Graphite Felt Modified by Atomic Layer Deposition with TiO2 Nanocoating Exhibits Super-Hydrophilicity, Low Charge-Transform Resistance, and High Electrochemical Activity
Abstract
1. Introduction
2. Materials and Methods
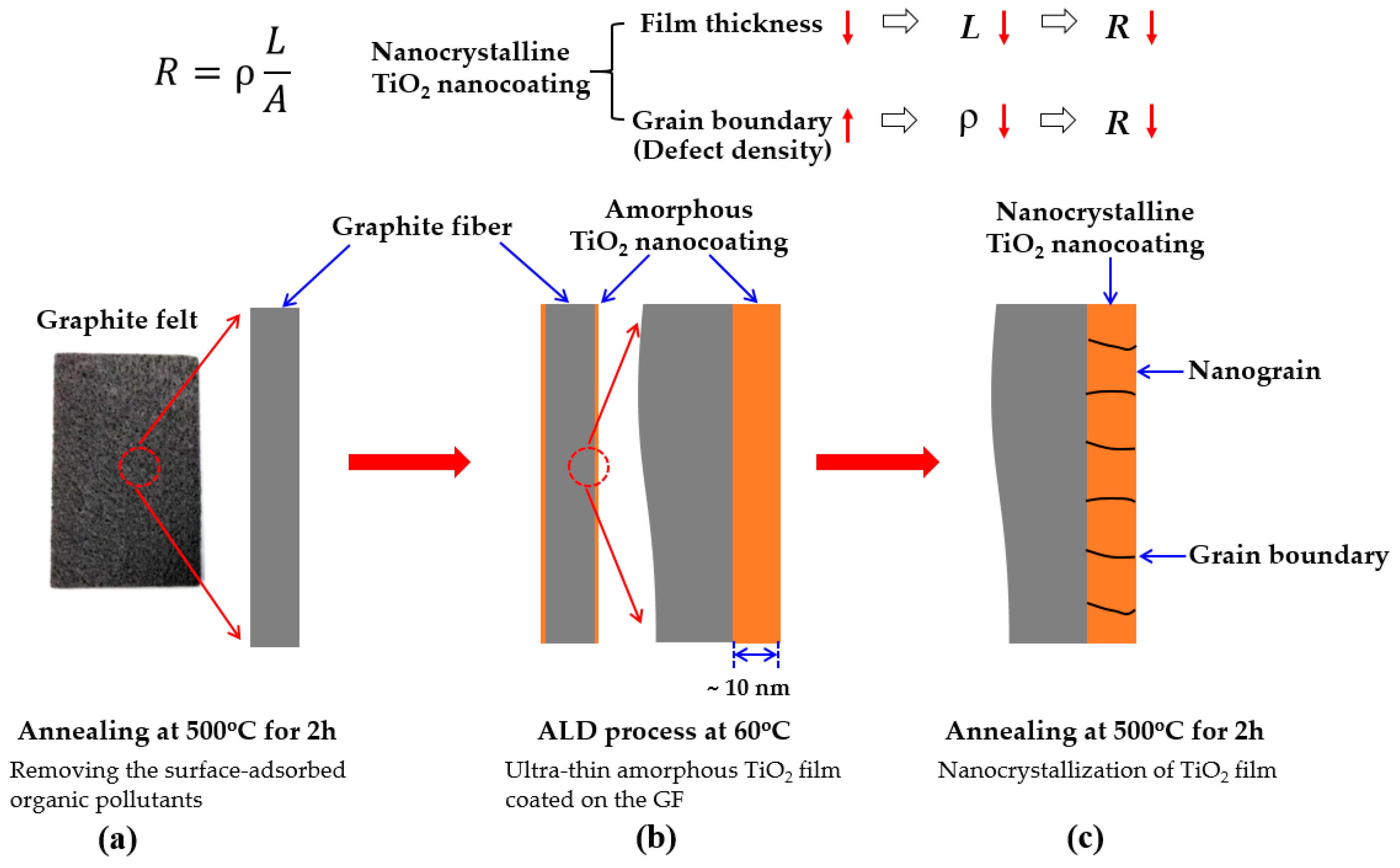
2.1. Surface Modification of GF
- (1)
- Removing the surface-adsorbed organic pollutants of GF: the original GF was introduced into the ALD reactor and then the GF was annealed at 500 °C for 2 h in the ALD reactor. The purpose of this step is to remove organic pollutants adsorbed on the surface of GF by thermal decomposition, to ensure that the GF has a clean and hydrophilic surface for ALD process.
- (2)
- Ultra-thin amorphous TiO2 film coated on the GF surface by ALD: a 10-nm-thick TiO2 film was coated on the GF surface by ALD with 100 ALD-cycles at 60 °C for which the details of the ALD process were described in our previous works [54,55,56]. Briefly, TiCl4 and H2O were used as the precursors, Ar was used as the purge gas, and the growth rate is about 0.1 nm per cycle. In this step, an ultra-thin amorphous TiO2 film is uniformly coated on all the surfaces of the GF to form ALD-TiO2/GF sample.
- (3)
- Nanocrystallization of TiO2 film: TiO2 grown at the low temperature of 60 °C has an amorphous structure. In order to improve the corrosion resistance and activity of TiO2, a post-annealing process was performed at 500 °C for 2h in the ALD reactor to transform the TiO2 surface coating from an amorphous into an anatase crystal structure.
Design Concept of This Study
2.2. Characterizations
2.3. Electrochemical Analysis
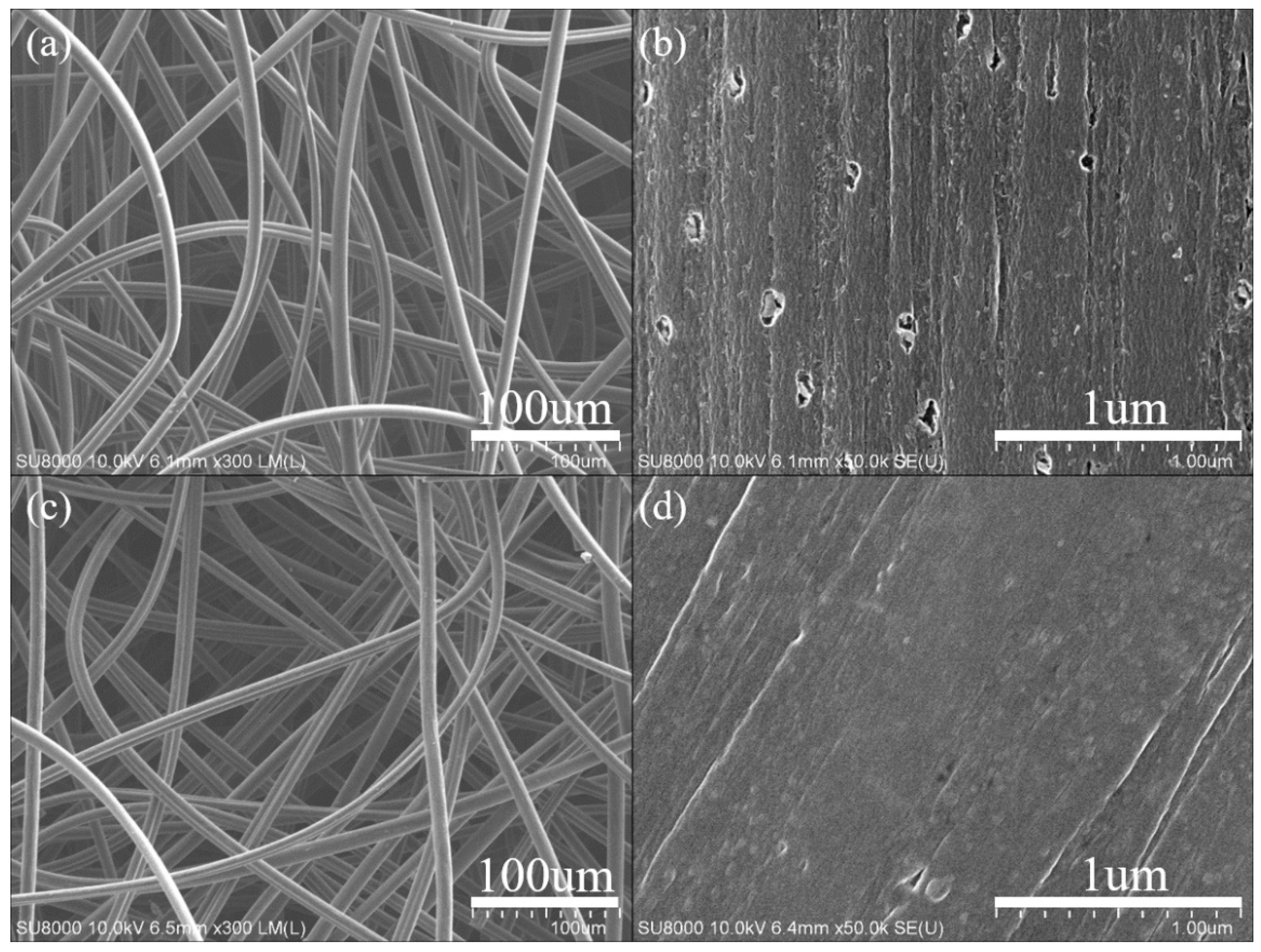
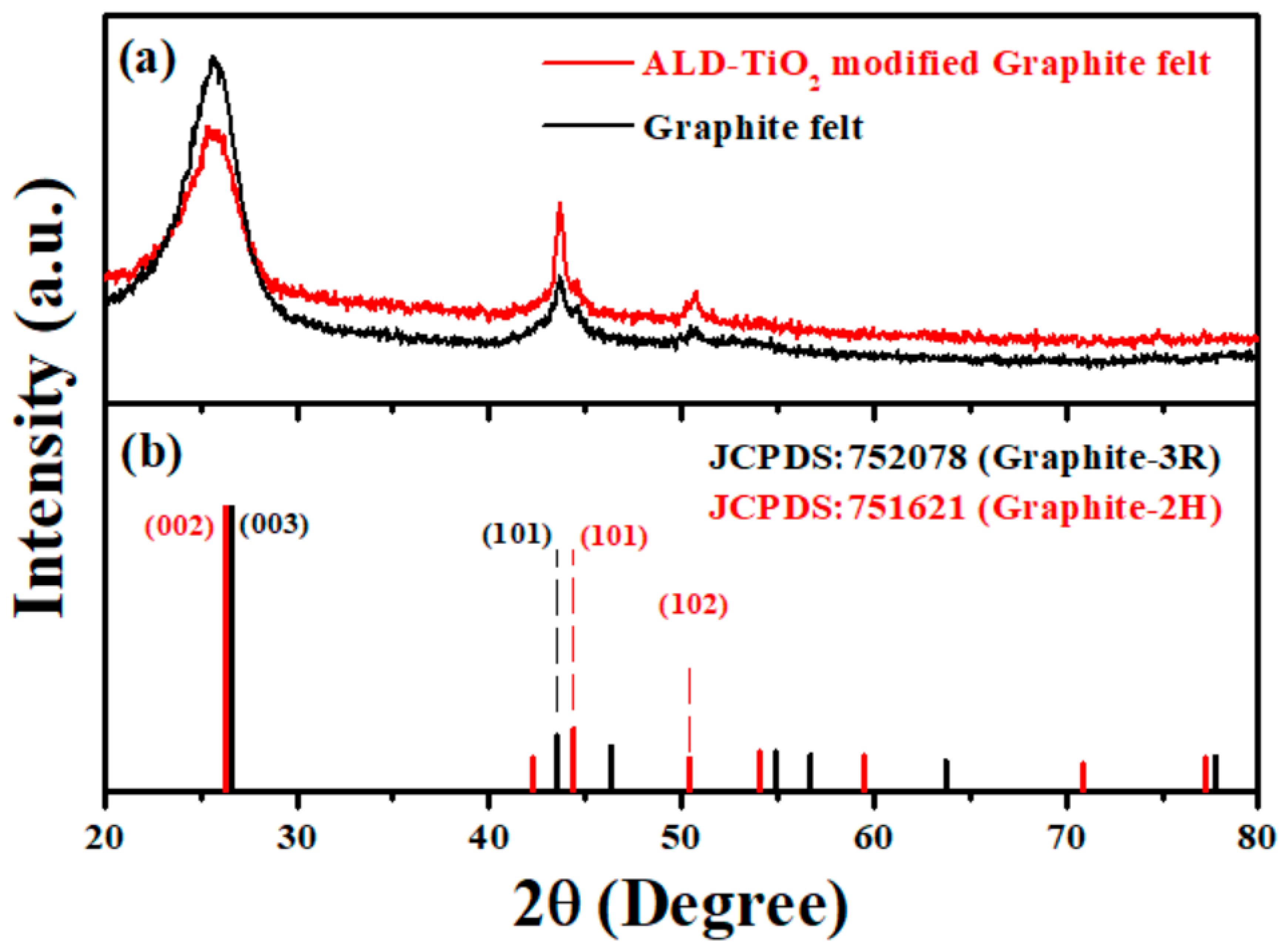
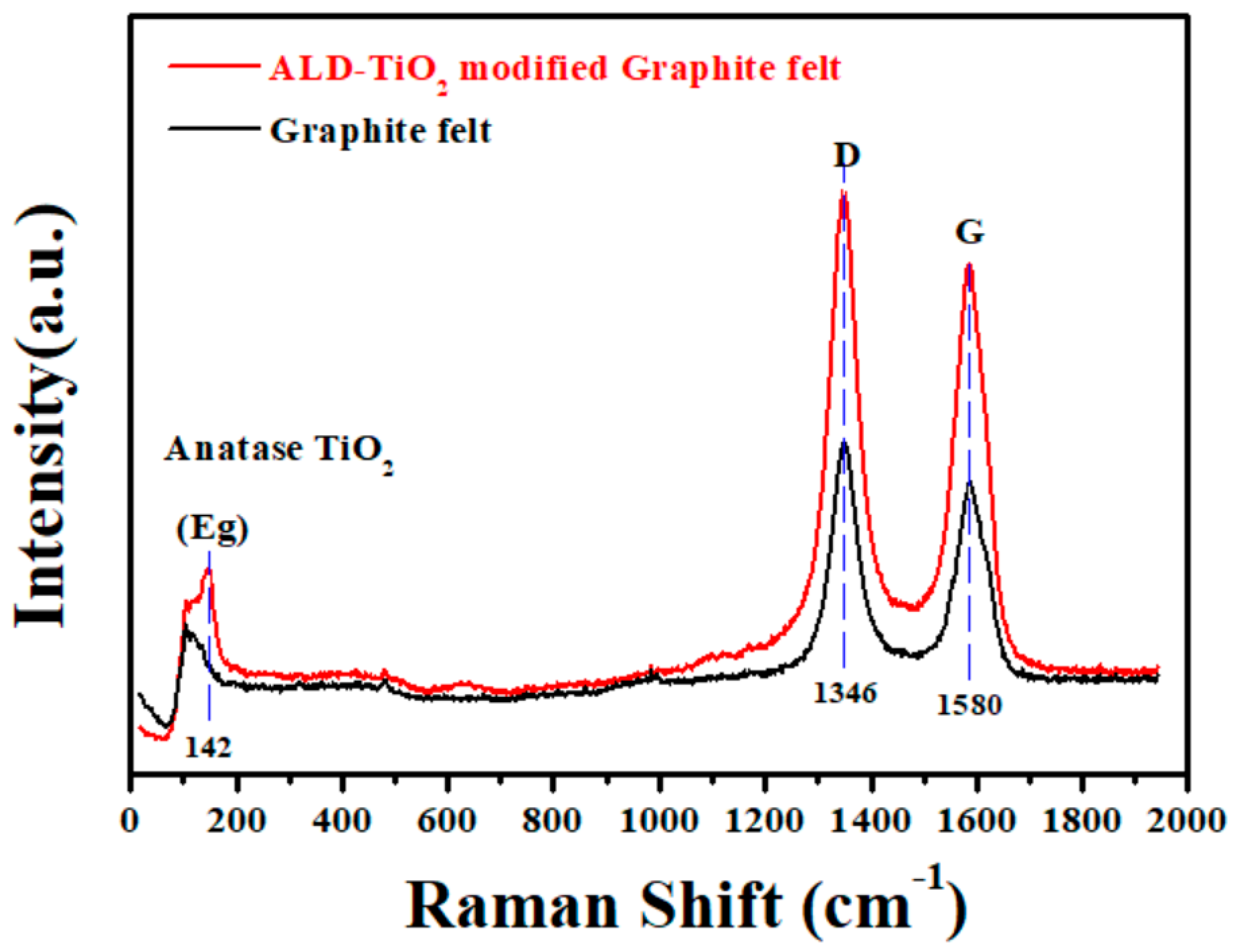
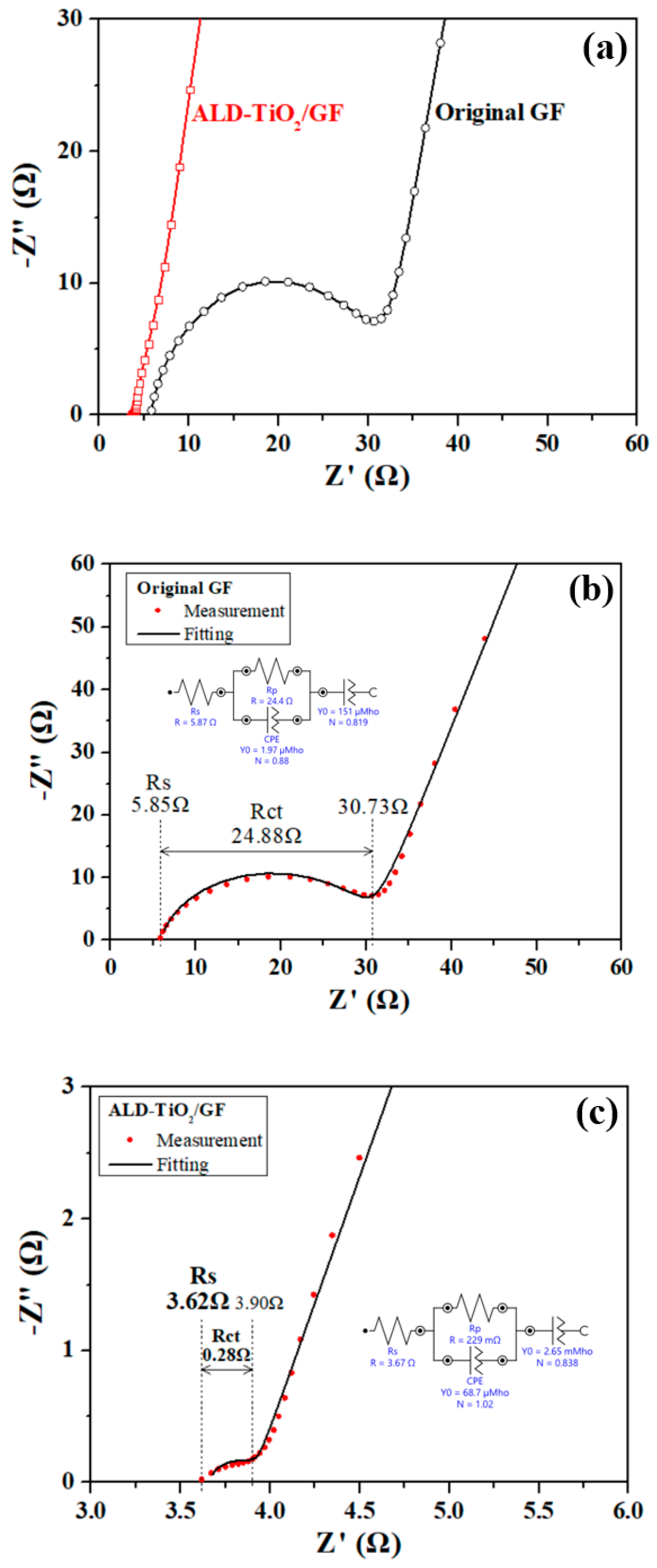
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shao, Y.; Cheng, Y.; Duan, W.; Wang, W.; Lin, Y.; Wang, Y.; Liu, J. Nanostructured electrocatalysts for PEM fuel cells and redox flow batteries: A selected review. ACS Catal. 2015, 5, 7288–7298. [Google Scholar] [CrossRef]
- Zhang, Z.; Xi, J.; Zhou, H.; Qiu, X. KOH etched graphite felt with improved wettability and activity for vanadium flow batteries. Electrochim. Acta 2016, 218, 15–23. [Google Scholar] [CrossRef]
- Wu, L.; Wang, J.; Shen, Y.; Liu, L.; Xi, J. Electrochemical evaluation methods of vanadium flow battery electrodes. Phys. Chem. Chem. Phys. 2017, 19, 14708–14717. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, L.F.; Walsh, F.C.; Nava, J.L.; León, C.P.D. Graphite felt as a versatile electrode material: Properties, reaction environment, performance and applications. Electrochim. Acta 2017, 258, 1115–1139. [Google Scholar] [CrossRef]
- Kabtamu, D.M.; Chen, J.Y.; Chang, Y.C.; Wang, C.H. Water-activated graphite felt as a high-performance electrode for vanadium redox flow batteries. J. Power Sources 2017, 341, 270–279. [Google Scholar] [CrossRef]
- Jianga, F.; Heb, Z.; Guoa, D.; Zhoua, X. Carbon aerogel modified graphite felt as advanced electrodes for vanadium redox flow batteries. J. Power Sources 2019, 440, 227114. [Google Scholar] [CrossRef]
- Hea, M.; Zheng, Y.; Du, Q. Three-dimensional polypyrrole/MnO2 composite networks deposited on graphite felt as free-standing electrode for supercapacitors. Mater. Lett. 2013, 104, 48–52. [Google Scholar] [CrossRef]
- Díaz, P.; González, Z.; Santamaría, R.; Granda, M.; Menéndez, R.; Blanco, C. Enhanced energy density of carbon-based supercapacitors using cerium (III) sulphate as inorganic redox electrolyte. Electrochim. Acta 2015, 168, 277–284. [Google Scholar] [CrossRef]
- Park, C.; Hwang, J.; Hwang, Y.T.; Song, C.; Ahn, S.; Kim, H.S.; Ahn, H. Intense pulsed white light assisted fabrication of Co-CoOx core-shell nanoflakes on graphite felt for flexible hybrid supercapacitors. Electrochim. Acta 2017, 246, 757–765. [Google Scholar] [CrossRef]
- Shen, P.; Wang, Z.; Yang, C.; Zhao, L.; Liu, T.; Shen, M.; Li, J.; Qian, D. Enhanced electrochemical property of graphite felt@Co2(OH)2CO3 via Ni-P electrodeposition for flexible supercapacitors. Electrochim. Acta 2018, 283, 1568–1577. [Google Scholar] [CrossRef]
- Pu, K.B.; Lu, C.X.; Zhang, K.; Zhang, H.; Chen, Q.Y.; Wang, Y.H. In situ synthesis of polypyrrole on graphite felt as bio-anode to enhance the start-up performance of microbial fuel cells. Bioprocess Biosyst. Eng. 2020, 43, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhai, H.; Liu, B.; Ji, M.; Li, J. Carbon nanomaterial-modified graphite felt as an anode enhanced the power production and polycyclic aromatic hydrocarbon removal in sediment microbial fuel cells. Sci. Total Environ. 2020, 713, 136483. [Google Scholar] [CrossRef] [PubMed]
- Palmore, G.T.R.; Bertschy, H.; Bergens, S.H.; Whitesides, G.M. A methanol/dioxygen biofuel cell that uses NAD+-dependent dehydrogenases as catalysts: Application of an electro-enzymatic method to regenerate nicotinamide adenine dinucleotide at low overpotentials. J. Electroanal. Chem. 1998, 443, 155–161. [Google Scholar] [CrossRef]
- Séamus, F.D.; Higson, P.J. Biofuel cells—Recent advances and applications. Biosens. Bioelectron. 2007, 22, 1224–1235. [Google Scholar]
- Liu, X.; Yang, D.; Zhou, Y.; Zhang, J.; Luo, L.; Meng, S.; Chen, S.; Tan, M.; Li, Z.; Tang, L. Electrocatalytic properties of N-doped graphite felt in electro-Fenton process and degradation mechanism of levofloxacin. Chemosphere 2017, 182, 306–315. [Google Scholar] [CrossRef]
- Liang, L.; Yu, F.; An, Y.; Liu, M.; Zhou, M. Preparation of transition metal composite graphite felt cathode for efficient heterogeneous electro-Fenton process. Environ. Sci. Pollut. Res. 2017, 24, 1122–1132. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, S.; Zhou, M.; Liang, L.; Ren, G. Removal of tetracycline by coupling of flow-through electro-Fenton and in-situ regenerative active carbon felt adsorption. Chem. Eng. J. 2018, 335, 685–692. [Google Scholar] [CrossRef]
- Le, T.X.; Bechelany, M.; Cretin, M. Carbon felt based-electrodes for energy and environmental applications: A review. Carbon 2017, 122, 564–591. [Google Scholar]
- Kozbial, A.; Zhou, F.; Li, Z.; Liu, H.; Li, L. Are graphitic surfaces hydrophobic? Acc. Chem. Res. 2016, 49, 2765–2773. [Google Scholar] [CrossRef]
- Chen, J.Z.; Liao, W.Y.; Hsieh, W.Y.; Hsu, C.C.; Chen, Y.S. All-vanadium redox flow batteries with graphite felt electrodes treated by atmospheric pressure plasma jets. J. Power Sources 2015, 274, 894–898. [Google Scholar] [CrossRef]
- Dixon, D.; Babu, D.J.; Langner, J.; Bruns, M.; Pfaffmann, L.; Bhaskar, A. Effect of oxygen plasma treatment on the electrochemical performance of the rayon and polyacrylonitrile based carbon felt for the vanadium redox flow battery application. J. Power Sources 2016, 332, 240–248. [Google Scholar] [CrossRef]
- Sun, B.; Skyllas-Kazacos, M. Chemical modification of graphite electrode materials for vanadium redox flow battery application-part II. Acid treatments. Electrochim. Acta 1992, 37, 2459–2465. [Google Scholar] [CrossRef]
- Yue, L.; Li, W.; Sun, F.; Zhao, L.; Xing, L. Highly hydroxylated carbon fibres as electrode materials of all-vanadium redox flow battery. Carbon 2010, 48, 3079–3090. [Google Scholar] [CrossRef]
- Flox, C.; Rubio-García, J.; Skoumal, M.; Andreu, T.; Morante, J.R. Thermo-chemical treatments based on NH3/O2 for improved graphite-based fiber electrodes in vanadium redox flow batteries. Carbon 2013, 60, 280–288. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, H.; Li, X.; Liu, T.; Xing, F. Vanadium flow battery for energy storage: Prospects and challenges. J. Phys. Chem. Lett. 2013, 4, 1281–1294. [Google Scholar] [CrossRef]
- Di Blasi, A.; Briguglio, N.; Di Blasi, O.; Antonucci, V. Charge-discharge performance of carbon fiber-based electrodes in single cell and short stack for vanadium redox flow battery. Appl. Energy 2014, 125, 114–122. [Google Scholar] [CrossRef]
- Hidalgo, D.; Tommasi, T.; Bocchini, S.; Chiolerio, A.; Chiodoni, A.; Mazzarino, I. Surface modification of commercial carbon felt used as anode for Microbial Fuel Cells. Energy 2016, 99, 193–201. [Google Scholar] [CrossRef]
- Sun, B.; Skyllas-Kazacos, M. Modification of graphite electrode materials for vanadium redox flow battery application-I. Thermal treatment. Electrochim. Acta 1992, 37, 1253–1260. [Google Scholar] [CrossRef]
- González, Z.; Botas, C.; Álvarez, P.; Roldán, S.; Blanco, C.; Santamaría, R.; Granda, M.; Menéndez, R. Thermally reduced graphite oxide as positive electrode in vanadium redox flow batteries. Carbon 2012, 50, 828–834. [Google Scholar] [CrossRef]
- Pezeshki, A.M.; Clement, J.T.; Veith, G.M.; Zawodzinski, T.A.; Mench, M.M. High performance electrodes in vanadium redox flow batteries through oxygen-enriched thermal activation. J. Power Sources 2015, 294, 333–338. [Google Scholar] [CrossRef]
- Le, T.X.H.; Charmette, C.; Bechelany, M.; Cretin, M. Facile preparation of porous carbon cathode to eliminate paracetamol in aqueous medium using electro-Fenton system. Electrochim. Acta 2016, 188, 378–384. [Google Scholar] [CrossRef]
- Liu, T.; Li, X.; Xu, C.; Zhang, H. Activated carbon fiber paper based electrodes with high electrocatalytic activity for vanadium flow batteries with improved power density. ACS Appl. Mater. Interfaces 2017, 9, 4626–4633. [Google Scholar] [CrossRef]
- Wang, P.; Lai, B.; Li, H.; Du, Z. Deposition of Fe on graphite felt by thermal decomposition of Fe(CO)5 for effective cathodic preparation of microbial fuel cells. Bioresour. Technol. 2013, 134, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hu, W.; Hong, J.; Sandoe, S. Electrochemical disinfection of simulated ballast water on PbO2/graphite felt electrode. Mar. Pollut. Bull. 2016, 105, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liang, P.; Yang, X.; Jiang, Y.; Bian, Y.; Chen, C.; Zhanga, X.; Huanga, X. Binder-free graphene and manganese oxide coated carbon felt anode for high-performance microbial fuel cell. Biosens. Bioelectron. 2016, 81, 32–38. [Google Scholar] [CrossRef]
- Mauricio Rosolen, J.; Patrick Poá, C.H.; Tronto, S.; Marchesin, M.S.; Silva, S.R.P. Electron field emission of carbon nanotubes on carbon felt. Chem. Phys. Lett. 2006, 424, 151–155. [Google Scholar] [CrossRef]
- Rosolen, J.M.; Tronto, S.; Marchesin, M.S.; Almeida, E.C.; Ferreira, N.G.; Patrick Poa, C.H.; Ravi, S.; Silvad, P. Electron field emission from composite electrodes of carbon nanotubes-boron-doped diamond and carbon felts. Appl. Phys. Lett. 2006, 88, 083116. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Cochell, T.; Manthiram, A. Nitrogen-doped carbon nanotube/graphite felts as advanced electrode materials for vanadium redox flow batteries. J. Phys. Chem. Lett. 2012, 3, 2164–2167. [Google Scholar] [CrossRef]
- Chang, Y.; Deng, L.; Meng, X.; Zhang, W.; Wang, C.; Wang, Y.; Zhao, S.; Lin, L.; Crittenden, J.C. Closed-loop electrochemical recycling of spent copper (II) from etchant wastewater using a carbon nanotube modified graphite felt anode. Environ. Sci. Technol. 2018, 52, 5940–5948. [Google Scholar] [CrossRef]
- Wang, W.H.; Wang, X.D. Investigation of Ir-modified carbon felt as the positive electrode of an all-vanadium redox flow battery. Electrochim. Acta 2007, 52, 6755–6762. [Google Scholar] [CrossRef]
- González, Z.; Sánchez, A.; Blanco, C.; Granda, M.; Menéndez, R.; Santamaría, R. Enhanced performance of a Bi-modified graphite felt as the positive electrode of a vanadium redox flow battery. Electrochem. Commun. 2011, 13, 1379–1382. [Google Scholar] [CrossRef]
- Solmaz, R.; Gündoğdu, A.; Döner, A.; Kardaş, G. The Ni-deposited carbon felt as substrate for preparation of Pt-modified electrocatalysts: Application for alkaline water electrolysis. Int. J. Hydrogen Energy 2012, 37, 8917–8922. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, T.S.; Zeng, L.; Zhou, X.L.; Zeng, Y.K. Copper nanoparticle-deposited graphite felt electrodes for all vanadium redox flow batteries. Appl. Energy 2016, 180, 386–391. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, H.; Xu, P.; Wu, X.; Dong, Y.; Lu, L. Electrochemical catalytic activity of tungsten trioxide-modified graphite felt toward VO2+/VO2+ redox reaction. Electrochim. Acta 2014, 132, 37–41. [Google Scholar] [CrossRef]
- Xiang, Y.; Daoud, W.A. Investigation of an advanced catalytic effect of cobalt oxide modification on graphite felt as the positive electrode of the vanadium redox flow battery. J. Power Sources 2019, 415, 175–183. [Google Scholar] [CrossRef]
- Vázquez-Galván, J.; Flox, C.; Fábega, C.; Ventosa, E.; Parra, A.; Andreu, T.; Morate, J.R. Hydrogen-treated rutile TiO2 shell in graphite-core structure as a negative electrode for high-performance vanadium redox flow batteries. ChemSusChem 2017, 10, 2089–2098. [Google Scholar] [CrossRef]
- Bayeh, A.W.; Kabtamu, D.M.; Chang, Y.C.; Chen, G.C.; Chen, H.Y.; Liu, T.R.; Wondimu, T.H.; Wang, K.C.; Wang, C.H. Hydrogen-treated defect-rich W18O49 nanowire-modified graphite felt as high-performance electrode for vanadium redox flow battery. ACS Appl. Energy Mater. 2019, 2, 2541–2551. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. Atomic layer deposition (ALD): From precursors to thin film structures. Thin Solid Film. 2002, 409, 138–146. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.B.R.; Maeng, W.J. Applications of atomic layer deposition to nanofabrication and emerging nanodevices. Thin Solid Film. 2009, 517, 2563–2580. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Moya, A.; Kemnade, N.; Osorio, M.R.; Cherevan, A.; Granados, D.; Eder, D.; Vilatela, J.J. Large area photoelectrodes based on hybrids of CNT fibres and ALD-grown TiO2. J. Mater. Chem. A 2017, 5, 24695–24706. [Google Scholar] [CrossRef]
- Li, M.; Zu, M.; Yu, J.; Cheng, H.; Li, Q.; Li, B. Controllable synthesis of core-sheath structured aligned carbon nanotube/titanium dioxide hybrid fibers by atomic layer deposition. Carbon 2017, 123, 151–157. [Google Scholar] [CrossRef]
- Geppert, T.N.; Bosund, M.; Putkonen, M.; Stühmeier, B.M.; Pasanen, A.T.; Heikkilä, P.; Gasteiger, H.A.; El-Sayed, H.A. HOR activity of Pt-TiO2-Y at unconventionally high potentials explained: The influence of SIMI on the electrochemical behavior of Pt. J. Electrochem. Soc. 2020, 167, 084517. [Google Scholar] [CrossRef]
- Lee, W.J.; Hon, M.H. Space-limited crystal growth mechanism of TiO2 films by atomic layer deposition. J. Phys. Chem. C 2010, 114, 6917–6921. [Google Scholar] [CrossRef]
- Lee, W.J.; Hon, M.H.; Chung, Y.W.; Lee, J.H. A three-dimensional nanostructure consisting of hollow TiO2 spheres fabricated by atomic layer deposition. Jpn. J. Appl. Phys. 2011, 50, 06GH06. [Google Scholar] [CrossRef]
- Lee, W.J.; Hon, M.H. An ultraviolet photo-detector based on TiO2/water solid-liquid heterojunction. Appl. Phys. Lett. 2011, 99, 251102. [Google Scholar] [CrossRef]
- Reich, S.; Thomsen, C. Raman spectroscopy of graphite. Phil. Trans. R. Soc. Lond. A 2004, 362, 2271–2288. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.; Zhang, J.; Pan, C. Raman spectroscopy: A new approach to measure the percentage of anatase TiO2 exposed (001) facets. J. Phys. Chem. C 2012, 116, 7515–7519. [Google Scholar] [CrossRef]
- Toro, R.G.; Diab, M.; de Caro, T.; Al-Shemy, M.; Adel, A.; Caschera, D. Study of the effect of titanium dioxide hydrosol on the photocatalytic and mechanical properties of paper sheets. Materials 2020, 13, 1326. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Goto, T.; Cho, S.; Lee, S.W.; Kakihana, M.; Sekino, T. Effects of annealing temperature on the crystal structure, morphology, and optical properties of peroxo-titanate nanotubes prepared by peroxo-titanium complex ion. Nanomaterials 2020, 10, 1331. [Google Scholar] [CrossRef] [PubMed]
- Rani, J.R.; Thangavel, R.; Oh, S.I.; Woo, J.M.; Das, N.C.; Kim, S.Y.; Lee, Y.S.; Jang, J.H. High volumetric energy density hybrid supercapacitors based on reduced graphene oxide scrolls. ACS Appl. Mater. Interfaces 2017, 9, 22398–22407. [Google Scholar] [CrossRef] [PubMed]
- Massaglia, G.; Fiorello, I.; Sacco, A.; Margaria, V.; Pirri, C.F.; Quaglio, M. Biohybrid cathode in single chamber microbial fuel cell. Nanomaterials 2019, 9, 36. [Google Scholar] [CrossRef]
- Abe, Y.; Hori, N.; Kumagai, S. Electrochemical impedance spectroscopy on the performance degradation of LiFePO4/graphite lithium-ion battery due to charge-discharge cycling under different C-rates. Energies 2019, 12, 4507. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-J.; Wu, Y.-T.; Liao, Y.-W.; Liu, Y.-T. Graphite Felt Modified by Atomic Layer Deposition with TiO2 Nanocoating Exhibits Super-Hydrophilicity, Low Charge-Transform Resistance, and High Electrochemical Activity. Nanomaterials 2020, 10, 1710. https://doi.org/10.3390/nano10091710
Lee W-J, Wu Y-T, Liao Y-W, Liu Y-T. Graphite Felt Modified by Atomic Layer Deposition with TiO2 Nanocoating Exhibits Super-Hydrophilicity, Low Charge-Transform Resistance, and High Electrochemical Activity. Nanomaterials. 2020; 10(9):1710. https://doi.org/10.3390/nano10091710
Chicago/Turabian StyleLee, Wen-Jen, Yu-Ting Wu, Yi-Wei Liao, and Yen-Ting Liu. 2020. "Graphite Felt Modified by Atomic Layer Deposition with TiO2 Nanocoating Exhibits Super-Hydrophilicity, Low Charge-Transform Resistance, and High Electrochemical Activity" Nanomaterials 10, no. 9: 1710. https://doi.org/10.3390/nano10091710
APA StyleLee, W.-J., Wu, Y.-T., Liao, Y.-W., & Liu, Y.-T. (2020). Graphite Felt Modified by Atomic Layer Deposition with TiO2 Nanocoating Exhibits Super-Hydrophilicity, Low Charge-Transform Resistance, and High Electrochemical Activity. Nanomaterials, 10(9), 1710. https://doi.org/10.3390/nano10091710




