The Influence of Photoactive Heterostructures on the Photocatalytic Removal of Dyes and Pharmaceutical Active Compounds: A Mini-Review
Abstract
1. Introduction
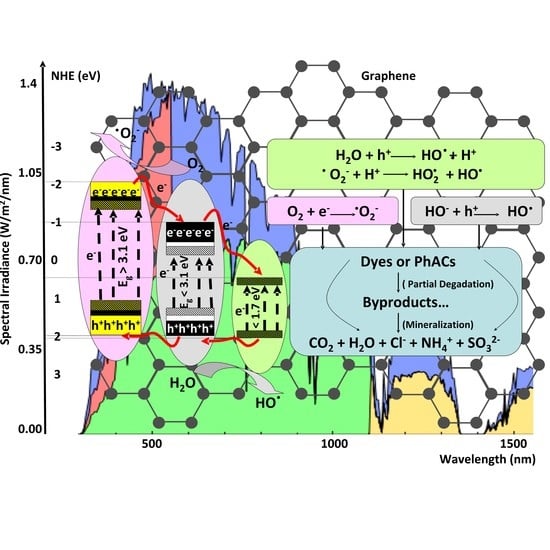
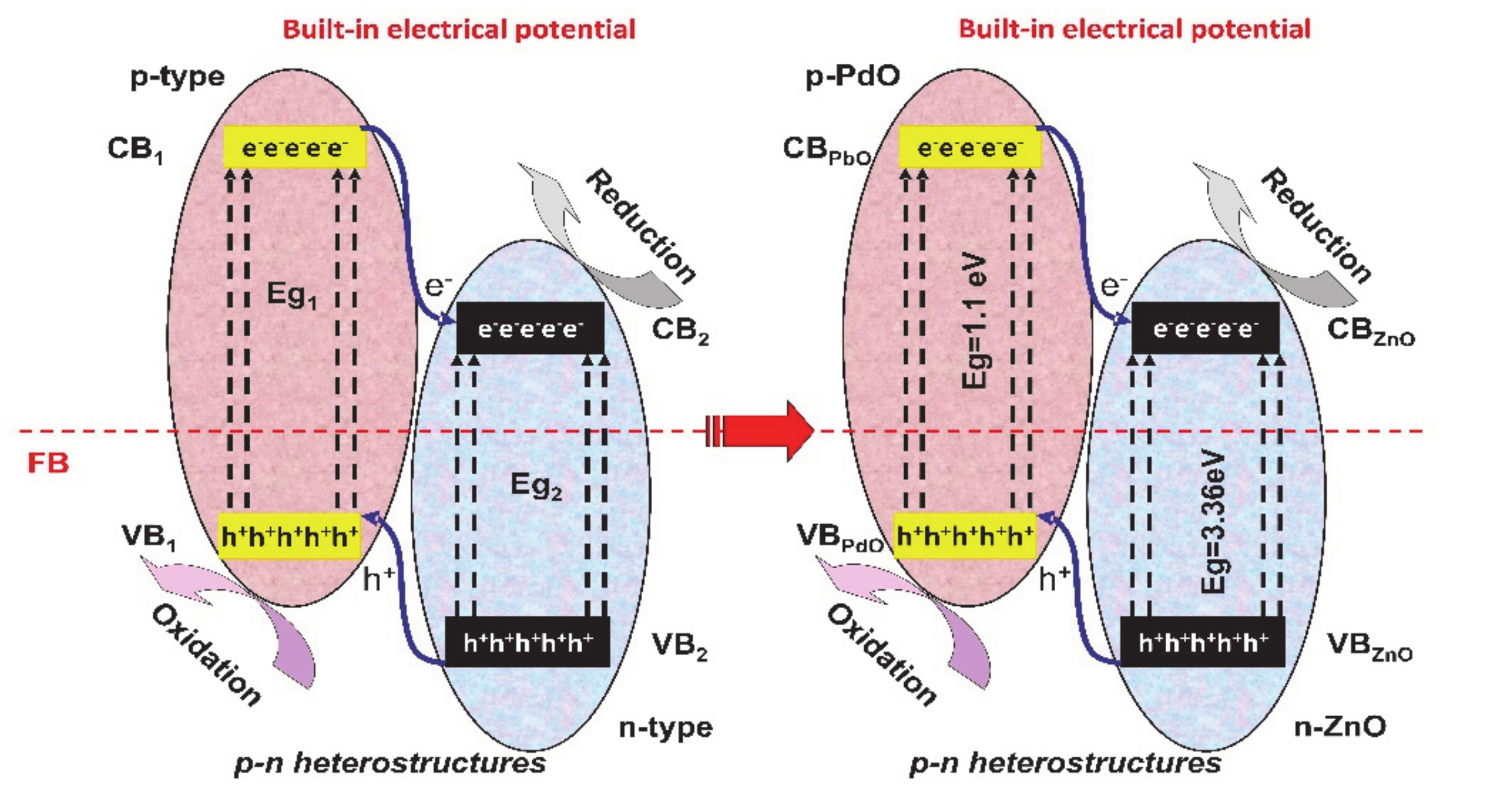
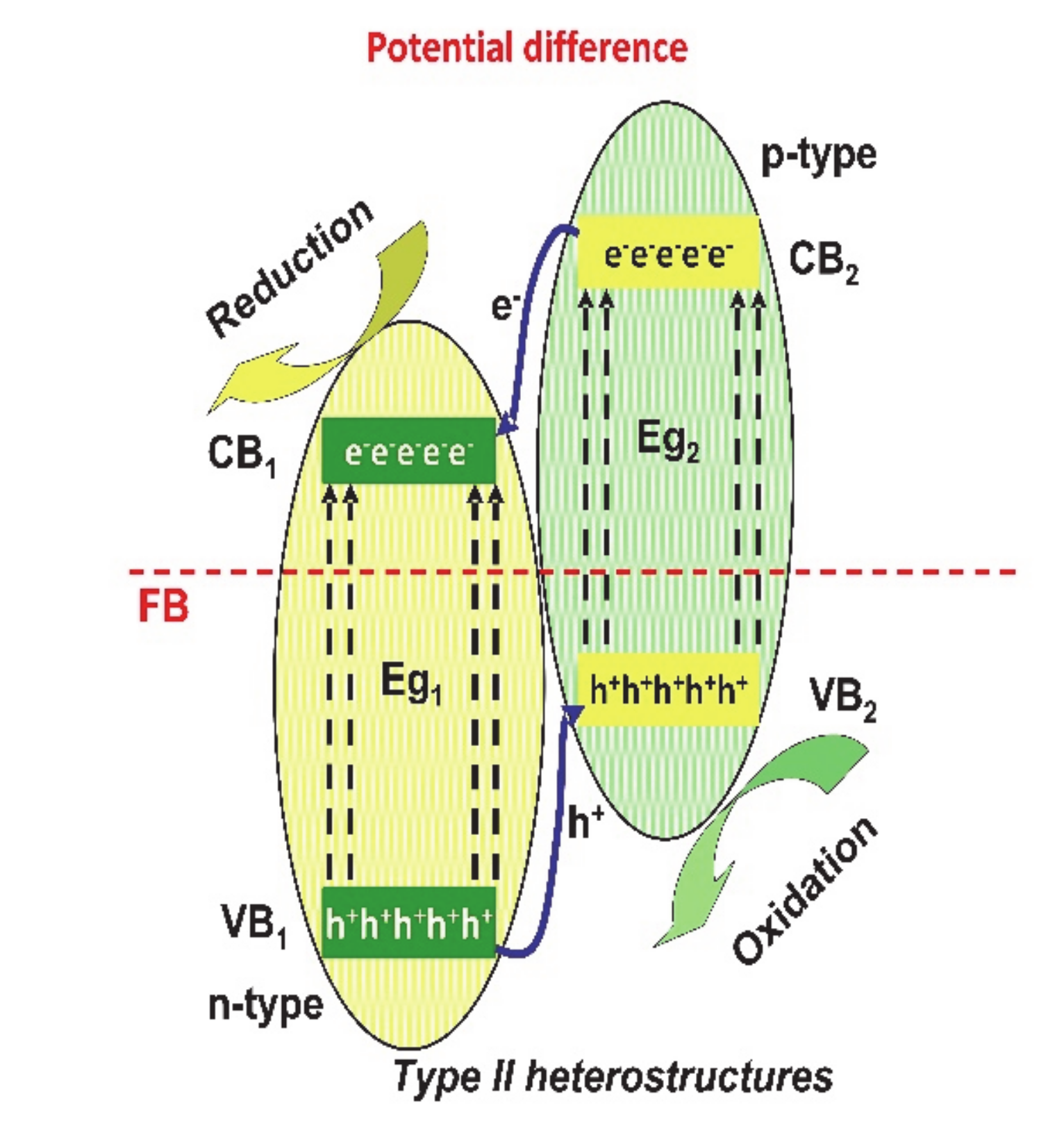
2. Heterostructure Mechanisms for Photocatalytic Application
3. Photocatalytic Organic Pollutants Removal by Heterostructures
3.1. Dyes
3.2. Pharmaceutical Active Compounds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, D.; Ngo, H.H.; Wei, D. A critical review on antibiotics and hormones in swine wastewater: Water pollution problems and control approaches. J. Hazard. Mater. 2020, 387, 121682. [Google Scholar] [CrossRef] [PubMed]
- Gautam, K.; Anbumani, S. Ecotoxicological effects of organic micro-pollutants on the environment. In Current Developments in Biotechnology and Bioengineering, 1st ed.; Varjani, S., Pandey, A., Tyagi, R.D., Ngo, H.H., Larroche, C., Eds.; Elsevier: New York, NY, USA, 2020; pp. 481–501. [Google Scholar]
- Duarte, R.M.; Matos, J.T.; Senesi, N. Organic pollutants in soils. In Soil Pollutions, 1st ed.; Duarte, A.C., Cachada, A., Rocha-Santos, T., Eds.; Elsevier: New York, NY, USA, 2018; pp. 103–126. [Google Scholar]
- Wood, D.; Shaw, S.; Cawte, T.; Shanen, E.; Heyst, B.V. An overview of photocatalyst immobilization methods for air pollution remedation. Chem. Eng. J. 2020, 391, 123490. [Google Scholar] [CrossRef]
- Hader, D.P.; Banaszak, A.T.; Helbling, E.W. Anthropogenic pollution of aquatic ecosystems: Emerging problems with global implications. Sci. Total Environ. 2020, 713, 136586. [Google Scholar] [CrossRef] [PubMed]
- Libralato, G.; Lofrano, G.; Siciliano, A.; Gambino, E.; Boccia, G.; Federica, C.; Francesco, A.; Galdiero, E.; Gesuele, R.; Guida, M. Toxicity assessment of wastewater after advanced oxidation processes for emerging contaminants’ degradation. In Visible Light Active Structured Photocatalysts for the Removal of Emerging Contaminants, 1st ed.; Sacco, O., Vaiano, V., Eds.; Elsevier: New York, NY, USA, 2020; pp. 195–211. [Google Scholar]
- Kaplan, A.; Mamane, H.; Lester, Y.; Avisar, D. Trace organic compound removal from wastewater reverse-osmosis concentrate by advanced oxidation processes with UV/O3/H2O2. Materials 2020, 13, 2785. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Zeng, L.; Qin, W.; Feng, J. Measures for reducing nitrate leaching in orchards: A review. Environ. Pollut. 2020, 263, 114553. [Google Scholar] [CrossRef]
- Serban, I.; Enesca, A. Metal Oxides-Based Semiconductors for Biosensors Applications. Front. Chem. 2020, 8, 354. [Google Scholar] [CrossRef]
- Tahreen, A.; Jami, M.S.; Ali, F. Role of electrocoagulation in wastewater treatment: A developmental review. J. Water Proc. Eng. 2020, 37, 101440. [Google Scholar] [CrossRef]
- Guo, W.; Umar, A.; Du, Y.; Wang, L.; Pei, M. Surface Modification of Bentonite with Polymer Brushes and Its Application as an Efficient Adsorbent for the Removal of Hazardous Dye Orange I. Nanomaterials 2020, 10, 1112. [Google Scholar] [CrossRef]
- Enesca, A.; Andronic, L.; Duta, A. The influence of surfactants on the crystalline structure, electrical and photocatalytic properties of hybrid multi-structured (SnO2, TiO2 and WO3) thin films. Appl. Surf. Sci. 2012, 258, 4339–4346. [Google Scholar] [CrossRef]
- Cornejo, O.M.; Murrieta, M.F.; Castañeda, L.F.; Nava, J.L. Characterization of the reaction environment in flow reactors fitted with BDD electrodes for use in electrochemical advanced oxidation processes: A critical review. Electrochim. Acta 2020, 33120, 135373. [Google Scholar] [CrossRef]
- Malakootian, M.; Shahesmaeili, A.; Faraji, M.; Amiri, H.; Martinez, S.S. Advanced oxidation processes for the removal of organophosphorus pesticides in aqueous matrices: A systematic review and meta-analysis. Process Saf. Environ. 2020, 134, 292–307. [Google Scholar] [CrossRef]
- Han, M.; Duan, X.; Cao, G.; Zhuc, S.; Ho, S.H. Graphitic nitride-catalyzed advanced oxidation processes (AOPs) for landfill leachate treatment: A mini review. Process Saf. Environ. 2020, 139, 230–240. [Google Scholar] [CrossRef]
- Yang, L.; He, L.; Xue, J.; Ma, Y.; Zhang, Z. UV/SO32− based advanced reduction processes of aqueous contaminants: Current status and prospects. Chem. Eng. J. 2020, 3971, 125412. [Google Scholar] [CrossRef]
- Bresolin, B.M.; Park, Y.; Bahnemann, D.W. Recent Progresses on Metal Halide Perovskite-Based Material as Potential Photocatalyst. Catalysts 2020, 10, 709. [Google Scholar] [CrossRef]
- Chen, L.; Tang, J.; Song, L.N.; Chen, P.; Yin, S.F. Heterogeneous photocatalysis for selective oxidation of alcohols and hydrocarbons. Appl. Catal. B 2019, 242, 379–388. [Google Scholar] [CrossRef]
- Chauhan, D.K.; Jain, S.; Battula, V.R.; Kailasam, K. Organic motif’s functionalization via covalent linkage in carbon nitride: An exemplification in photocatalysis. Carbon 2019, 152, 40–58. [Google Scholar] [CrossRef]
- Hu, X.; Ma, Q.; Wang, X.; Yang, Y.; Liu, N.; Zhang, C.; Kawazoe, N.; Chen, G.; Yang, Y. Layered Ag/Ag2O/BiPO4/Bi2WO6 heterostructures by two-step method for enhanced photocatalysis. J. Catal. 2020, 387, 28–38. [Google Scholar] [CrossRef]
- Ojha, D.P.; Karki, H.P.; Song, J.H.; Kim, H.J. Amine-assisted synthesis of FeWO4 nanorodg-C3N4 for enhanced visible light-driven Z-scheme photocatalysis. Compos. Part B Eng. 2019, 1601, 277–284. [Google Scholar] [CrossRef]
- Wetchakun, K.; Wetchakun, N.; Sakulsermsuk, S. An overview of solar/visible light-driven heterogeneous photocatalysis for water purification: TiO2- and ZnO-based photocatalysts used in suspension photoreactors. J. Ind. Eng. Chem. 2019, 7125, 19–49. [Google Scholar] [CrossRef]
- Yao, S.; Wang, J.; Zhou, X.; Zhou, S.; Pu, X.; Li, W. One-pot low-temperature synthesis of BiOX/TiO2 hierarchic composites of adsorption coupled with photocatalysis for quick degradation of colored and colorless organic pollutants. Adv. Powder Technol. 2020, 31, 1924–1932. [Google Scholar] [CrossRef]
- Kant, S.; Pathania, D.; Singh, P.; Dhiman, P.; Kumar, A. Removal of malachite green and methylene blue by Fe0.01Ni0.01Zn0.98O/polyacrylamide nanocompositeusing coupled adsorption and photocatalysis. Appl. Catal. B 2014, 147, 340–352. [Google Scholar] [CrossRef]
- Ma, Y.; Xiong, H.; Zhao, Z.; Yu, Y.; Zhou, D.; Dong, S. Model-based evaluation of tetracycline hydrochloride removal and mineralization in an intimately coupled photocatalysis and biodegradation reactor. Chem. Eng. J. 2018, 351, 967–975. [Google Scholar] [CrossRef]
- Li, F.; Lan, X.; Wang, L.; Kong, X.; Xu, P.; Tai, Y.; Liu, G.; Shi, J. An efficient photocatalyst coating strategy for intimately coupled photocatalysis and biodegradation (ICPB): Powder spraying method. Chem. Eng. J. 2020, 383, 123092. [Google Scholar] [CrossRef]
- Ceretta, M.B.; Vieira, Y.; Wolski, E.A.; Foletto, E.L.; Silvestri, S. Biological degradation coupled to photocatalysis by ZnO/polypyrrole composite for the treatment of real textile wastewater. J. Water Proc. Eng. 2020, 35, 101230. [Google Scholar] [CrossRef]
- Grcic, I.; Vrsaljko, D.; Katancic, Z.; Papic, S. Purification of household grey water loaded with hair colorants by solar photocatalysis using TiO2-coated textile fibers coupled flocculation with chitosan. J. Water Proc. Eng. 2015, 5, 15–27. [Google Scholar] [CrossRef]
- Xu, T.; Wang, P.; Wang, D.; Zhao, K.; Wei, M.; Liu, X.; Liu, H.; Cao, J.; Fan, H.; Yang, L. Ultrasound-assisted synthesis of hyper-dispersed type-II tubular Fe3O4@SiO2@ZnO/ZnS core/shell heterostructure for improved visible-light photocatalysis. J. Alloy. Compd. 2020, 838, 155689. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Quan, X.; Wu, Y. Enhanced generation of oxidative species and phenol degradation in a discharge plasma system coupled with TiO2 photocatalysis. Appl. Catal. B 2008, 83, 72–77. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Ma, J.; Peng, Y.; Wang, A. A review on bidirectional analogies between the photocatalysis and antibacterial properties of ZnO. J. Alloy. Compd. 2019, 78330, 898–918. [Google Scholar] [CrossRef]
- Li, H.; Zhang, N.; Zhao, F.; Liu, T.; Wang, Y. Facile Fabrication of a Novel Au/Phosphorus-Doped g-C3N4 Photocatalyst with Excellent Visible Light Photocatalytic Activity. Catalysts 2020, 10, 701. [Google Scholar] [CrossRef]
- Enesca, A.; Yamaguchi, Y.; Terashima, C.; Fujishima, A.; Nakata, K.; Duta, A. Enhanced UV-Vis photocatalytic performance of the CuInS2/TiO2/SnO2 hetero-structure for air decontamination. J. Catal. 2017, 350, 174–181. [Google Scholar] [CrossRef]
- Roques-Carmes, T.; Alem, H.; Hamieh, T.; Toufaily, J.; Frochot, C.; Villieras, F. Different strategies of surface modification to improve the photocatalysis properties: Pollutant adsorption, visible activation, and catalyst recovery. In Handbook of Smart Photocatalytic Materials, 1st ed.; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: New York, NY, USA, 2020; pp. 39–57. [Google Scholar]
- Zhang, X.; Teng, S.Y.; Loy, A.C.M.; How, B.S.; Leong, W.D.; Tao, X. Transition Metal Dichalcogenides for the Application of Pollution Reduction: A Review. Nanomaterials 2020, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Mouchaal, Y.; Enesca, A.; Mihoreanu, C.; Khelil, A.; Duta, A. Tuning the opto-electrical properties of SnO2 thin films by Ag+1 and In+3 co-doping. Mater. Sci. Eng. B 2015, 199, 22–29. [Google Scholar] [CrossRef]
- Song, B.; Zeng, Z.; Zeng, G.; Gong, J.; Tang, X. Powerful combination of g-C3N4 and LDHs for enhanced photocatalytic performance: A review of strategy, synthesis, and applications. Adv. Colloid Interface Sci. 2019, 272, 101999. [Google Scholar] [CrossRef] [PubMed]
- Salgado, B.C.; Cardeal, R.A.; Valentini, A. Photocatalysis and Photodegradation of Pollutants. In Nanomaterials Applications for Environmental Matrices, 1st ed.; Nascimento, R.F., Ferreira, O.P., De Paula, A.J., Neto, V.O., Eds.; Elsevier: New York, NY, USA, 2019; pp. 449–488. [Google Scholar]
- Enesca, A.; Andronic, L.; Duta, A. Optimization of Opto-Electrical and Photocatalytic Properties of SnO2 Thin Films Using Zn2+ and W6+ Dopant Ions. Catal. Lett. 2012, 142, 224–230. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, C.; Xiong, W.; Zeng, G.; Huang, D.; Zhang, C.; Wang, W.; Song, B.; Tang, X.; Li, X.; et al. Recent advances in application of graphitic carbon nitride-based catalysts for degrading organic contaminants in water through advanced oxidation processes beyond photocatalysis: A critical review. Water Res. 2020, 184, 116200. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, C.; Qi, Y.; Chen, Z.; Xu, Q. CO2-Induced Defect Engineering: A New Protocol by Doping Vacancies in 2D Heterostructures for Enhanced Visible-Light Photocatalysis. Appl. Surf. Sci. 2017, 419, 573–579. [Google Scholar] [CrossRef]
- Pedanekar, R.S.; Shaikh, S.K.; Rajpure, K.Y. Thin film photocatalysis for environmental remediation: A status review. Curr. Appl. Phys. 2020, 20, 931–952. [Google Scholar] [CrossRef]
- He, W.; Sun, Y.; Jiang, G.; Li, Y.; Dong, F. Defective Bi4MoO9/Bi metal core/shell heterostructure: Enhanced visible light photocatalysis and reaction mechanism. Appl. Catal. B 2018, 239, 619–627. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, J.; Xu, L.; Li, X.; Huang, S. A room-temperature methane sensor based on Pd-decorated ZnO/rGO hybrids enhanced by visible light photocatalysis. Sens. Actuat. B 2020, 3041, 127334. [Google Scholar] [CrossRef]
- Andronic, L.; Enesca, A.; Cazan, C.; Visa, M. TiO2-active carbon composites for wastewater photocatalysis. J. Sol-Gel Sci. Technol. 2014, 71, 399–405. [Google Scholar] [CrossRef]
- Irani, E.; Amoli-Diva, M. Hybrid adsorption–photocatalysis properties of quaternary magneto-plasmonic ZnO/MWCNTs nanocomposite for applying synergistic photocatalytic removal and membrane filtration in industrial wastewater treatment. J. Photochem. Photobiol. A Chem. 2020, 39115, 112359. [Google Scholar] [CrossRef]
- Kusmierek, E. Semiconductor Electrode Materials Applied in Photoelectrocatalytic Wastewater Treatment—An Overview. Catalysts 2020, 10, 439. [Google Scholar] [CrossRef]
- Xiao, W.Z.; Xu, L.; Rong, Q.Y.; Dai, X.Y.; Wang, L.L. Two-dimensional H-TiO2/MoS2(WS2) van der Waals heterostructures for visible-light photocatalysis and energy conversion. Appl. Surf. Sci. 2020, 504, 144425. [Google Scholar] [CrossRef]
- Kanakkillam, S.S.; Krishnan, B.; Avellaneda, D.A.; Shaji, S. Surfactant free stable cobalt oxide nanocolloid in water by pulsed laser fragmentation and its thin films for visible light photocatalysis. Colloid. Surface. A 2020, 5945, 124657. [Google Scholar] [CrossRef]
- Rafiq, U.; Majid, K. Mitigating the charge recombination by the targeted synthesis of Ag2WO4/Bi2Fe4O9 composite: The facile union of orthorhombic semiconductors towards efficient photocatalysis. J. Alloy. Compd. 2020, 84225, 155876. [Google Scholar] [CrossRef]
- Jia, S.; Xu, M.; Chen, S.; Yan, J.; Ma, X. A hierarchical sandwich-structured MoS2/SnO2/CC heterostructure for high photocatalysis performance. Mater. Lett. 2019, 236, 697–701. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Meng, X.; Shen, T.; Sun, J.; Han, W.; Wang, X. Effects of Particle Size on the Structure and Photocatalytic Performance by Alkali-Treated TiO2. Nanomaterials 2020, 10, 546. [Google Scholar] [CrossRef]
- Estahbanati, M.R.K.; Feilizadeh, M.; Babin, A.; Mei, B.; Iliuta, M.C. Selective photocatalytic oxidation of cyclohexanol to cyclohexanone: A spectroscopic and kinetic study. Chem. Eng. J. 2020, 38215, 122732. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, X.; Li, X.; Shao, C.; Liu, Y. Bismuth oxychloride (BiOCl)/copper phthalocyanine (CuTNPc) heterostructures immobilized on electrospun polyacrylonitrile nanofibers with enhanced activity for floating photocatalysis. J. Colloid Interface Sci. 2018, 525, 187–195. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Li, N.; Xu, Q.; Lu, J. Efficient reduction of Cr(VI) by a BMO/Bi2S3 heterojunction via synergistic adsorption and photocatalysis under visible light. J. Hazard. Mater. 2020, 4005, 123243. [Google Scholar] [CrossRef]
- Mintcheva, N.; Gicheva, G.; Panayotova, M.; Kulinich, S.A. Room-Temperature Synthesis of ZnS Nanoparticles Using Zinc Xanthates as Molecular Precursors. Materials 2020, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, C.; Gu, Y.; Cheng, W.; Zhao, C. Novel electronic properties of a new MoS2/TiO2 heterostructure and potential applications in solar cells and photocatalysis. Appl. Surf. Sci. 2017, 414, 34–40. [Google Scholar] [CrossRef]
- González-Burciaga, L.A.; Núñez-Núñez, C.M.; Morones-Esquivel, M.M.; Avila-Santos, M.; Lemus-Santana, A.; Proal-Nájera, J.B. Characterization and Comparative Performance of TiO2 Photocatalysts on 6-Mercaptopurine Degradation by Solar Heterogeneous Photocatalysis. Catalysts 2020, 10, 118. [Google Scholar] [CrossRef]
- Liu, N.; Lu, N.; Yu, H.T.; Chen, S.; Quan, X. Efficient day-night photocatalysis performance of 2D/2D Ti3C2/Porous g-C3N4 nanolayers composite and its application in the degradation of organic pollutants. Chemosphere 2020, 246, 125760. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, G.; Wang, J.; Hu, Z.; Su, Y. Step-scheme NiO/BiOI heterojunction photocatalyst for rhodamine photodegradation. App. Surf. Sci. 2020, 511, 145499. [Google Scholar] [CrossRef]
- Kumar, R.S.; Min, K.S.; Lee, S.H.; Mergu, N.; Son, Y.A. Synthesis of novel panchromatic porphyrin-squaraine dye and application towards TiO2 combined photocatalysis. J. Photochem. Photobiol. A 2020, 397, 112595. [Google Scholar] [CrossRef]
- Tsai, C.G.; Tseng, W.J. Preparation of TiN–TiO2 composite nanoparticles for organic dye adsorption and photocatalysis. Ceram. Int. 2020, 46, 14529–14535. [Google Scholar] [CrossRef]
- Hernández-Carrillo, M.A.; Torres-Ricárdez, R.; García-Mendoza, M.F.; Ramírez-Morales, E.; Pérez-Hernández, G. Eu-modified ZnO nanoparticles for applications in photocatalysis. Catal. Today 2020, 3491, 191–197. [Google Scholar] [CrossRef]
- Cao, L.; Li, Y.F.; Tong, Y.; Yang, R.; Sun, L.; Cao, O.; Chen, R. A novel Bi12TiO20/g-C3N4 hybrid catalyst with a bionic granum configuration for enhanced photocatalytic degradation of organic pollutants. J. Hazard. Mater. 2019, 379, 120808. [Google Scholar] [CrossRef]
- Shende, A.G.; Tiwari, C.S.; Bhoyar, T.H.; Vidyasagar, D.; Umare, S.S. BWO nano-octahedron coupled with layered g-C3N4: An efficient visible light active photocatalyst for degradation of cationic/anionic dyes, and N2 reduction. J. Molec. Liq. 2019, 296, 111771. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Mei, J.; Sarina, S.; Wu, Z.; Liao, T.; Yan, C.; Sun, Z. Strongly interfacial-coupled 2D-2D TiO2/g-C3N4 heterostructure for enhanced visible-light induced synthesis and conversion. J. Hazard. Mater. 2020, 394, 122529. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Lan, H.; Zhang, M.; Zhu, H.; Bu, M. Preparation of heterostructure g-C3N4/ZnO nanorods for high photocatalytic activity on different pollutants (MB, RhB, Cr(VI) and eosin). Ceram. Int. 2020, 46, 12192–12199. [Google Scholar] [CrossRef]
- Fu, S.; Yuan, W.; Liu, X.; Yan, Y.; Liu, H.; Li, L.; Zhao, F.; Zhou, J. A novel 0D/2D WS2/BiOBr heterostructure with rich oxygen vacancies for enhanced broad-spectrum photocatalytic performance. J. Colloid. Interf. Sci. 2020, 569, 150–163. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhang, H.; Wu, Y.; Jia, Y.; Jin, R.; Gao, S. CTAB-assisted solvothermal construction of hierarchical Bi2MoO6/Bi5O7Br with improved photocatalytic performances. Sep. Purif. Technol. 2020, 242, 116775. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, Z.; Feng, Y.; Lin, S.; Li, H.; Gao, X. Surface oxygen vacancy modified Bi2MoO6/MIL-88B(Fe) heterostructure with enhanced spatial charge separation at the bulk & interface. Appl. Catal. B 2020, 268, 118740. [Google Scholar]
- Bao, L.; Yang, F.; Cheng, D.; Pan, X.; Zhang, H.; Zhao, F.; Zhao, S.; Tegus, O. Modified electronic structure of Ta2O5 via surface decorated with Ta3B2 nanodots for enhanced photocatalytic activity. App. Surf. Sci. 2020, 513, 145767. [Google Scholar] [CrossRef]
- Jin, J.; Xie, Y. Ultraviolet light induced oxygen vacancy-rich BiPO4−x/Bi2S3 nanorods with enhanced photocatalytic activity and mechanism. Res. Chem. Intermed. 2019, 45, 5609–5623. [Google Scholar] [CrossRef]
- Mandal, B.; Panda, J.; Paul, P.K.; Sarkar, R.; Tudu, B. MnFe2O4 decorated reduced graphene oxide heterostructures: Nanophotocatalyst for methylene blue dye degradation. Vacuum 2020, 173, 109150. [Google Scholar] [CrossRef]
- Alido, J.P.M.; Sari, F.N.I.; Ting, Y.M. Synthesis of Ag/hybridized 1T-2H MoS2/TiO2 heterostructure for enhanced visible-light photocatalytic activity. Ceram. Int. 2019, 45, 23651–23657. [Google Scholar] [CrossRef]
- Tian, Q.; Fang, G.; Ding, L.; Ran, M.; Zhang, H.; Pan, A.; Shen, K.; Deng, Y. ZnAl2O4/Bi2MoO6 heterostructures with enhanced photocatalytic activity for the treatment of organic pollutants and eucalyptus chemimechanical pulp wastewater. Mater. Chem. Phys. 2020, 241, 122299. [Google Scholar] [CrossRef]
- Çinar, B.; Kerimoglu, I.; Tonbül, B.; Demirbüken, A.; Dursun, S.; Kaya, I.C.; Kalem, V.; Akyildiz, H. Hydrothermal/electrospinning synthesis of CuO plate-like particles/TiO2 fibers heterostructures for high-efficiency photocatalytic degradation of organic dyes and phenolic pollutants. Mater. Sci. Semic. Proces. 2020, 109, 104919. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, J.; Geng, J.; Bao, R.; Wang, Z.; Xia, J.; Li, H. Perovskite LaNiO3/TiO2 step-scheme heterojunction with enhanced photocatalytic activity. Appl. Surf. Sci. 2020, 503, 144287. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Z.; Kong, X.; He, F.; Zhao, R.; Wua, R.; Wei, T.; Wang, L.; Feng, J. A novel P-N heterojunction with staggered energy level based on ZnFe2O4 decorating SnS2 nanosheet for efficient photocatalytic degradation. App. Surf. Sci. 2020, 510, 145442. [Google Scholar] [CrossRef]
- Yan, H.; Zhu, Z.; Long, Y.; Li, W. Single-source-precursor-assisted synthesis of porous WO3/g-C3N4 with enhanced photocatalytic property. Colloid. Surfaces A 2019, 582, 123857. [Google Scholar] [CrossRef]
- Neto, N.F.A.; Lima, A.B.; Bomio, M.R.D.; Motta, F.V. Microwave-assisted hydrothermal synthesis of Ag2Mo1−xWxO4 (x = 0, 0.25, 0.50, 0.75 and 1 mol%) heterostructures for enhanced photocatalytic degradation of organic dyes. J. Alloy. Compd. 2020, 844, 156007. [Google Scholar]
- Wei, Y.; Huang, Y.; Fang, Y.; Zhao, Y.; Luo, D.; Guo, Q.; Fan, L.; Wu, J. Hollow mesoporous TiO2/WO3 sphere heterojunction with high visiblelight-driven photocatalytic activity. Mater. Res. Bull. 2019, 119, 110571. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, L.; Xin, A.; Yu, Y.; Wang, L.; Zhang, W. Visible light response and heterostructure of composite CdS@ZnSeZnO to enhance its photocatalytic activity. J. Alloys Compd. 2020, 813, 152190. [Google Scholar] [CrossRef]
- Yulizar, Y.; Apriandanua, D.O.B.; Ashna, R.I. La2CuO4-decorated ZnO nanoparticles with improved photocatalytic activity for malachite green degradation. Chem. Phys. Lett. 2020, 755, 137749. [Google Scholar] [CrossRef]
- Jia, J.; Du, X.; Zhang, Q.; Liu, E.; Fan, J. Z-scheme MgFe2O4/Bi2MoO6 heterojunction photocatalyst with enhanced visible light photocatalytic activity for malachite green removal. Appl. Surf. Sci. 2019, 492, 527–539. [Google Scholar] [CrossRef]
- Isari, A.A.; Hayati, F.; Kakavandi, B.; Rostami, M.; Dehghanifard, E. Cu co-doped TiO2@functionalized SWCNT photocatalyst coupled with ultrasound and visible-light: An effective sono-photocatalysis process for pharmaceutical wastewaters treatment. Chem. Eng. J. 2020, 392, 123685. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Zhang, W.; Liu, Z.; Chen, M. Advances in photocatalysis based on fullerene C60 and its derivatives: Properties, mechanism, synthesis, and applications. Appl. Catal. B 2020, 26515, 118579. [Google Scholar] [CrossRef]
- Ahmad, K.; Ghatak, H.R.; Ahuja, S.M. A review on photocatalytic remediation of environmental pollutants and H2 production through water splitting: A sustainable approach. Environ. Technol. Innov. 2020, 19, 100893. [Google Scholar] [CrossRef]
- Kovacic, M.; Papac, J.; Kusic, H.; Karamanis, P.; Bozic, A.L. Degradation of polar and non-polar pharmaceutical pollutants in water by solar assisted photocatalysis using hydrothermal TiO2-SnS2. Chem. Eng. J. 2020, 382, 122826. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, X.; Jiang, L.; Zhang, J.; Zeng, G. Powerful combination of 2D g-C3N4 and 2D nanomaterials for photocatalysis: Recent advances. Chem. Eng. J. 2020, 39015, 124475. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 27015, 110906. [Google Scholar] [CrossRef]
- Acharya, L.; Nayak, S.; Pattnaik, S.P.; Acharya, R.; Parida, K. Resurrection of boron nitride in p-n type-II boron nitride/B-doped-g-C3N4 nanocomposite during solid-state Z-scheme charge transfer path for the degradation of tetracycline hydrochloride. J. Colloid Interface Sci. 2020, 566, 211–223. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, W.; Zhu, J.; Fu, L.; Li, D.; Zhou, L. Enhanced visible light photocatalytic activity of g-C3N4 decorated ZrO2−x nanotubes heterostructure for degradation of tetracycline hydrochloride. J. Hazard. Mater. 2020, 384, 121275. [Google Scholar] [CrossRef]
- Shi, W.; Liu, C.; Li, M.; Lin, X.; Guo, F.; Shi, J. Fabrication of ternary Ag3PO4/Co3(PO4)2/g-C3N4 heterostructure with following Type II and Z-Scheme dual pathways for enhanced visible-light photocatalytic activity. J. Hazard. Mater. 2020, 389, 121907. [Google Scholar] [CrossRef]
- Kang, J.; Jin, C.; Li, Z.; Wang, M.; Chen, Z.; Wang, Y. Dual Z-scheme MoS2/g-C3N4/Bi24O31Cl10 ternary heterojunction photocatalysts for enhanced visible-light photodegradation of antibiotic. J. Alloys Compd. 2020, 825, 153975. [Google Scholar] [CrossRef]
- Wang, L.; Yang, G.; Wang, D.; Lu, C.; Guan, W.; Li, Y.; Deng, J.; Crittenden, J. Fabrication of the flower-flake-like CuBi2O4/Bi2WO6 heterostructure as efficient visible-light driven photocatalysts: Performance, kinetics and mechanism insight. Appl. Surf. Sci. 2019, 495, 143521. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, H.; Li, D.; Zou, Z.; Xia, Z. Microwave-assisted synthesis of La(OH)3/BiOCl n-n heterojunctions with high oxygen vacancies and its enhanced photocatalytic properties. Chem. Phys. Lett. 2019, 736, 136805. [Google Scholar] [CrossRef]
- Huang, J.; Chen, W.; Yu, X.; Fu, X.; Zhu, Y.; Zhang, Y. Fabrication of a ternary BiOCl/CQDs/rGO photocatalyst: The roles of CQDs and rGO in adsorption-photocatalytic removal of ciprofloxacin. Colloid. Surf. A 2020, 597, 124758. [Google Scholar] [CrossRef]
- Lu, C.; Guo, F.; Yan, Q.; Zhang, Z.; Li, D.; Wang, L.; Zhou, Y. Hydrothermal synthesis of type II ZnIn2S4/BiPO4 heterojunction photocatalyst with dandelion-like microflower structure for enhanced photocatalytic degradation of tetracycline under simulated solar light. J. Alloys Compd. 2019, 811, 151976. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, H.; Ma, X.; Zhang, X.; Zhu, Y.; Zhang, A.; Cao, Y.; Yang, P. Construction of AgI/Bi2MoO6/AgBi(MoO4)2 multi-heterostructure composite nanosheets for visible-light photocatalysis. Mater. Today Commun. 2020, 23, 100903. [Google Scholar] [CrossRef]
- Chen, M.; Dai, Y.; Guo, J.; Yang, H.; Liu, D.; Zhai, Y. Solvothermal synthesis of biochar@ZnFe2O4/BiOBr Z-scheme heterojunction for efficient photocatalytic ciprofloxacin degradation under visible light. Appl. Surf. Sci. 2019, 493, 1361–1367. [Google Scholar] [CrossRef]
- Bariki, R.; Majhi, D.; Das, K.; Behera, A.; Mishra, B.G. Facile synthesis and photocatalytic efficacy of UiO-66/CdIn2S4 nanocomposites with flowerlike 3D-microspheres towards aqueous phase decontamination of triclosan and H2 evolution. Appl. Catal. B 2020, 270, 118882. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Czech, B.; Goktaş, A.C.; Yubuta, K.; Kadirova, Z.C. SnO2@ZnS photocatalyst with enhanced photocatalytic activity for the degradation of selected pharmaceuticals and personal care products in model wastewater. J. Alloy. Compd. 2020, 827, 154339. [Google Scholar] [CrossRef]
- Li, M.; Xu, G.; Guan, Z.; Wang, Y.; Yu, H.; Yu, Y. Synthesis of Ag/BiVO4/rGO composite with enhanced photocatalytic degradation of triclosan. Sci. Total Environ. 2019, 664, 230–239. [Google Scholar] [CrossRef]
- Chang, C.; Yang, H.; Mu, W.; Cai, Y.; Wang, L.; Yang, L.; Qin, H. In situ fabrication of bismuth oxyiodide (Bi7O9I3/Bi5O7I) n-n heterojunction for enhanced degradation of triclosan (TCS) under simulated solar light irradiation. Appl. Catal. B. 2019, 254, 647–658. [Google Scholar] [CrossRef]
- Yu, T.; Wu, W.W.; Liu, L.; Gao, C.; Yang, T. Novel ternary p-ZnIn2S4/rGO/n-g-C3N4 Z-scheme nanocatalyst with enhanced antibiotic degradation in a dark self-biased fuel cell. Ceram. Int. 2020, 46, 9567–9574. [Google Scholar] [CrossRef]
- Yentür, G.; Dükkanci, M. Synthesis of Visible-Light Heterostructured Photocatalyst of Ag/AgCl Deposited on (040) Facet of Monoclinic BiVO4 for Efficient Carbamazepine Photocatalytic Removal. Appl. Surf. Sci. 2020, in press. [Google Scholar] [CrossRef]
- Yentür, G.; Dükkancı, M. Fabrication of magnetically separable plasmonic composite photocatalyst of Ag/AgBr/ZnFe2O4 for visible light photocatalytic oxidation of carbamazepine. Appl. Surf. Sci. 2020, 510, 145374. [Google Scholar] [CrossRef]
- Hu, Z.; Cai, X.; Wang, Z.; Li, S.; Wang, Z.; Xie, X. Construction of carbon-doped supramolecule-based g-C3N4/TiO2 composites for removal of diclofenac and carbamazepine: A comparative study of operating parameters, mechanisms, degradation pathways. J. Hazard. Mater. 2019, 380, 120812. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Y.; Li, L. Facet-engineered surface and interface design of WO3/Bi2WO6 photocatalyst with direct Z-scheme heterojunction for efficient salicylic acid removal. Appl. Surf. Sci. 2020, 508, 144796. [Google Scholar] [CrossRef]
- Plodinec, M.; Grcic, I.; Willinger, M.G.; Hammud, A.; Huang, X.; Panzic, I.; Gajovi, A. Black TiO2 nanotube arrays decorated with Ag nanoparticles for enhanced visible-light photocatalytic oxidation of salicylic acid. J. Alloy. Compd. 2019, 776, 883–896. [Google Scholar] [CrossRef]
- Malefane, M.E.; Feleni, U.; Mafa, P.J.; Kuvarega, A.T. Fabrication of direct Z-scheme Co3O4/BiOI for ibuprofen and trimethoprim degradation under visible light irradiation. Appl. Surf. Sci. 2020, 514, 145940. [Google Scholar] [CrossRef]
- Deng, Y.; Feng, C.; Tang, L.; Zhou, Y.; Chen, Z.; Feng, H.; Wang, J.; Yu, J.; Liu, Y. Ultrathin low dimensional heterostructure composites with superior photocatalytic activity: Insight into the multichannel charge transfer mechanism. Chem. Eng. J. 2020, 393, 124718. [Google Scholar] [CrossRef]
- Liu, N.; Wang, J.; Wu, J.; Li, Z.; Huang, W.; Zheng, Y.; Lei, J.; Zhang, X.; Tang, L. Magnetic Fe3O4@MIL-53(Fe) nanocomposites derived from MIL-53(Fe) for the photocatalytic degradation of ibuprofen under visible light irradiation. Mater. Res. Bull. 2020, 132, 111000. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Yang, Y.; Zhou, Z.; Shang, Y.; Zhuang, X.; Zhang, T. Two-stage calcination composite of Bi2O3-TiO2 supported on powdered activated carbon for enhanced degradation of sulfamethazine under solar irradiation. J. Water Proc. Eng. 2020, 35, 101220. [Google Scholar] [CrossRef]
- Liang, M.; Yu, Y.; Wang, Y.; Yu, Y. Remarkably efficient charge transfer through a double heterojunction mechanism by a CdS-SnS-SnS2/rGO composite with excellent photocatalytic performance under visible light. J. Hazard. Mater. 2020, 391, 121016. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; Zeng, X.; Lo, I. Development of g-C3N4/TiO2/Fe3O4@SiO2 heterojunction via sol-gel route: A magnetically recyclable direct contact Z-scheme nanophotocatalyst for enhanced photocatalytic removal of ibuprofen from real sewage effluent under visible light. Chem. Eng. J. 2018, 353, 645–656. [Google Scholar] [CrossRef]
- Ji, H.; Du, P.; Zhao, D.; Li, S.; Sun, F.; Duin, E.C.; Liu, W. 2D/1D graphitic carbon nitride/titanate nanotubes heterostructure for efficient photocatalysis of sulfamethazine under solar light: Catalytic “hot spots” at the rutile–anatase–titanate interfaces. Appl. Catal. B 2020, 263, 118357. [Google Scholar] [CrossRef]
- Di, G.; Zhu, Z.; Zhang, H.; Qiu, Y.; Yin, D.; Crittenden, J. Simultaneous sulfamethazine oxidation and bromate reduction by Pd mediated Z-scheme Bi2MoO6/g-C3N4 photocatalysts: Synergetic mechanism and degradative pathway. Chem. Eng. J. 2020, 401, 126061. [Google Scholar] [CrossRef]
- Cao, Y.; Fang, Y.; Lei, X.; Tan, B.; Hu, X.; Liu, B.; Chen, Q. Fabrication of novel CuFe2O4/MXene hierarchical heterostructures for enhanced photocatalytic degradation of sulfonamides under visible light. J. Hazard. Mater. 2020, 387, 122021. [Google Scholar] [CrossRef] [PubMed]
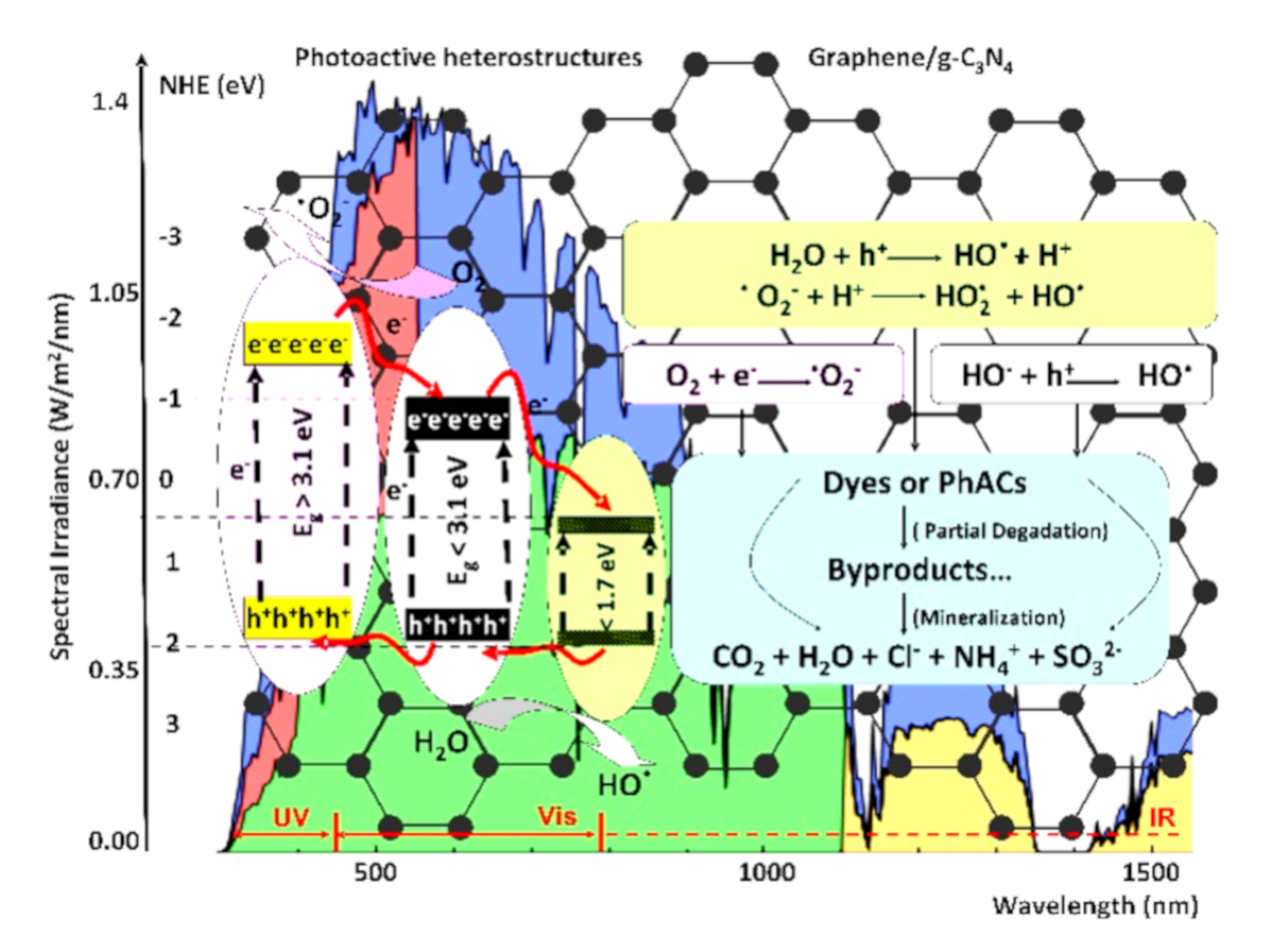
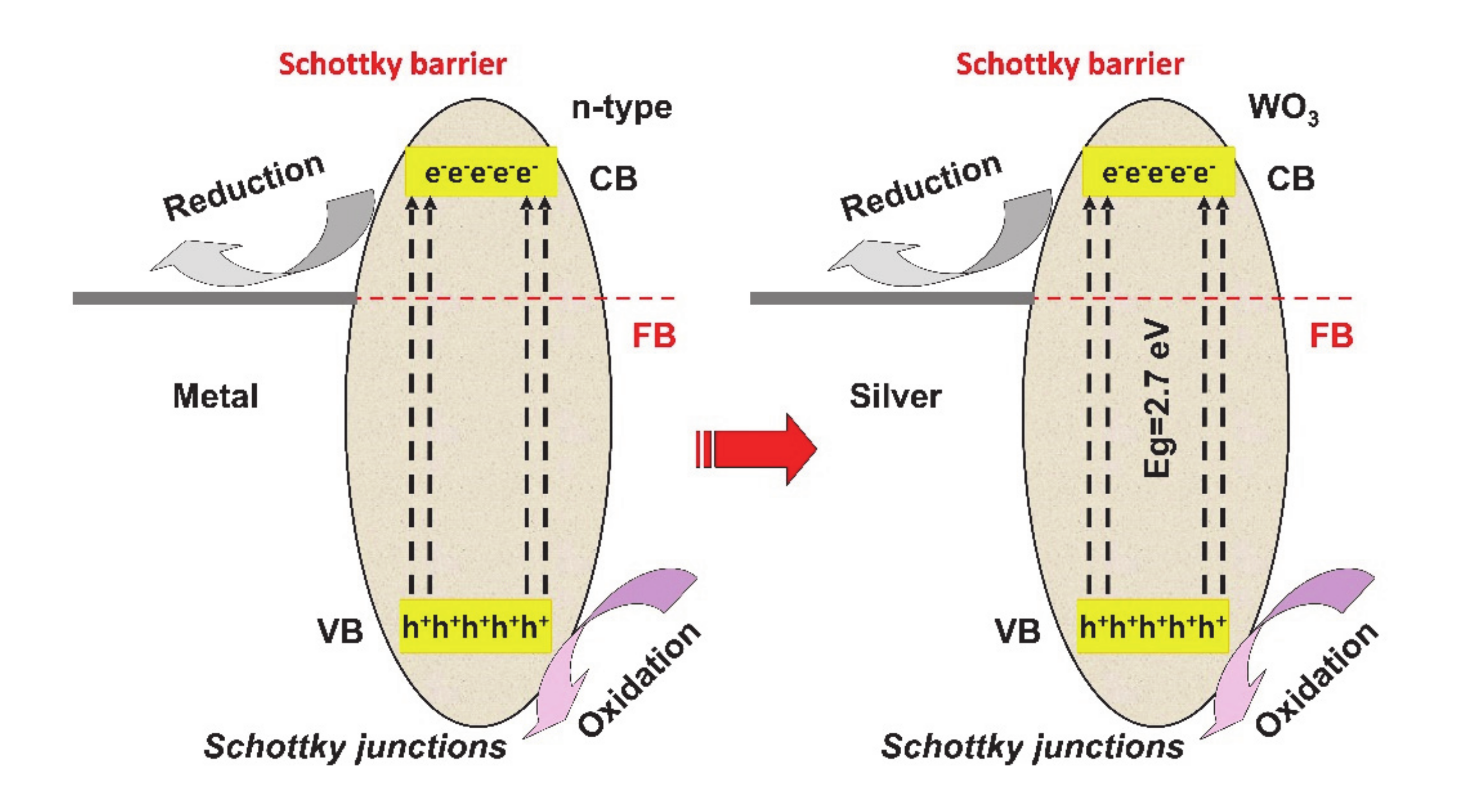
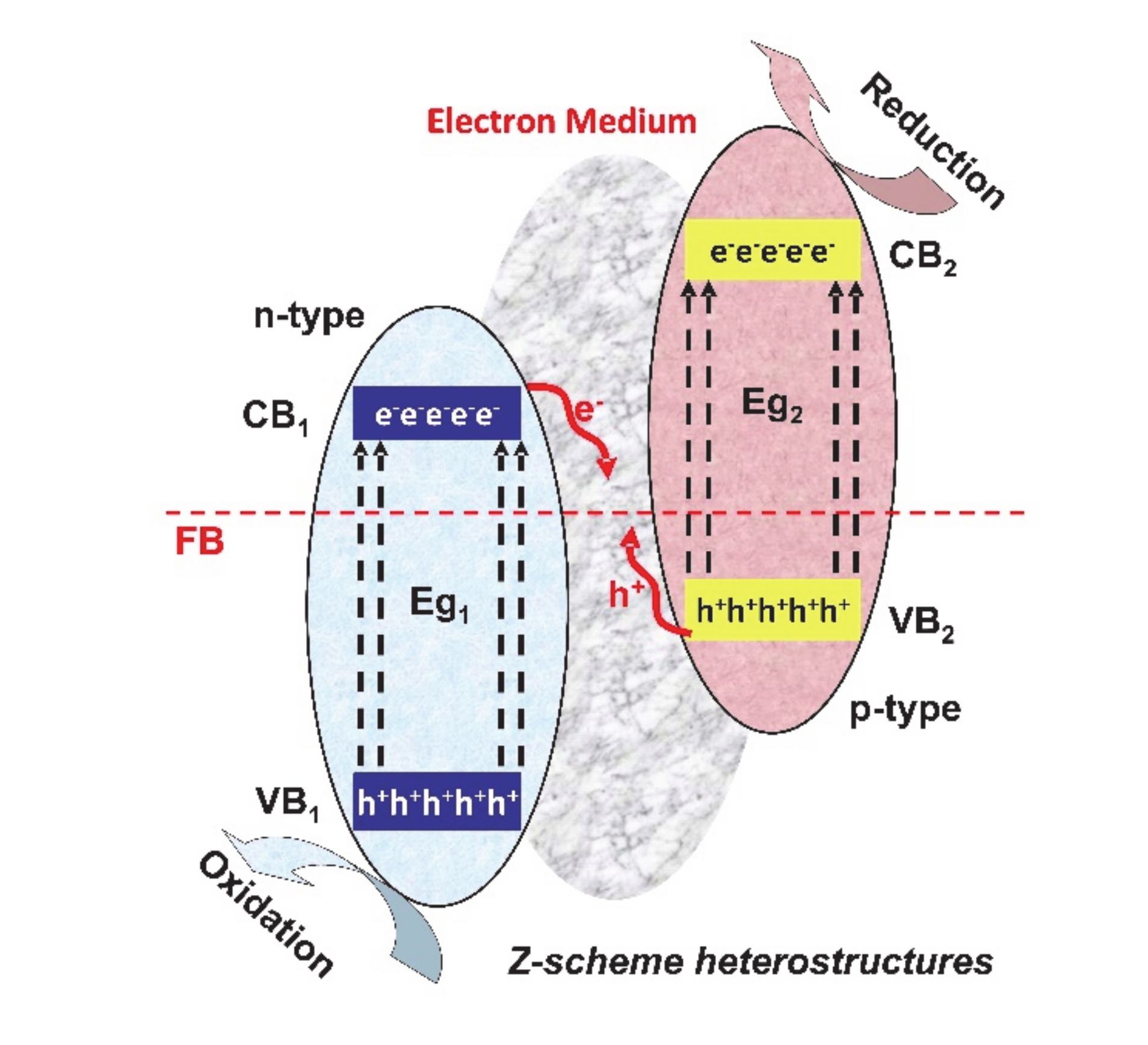
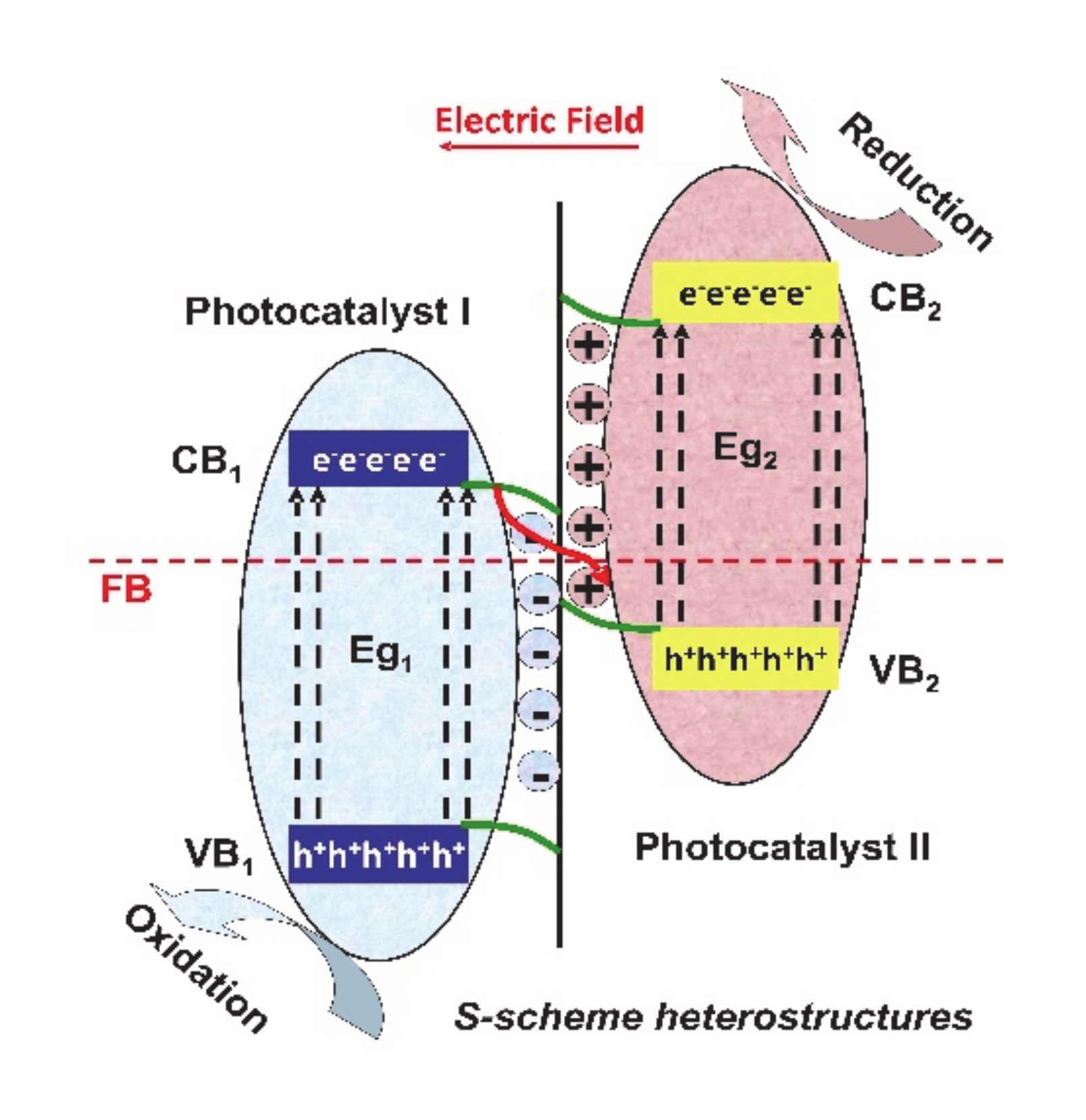
| Heterostructure Composition | Synthesis Method | Morphology/Crystallinity | Pollutant and Concentration (mg/L) | Radiation Type, Intensity (I), Irradiation Time (t) | Efficiency and Rate Constant (min−1) | Ref. |
|---|---|---|---|---|---|---|
| Bi12TiO20/g-C3N4 | Ultrasonication | Multilayer structure/cubic Bi12TiO20 | RhB = 10 MO = 20 | Vis I = 500 W t = 50 min | 97% (RhB), 90% (MO)/0.0537 (RhB), 0.0328 (MO) | [64] |
| Bi3.84W0.16O6.24 (BWO)/g-C3N4 | Ultrasonication | Sheets/cubic BWO | RhB = 10 | Vis I = 100 W t = 50 min | 99.8%/ 0.0562 | [65] |
| Bi2MoO6/Bi5O7Br/TiO2 | Solvothermal | Tube arrays/anatase TiO2, orthorhombic Bi2MoO6 | RhB = 10 MO = 16 MB = 6.5 | Vis I = 500 W t = 180 min | 73.43% (RhB) 47.77% (MO) 93.81% (MB)/0.00742 (RhB) 0.00354 (MO) 0.00225 (MB) | [69] |
| Bi2MoO6/Fe3O | Solvothermal | Flower/orthorhombic Bi2MoO6 | RhB = 20 | Vis I = 350 W t = 120 min | 99.5%/ 0.0364 | [70] |
| NiO/BiOI | Solvothermal | Foam/crystalline NiO | RhB = 4.8 | Vis I = 300 W t = 60 min | 90%/ 0.0572 | [60] |
| TiO2/g-C3N4 | Hydrothermal | 2D sheet/pristine 2D-TiO2 | RhB = 10 | Vis I = 500 W t = 60 min | 85%/ 0.03 | [66] |
| WS2/BiOBr | Hydrothermal | Plates/tetragonal BiOBr | RhB = 20 | Vis I = 500 W t = 100 min | 95%/ np * | [68] |
| g-C3N4/ZnO | Hydrothermal | Rod/hexagonal wurtzite ZnO | RhB = 10 MB = 10 | Vis I = 300 W t = 70 min | 98% (MB), 98.5% (RhB)/ np | [67] |
| BiPO4−x/B2S3 | Hydrothermal | Sheet/monoclinic BiPO4 and orthorhombic Bi2S3 | MB = 5 | Vis I = 300 W t = 360 min | 98%/ 0.0222 | [72] |
| MnFe2O4/rGO | Coprecipitation | Spherical/cubic MnFe2O4 | MB = 10 | UV I = 40 W t = 60 min | 97%/ 0.0589 | [73] |
| ZnAl2O4/Bi2MoO6 | Coprecipitation | Sheet/koechlinite Bi2MoO6 and gahnite ZnAl2O4 | MB = 30 | UV I = 100 W t = 180 min | 86.36%/ 0.638 | [75] |
| Ag/hybridized 1T-2H MoS2/TiO2 | Chemical reduction | Flower/anatase TiO2 | MB = 20 | UV I = 235 W t = 60 min | 96.8%/ 0.0539 | [74] |
| Ta3B2@Ta2O5 | In situ | Powder/crystalline Ta3B2 and Ta2O5 | MB = 50 | Vis I = 500 W t = 180 min | 80%/ np | [71] |
| CuO–TiO2 | Ultrasonication | Fiber/anatase TiO2 and monoclinic CuO | MB = 1 | UVc, Vis IUVc = 96 W IVis = 250 W tUVc = 30 min tVis = 240 min | 99% (UVc) 98% (Vis)/ 0.135 (UVc) 0.015 (Vis) | [76] |
| WO3/g-C3N4 | Polymerization | Sheet/crystalline WO3/g-C3N4 | MO = 10 | Vis I = 300 W t = 120 min | 93%/ 0.0213 | [79] |
| LaNiO3/TiO2 | Sol–gel | Particles/perovskite LaNiO3, anatase and rutile TiO2 | MO = 10 MO = 20 | Vis I = 300 W t = 150 min | 100% (10 mg/L) 92% (20 mg/L)/ np | [77] |
| ZnFe2O4/SnS2 | Solvothermal | Particles/crystalline ZnFe2O4 and SnS2 | MO = 50 | Vis I = 300 W t = 20 min | 99%/ 0.214 | [78] |
| Ag2Mo1−xWxO4 | Microwave-assisted hydrothermal | Rod/cubic Ag2MoO4, orthorhombic Ag2WO4 | MO = 5 | UVc I = 90 W t = 140 min | 45%/ 0.0058 | [80] |
| TiO2/WO3 | One pot | Hollow sphere/anatase TiO2 and monoclinic WO3 | MG = 50 | Vis I = 300 W t = 60 min | 98%/ 0.0746 | [81] |
| La2CuO4-decorated ZnO | In situ extraction | Particles/crystalline ZnO, orthorhombic La2CuO4 | MG = 25 | Vis I = 125 W t = 120 min | 91%/ 0.063 | [83] |
| MgFe2O4/Bi2MoO6 | Hydrothermal | Plates/crystalline Bi2MoO6 and MgFe2O4 | MG = 20 | Vis I = 300 W t = 120 min | 97%/ 0.0113 | [84] |
| CdS@ZnS@ZnO | Hydrothermal | Spherical/cubic ZnS, hexagonal CdS and ZnO | MG = 50 | UV, Vis IUV = 125 W IVis = 400 W tUV = 30 min tVis = 180 min | 95% (UV) 65% (Vis)/ np | [82] |
| Heterostructure Composition | Synthesis Method | Morphology/Crystallinity | Pollutant and Concentration (mg/L) | Radiation Type, Intensity (I), Irradiation Time (t) | Efficiency and Rate Constant (min−1) | Ref. |
|---|---|---|---|---|---|---|
| BN/B-doped-g-C3N4 | In situ growth | Sheet/hexagonal BN | TC = 10 | Vis I = 300 W t = 60 min | 88.1%/ 0.034 | [91] |
| WO3/g-C3N4 | Polymerization | Sheet/crystalline WO3/g-C3N4 | TC = 10 | Vis I = 300 W t = 180 min | 97%/ np * | [79] |
| Ag3PO4/Co3(PO4)2/g-C3N4 | Precipitation | 3D flower/crystalline Co3(PO4)2, g-C3N4 and Ag3PO4 | TC = 10 | Vis I = 300 W t = 120 min | 88%/ 0.0159 | [93] |
| g-C3N4-decorated ZrO2−x | Anodic oxidation and PVD | Tube/tetragonal and monoclinic zirconia | TC = 10 | Vis I = 300 W t = 60 min | 90.6%/ 0.0474 | [92] |
| ZnIn2S4/BiPO4 | Hydrothermal | Flower/monoclinic BiPO4 | TC = 40 | Vis I = 300 W t = 90 min | 84%/ 0.0201 | [98] |
| AgI/Bi2MoO6/AgBi(MoO4)2 | Hydrothermal | Sheets/crystalline AgI, Bi2MoO6 and AgBi(MoO4)2 | TC = 5 | Vis I = 400 W t = 90 min | 91.9%/ 0.0097 | [99] |
| MoS2/g-C3N4/Bi24O31Cl10 | Calcination | Sheet/monoclinic Bi24O31Cl10 and MoS2 | TC = 20 | Vis I = 300 W t = 50 min | 97.5%/ 0.0642 | [94] |
| CuBi2O4/Bi2WO6 | Hydrothermal | Pseudo-sphere/tetragonal CuBi2O4 and orthorhombic Bi2WO6 | TC = 20 | Vis I = 300 W t = 120 min | 93%/ 0.0286 | [95] |
| La(OH)3/BiOCl | Microwave | Sheet/crystalline BiOCl | TC = 20 | Vis I = 5 W t = 60 min | 85%/ 0.037 | [96] |
| WS2/BiOBr | Hydrothermal | Plates/tetragonal BiOBr | TC = 20 CIP = 20 | Vis I = 500 W t = 100 min | 96% (TC) 92% (CIP)/ np (TC) 0.01708 (CIP) | [68] |
| BiOCl/CQDs/rGO | Hydrothermal | Sheet/tetragonal BiOCl | CIP = 20 | Vis I = 300 W t = 100 min | 87%/ 0.0146 | [97] |
| LaNiO3/TiO2 | In situ sol–gel | Granular/anatase TiO2 and perovskite LaNiO3 | CIP = 10 | UV, Vis IUV, Vis = 300 W t = 180 min | 90% (UV) 55% (Vis)/ np | [77] |
| PVPbiochar@ZnF2O4/BiOBr | Solvothermal | Sheet/tetragonal BiOBr, spinel ZnFe2O4 | CIP = 15 | Vis I = 300 W t = 60 min | 84%/ np | [100] |
| UiO-66/CdIn2S4 | Solvothermal | 3D flower/pristine CIS | TCS = 10 | Vis I = 150 W t = 180 min | 92%/ 0.0094 | [101] |
| Ag/BiVO4/rGO | Hydrothermal | Irregular Particles/monoclinic BiVO4 | TCS = 10 | Vis I = 300 W t = 120 min | 100%/ np | [103] |
| SnO2@ZnS | Hydrothermal | Sheet/cubic ZnS, tetragonal SnO2 | TCS = 10 | Vis I = 500 W t = 120 min | 40%/ 0.0033 | [102] |
| Bi7O9I3/Bi5O7I | Calcination | Bone-stick/crystalline Bi7O9I3 and Bi5O7I | TCS = 20 | Vis I = 500 W t = 180 min | 89.28%/ 0.0168 | [104] |
| p-ZnIn2S4/rGO/n-g-C3N4 | Hydrothermal | Sheet/crystalline ZnIn2S4 | TCS = 50 | UV, Vis IUV = 20 W IVis = 2 W t = 120 min | 100% (UV) 97% (Vis)/ np | [105] |
| Ag/AgCl/BiVO4 | Ultrasonication | Octahedral particle/monoclinic BiVO4, crystalline AgCl and Ag | CBZ = 10 | Vis I = 93.38 W t = 240 min | 70.6%/ np | [106] |
| g-C3N4/TiO2 | Calcination | Sheet/crystalline g-C3N4, anatase TiO2 | CBZ = 10 | Vis I = 50 W t = 360 min | 99.77%/ 0.1796 | [108] |
| Ag/AgBr/ZnFe2O4 | Ultrasonication | Spherical/cubic AgBr and ZnFe2O4 | CBZ = 10 | Vis I = 93.38 W t = 240 min | 22.7%/ np | [107] |
| Bi12TiO20/g-C3N4 | Ultrasonication | Spherical/cubic Bi12TiO20 | SA = 10 | Vis I = 500 W t = 50 min | 50%/ np | [64] |
| WO3/Bi2WO6 | Hydrothermal | Flower/orthorhombic Bi2WO6 | SA = 5 | Vis I = 300 W t = 360 min | 74.5%/ 0.00435 | [109] |
| TiO2-NT’s@Ag-HA | Photoreduction | Tubes/anatase TiO2 | SA = 28 | Full Spectrum (FS), Vis IFS = 120 W IVis = 100 W t = 240 min | 75% (FS) 30% (Vis)/ 0.00581 (FS) 0.00129 (Vis) | [110] |
| TiO2/WO3 | One pot | Hollow sphere/anatase TiO2 and monoclinic WO3 | SA = 50 | UV I = 300 W t = 60 min | 42%/ np | [81] |
| CdS-SnS-SnS2/rGO | Solvothermal | Sheet/hexagonal CdS, SnS2 and orthorhombic SnS | IBF = 100 | Vis I = 300 W t = 60 min | 84.4%/ 0.0257 | [115] |
| Bi2O3-TiO2/carbon | Calcination | Particle/anatase and rutile TiO2 | IBF = 20 | Vis I = 300 W t = 120 min | 100%/ 0.0290 | [114] |
| W18O49/g-C3N4 | Hydrothermal | Sheet/monoclinic W18O49 | IBF = 10 | Vis, NIR IVis, NIR = 300 W tVis = 60 min tNIR = 120 min | 96.3% (Vis) 39.2% (NIR)/0.0464 (Vis) 0.0027 (NIR) | [112] |
| Fe3O4@MIL-53(Fe) | Calcination | Particles with polyhedron structure/crystalline Fe3O4 | IBF = 10 | Vis I = 500 W t = 60 min | 99%/ 0.0471 | [113] |
| Co3O4/BiOI | Solvothermal | Plates/crystalline Co3O4, Tetragonal BiOI | IBF = 10 | Vis I = 60 W t = 60 min | 93.87%/ 0.0945 | [111] |
| g-C3N4/TiO2/Fe3O4@SiO2 | Sol–gel | Sheet/standard magnetite, anatase TiO2 | IBF = 2 | Vis I = 64 W t = 15 min | 97%/ np | [116] |
| g-C3N4/TNTs | Hydrothermal | Sheet/anatase and rutile TiO2 | SMZ = 5 | Vis I = 450 W t = 300 min | 100%/ 0.0193 | [117] |
| Pd-Bi2MoO6/g-C3N4 | Precipitation | Flake/crystalline Bi2MoO6 and g-C3N4 | SMZ = 5 | Vis I = 36 W t = 90 min | 98.8%/ 0.0440 | [118] |
| CuFe2O4/Ti3C2 | Hydrothermal | Sheet/spinel CuFe2O4 | SMZ = 40 | Vis I = 300 W t = 60 min | 70%/ 0.0128 | [119] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enesca, A.; Andronic, L. The Influence of Photoactive Heterostructures on the Photocatalytic Removal of Dyes and Pharmaceutical Active Compounds: A Mini-Review. Nanomaterials 2020, 10, 1766. https://doi.org/10.3390/nano10091766
Enesca A, Andronic L. The Influence of Photoactive Heterostructures on the Photocatalytic Removal of Dyes and Pharmaceutical Active Compounds: A Mini-Review. Nanomaterials. 2020; 10(9):1766. https://doi.org/10.3390/nano10091766
Chicago/Turabian StyleEnesca, Alexandru, and Luminita Andronic. 2020. "The Influence of Photoactive Heterostructures on the Photocatalytic Removal of Dyes and Pharmaceutical Active Compounds: A Mini-Review" Nanomaterials 10, no. 9: 1766. https://doi.org/10.3390/nano10091766
APA StyleEnesca, A., & Andronic, L. (2020). The Influence of Photoactive Heterostructures on the Photocatalytic Removal of Dyes and Pharmaceutical Active Compounds: A Mini-Review. Nanomaterials, 10(9), 1766. https://doi.org/10.3390/nano10091766