pH-Responsive Nanoparticles for Cancer Immunotherapy: A Brief Review
Abstract
1. Introduction
1.1. Modulation of Anticancer Immunity
1.2. Ongoing Challenges in Cancer Immunotherapy
2. Cancer Immunotherapy with Nanoparticles
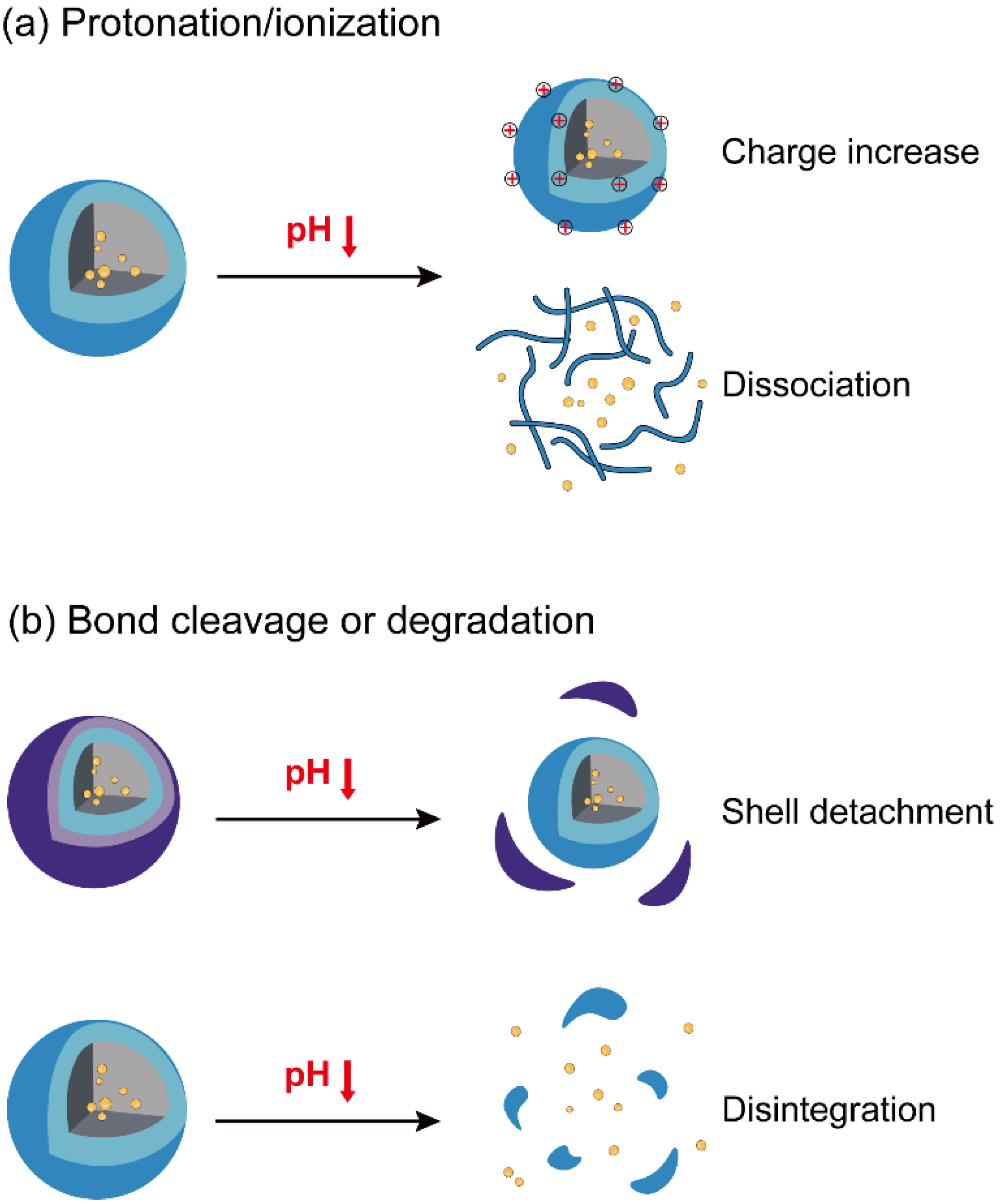
3. pH-Responsive Nanoparticles for Cancer Immunotherapy
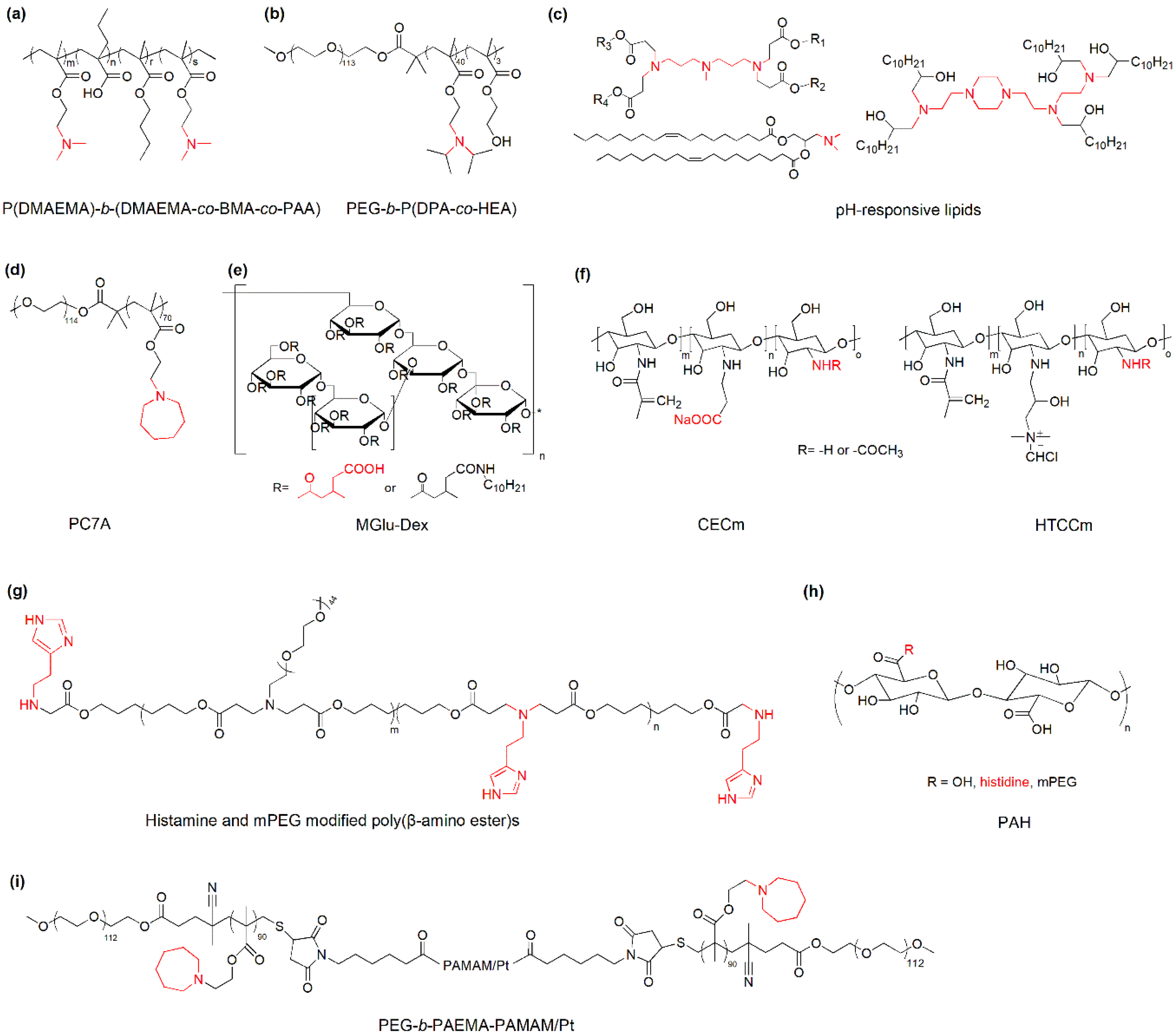
3.1. Nanoparticles with pH-Responsive Protonation/Ionization for Cancer Immunotherapy
3.2. Nanoparticles with pH-Responsive Bond Cleavage or Degradation for Cancer Immunotherapy
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Martin, J.D.; Cabral, H.; Stylianopoulos, T.; Jain, R.K. Improving cancer immunotherapy using nanomedicines: Progress, opportunities and challenges. Nat. Rev. Clin. Oncol. 2020, 17, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.J.; Dane, E.L. Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 2020, 20, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.S. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer 2019, 19, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Son, S.; Park, K.S.; Zou, W.; Shea, L.D.; Moon, J.J. Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 2019, 4, 398–414. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013, 39, 38–48. [Google Scholar] [CrossRef]
- Chi, H.; Li, C.; Zhao, F.S.; Zhang, L.; Ng, T.B.; Jin, G.; Sha, O. Anti-tumor activity of toll-like receptor 7 agonists. Front. Pharmacol. 2017, 8, 304. [Google Scholar] [CrossRef]
- Ramanjulu, J.M.; Pesiridis, G.S.; Yang, J.; Concha, N.; Singhaus, R.; Zhang, S.Y.; Tran, J.L.; Moore, P.; Lehmann, S.; Eberl, H.C.; et al. Design of amidobenzimidazole sting receptor agonists with systemic activity. Nature 2018, 564, 439–443. [Google Scholar] [CrossRef]
- Paquette, R.L.; Hsu, N.C.; Kiertscher, S.M.; Park, A.N.; Tran, L.; Roth, M.D.; Glaspy, J.A. Interferon-alpha and granulocyte-macrophage colony-stimulating factor differentiate peripheral blood monocytes into potent antigen-presenting cells. J. Leukoc. Biol. 1998, 64, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A. Il-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Restifo, N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015, 348, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene ciloleucel car t-cell therapy in refractory large b-cell lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in children and young adults with b-cell lymphoblastic leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Hajj, K.A.; Ball, R.L.; Deluty, S.B.; Singh, S.R.; Strelkova, D.; Knapp, C.M.; Whitehead, K.A. Branched-tail lipid nanoparticles potently deliver mrna in vivo due to enhanced ionization at endosomal pH. Small 2019, 15, 1805097. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric antigen receptor t-cell therapy—Assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef]
- Zhao, T.; Huang, G.; Li, Y.; Yang, S.C.; Ramezani, S.; Lin, Z.Q.; Wang, Y.G.; Ma, X.P.; Zeng, Z.Q.; Luo, M.; et al. A transistor-like ph nanoprobe for tumour detection and image-guided surgery. Nat. Biomed. Eng. 2017, 1, UNSP0006. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.P.; Wang, Y.G.; Zhao, T.; Li, Y.; Su, L.C.; Wang, Z.H.; Huang, G.; Sumer, B.D.; Gao, J.M. Ultra-ph-sensitive nanoprobe library with broad ph tunability and fluorescence emissions. J. Am. Chem. Soc. 2014, 136, 11085–11092. [Google Scholar] [CrossRef] [PubMed]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and biomaterials in ph-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Lane, L.A.; Nie, S. Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J. Control. Release 2015, 219, 205–214. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, H.; Radosz, M.; Van Kirk, E.; Murdoch, W.I. Ph-responsive nanoparticles for cancer drug delivery. In Drug Delivery Systems; Jain, K.K., Ed.; Humana Press: Totowa, NJ, USA, 2008; Volume 437, pp. 183–216. [Google Scholar]
- Dai, L.; Li, K.; Li, M.; Zhao, X.; Luo, Z.; Lu, L.; Luo, Y.; Cai, K. Size/charge changeable acidity-responsive micelleplex for photodynamic-improved pd-l1 immunotherapy with enhanced tumor penetration. Adv. Funct. Mater. 2018, 28, 1707249. [Google Scholar] [CrossRef]
- Yang, G.B.; Xu, L.G.; Xu, J.; Zhang, R.; Song, G.S.; Chao, Y.; Feng, L.Z.; Han, F.X.; Dong, Z.L.; Li, B.; et al. Smart nanoreactors for ph-responsive tumor homing, mitochondria-targeting, and enhanced photodynamic-immunotherapy of cancer. Nano Lett. 2018, 18, 2475–2484. [Google Scholar] [CrossRef]
- Wang, C.; Li, P.; Liu, L.; Pan, H.; Li, H.; Cai, L.; Ma, Y. Self-adjuvanted nanovaccine for cancer immunotherapy: Role of lysosomal rupture-induced ros in mhc class i antigen presentation. Biomaterials 2016, 79, 88–100. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, X.M.; Jia, J.L.; Zhang, W.F.; Yang, T.Y.; Wang, L.Y.; Ma, G.H. Ph-responsive poly(d,l-lactic-co-glycolic acid) nanoparticles with rapid antigen release behavior promote immune response. ACS Nano 2015, 9, 4925–4938. [Google Scholar] [CrossRef]
- Zheng, D.-W.; Chen, J.-L.; Zhu, J.-Y.; Rong, L.; Li, B.; Lei, Q.; Fan, J.-X.; Zou, M.-Z.; Li, C.; Cheng, S.-X.; et al. Highly integrated nano-platform for breaking the barrier between chemotherapy and immunotherapy. Nano Lett. 2016, 16, 4341–4347. [Google Scholar] [CrossRef]
- Hu, Y.; Litwin, T.; Nagaraja, A.R.; Kwong, B.; Katz, J.; Watson, N.; Irvine, D.J. Cytosolic delivery of membrane-impermeable molecules in dendritic cells using ph-responsive core-shell nanoparticles. Nano Lett. 2007, 7, 3056–3064. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.E.; Wang-Bishop, L.; Becker, K.W.; Wilson, J.T. Delivery of 5′-triphosphate rna with endosomolytic nanoparticles potently activates rig-i to improve cancer immunotherapy. Biomater. Sci. 2019, 7, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, T.; Liu, J.; Yu, H.; Jiao, S.; Feng, B.; Zhou, F.; Fu, Y.; Yin, Q.; Zhang, P.; et al. Acid-activatable versatile micelleplexes for pd-l1 blockade-enhanced cancer photodynamic immunotherapy. Nano Lett. 2016, 16, 5503–5513. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.Q.; Lee, G.Y.; Ding, J.X.; Li, W.L.; Shi, J.J. Biomedical applications of mrna nanomedicine. Nano Res. 2018, 11, 5281–5309. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.F.; Xiong, H.; Zhang, X.Y.; Cheng, Q.; Siegwart, D.J. Systemic mrna delivery to the lungs by functional polyester-based carriers. Biomacromolecules 2017, 18, 4307–4315. [Google Scholar] [CrossRef]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective organ targeting (sort) nanoparticles for tissue-specific mrna delivery and crispr-cas gene editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar]
- Luo, M.; Wang, H.; Wang, Z.; Cai, H.; Lu, Z.; Li, Y.; Du, M.; Huang, G.; Wang, C.; Chen, X.; et al. A sting-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 2017, 12, 648–654. [Google Scholar]
- Yuba, E.; Tajima, N.; Yoshizaki, Y.; Harada, A.; Hayashi, H.; Kono, K. Dextran derivative-based ph-sensitive liposomes for cancer immunotherapy. Biomaterials 2014, 35, 3091–3101. [Google Scholar] [CrossRef]
- Song, Q.; Yin, Y.; Shang, L.; Wu, T.; Zhang, D.; Kong, M.; Zhao, Y.; He, Y.; Tan, S.; Guo, Y.; et al. Tumor microenvironment responsive nanogel for the combinatorial antitumor effect of chemotherapy and immunotherapy. Nano Lett. 2017, 17, 6366–6375. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.X.; Qiao, S.L.; An, H.W.; Ma, Y.; Qiao, Z.Y.; Rajapaksha, R.P.; Wang, H. Polymeric nanoparticles promote macrophage reversal from m2 to m1 phenotypes in the tumor microenvironment. Biomaterials 2017, 112, 153–163. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Z.; Jiang, Y.; Zhang, D.; Chen, J.; Dong, L.; Zhang, J. Targeted delivery of oligonucleotides into tumor-associated macrophages for cancer immunotherapy. J. Control. Release 2012, 158, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Li, H.J.; Chen, K.G.; Wang, Y.C.; Yang, X.Z.; Lian, Z.X.; Du, J.Z.; Wang, J. Spatial targeting of tumor-associated macrophages and tumor cells with a ph-sensitive cluster nanocarrier for cancer chemoimmunotherapy. Nano Lett. 2017, 17, 3822–3829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, G.; Zhang, J.; Song, H.; Niu, J.; Shi, S.; Huang, P.; Wang, Y.; Wang, W.; Li, C.; et al. Targeted antigen delivery to dendritic cell via functionalized alginate nanoparticles for cancer immunotherapy. J. Control. Release 2017, 256, 170–181. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, C.J.; Benner, N.L.; Haabeth, O.A.; Waymouth, R.M.; Wender, P.A. Enhanced mrna delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters. Proc. Natl. Acad. Sci. USA 2018, 115, E5859–E5866. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Niu, M.; O’Mary, H.; Cui, Z. Targeting of tumor-associated macrophages made possible by peg-sheddable, mannose-modified nanoparticles. Mol. Pharm. 2013, 10, 3525–3530. [Google Scholar] [CrossRef]
- Feng, B.; Zhou, F.; Hou, B.; Wang, D.; Wang, T.; Fu, Y.; Ma, Y.; Yu, H.; Li, Y. Binary cooperative prodrug nanoparticles improve immunotherapy by synergistically modulating immune tumor microenvironment. Adv. Mater. 2018, 30, e1803001. [Google Scholar] [CrossRef]
- Song, Y.; Tang, C.; Yin, C. Combination antitumor immunotherapy with vegf and pigf sirna via systemic delivery of multi-functionalized nanoparticles to tumor-associated macrophages and breast cancer cells. Biomaterials 2018, 185, 117–132. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, L.; Zhang, S.; Wan, G.; Chen, B.; Zhou, P.; Zhang, N.; Wang, Y. Dual ph-responsive multifunctional nanoparticles for targeted treatment of breast cancer by combining immunotherapy and chemotherapy. Acta Biomater. 2018, 66, 310–324. [Google Scholar] [CrossRef]
- Solbrig, C.M.; Saucier-Sawyer, J.K.; Cody, V.; Saltzman, W.M.; Hanlon, D.J. Polymer nanoparticles for immunotherapy from encapsulated tumor-associated antigens and whole tumor cells. Mol. Pharmaceut. 2007, 4, 47–57. [Google Scholar] [CrossRef]
- Molavi, L.; Mahmud, A.; Hamdy, S.; Hung, R.W.; Lai, R.; Samuel, J.; Lavasanifar, A. Development of a poly(d,l-lactic-co-glycolic acid) nanoparticle formulation of stat3 inhibitor jsi-124: Implication for cancer immunotherapy. Mol. Pharm. 2010, 7, 364–374. [Google Scholar] [CrossRef]
- Kwon, Y.J.; James, E.; Shastri, N.; Frechet, J.M.J. In vivo targeting of dendritic cells for activation of cellular immunity using vaccine carriers based on ph-responsive microparticles. Proc. Natl. Acad. Sci. USA 2005, 102, 18264–18268. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Sehgal, D.; Kucaba, T.A.; Ferguson, D.M.; Griffith, T.S.; Panyam, J. Acidic ph-responsive polymer nanoparticles as a tlr7/8 agonist delivery platform for cancer immunotherapy. Nanoscale 2018, 10, 20851–20862. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Feng, X.; Yang, X.; Sun, W.; Jin, Y.; Liu, H.; Ge, K.; Li, Z.; Zhang, J. A simple and powerful co-delivery system based on ph-responsive metal-organic frameworks for enhanced cancer immunotherapy. Biomaterials 2017, 122, 23–33. [Google Scholar] [CrossRef] [PubMed]


| pH-Responsive Components | Immunotherapeutic Drugs | Disease Models | Refs. | |
|---|---|---|---|---|
| pH-responsive protonation/ionization | Amines | 3pRNA | CT26 colon cancer | [33] |
| Photosensitizer, siPD-L1 | B16-F10 melanoma | [34] | ||
| Luciferase mRNA | C57BL/6 mice | [17] | ||
| OVA | C57BL/6 mice, INF-α/βR−/−mice et al. | [38] | ||
| BLZ-945, Pt-prodrug | B16 melanoma, CT26 colon cancer | [43] | ||
| IL-12 | B16-F10 tumor | [41] | ||
| CpG ODN, anti-IL-10 ODN, anti-IL-10 receptor ODN | Hepa 1–6 tumor | [42] | ||
| Carboxyl groups | OVA | E.G7-OVA tumor | [39] | |
| Amines and Carboxyl groups | PTX and IL-2 | Murine melanoma | [40] | |
| Acid-liable bonds | Schiff base bonds | OVA | E.G7 tumor | [44] |
| Hydrazone bonds | OVA | Melanoma | [29] | |
| TLR7/8 agonist, R848, DOX | 4T1 tumor | [49] | ||
| / | C57BL/6 mice | [46] | ||
| Amide bonds | siPD-L1, photosensitizer | B16-F10 tumor | [27] | |
| Oxaliplatin, NLG919 | 4T1 tumor | [47] | ||
| Ester bonds | EGFP and luciferase mRNA | Multiple cell lines, BALB/c mice | [45] | |
| gp100, OVA et al., and JSI-124 | B16-F10 tumor | [50], [51] | ||
| Benzamide bonds | siVEGF and siPIGF | 4T1, lung metastasis tumor | [48] | |
| NH4HCO3, NaHCO3 | OVA, agonist 522 | C57BL/6 mice, B16-F10 tumor | [30,53] | |
| Metal–ligand bonds | OVA, CpG | B16 melanoma | [54] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Ding, H. pH-Responsive Nanoparticles for Cancer Immunotherapy: A Brief Review. Nanomaterials 2020, 10, 1613. https://doi.org/10.3390/nano10081613
Yan Y, Ding H. pH-Responsive Nanoparticles for Cancer Immunotherapy: A Brief Review. Nanomaterials. 2020; 10(8):1613. https://doi.org/10.3390/nano10081613
Chicago/Turabian StyleYan, Yunfeng, and Hangwei Ding. 2020. "pH-Responsive Nanoparticles for Cancer Immunotherapy: A Brief Review" Nanomaterials 10, no. 8: 1613. https://doi.org/10.3390/nano10081613
APA StyleYan, Y., & Ding, H. (2020). pH-Responsive Nanoparticles for Cancer Immunotherapy: A Brief Review. Nanomaterials, 10(8), 1613. https://doi.org/10.3390/nano10081613





