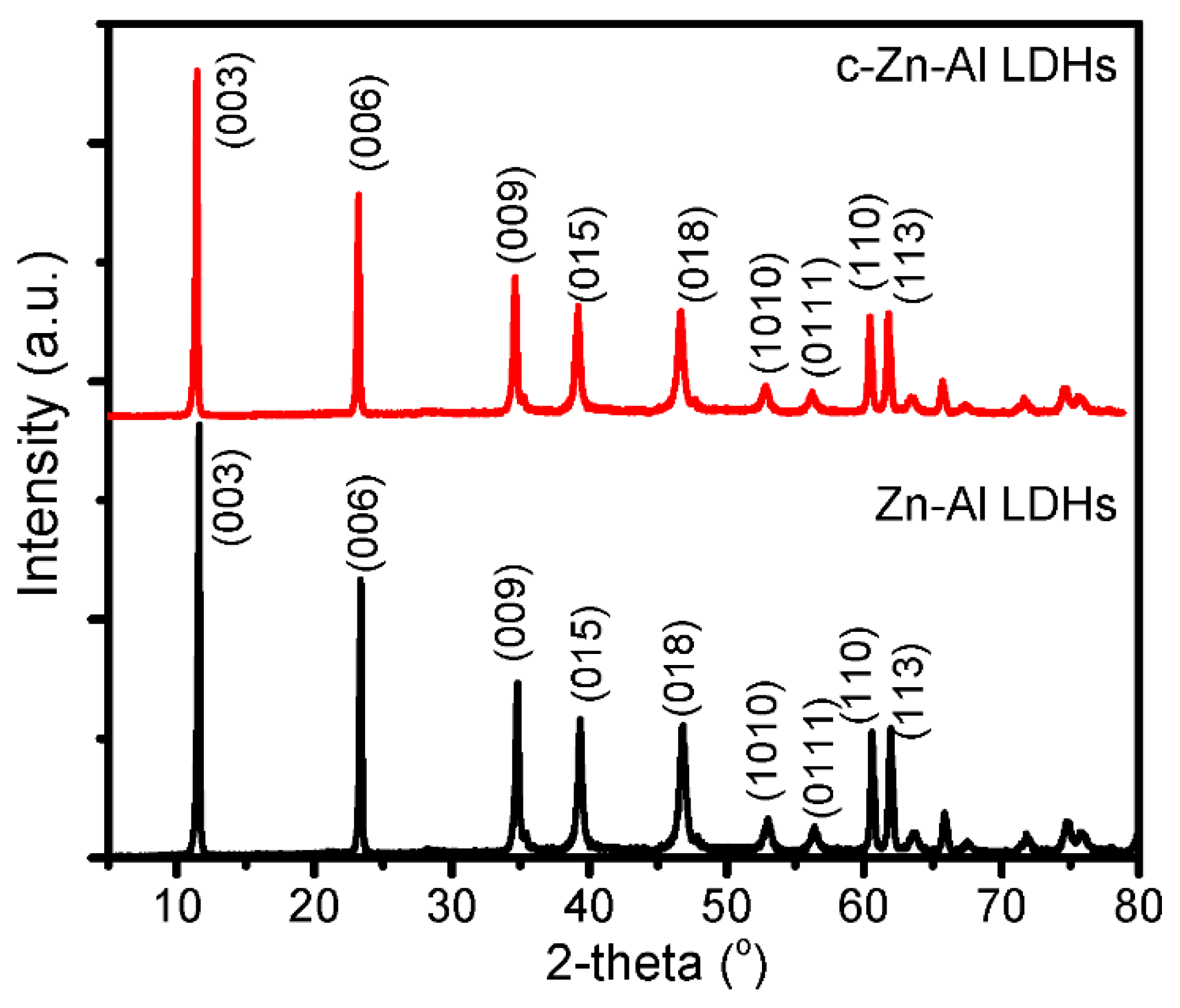




| 2θ | Zn-Al LDHs | c-Zn-Al LDHs | ||
|---|---|---|---|---|
| FWHM (unit) | Crystallite Size (nm) | FWHM (unit) | Crystallite Size (nm) | |
| 11.1 | 1.018 | 87.1 | 1.094 | 81.1 |
| 22.3 | 1.816 | 58.7 | 1.533 | 49.5 |
| 34.7 | 0.938 | 98.5 | 1.476 | 62.6 |
| Crystallite size (nm) | 81.4 | 64.4 | ||
| Surface Parameter | Zn-Al LDHs | c-Zn-Al LDHs |
|---|---|---|
| BET specific surface area (m2/g) | 38.65 | 71.28 |
| Pore volume (cc/g) | 2.4 × 10−2 | 8.9 × 10−2 |
| Pore radius (Å) | 8.7 | 12.6 |
| Process/Material | R2 of the Pseudo-First-Order Kinetics | R2 of the Pseudo-Second-Order Kinetics | Kinetics Constant (Pseudo-First-Order Kinetics-1/min) | DE at 120 min (%) | |
|---|---|---|---|---|---|
| Adsorption | Zn-Al LDHs | 0.968 | 0.956 | 1.04 × 10−3 | 17.54 |
| c-Zn-Al LDHs | 0.994 | 0.991 | 1.72 × 10−3 | 18.53 | |
| Photooxidation | Zn-Al LDHs | 0.997 | 0.905 | 2.96 × 10−3 | 41.28 |
| c-Zn-Al LDHs | 0.998 | 0.984 | 3.37 × 10−3 | 45.57 |
| Adsorbent | Freundlich Isotherm Parameters | Langmuir Isotherm Parameters | |||||
|---|---|---|---|---|---|---|---|
| KF (L/g) | 1/n | R2 | qm (mg/g) | KL (L/mg) | RL | R2 | |
| Zn-Al LDHs | 2.45 | 4.51 | 0.98 | 7.89 | 7.39 × 10−3 | 0.79 | 0.33 |
| c-Zn-Al LDHs | 2.64 | 4.46 | 0.97 | 9.02 | 9.03 ×10−3 | 0.98 | 0.55 |
| Photocatalyst | Remark | Light Wavelength/Source | Reference |
|---|---|---|---|
| ZnO nanoparticles | DE of 85% for 120 min photooxidation of MV 12.5 ppm by 1 g/L of photocatalyst | Sunlight | [38] |
| Zn-Cr LDHs | DE of 36% for 120 min photooxidation of 30 ppm MV by 1 g/L of photocatalyst | Solar simulator | [39] |
| PbTiO3 | Photooxidation treatment for 120 min gave the DE of 90% on the initial MV concentration of 5 ppm and the photocatalyst dose of 3 g/30 mL | 296 nm | [36] |
| ZnO nanorods | Photooxidation treatment for 120 min gave the DE of 90% for 10 ppm of MV and the dosage of 0.3 g/L | Sunlight | [34] |
| ZnO | Photooxidation treatment for 120 min gave the DE of 95% on the initial MV concentration of 10 ppm and the photocatalyst dose of 1.5 g/L | 365 nm | [40] |
| ZnO | Photooxidation treatment for 120 min gave the DE of 96% on the initial MV concentration of 10 ppm and the photocatalyst dose of 1.5 g/L | Xenon lamp (Visible) | [41] |
| Zn-Al LDHs | Photooxidation treatment for 120 min gave the DE of 95.9% on the initial MV concentration of 10 ppm and the photocatalyst dose of 1.0 g/L | 296 nm | This work |
| Parameter | Value |
|---|---|
| pH | 6.2 |
| TOC (mg/L) | 1024 |
| COD (mg/L) | 655 |
| UV254 | 2.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatimah, I.; Yahya, A.; Iqbal, R.M.; Tamyiz, M.; Doong, R.-a.; Sagadevan, S.; Oh, W.-C. Enhanced Photocatalytic Activity of Zn-Al Layered Double Hydroxides for Methyl Violet and Peat Water Photooxidation. Nanomaterials 2022, 12, 1650. https://doi.org/10.3390/nano12101650
Fatimah I, Yahya A, Iqbal RM, Tamyiz M, Doong R-a, Sagadevan S, Oh W-C. Enhanced Photocatalytic Activity of Zn-Al Layered Double Hydroxides for Methyl Violet and Peat Water Photooxidation. Nanomaterials. 2022; 12(10):1650. https://doi.org/10.3390/nano12101650
Chicago/Turabian StyleFatimah, Is, Amri Yahya, Rendy Muhamad Iqbal, Muchammad Tamyiz, Ruey-an Doong, Suresh Sagadevan, and Won-Chun Oh. 2022. "Enhanced Photocatalytic Activity of Zn-Al Layered Double Hydroxides for Methyl Violet and Peat Water Photooxidation" Nanomaterials 12, no. 10: 1650. https://doi.org/10.3390/nano12101650
APA StyleFatimah, I., Yahya, A., Iqbal, R. M., Tamyiz, M., Doong, R.-a., Sagadevan, S., & Oh, W.-C. (2022). Enhanced Photocatalytic Activity of Zn-Al Layered Double Hydroxides for Methyl Violet and Peat Water Photooxidation. Nanomaterials, 12(10), 1650. https://doi.org/10.3390/nano12101650










