Perspectives on Nickel Hydroxide Electrodes Suitable for Rechargeable Batteries: Electrolytic vs. Chemical Synthesis Routes
Abstract
:1. Introduction
2. Description of Basic Materials of Nickel Metal Hydride Batteries
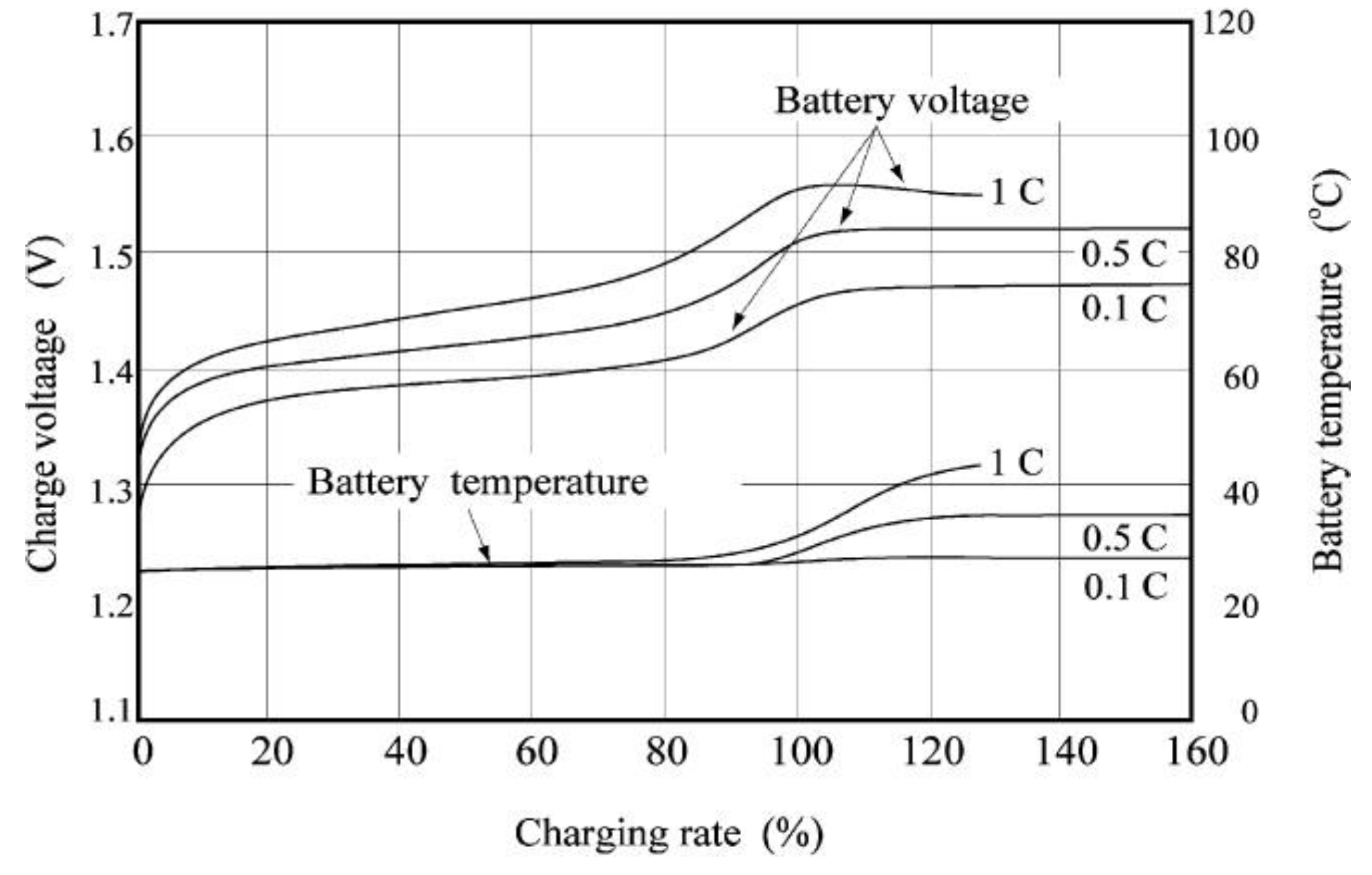
2.1. Performance Characteristics of Ni-MH Battery
- Energy/size: 140–300 Wh/L
- Energy/weight: 40–80 Wh/kg
- Power/weight: 250–1000 W/kg
- Self-discharge rate: 30%/month
- Charge/discharge efficiency: 66%
- Nominal cell voltage: 1.32 V
- Cycle durability: 500–1000 cycles
2.1.1. Pocket Plate Technology
2.1.2. Tubular Plate Technology
2.1.3. Sintered Plate Technology
2.1.4. Plastic Bonded Electrodes
2.2. The Chemistry of Nickel Hydroxide
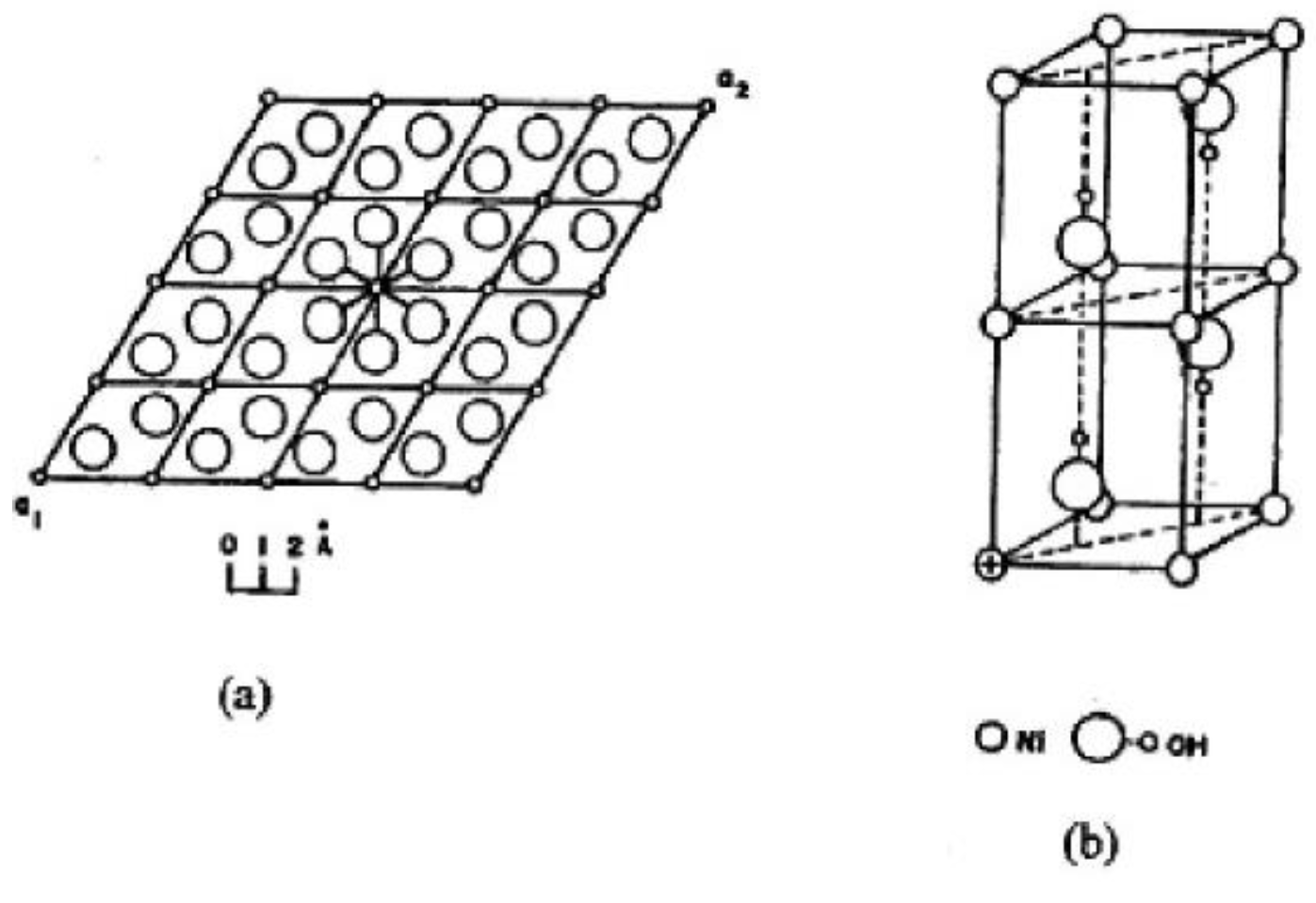
2.2.1. The Structure of Nickel Hydroxide and Its Various Forms
2.2.2. β-Ni(OH)2
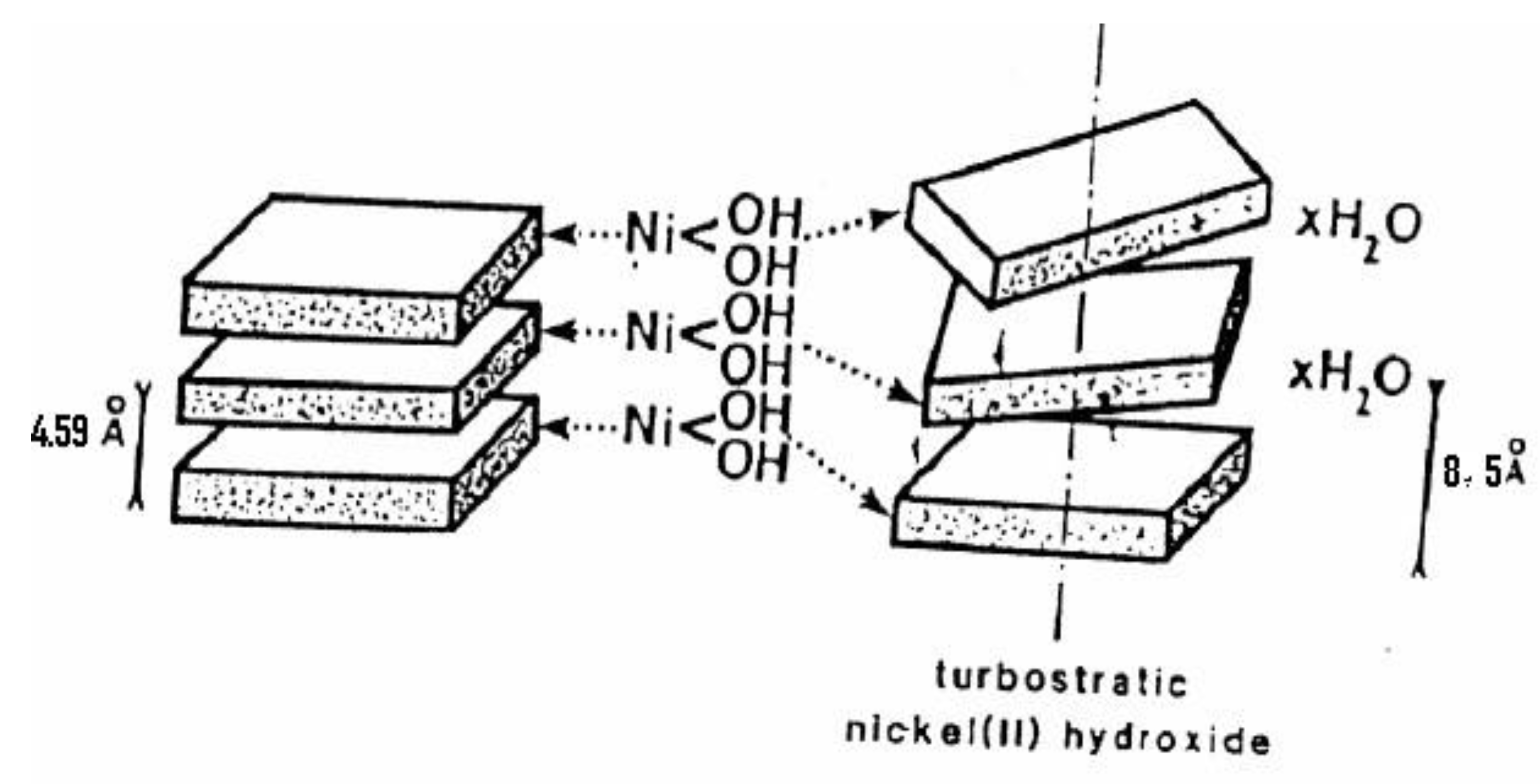
2.2.3. α-Ni(OH)2
2.2.4. β-NiOOH
2.2.5. γ–NiOOH
2.3. Properties of Nickel Hydroxide
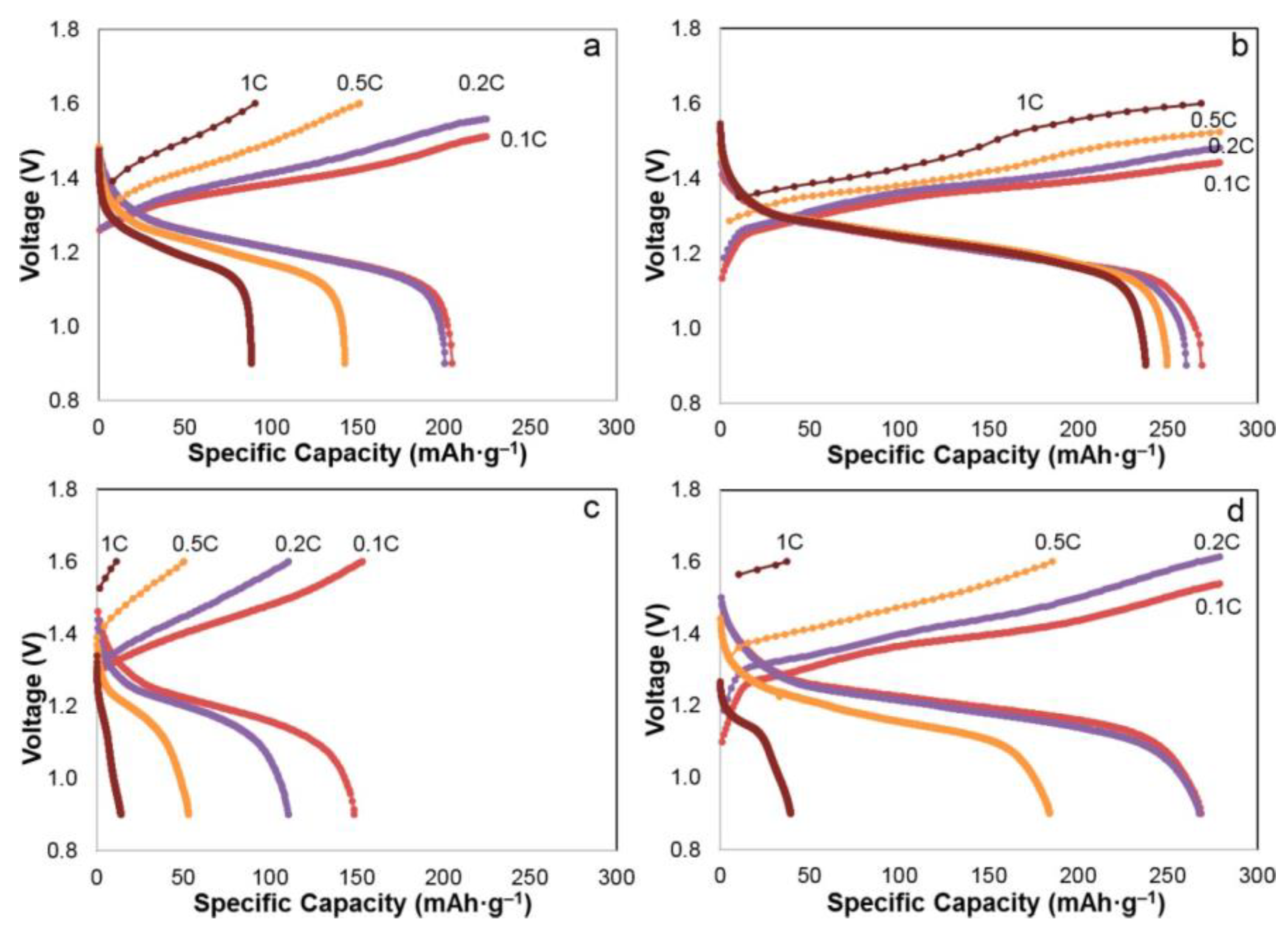
2.3.1. Discharge Capacity
2.3.2. Density
2.3.3. Ni-Based Electrocatalyst for Oxygen Evolution Reaction
3. Production of Nickel Hydroxide
Criteria for Preparation
4. Technologies for Preparation of Battery Grade Nickel Hydroxide
4.1. Chemical Method of Preparation
4.1.1. Alkali Induced Precipitation
4.1.2. Ammonia Induced Precipitation
4.1.3. Homogeneous Precipitation by Urea Hydrolysis
4.1.4. Other Precipitation Methods
4.1.5. Subsequent Treatment of Precipitated Nickel Hydroxide
4.2. Electrochemical Method of Preparation
4.2.1. Reactions for Electrochemical Precipitation of Nickel Hydroxide
4.2.2. Electrolytic Preparation of Nickel Hydroxide Thin Films
4.2.3. Nickel Hydroxide Electrodes by Electrochemical Impregnation
- (i)
- Low current density (5 mA/cm2) with low (Ni++) (~0.3 M) and a soluble anode,
- (ii)
- High current density (175 mA/cm2) with (Ni++): 4 M,
- (iii)
- Nickel nitrate and cobalt nitrate dissolved in 1:1 ethanol-water mixture and
- (iv)
- Ni(NO3)2 + Co(NO3)2 + NaNO2 at pH = 3 with platinized platinum, anode.
4.2.4. Bulk Production of Nickel Hydroxide by Electrochemical Method
4.2.5. Quantitative Assessment of Electrochemical Precipitation of Nickel Hydroxide
5. Emerging Technology Developments and Future Scope
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beck, F.; Rüetschi, P. Rechargeable batteries with aqueous electrolytes. Electrochim. Acta 2000, 45, 2467–2482. [Google Scholar] [CrossRef]
- Deutscher, R.L.; Florence, T.M.; Woods, R. Investigations on an aqueous lithium secondary cell. J. Power Sources 1995, 55, 41–46. [Google Scholar] [CrossRef]
- Fan, X.; Liu, B.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Lv, X.; Xie, Y.; Chen, B.; Hu, W.; et al. Battery Technologies for Grid-Level Large-Scale Electrical Energy Storage. Trans. Tianjin Univ. 2020, 26, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef] [PubMed]
- Mabbett, I.; Glover, C.F.; Malone, J.H.; Worsley, D.A. Comparison of Pocket Plate and Sintered Plate Ni Electrodes Used in Energy Storage Technologies for PV Back Up Applications. ECS Trans. 2013, 50, 25–35. [Google Scholar] [CrossRef]
- Takaki, M.; Fujioka, N.; Ikoma, M. Development of small and medium sized sealed-type nickel/metal-hydride battery for electric vehicle. In Proceedings of the 14th International Electric Vehicle Symposium, Orlando, FL, USA, 15–17 December 1997. [Google Scholar]
- Ohta, K.; Hayashi, K.; Matsuda, H.; Toyoguchi, Y.; Ikoma, M. Nickel hydroxide: Improvement of charge efficiency at high temperature. In Hydrogen and Metal Hydride Batteries; Bennett, P.D., Sakai, T., Eds.; The Electrochemical Society: Pennington, NJ, USA, 1994; pp. 296–302. [Google Scholar]
- Fritts, D.H. The Mechanics of Electrochemically Coprecipitated Cobalt Hydroxide in Nickel Hydroxide Electrodes. J. Electrochem. Soc. 1982, 129, 118. [Google Scholar] [CrossRef]
- McBreen, J. “Nickel Hydroxides”, Handbook of Battery Materials; Besenhard, J.O., Ed.; V.C.H Publications: Weinheim, Germany, 1997. [Google Scholar]
- McBreen, J. The Nickel Oxide Electrode in: Modern Aspects of Electrochemistry No. 21; White, R.E., Bockris, J.O.M., Conway, B.E., Eds.; Plenum Publishing Corporation: New York, NY, USA, 1989; pp. 29–58. [Google Scholar]
- Chani, V.I.; Yang, Q.; Wilkinson, D.S.; Weatherly, G.C. Effect of substrate pre-coating on adhesion of sintered nickel plaques for electrode application in rechargeable batteries. J. Power Sources 2005, 142, 370–381. [Google Scholar] [CrossRef]
- Vassal, N.; Salmon, E.; Fauvarque, J. Nickel/Metal Hydride Secondary Batteries Using an Alkaline Solid Polymer Electrolyte. J. Electrochem. Soc. 2019, 146, 20–26. [Google Scholar] [CrossRef]
- El Kharbachi, A.; Dematteis, E.M.; Shinzato, K.; Stevenson, S.C.; Bannenberg, L.J.; Heere, M.; Zlotea, C.; Szilágyi, P.Á.; Bonnet, J.-P.; Grochala, W.; et al. Metal Hydrides and Related Materials. Energy Carriers for Novel Hydrogen and Electrochemical Storage. J. Phys. Chem. C 2020, 124, 7599–7607. [Google Scholar] [CrossRef] [Green Version]
- Shukla, A.; Venugopalan, S.; Hariprakash, B. Nickel-based rechargeable batteries. J. Power Sources 2001, 100, 125–148. [Google Scholar] [CrossRef]
- Taniguchi, A. Development of nickel/metal-hydride batteries for EVs and HEVs. J. Power Sources 2001, 100, 117–124. [Google Scholar] [CrossRef]
- Hui, L.; Yunchang, D.; Jiongliang, Y.; Zeyun, W. Study of the electrochemical performance of nickel hydroxide. J. Power Sources 1995, 57, 137–140. [Google Scholar] [CrossRef]
- Imoto, T.; Kimoto, M.; Matuura, Y.; Higashiyama, N.; Fujitani, S.; Nishio, K. Denki Kagaku. Electrochemistry 1998, 4, 432. [Google Scholar]
- Ikoma, M.; Umeo, R.; Kawano, H.; Yanagihara, N.; Ito, Y.; Matsumoto, I. Proceedings of the Extended Abstracts of the 27th Battery Symposium, Osaka, Japan, 25–27 November 1986; pp. 89–90.
- Morioka, Y. State-of-the-art of alkaline rechargeable batteries. J. Power Sources 2001, 100, 107–116. [Google Scholar] [CrossRef]
- Yan, S.; Meng, T.; Young, K.; Nei, J. A Ni/MH Pouch Cell with High-Capacity Ni(OH)2. Batter 2017, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Lai, W.; Yan, G.; Wu, J. Study of preparation technology for high performance AA size Ni–MH batteries. J. Alloys Compd. 1999, 293, 784–787. [Google Scholar] [CrossRef]
- Kim, T.; Choi, J.; Ryu, H.; Cho, G.; Kim, K.; Ahn, J.; Cho, K.; Ahn, H.-J. Electrochemical properties of sodium/pyrite battery at room temperature. J. Power Sources 2007, 174, 1275–1278. [Google Scholar] [CrossRef]
- Brodd, R.J. Nickel-Based Battery Systems Nickel-Based Battery Systems; Springer Science and Business Media LLC: New York, NY, USA, 2012; pp. 6938–6950. [Google Scholar]
- Stumbock, M.J. Method of Making Ni-Cd Batteries. U.S. Patent 2,667,526, 26 January 1954. [Google Scholar]
- Chang, S.; Young, K.; Lien, Y.L. Reviews of European Patents on Nickel/Metal Hydride Batteries. Batteries 2017, 3, 25. [Google Scholar] [CrossRef]
- Cheng, F.; Liang, J.; Tao, Z.; Chen, J. Functional Materials for Rechargeable Batteries. Adv. Mater. 2011, 23, 1695–1715. [Google Scholar] [CrossRef]
- Brzózka, A.; Fic, K.; Bogusz, J.; Brudzisz, A.; Marzec, M.M.; Gajewska, M.; Sulka, G.D. Polypyrrole–Nickel Hydroxide Hybrid Nanowires as Future Materials for Energy Storage. Nanomaterials 2019, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Acharya, R.; Subbaiah, T.; Anand, S.; Das, R. Effect of precipitating agents on the physicochemical and electrolytic characteristics of nickel hydroxide. Mater. Lett. 2003, 57, 3089–3095. [Google Scholar] [CrossRef]
- Bode, H.; Dehmelt, K.; Witte, J. Zur kenntnis der nickel hydroxide elektrode-I. Über das nickel (II)-hydroxidhydrat. Electrochim. Acta 1966, 11, 1079–1087. [Google Scholar] [CrossRef]
- Ramesh, T.N. Crystallite size effects in stacking faulted nickel hydroxide and its electrochemical behaviour. Mater. Chem. Phys. 2009, 114, 618–623. [Google Scholar] [CrossRef]
- Ramesh, T.; Kamath, P.V. Synthesis of nickel hydroxide: Effect of precipitation conditions on phase selectivity and structural disorder. J. Power Sources 2006, 156, 655–661. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, T.; Kamath, P.V. Planar defects in layered hydroxides: Simulation and structure refinement of β-nickel hydroxide. Mater. Res. Bull. 2008, 43, 3227–3233. [Google Scholar] [CrossRef]
- Ramesh, T.N.; Kamath, P.V.; Shivakumara, C. Correlation of Structural Disorder with the Reversible Discharge Capacity of Nickel Hydroxide Electrode. J. Electrochem. Soc. 2005, 152, A806. [Google Scholar] [CrossRef]
- Chen, Y.; Rui, K.; Zhu, J.; Dou, S.X.; Sun, W. Recent Progress on Nickel-Based Oxide/(Oxy)Hydroxide Electrocatalysts for the Oxygen Evolution Reaction. Chem. Eur. J. 2018, 25, 703–713. [Google Scholar] [CrossRef]
- Deng, J.; Nellist, M.R.; Stevens, M.B.; Dette, C.; Wang, Y.; Boettcher, S.W. Morphology Dynamics of Single-Layered Ni(OH)2/NiOOH Nanosheets and Subsequent Fe Incorporation Studied by in Situ Electrochemical Atomic Force Microscopy. Nano Lett. 2017, 17, 6922–6926. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, S.; Yue, X.; Du, H.; Shen, P.K. Mo- and Fe-Modified Ni(OH)2/NiOOH Nanosheets as Highly Active and Stable Electrocatalysts for Oxygen Evolution Reaction. ACS Catal. 2018, 8, 2359–2363. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.; Bock, C.; MacDougall, B.R. Nickel hydroxides and related materials: A review of their structures, synthesis and properties. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471, 20140792. [Google Scholar] [CrossRef]
- Kumar, C.R.R.; Kotteeswaran, P.; Raju, V.B.; Murugan, A.; Santosh, M.S.; Nagaswarupa, H.P.; Prashantha, S.C.; Kumar, M.R.A. Effects of precipitation pH values on the electrochemical properties of β-nickel hydroxide materials. IOSR J. Appl. Chem. 2015, 8, 45–51. [Google Scholar] [CrossRef]
- Huang, L.-F.; Hutchison, M.; Santucci, R.; Scully, J.R.; Rondinelli, J.M. Improved Electrochemical Phase Diagrams from Theory and Experiment: The Ni–Water System and Its Complex Compounds. J. Phys. Chem. C 2017, 121, 9782–9789. [Google Scholar] [CrossRef]
- Dima, G.; De Vooys, A.; Koper, M. Electrocatalytic reduction of nitrate at low concentration on coinage and transition-metal electrodes in acid solutions. J. Electroanal. Chem. 2003, 554, 15–23. [Google Scholar] [CrossRef]
- Dong, L.; Chu, Y.; Sun, W. Controllable Synthesis of Nickel Hydroxide and Porous Nickel Oxide Nanostructures with Different Morphologies. Chem.-A Eur. J. 2008, 14, 5064–5072. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Axe, L.; Boonfueng, T.; Tyson, T.A.; Trivedi, P.; Pandya, K. Ni(II) complexation to amorphous hydrous ferric oxide: An X-ray absorption spectroscopy study. J. Colloid Interface Sci. 2007, 314, 10–17. [Google Scholar] [CrossRef]
- Wang, X.; Luo, H.; Parkhutik, P.; Millan, A.-C.; Matveeva, E. Studies of the performance of nanostructural multiphase nickel hydroxide. J. Power Sources 2003, 115, 153–160. [Google Scholar] [CrossRef]
- Xu, P.; Han, X.; Zhang, B.; Lv, Z.; Liu, X. Characterization of an ultrafine β-nickel hydroxide from supersonic co-precipitation method. J. Alloys Compd. 2007, 436, 369–374. [Google Scholar] [CrossRef]
- Song, Q.; Tang, Z.; Guo, H.; Chan, S.L.I. Structural characteristics of nickel hydroxide synthesized by a chemical precipitation route under different pH values. J. Power Sources 2002, 112, 428–434. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Venugopal, N.; Reddy, B.R.; Rao, M.M. Optimum conditions to prepare high yield, phase pure α-Ni(OH)2 nanoparticles by urea hydrolysis and electrochemical ageing in alkali solutions. J. Power Sources 2005, 150, 272–275. [Google Scholar] [CrossRef]
- Tarutani, N.; Tokudome, Y.; Jobbagy, M.; Soler-Illia, G.J.A.A.; Tang, Q.; Müller, M.; Takahashi, M.; Mueller, M. Highly Ordered Mesoporous Hydroxide Thin Films through Self-Assembly of Size-Tailored Nanobuilding Blocks: A Theoretical-Experimental Approach. Chem. Mater. 2018, 31, 322–330. [Google Scholar] [CrossRef]
- Numan, A.; Duraisamy, N.; Omar, F.S.; Gopi, D.; Ramesh, K.; Ramesh, S. Sonochemical synthesis of nanostructured nickel hydroxide as an electrode material for improved electrochemical energy storage application. Prog. Nat. Sci. 2017, 27, 416–423. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.; Xi, S.; Lee, W.S.V.; Zhang, J.; Wu, Z.; Wang, J.O.; Hu, T.; Liu, L.; Han, Y.; et al. Strain stabilized nickel hydroxide nanoribbons for efficient water splitting. Energy Environ. Sci. 2020, 13, 229–237. [Google Scholar] [CrossRef]
- Subbaiah, T.; Mallick, S.; Mishra, K.; Sanjay, K.; Das, R. Electrochemical precipitation of nickel hydroxide. J. Power Sources 2002, 112, 562–569. [Google Scholar] [CrossRef]
- Ash, B.; Kheti, J.; Sanjay, K.; Subbaiah, T.; Anand, S.; Paramguru, R. Physico-chemical and electro-chemical properties of nickel hydroxide precipitated in the presence of metal additives. Hydrometallurgy 2006, 84, 250–255. [Google Scholar] [CrossRef]
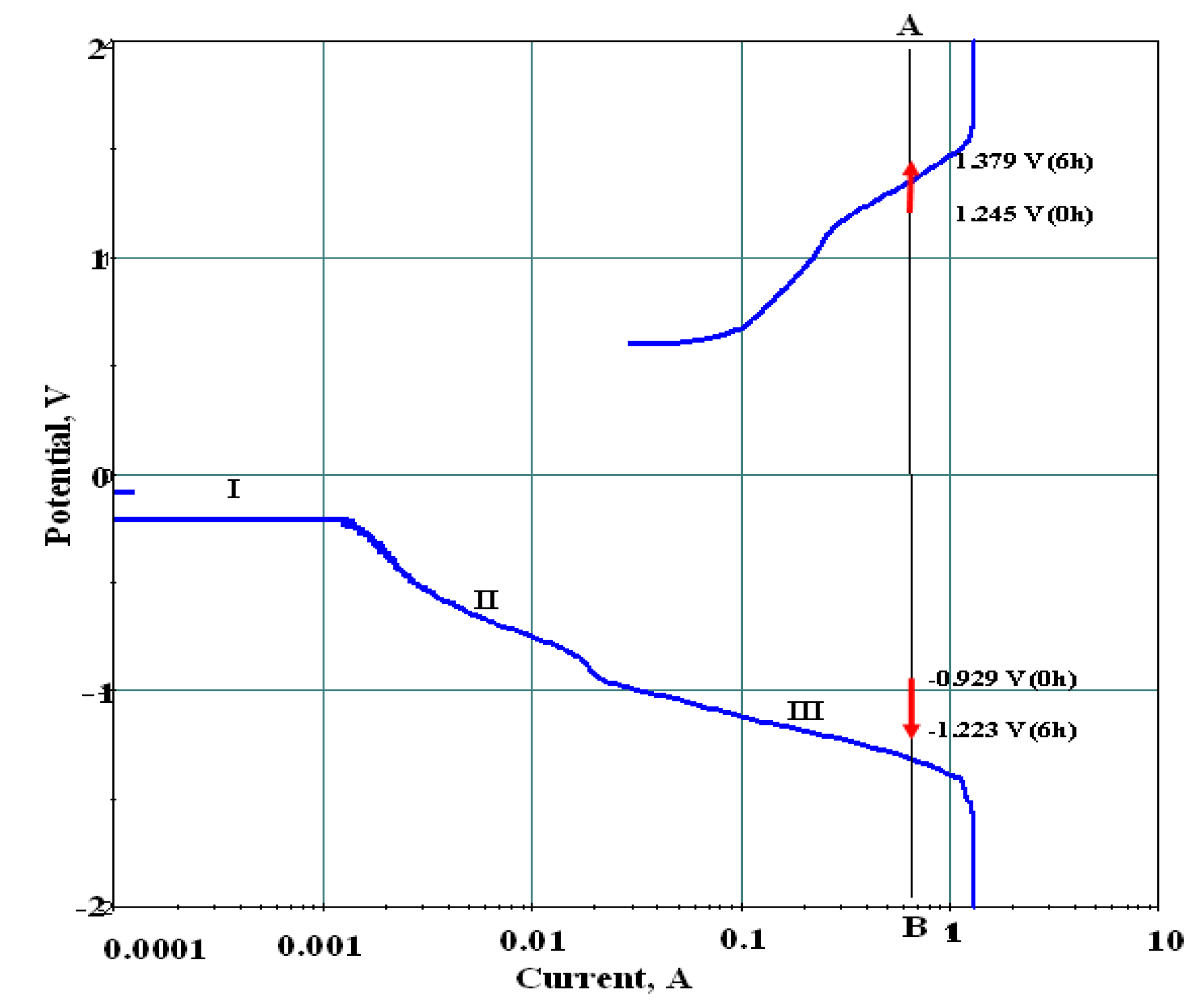
- Ash, B.; Paramguru, R.K.; Mishra, B.K. Electrode reactions during electrolytic preparation of nickel hydroxide. Electrochem. Commun. 2010, 12, 48–51. [Google Scholar] [CrossRef]
- Ash, B.; Mishra, K.G.; Subbaiah, T.; Paramguru, R.K.; Mishra, B.K. Electrochemical studies on electrolytic preparation of battery grade nickel hydroxide—Effect of (OH)− to Ni2+ ratio. J. Power Sources 2015, 275, 55–63. [Google Scholar] [CrossRef]
- Ash, B.; Paramguru, R.K.; Mishra, B.K. A Fundamental Investigation of Electrochemical Preparation of Battery Grade Nickel Hydroxide. Adv. Mater. Res. 2010, 117, 15–20. [Google Scholar] [CrossRef]
- Cattarin, S.; Tributsch, H.; Stimming, U. Reduction of H2O2 at CuInSe2 (Photo)cathodes I. Characterization of the Electrode-Electrolyte Interface. J. Electrochem. Soc. 1992, 139, 1320. [Google Scholar] [CrossRef]
- Corrigan, D.A.; Bendert, R.M. ChemInform Abstract: Effect of Coprecipitated Metal Ions on the Electrochemistry of Nickel Hydroxide Thin Films: Cyclic Voltammetry in 1 M KOH. J. Electrochem. Soc. 1989, 20, 723–728. [Google Scholar] [CrossRef]
- Streinz, C.C.; Hartman, A.P.; Motupally, S.; Weidner, J.W. The Effect of Current and Nickel Nitrate Concentration on the Deposition of Nickel Hydroxide Films. J. Electrochem. Soc. 1995, 142, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Streinz, C.C.; Motupally, S.; Weidner, J.W. The Effect of Temperature and Ethanol on the Deposition of Nickel Hydroxide Films. J. Electrochem. Soc. 1995, 142, 4051–4056. [Google Scholar] [CrossRef]
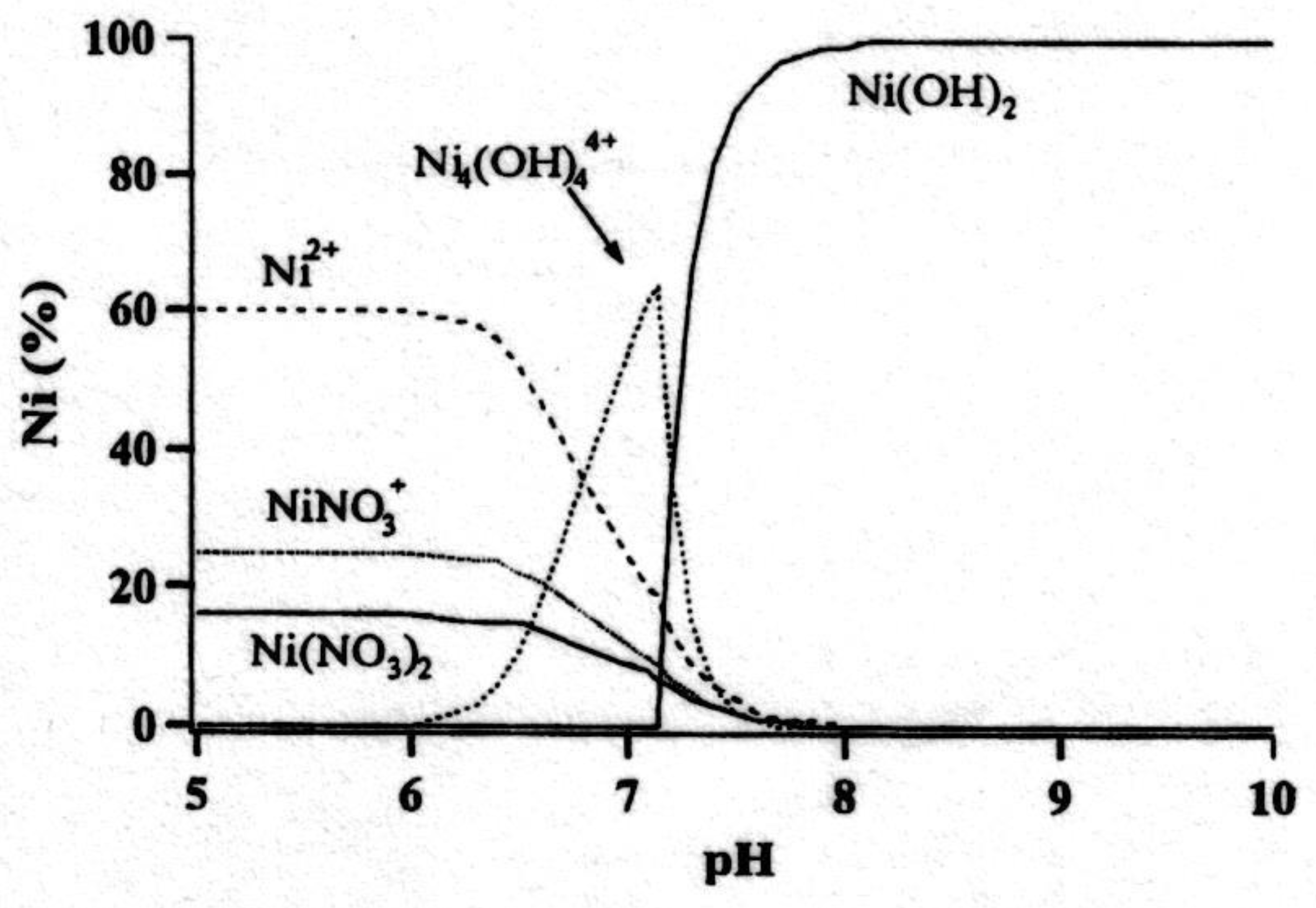
- Carter, J.C.; Khulbe, P.K.; Gray, J.; Van Zee, J.; Angel, S.M. Raman spectroscopic evidence supporting the existence of Ni4(OH)44+ in aqueous, Ni(NO3)2 solutions. Anal. Chim. Acta 2004, 514, 241–245. [Google Scholar] [CrossRef]
- Fierro, C.; Zallen, A.; Koch, J.; Fetcenko, M.A. The Influence of Nickel-Hydroxide Composition and Microstructure on the High-Temperature Performance of Nickel Metal Hydride Batteries. J. Electrochem. Soc. 2006, 153, A492–A496. [Google Scholar] [CrossRef]
- Ta, K.P.; Newman, J. Proton Intercalation Hysteresis in Charging and Discharging Nickel Hydroxide Electrodes. J. Electrochem. Soc. 1999, 146, 2769. [Google Scholar] [CrossRef]
- He, X.; Li, J.; Cheng, H.; Jiang, C.; Wan, C. Controlled crystallization and granulation of nano-scale β-Ni(OH)2 cathode materials for high power Ni-MH batteries. J. Power Sources 2005, 152, 285–290. [Google Scholar] [CrossRef]
- Minakshi, M.; Singh, P.; Sharma, N.; Blackford, M.; Ionescu, M. Lithium Extraction−Insertion from/into LiCoPO4 in Aqueous Batteries. Ind. Eng. Chem. Res. 2011, 50, 1899–1905. [Google Scholar] [CrossRef]
- Minakshi, M.; Barmi, M.; Mitchell, D.; Barlow, A.J.; Fichtner, M. Effect of oxidizer in the synthesis of NiO anchored nanostructure nickel molybdate for sodium-ion battery. Mater. Today Energy 2018, 10, 1–14. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Guo, Z. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering. Sci. Adv. 2019, 5, eaav7412. [Google Scholar] [CrossRef] [Green Version]
- El Kharbachi, A.; Zavorotynska, O.; Latroche, M.; Cuevas, F.; Yartys, V.A.; Fichtner, M. Exploits, advances and challenges benefiting beyond Li-ion battery technologies. J. Alloys Compd. 2020, 817, 153261. [Google Scholar] [CrossRef]

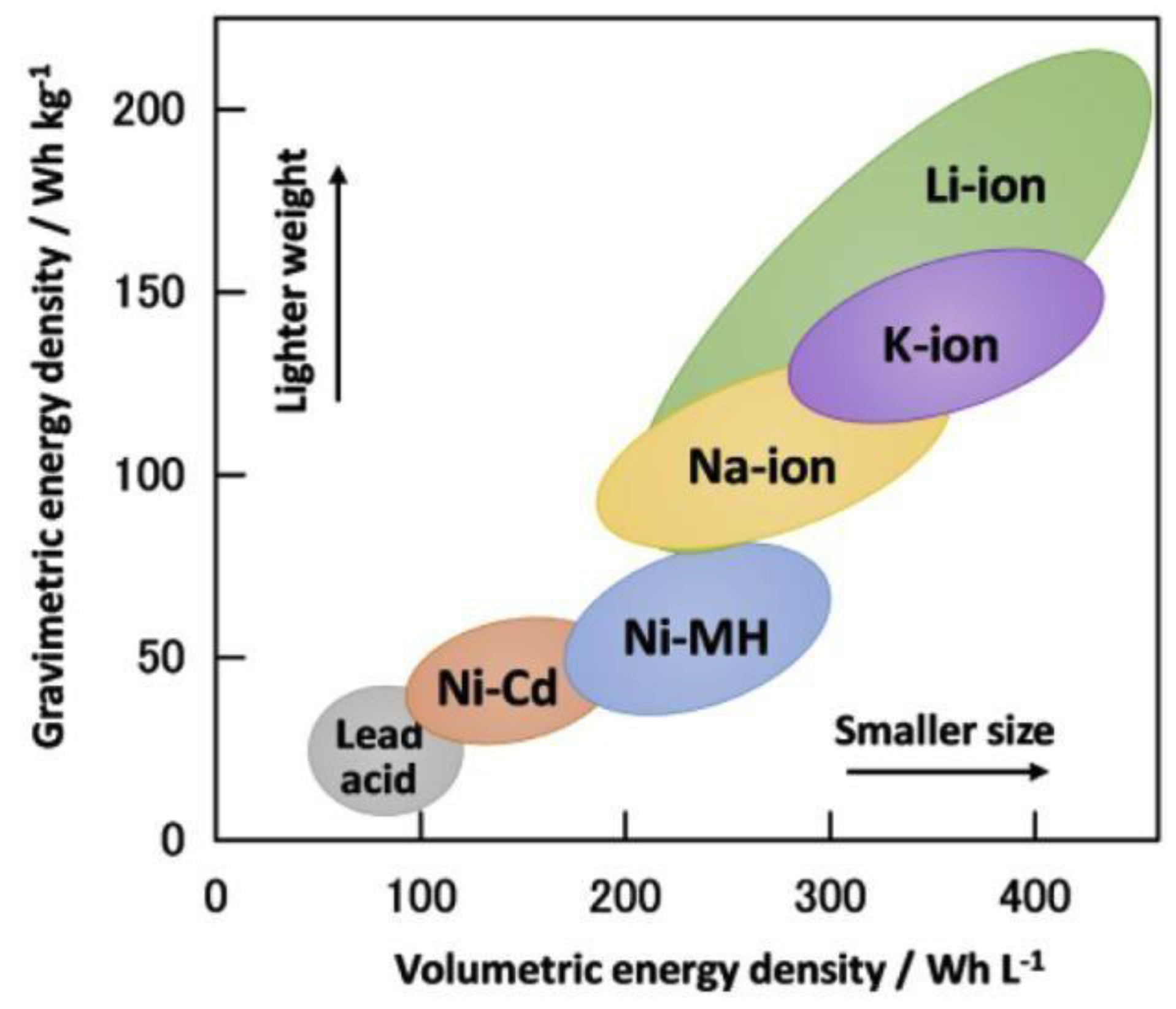
| Rechargeable Battery System | Electrolyte | Specific Energy (Wh/kg) | Cycle Life | Nominal Voltage (V) |
|---|---|---|---|---|
| Lead Acid | aq. H2SO4 | 30–50 | 200–300 | 2.0 |
| Ni-Cd | aq. KOH | 45–80 | 1000 | 1.2 |
| Ni-MH | aq. KOH | 60–120 | 300–500 | 1.2 |
| Li-ion (LiCoO2) | LiPF6 in propylene carbonate/diethyl carbonate | 150–250 | 500–1000 | 3.6 |
| Li-ion (LiFePO4) | LiPF6 in ethylene carbonate | 90–120 | 1000–2000 | 3.3 |
| Li-ion (LiMnO2) | LiClO4 in ethylene carbonate | 100–150 | 500–1000 | 3.7 |
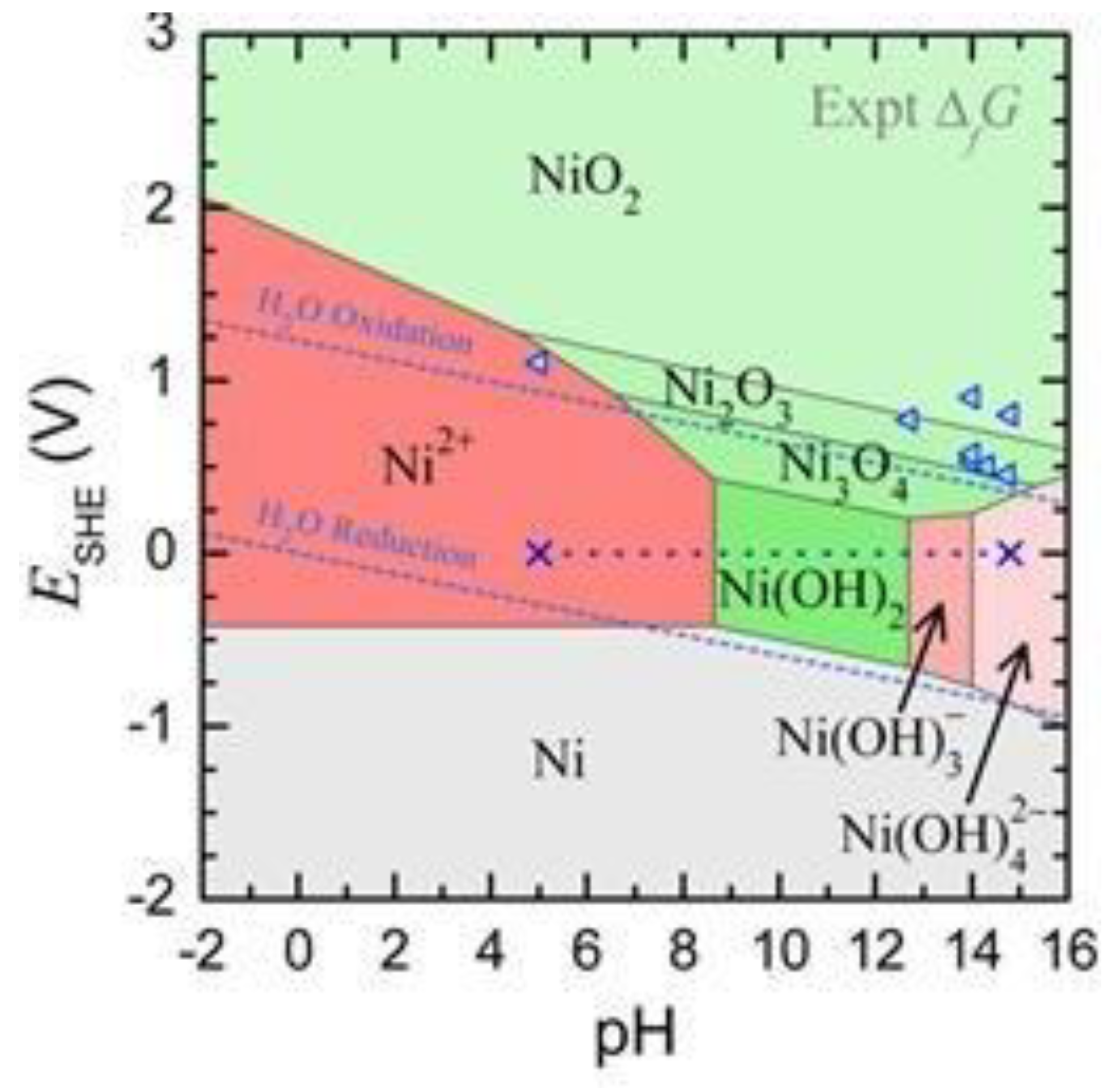
| pH | Electrochemically Formed Ni Structure |
|---|---|
| 2.9 | Ni2+ |
| 4.9 | Ni2+ |
| 5.4 | Ni2+ |
| 8.4 | Ni(OH)2 + NiO + Ni2+ |
| 14 | Ni(OH)3− |
| Sl. No. | Experimental Condition | (OH−)/(Ni++) | % Ni | No. of Water Content (x) | # TD, g/cc | d50, µm | Surface Area, m2/g | @ DC, mAh/g | |
|---|---|---|---|---|---|---|---|---|---|
| (Ni++), M | * CD, A/m2 | ||||||||
| 1 | 1.02 | 500 | 6.60 | 58.0 | 0.48 | 1.19 | 44.38 | 67 | 120 |
| 2 | 0.34 | 200 | 6.72 | 54.5 | 0.83 | 1.15 | 34.85 | 110 | 125 |
| Sl. No. | Experimental Condition | (OH–)/(Ni++) | % Ni | No. of Water Content (x) | # TD, g/cc | d50, µm | Surface Area, m2/g | @ DC, mAh/g | |
|---|---|---|---|---|---|---|---|---|---|
| (Ni++), M | * CD, A/m2 | ||||||||
| 1 | 1.02 | 50 | 0.45 | 50.5 | 1.30 | 1.18 | 10.56 | 238 | 200 |
| 2 | 1.19 | 200 | 2.34 | 52.0 | 1.12 | 1.46 | 19.43 | 151 | 140 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ash, B.; Nalajala, V.S.; Popuri, A.K.; Subbaiah, T.; Minakshi, M. Perspectives on Nickel Hydroxide Electrodes Suitable for Rechargeable Batteries: Electrolytic vs. Chemical Synthesis Routes. Nanomaterials 2020, 10, 1878. https://doi.org/10.3390/nano10091878
Ash B, Nalajala VS, Popuri AK, Subbaiah T, Minakshi M. Perspectives on Nickel Hydroxide Electrodes Suitable for Rechargeable Batteries: Electrolytic vs. Chemical Synthesis Routes. Nanomaterials. 2020; 10(9):1878. https://doi.org/10.3390/nano10091878
Chicago/Turabian StyleAsh, Baladev, Venkata Swamy Nalajala, Ashok Kumar Popuri, Tondepu Subbaiah, and Manickam Minakshi. 2020. "Perspectives on Nickel Hydroxide Electrodes Suitable for Rechargeable Batteries: Electrolytic vs. Chemical Synthesis Routes" Nanomaterials 10, no. 9: 1878. https://doi.org/10.3390/nano10091878
APA StyleAsh, B., Nalajala, V. S., Popuri, A. K., Subbaiah, T., & Minakshi, M. (2020). Perspectives on Nickel Hydroxide Electrodes Suitable for Rechargeable Batteries: Electrolytic vs. Chemical Synthesis Routes. Nanomaterials, 10(9), 1878. https://doi.org/10.3390/nano10091878






