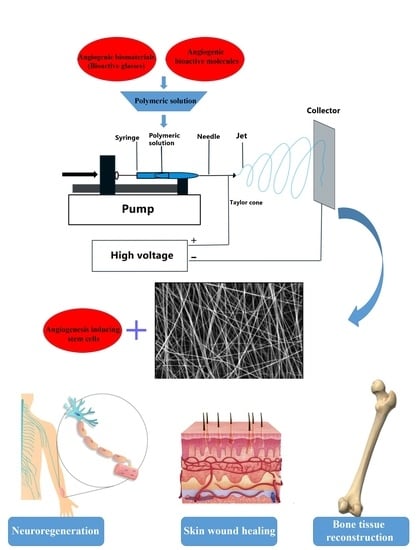
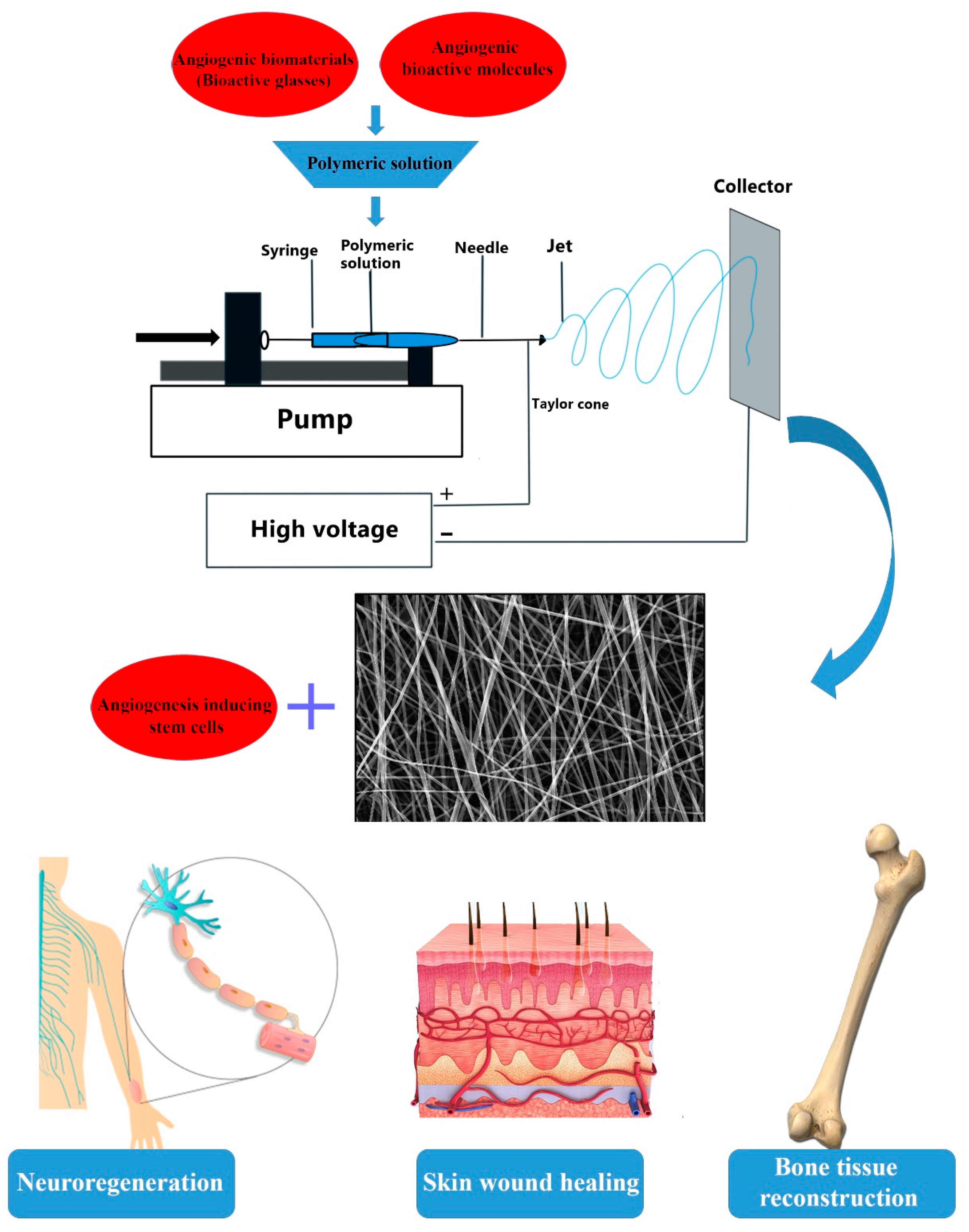
Electrospun Nanofibers for Improved Angiogenesis: Promises for Tissue Engineering Applications
Abstract
1. Introduction
2. Angiogenesis: A Critical Procedure in Tissue Engineering
3. Electrospun Nanofibers: A Brief Overview
4. Electrospun Nanofibers Meet Angiogenesis
4.1. Nanofibers as Delivery Systems of Angiogenic Substances
4.1.1. Pro-Angiogenic Growth Factor/Hormone-Loaded Nanofibrous Scaffolds
4.1.2. Nanofibers Incorporating Phytochemicals and Other Bioactive Molecules
4.2. Bioactive Glass- and Bioceramic-Incorporated Electrospun Scaffolds
4.3. Cell-Laden Nanofibers for Pro-Angiogenesis Strategies
5. Angiogenic Nanofibrous Scaffolds in Tissue Engineering
5.1. Angiogenic Nanofibers for Hard Tissue Engineering
5.2. Angiogenic Nanofibers for Soft Tissue Regeneration
5.2.1. Angiogenic Nanofibrous Mats for Skin Regeneration
5.2.2. Angiogenic Fibers for Neuroregeneration
| Polymer | Fiber Diameter | Therapeutic Element | Target Tissue | Remarks | Ref. |
|---|---|---|---|---|---|
| PLCL | 1.16 ± 0.18 μm | Human fibroblast-derived ECM | Skin | Higher proliferation and vascular morphogenesis of HUVECs seeded on the scaffold A promising role on wound healing by increased wound closure rate, mature blood vessel density, and regenerated epidermis and skin appendages after 4 weeks post-implantation | [276] |
| PLGA | Angiogenin and curcumin | Skin | Maintained bioactivity and sustained release of curcumin and angiogenin in 6 days and 20 days, respectively | [38] | |
| PCL | 100 ± 20 nm | Si and Zn ions and CiH | Skin | Releasing Si ions promoted angiogenesis and skin regeneration after 14 days post-implantation Zn ions and ciprofloxacin hydrochloride (CiH) resulted in enhanced hair follicle regeneration and antibacterial activity | [277] |
| PCL | According to the incubation time with acetone, 2.4 ± 0.7, 1.1 ± 0.3, 0.5 ± 0.1 µm for 10 min, 1 h, and 6 h, respectively | Vasoactive intestinal peptide | Skin | Enhanced cell adhesion and proliferation Promoted wound healing by increased granulation tissue formation and angiogenesis, but not significant re-epithelialization | [278] |
| Gelatin and PLGA | PEGylated curcumin cobalt nanoparticles | Skin | Enhanced endothelial cell proliferation and VEGF production | [241] | |
| PCL | 218.24 ± 35.21 nm | Placental-derived bioactive molecules | Skin | Promoted adhesion, infiltration, and proliferation of fibroblasts and keratinocytes and enhanced vascularization | [163] |
| Cellulose acetate/gelatin | 316 ± 115 nm | Nanohydroxyapa-tite (nHA) | Skin | 25 mg nHA loaded in cellulose acetate/gelatin (CA/Gel) showed the highest collagen synthesis, re-epithelialization, neovascularization, and the greatest wound closure value (93.5 ± 1.6%) compared with 12.5 and 50 mg nHA | [279] |
| PVA and chitosan | 716.5 ± 76.1 nm | Desferrioxamine | Diabetic wound | Upregulated expression of HIF-1α, VEGF, and SDF-1α Promoted interaction of fibroblasts and endothelial cells | [18] |
| Collagen, hyaluronic acid, and gelatin nanoparticles | 486 ±151 nm and 534 ±128 nm for HA and COL nanofibers, respectively | VEGF, PDGF, bFGF, and EGF | Diabetic wound | Sustained release of growth factors up to one month owing to encapsulation of the gelatin nanoparticlesThe scaffold possesses similar mechanical properties to native skin | [133] |
| Heparin mimetic peptide | Diabetic wound | Accelerated wound closure and granulation tissue formation Greater expression of alpha-smooth muscle actin (α-SMA) and VEGF | [259] | ||
| Chitosan | 50–200 nm | Skin | Greater cell adhesion and cell proliferation, faster regeneration of dermis and epidermis components, and well-vascularization compared with chitosan films and sponges | [254] | |
| Polydioxanone (PDS) | 1–17 µm at the concentration of 125 mg/mL PDS | Alginate beads encapsulated with NGF and chondroitinase ABC | Nerve tissue | More cellular infiltration owing to aligned nanofibers using air-gap electrospinning Vascular network formation after 3 weeks post-implantation Regenerating axons following spinal cord injury owing to trophic support and directional guidance of a scaffold | [273] |
| Poly-L/DL lactic acid (PLA70/30) | 657 ± 101 nm for random and 568 ± 81 nm for aligned nanofibers | Damaged brain | Radially aligned nanofibers supported neuronal migration Long-term viability and integration of the newly generated neurons | [111] | |
| Silk fibroin | 1.8 ± 0.5 µm | BDNF, VEGF | Nerve tissue | De novo innervation and neovascularization indicated by a positive endothelial marker (von Willebrand factor) and innervation marker (S-100 protein) without inflammation Promoted nerve regeneration and angiogenesis after 8 weeks post-implantation | [272] |
| PCL/gelatin | 400–700 nm | bFGF | Bone | A more controlled release of heparin-mediated bFGF up to 24 h Increased proliferation and migration of hMSCs and tubulogenesis of HUVECs Heparinized nanofibers incorporated with 50 or 100 ng/mL bFGF showed a two- and threefold increase in new bone formation, respectively | [245] |
| PLA | 830.3 ± 211.9 nm and 853.7 ± 238.6 nm for the uncoated and coated fibers, respectively | Surface coating of polydopamine | Bone | Greater ALP activity and osteocalcin of hADSC cultured on the scaffold Up-regulation of ang-1 and vWF proteins | [171] |
| PCL | 580 ± 80 nm | Ceramic nanoparticles including Si4+, Ca2+, and PO43- | Bone | Enhanced bioactivity of PCL nanofibers owing to greater apatite formation Reduced contact angle of PCL-Ca-Si (63° ± 3°) compared with only the PCL scaffold (120° ± 10°) | [48] |
| PLGA | 588.9 ± 110.3 nm | Heparin-mediated immobilization of VEGF Co-culture of HUVECs with human/rat MSCs | Bone | Sustained release of VEGF in conjugation with heparin Enhanced angiogenesis, which was detected by CD31 immunostaining after 3 weeks | [244] |
| PCL | In situ silica gelation | Bone | Enhanced water wettability and sustained release of silicon ions (28 ppm silicon ions in 14 days) Promoted tubulogenesis of HUVECs Up-regulation of pro-angiogenic markers including CD31, VEGF-A, PDGF-B, and eNOS and osteogenic markers such as COL1 A1, RUNX2, OSTX, and BMP2 | [280] | |
| Methylmethacrylate (MMA), hexylmethacrylate (HMA), and (trimethoxysilylprolyl) methacrylate (siMA) | Below 500 nm | Mg implant-coated with electrospun nanofiber containing NO | Bone | Stable and local delivery of NO for targeted tubulogenesis of HUVECs | [281] |
| PLLA/chitosan | Not mentioned | Icariin, deferoxamine, and polydopamine | Bone | Promoted cell adhesion, proliferation, osteogenic differentiation, and mineralization of MC3T3-E1 through upregulation of Runx-2, ALP, COL 1, and osteocalcin Up-regulation of angiogenic markers of HUVECs including eNOS, HIF-1α, VEGF, and CD31 | [43] |
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Nomi, M.; Atala, A.; De Coppi, P.; Soker, S. Principals of neovascularization for tissue engineering. Mol. Asp. Med. 2002, 23, 463–483. [Google Scholar] [CrossRef]
- Sarker, M.; Naghieh, S.; McInnes, A.D.; Schreyer, D.J.; Chen, X. Strategic design and fabrication of nerve guidance conduits for peripheral nerve regeneration. Biotechnol. J. 2018, 13, 1700635. [Google Scholar] [CrossRef]
- Frerich, B.; Lindemann, N.; Kurtz-Hoffmann, J.; Oertel, K. In vitro model of a vascular stroma for the engineering of vascularized tissues. Int. J. Oral Maxillofac. Surg. 2001, 30, 414–420. [Google Scholar] [CrossRef]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef]
- Arroyo, A.G.; Iruela-Arispe, M.L. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc. Res. 2010, 86, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef]
- Mahabeleshwar, G.H.; Feng, W.; Reddy, K.; Plow, E.F.; Byzova, T.V. Mechanisms of integrin–vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ. Res. 2007, 101, 570–580. [Google Scholar] [CrossRef]
- Somanath, P.R.; Ciocea, A.; Byzova, T.V. Integrin and growth factor receptor alliance in angiogenesis. Cell Biochem. Biophys. 2009, 53, 53–64. [Google Scholar] [CrossRef]
- Kim, S.-H.; Turnbull, J.; Guimond, S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef]
- Herbert, S.P.; Stainier, D.Y. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell Biol. 2011, 12, 551–564. [Google Scholar] [CrossRef]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/pericyte interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Gaengel, K.; Genové, G.; Armulik, A.; Betsholtz, C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Nico, B.; Crivellato, E. The role of pericytes in angiogenesis. Int. J. Dev. Biol. 2011, 55, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injury 2011, 42, S3–S15. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Kargozar, S.; Ramakrishna, S.; Mozafari, M. Chemistry of biomaterials: Future prospects. Curr. Opin. Biomed. Eng. 2019, 10, 181–190. [Google Scholar] [CrossRef]
- Chen, H.; Jia, P.; Kang, H.; Zhang, H.; Liu, Y.; Yang, P.; Yan, Y.; Zuo, G.; Guo, L.; Jiang, M. Upregulating Hif-1α by hydrogel nanofibrous scaffolds for rapidly recruiting angiogenesis relative cells in diabetic wound. Adv. Healthc. Mater. 2016, 5, 907–918. [Google Scholar] [CrossRef]
- Gao, W.; Jin, W.; Li, Y.; Wan, L.; Wang, C.; Lin, C.; Chen, X.; Lei, B.; Mao, C. A highly bioactive bone extracellular matrix-biomimetic nanofibrous system with rapid angiogenesis promotes diabetic wound healing. J. Mater. Chem. B 2017, 5, 7285–7296. [Google Scholar] [CrossRef]
- Abebayehu, D.; Spence, A.J.; McClure, M.J.; Haque, T.T.; Rivera, K.O.; Ryan, J.J. Polymer scaffold architecture is a key determinant in mast cell inflammatory and angiogenic responses. J. Biomed. Mater. Res. Part A 2019, 107, 884–892. [Google Scholar] [CrossRef]
- Garg, K.; Pullen, N.A.; Oskeritzian, C.A.; Ryan, J.J.; Bowlin, G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 2013, 34, 4439–4451. [Google Scholar] [CrossRef] [PubMed]
- Madden, L.R.; Mortisen, D.J.; Sussman, E.M.; Dupras, S.K.; Fugate, J.A.; Cuy, J.L.; Hauch, K.D.; Laflamme, M.A.; Murry, C.E.; Ratner, B.D. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 15211–15216. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, O.; Ventre, M.; Netti, P. Functional porous hydrogels to study angiogenesis under the effect of controlled release of vascular endothelial growth factor. Acta Biomater. 2012, 8, 3294–3301. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J. Bone Joint Surg. 2001, 83, S105–S115. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adhes. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Gugutkov, D.; Gustavsson, J.; Cantini, M.; Salmeron-Sánchez, M.; Altankov, G. Electrospun fibrinogen–PLA nanofibres for vascular tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 2774–2784. [Google Scholar] [CrossRef]
- Mammadov, R.; Mammadov, B.; Guler, M.O.; Tekinay, A.B. Growth factor binding on heparin mimetic peptide nanofibers. Biomacromolecules 2012, 13, 3311–3319. [Google Scholar] [CrossRef]
- Sung, H.-J.; Meredith, C.; Johnson, C.; Galis, Z.S. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials 2004, 25, 5735–5742. [Google Scholar] [CrossRef]
- Mason, B.N.; Starchenko, A.; Williams, R.M.; Bonassar, L.J.; Reinhart-King, C.A. Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta Biomater. 2013, 9, 4635–4644. [Google Scholar] [CrossRef]
- Klumpp, D.; Rudisile, M.; Kühnle, R.I.; Hess, A.; Bitto, F.F.; Arkudas, A.; Bleiziffer, O.; Boos, A.M.; Kneser, U.; Horch, R.E. Three-dimensional vascularization of electrospun PCL/collagen-blend nanofibrous scaffolds in vivo. J. Biomed. Mater. Res. Part A 2012, 100, 2302–2311. [Google Scholar] [CrossRef]
- Kobayashi, H.; Terada, D.; Yokoyama, Y.; Moon, D.W.; Yasuda, Y.; Koyama, H.; Takato, T. Vascular-inducing poly (glycolic acid)-collagen nanocomposite-fiber scaffold. J. Biomed. Nanotechnol. 2013, 9, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Kenar, H.; Ozdogan, C.Y.; Dumlu, C.; Doger, E.; Kose, G.T.; Hasirci, V. Microfibrous scaffolds from poly (l-lactide-co-ε-caprolactone) blended with xeno-free collagen/hyaluronic acid for improvement of vascularization in tissue engineering applications. Mater. Sci. Eng. C 2019, 97, 31–44. [Google Scholar] [CrossRef]
- Montero, R.B.; Vial, X.; Nguyen, D.T.; Farhand, S.; Reardon, M.; Pham, S.M.; Tsechpenakis, G.; Andreopoulos, F.M. bFGF-containing electrospun gelatin scaffolds with controlled nano-architectural features for directed angiogenesis. Acta Biomater. 2012, 8, 1778–1791. [Google Scholar] [CrossRef]
- Del Gaudio, C.; Baiguera, S.; Boieri, M.; Mazzanti, B.; Ribatti, D.; Bianco, A.; Macchiarini, P. Induction of angiogenesis using VEGF releasing genipin-crosslinked electrospun gelatin mats. Biomaterials 2013, 34, 7754–7765. [Google Scholar] [CrossRef] [PubMed]
- Said, S.S.; Yin, H.; Elfarnawany, M.; Nong, Z.; O’Neil, C.; Leong, H.; Lacefield, J.C.; Mequanint, K.; Pickering, J.G. Fortifying Angiogenesis in Ischemic Muscle with FGF9-Loaded Electrospun Poly (Ester Amide) Fibers. Adv. Healthc. Mater. 2019, 8, 1801294. [Google Scholar] [CrossRef]
- Mulholland, E.J.; Ali, A.; Robson, T.; Dunne, N.J.; McCarthy, H.O. Delivery of RALA/siFKBPL nanoparticles via electrospun bilayer nanofibres: An innovative angiogenic therapy for wound repair. J. Control. Release 2019, 316, 53–65. [Google Scholar] [CrossRef]
- Rather, H.A.; Patel, R.; Yadav, U.C.; Vasita, R. Dual drug-delivering polycaprolactone-collagen scaffold to induce early osteogenic differentiation and coupled angiogenesis. Biomed. Mater. 2020, 15, 045008. [Google Scholar] [CrossRef]
- Mo, Y.; Guo, R.; Zhang, Y.; Xue, W.; Cheng, B.; Zhang, Y. Controlled dual delivery of angiogenin and curcumin by electrospun nanofibers for skin regeneration. Tissue Eng. Part A 2017, 23, 597–608. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Y.; Xue, Y.; Cheng, X.; Zhao, W.; Wang, J.; He, R.; Wan, Q.; Pei, X. Tazarotene released from aligned electrospun membrane facilitates cutaneous wound healing by promoting angiogenesis. ACS Appl. Mater. Interfaces 2019, 11, 36141–36153. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Baino, F.; Hoseini, S.J.; Verdi, J.; Asadpour, S.; Mozafari, M. Curcumin: Footprints on cardiac tissue engineering. Expert Opin. Biol. Ther. 2019, 19, 1199–1205. [Google Scholar] [CrossRef]
- Selvaprithviraj, V.; Sankar, D.; Sivashanmugam, A.; Srinivasan, S.; Jayakumar, R. Pro-angiogenic molecules for therapeutic angiogenesis. Curr. Med. Chem. 2017, 24, 3413–3432. [Google Scholar] [CrossRef] [PubMed]
- Rujitanaroj, P.-O.; Aid-Launais, R.; Chew, S.Y.; Le Visage, C. Polysaccharide electrospun fibers with sulfated poly (fucose) promote endothelial cell migration and VEGF-mediated angiogenesis. Biomater. Sci. 2014, 2, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wen, W.; Chen, S.; Zhou, C.; Luo, B. Preparation of Icariin and Deferoxamine functionalized poly (l-lactide)/chitosan micro/Nanofibrous membranes with synergistic enhanced Osteogenesis and angiogenesis. ACS Appl. Bio Mater. 2018, 1, 389–402. [Google Scholar] [CrossRef]
- Park, I.-S.; Mahapatra, C.; Park, J.S.; Dashnyam, K.; Kim, J.-W.; Ahn, J.C.; Chung, P.-S.; Yoon, D.S.; Mandakhbayar, N.; Singh, R.K.; et al. Revascularization and limb salvage following critical limb ischemia by nanoceria-induced Ref-1/APE1-dependent angiogenesis. Biomaterials 2020, 242, 119919. [Google Scholar] [CrossRef]
- Augustine, R.; Nethi, S.K.; Kalarikkal, N.; Thomas, S.; Patra, C.R. Electrospun polycaprolactone (PCL) scaffolds embedded with europium hydroxide nanorods (EHNs) with enhanced vascularization and cell proliferation for tissue engineering applications. J. Mater. Chem. B 2017, 5, 4660–4672. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hoseini, S.J.; Hamzehlou, S.; Darroudi, M.; Verdi, J.; Hasanzadeh, L.; Kim, H.-W.; Mozafari, M. Biomedical applications of nanoceria: New roles for an old player. Nanomedicine 2018, 13, 3051–3069. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hamblin, M.R.; Mozafari, M. Nanotechnology for angiogenesis: Opportunities and challenges. Chem. Soc. Rev. 2020, 49, 5008–5057. [Google Scholar] [CrossRef]
- Meka, S.R.K.; Agarwal, V.; Chatterjee, K. In situ preparation of multicomponent polymer composite nanofibrous scaffolds with enhanced osteogenic and angiogenic activities. Mater. Sci. Eng. C 2019, 94, 565–579. [Google Scholar] [CrossRef]
- Rahmani, A.; Hashemi-Najafabadi, S.; Eslaminejad, M.B.; Bagheri, F.; Sayahpour, F.A. The effect of modified electrospun PCL-nHA-nZnO scaffolds on osteogenesis and angiogenesis. J. Biomed. Mater. Res. Part A 2019, 107, 2040–2052. [Google Scholar] [CrossRef]
- Fu, Y.; Guan, J.; Guo, S.; Guo, F.; Niu, X.; Liu, Q.; Zhang, C.; Nie, H.; Wang, Y. Human urine-derived stem cells in combination with polycaprolactone/gelatin nanofibrous membranes enhance wound healing by promoting angiogenesis. J. Transl. Med. 2014, 12, 274. [Google Scholar] [CrossRef]
- Xu, K.; Cleaver, O. Tubulogenesis during blood vessel formation. Semin. Cell Dev. Biol. 2011, 229, 993–1004. [Google Scholar] [CrossRef]
- Dragoni, S.; Laforenza, U.; Bonetti, E.; Lodola, F.; Bottino, C.; Berra-Romani, R.; Carlo Bongio, G.; Cinelli, M.P.; Guerra, G.; Pedrazzoli, P. Vascular endothelial growth factor stimulates endothelial colony forming cells proliferation and tubulogenesis by inducing oscillations in intracellular Ca2+ concentration. Stem Cells 2011, 29, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Q.; Gao, W.; Zhang, Z.; Lou, Y.; Jin, H.; Chen, X.; Lei, B.; Xu, H.; Mao, C. Highly efficient local delivery of endothelial progenitor cells significantly potentiates angiogenesis and full-thickness wound healing. Acta Biomater. 2018, 69, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Therapeutics targeting angiogenesis: Genetics and epigenetics, extracellular miRNAs and signaling networks. Int. J. Mol. Med. 2013, 32, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 438, 932–936. [Google Scholar] [CrossRef]
- Rouwkema, J.; Khademhosseini, A. Vascularization and angiogenesis in tissue engineering: Beyond creating static networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Foresti, R.; Rossi, S.; Pinelli, S.; Alinovi, R.; Barozzi, M.; Sciancalepore, C.; Galetti, M.; Caffarra, C.; Lagonegro, P.; Scavia, G. Highly-defined bioprinting of long-term vascularized scaffolds with Bio-Trap: Complex geometry functionalization and process parameters with computer aided tissue engineering. Materialia 2020, 9, 100560. [Google Scholar] [CrossRef]
- Richards, D.; Jia, J.; Yost, M.; Markwald, R.; Mei, Y. 3D bioprinting for vascularized tissue fabrication. Ann. Biomed. Eng. 2017, 45, 132–147. [Google Scholar] [CrossRef]
- Blinder, Y.; Mooney, D.; Levenberg, S. Engineering approaches for inducing blood vessel formation. Curr. Opin. Chem. Eng. 2014, 3, 56–61. [Google Scholar] [CrossRef]
- Novosel, E.C.; Kleinhans, C.; Kluger, P.J. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 2011, 63, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhu, W.; Nowicki, M.; Zhou, X.; Khademhosseini, A.; Zhang, L.G. Hierarchical fabrication of engineered vascularized bone biphasic constructs via dual 3D bioprinting: Integrating regional bioactive factors into architectural design. Adv. Healthc. Mater. 2016, 5, 2174–2181. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Gu, Y.; Liu, L.; Tang, J.; Mao, J.; Xi, K.; Jiang, Z.; Zhou, Y.; Xu, Y.; Deng, L. Hierarchical micro/nanofibrous membranes of sustained releasing VEGF for periosteal regeneration. Biomaterials 2020, 227, 119555. [Google Scholar] [CrossRef]
- Haerst, M.; Ahrens, M.; Seitz, V.; Wintermantel, E. Electrospinning of commercially available medical silicone rubber. Biomed. Technol. 2014, s1, 59. [Google Scholar]
- Stocco, T.D.; Bassous, N.J.; Zhao, S.; Granato, A.E.; Webster, T.J.; Lobo, A.O. Nanofibrous scaffolds for biomedical applications. Nanoscale 2018, 10, 12228–12255. [Google Scholar] [CrossRef]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: Advances, challenges, and future prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef]
- Rim, N.G.; Shin, C.S.; Shin, H. Current approaches to electrospun nanofibers for tissue engineering. Biomed. Mater. 2013, 8, 014102. [Google Scholar] [CrossRef]
- Wang, J.; Windbergs, M. Functional electrospun fibers for the treatment of human skin wounds. Eur. J. Pharm. Biopharm. 2017, 119, 283–299. [Google Scholar] [CrossRef]
- Yadav, R.; Balasubramanian, K. Metallization of electrospun PAN nanofibers via electroless gold plating. RSC Adv. 2015, 5, 24990–24996. [Google Scholar] [CrossRef]
- Magisetty, R.; Kumar, P.; Gore, P.M.; Ganivada, M.; Shukla, A.; Kandasubramanian, B.; Shunmugam, R. Electronic properties of Poly (1, 6-heptadiynes) electrospun fibrous non-woven mat. Mater. Chem. Phys. 2019, 223, 343–352. [Google Scholar] [CrossRef]
- Tahalyani, J.; Datar, S.; Balasubramanian, K. Investigation of dielectric properties of free standing electrospun nonwoven mat. J. Appl. Polym. Sci. 2018, 135, 46121. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Progress in the field of electrospinning for tissue engineering applications. Adv. Mater. 2009, 21, 3343–3351. [Google Scholar] [CrossRef]
- Simon, S.; Balasubramanian, K. Facile immobilization of camphor soot on electrospun hydrophobic membrane for oil-water separation. Mater. Focus 2018, 7, 295–303. [Google Scholar] [CrossRef]
- Simon, S.; Malik, A.; Kandasubramanian, B. Hierarchical electrospun super-hydrophobic nanocomposites of fluoroelastomer. Mater. Focus 2018, 7, 194–206. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Braghirolli, D.I.; Steffens, D.; Pranke, P. Electrospinning for regenerative medicine: A review of the main topics. Drug Discov. Today 2014, 19, 743–753. [Google Scholar] [CrossRef]
- Badhe, Y.; Balasubramanian, K. Nanoencapsulated core and shell electrospun fibers of resorcinol formaldehyde. Ind. Eng. Chem. Res. 2015, 54, 7614–7622. [Google Scholar] [CrossRef]
- Bhalara, P.D.; Balasubramanian, K.; Banerjee, B.S. Spider–web textured electrospun composite of graphene for sorption of Hg (II) ions. Mater. Focus 2015, 4, 154–163. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Malik, R.; Garg, T.; Goyal, A.K.; Rath, G. Polymeric nanofibers: Targeted gastro-retentive drug delivery systems. J. Drug Target. 2015, 23, 109–124. [Google Scholar] [CrossRef]
- Kar, K.K.; Rana, S.; Pandey, J. Handbook of Polymer Nanocomposites Processing, Performance and Application; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Lee, J.; Kwon, H.; Seo, J.; Shin, S.; Koo, J.H.; Pang, C.; Son, S.; Kim, J.H.; Jang, Y.H.; Kim, D.E. Conductive fiber-based ultrasensitive textile pressure sensor for wearable electronics. Adv. Mater. 2015, 27, 2433–2439. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Electrospinning of gelatin with tunable fiber morphology from round to flat/ribbon. Mater. Sci. Eng. C 2017, 80, 371–378. [Google Scholar] [CrossRef]
- Ki, C.S.; Baek, D.H.; Gang, K.D.; Lee, K.H.; Um, I.C.; Park, Y.H. Characterization of gelatin nanofiber prepared from gelatin–formic acid solution. Polymer 2005, 46, 5094–5102. [Google Scholar] [CrossRef]
- Nadri, S.; Nasehi, F.; Barati, G. Effect of parameters on the quality of core-shell fibrous scaffold for retinal differentiation of conjunctiva mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2017, 105, 189–197. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Dai, L.; Yao, J. Scalable Fabrication of Nanoporous Carbon Fiber Films as Bifunctional Catalytic Electrodes for Flexible Zn-Air Batteries. Adv. Mater. 2016, 28, 3000–3006. [Google Scholar] [CrossRef]
- Bedane, A.H.; Eić, M.; Farmahini-Farahani, M.; Xiao, H. Theoretical modeling of water vapor transport in cellulose-based materials. Cellulose 2016, 23, 1537–1552. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; du Toit, L.C.; Ndesendo, V.M.K. A Review of the Effect of Processing Variables on the Fabrication of Electrospun Nanofibers for Drug Delivery Applications. J. Nanomater. 2013, 2013, 789289. [Google Scholar] [CrossRef]
- Chou, S.-F.; Carson, D.; Woodrow, K.A. Current strategies for sustaining drug release from electrospun nanofibers. J. Control. Release 2015, 220, 584–591. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, J.; Yu, G.; Cardenas, R.; Wei, S.; Wujcik, E.K.; Guo, Z. Coaxial electrospun fibers: Applications in drug delivery and tissue engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 654–677. [Google Scholar] [CrossRef]
- Yu, D.-G.; Li, X.-Y.; Wang, X.; Yang, J.-H.; Bligh, S.A.; Williams, G.R. Nanofibers fabricated using triaxial electrospinning as zero order drug delivery systems. ACS Appl. Mater. Interfaces 2015, 7, 18891–18897. [Google Scholar] [CrossRef]
- Han, D.; Steckl, A.J. Triaxial electrospun nanofiber membranes for controlled dual release of functional molecules. ACS Appl. Mater. Interfaces 2013, 5, 8241–8245. [Google Scholar] [CrossRef]
- Horst, M.; Madduri, S.; Milleret, V.; Sulser, T.; Gobet, R.; Eberli, D. A bilayered hybrid microfibrous PLGA–acellular matrix scaffold for hollow organ tissue engineering. Biomaterials 2013, 34, 1537–1545. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Direct fabrication of composite and ceramic hollow nanofibers by electrospinning. Nano Lett. 2004, 4, 933–938. [Google Scholar] [CrossRef]
- Ren, B.; Fan, M.; Liu, Q.; Wang, J.; Song, D.; Bai, X. Hollow NiO nanofibers modified by citric acid and the performances as supercapacitor electrode. Electrochim. Acta 2013, 92, 197–204. [Google Scholar] [CrossRef]
- Gupta, P.; Wilkes, G.L. Some investigations on the fiber formation by utilizing a side-by-side bicomponent electrospinning approach. Polymer 2003, 44, 6353–6359. [Google Scholar] [CrossRef]
- Gu, B.K.; Shin, M.K.; Sohn, K.W.; Kim, S.I.; Kim, S.J.; Kim, S.-K.; Lee, H.; Park, J.S. Direct fabrication of twisted nanofibers by electrospinning. Appl. Phys. Lett. 2007, 90, 263902. [Google Scholar] [CrossRef]
- Canejo, J.P.; Borges, J.P.; Godinho, M.H.; Brogueira, P.; Teixeira, P.I.; Terentjev, E.M. Helical twisting of electrospun liquid crystalline cellulose micro-and nanofibers. Adv. Mater. 2008, 20, 4821–4825. [Google Scholar] [CrossRef]
- Ji, L.; Medford, A.J.; Zhang, X. Porous carbon nanofibers loaded with manganese oxide particles: Formation mechanism and electrochemical performance as energy-storage materials. J. Mater. Chem. 2009, 19, 5593–5601. [Google Scholar] [CrossRef]
- Dayal, P.; Liu, J.; Kumar, S.; Kyu, T. Experimental and theoretical investigations of porous structure formation in electrospun fibers. Macromolecules 2007, 40, 7689–7694. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Rychter, M.; Baranowska-Korczyc, A.; Lulek, J. Progress and perspectives in bioactive agent delivery via electrospun vascular grafts. RSC Adv. 2017, 7, 32164–32184. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, Y.; Duan, G.; Mei, C.; Greiner, A.; Agarwal, S. Electrospun nanofiber reinforced composites: A review. Polym. Chem. 2018, 9, 2685–2720. [Google Scholar] [CrossRef]
- Hong, J.K.; Bang, J.Y.; Xu, G.; Lee, J.-H.; Kim, Y.-J.; Lee, H.-J.; Kim, H.S.; Kwon, S.-M. Thickness-controllable electrospun fibers promote tubular structure formation by endothelial progenitor cells. Int. J. Nanomed. 2015, 10, 1189. [Google Scholar]
- Rnjak-Kovacina, J.; Weiss, A.S. Increasing the pore size of electrospun scaffolds. Tissue Eng. Part B Rev. 2011, 17, 365–372. [Google Scholar] [CrossRef]
- Leong, M.F.; Rasheed, M.Z.; Lim, T.C.; Chian, K.S. In vitro cell infiltration and in vivo cell infiltration and vascularization in a fibrous, highly porous poly (D, L-lactide) scaffold fabricated by cryogenic electrospinning technique. J. Biomed. Mater. Res. Part A 2009, 91, 231–240. [Google Scholar] [CrossRef]
- Telemeco, T.; Ayres, C.; Bowlin, G.; Wnek, G.; Boland, E.; Cohen, N.; Baumgarten, C.; Mathews, J.; Simpson, D. Regulation of cellular infiltration into tissue engineering scaffolds composed of submicron diameter fibrils produced by electrospinning. Acta Biomater. 2005, 1, 377–385. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; Wise, S.G.; Li, Z.; Maitz, P.K.; Young, C.J.; Wang, Y.; Weiss, A.S. Tailoring the porosity and pore size of electrospun synthetic human elastin scaffolds for dermal tissue engineering. Biomaterials 2011, 32, 6729–6736. [Google Scholar] [CrossRef]
- Álvarez, Z.; Castaño, O.; Castells, A.A.; Mateos-Timoneda, M.A.; Planell, J.A.; Engel, E.; Alcántara, S. Neurogenesis and vascularization of the damaged brain using a lactate-releasing biomimetic scaffold. Biomaterials 2014, 35, 4769–4781. [Google Scholar] [CrossRef]
- Kang, L.; Jia, W.; Li, M.; Wang, Q.; Wang, C.; Liu, Y.; Wang, X.; Jin, L.; Jiang, J.; Gu, G. Hyaluronic acid oligosaccharide-modified collagen nanofibers as vascular tissue-engineered scaffold for promoting endothelial cell proliferation. Carbohydr. Polym. 2019, 223, 115106. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Lee, K.-P.; Huh, M.-I.; Eom, S.; Park, B.-U.; Kim, K.H.; Park, D.H.; Kim, D.S.; Kim, H.K. Development of an in vitro 3D choroidal neovascularization model using chemically induced hypoxia through an ultra-thin, free-standing nanofiber membrane. Mater. Sci. Eng. C 2019, 104, 109964. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, Y.; Wang, Z.; Wang, J.; Zhang, C.; Li, C.; Kong, D. The regeneration of macro-porous electrospun poly (ɛ-caprolactone) vascular graft during long-term in situ implantation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Walthers, C.M.; Nazemi, A.K.; Patel, S.L.; Wu, B.M.; Dunn, J.C. The effect of scaffold macroporosity on angiogenesis and cell survival in tissue-engineered smooth muscle. Biomaterials 2014, 35, 5129–5137. [Google Scholar] [CrossRef] [PubMed]
- Jundziłł, A.; Pokrywczyńska, M.; Adamowicz, J.; Kowalczyk, T.; Nowacki, M.; Bodnar, M.; Marszałek, A.; Frontczak-Baniewicz, M.; Mikułowski, G.; Kloskowski, T. Vascularization potential of electrospun poly (L-lactide-co-caprolactone) scaffold: The Impact for tissue engineering. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 1540. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Cho, B.; Martin, R.; Seu, M.; Zhang, C.; Zhou, Z.; Choi, J.S.; Jiang, X.; Chen, L.; Walia, G. Nanofiber-hydrogel composite–mediated angiogenesis for soft tissue reconstruction. Sci. Transl. Med. 2019, 11, eaau6210. [Google Scholar] [CrossRef]
- Lee, J.B.; Balikov, D.A.; Yang, J.W.; Kim, K.S.; Park, H.K.; Kim, J.K.; Kwon, I.K.; Bellan, L.M.; Sung, H.-J. Cationic nanocylinders promote angiogenic activities of endothelial cells. Polymers 2016, 8, 15. [Google Scholar] [CrossRef]
- Eghtesad, S.; Nurminskaya, M.V. Binding of pro-migratory serum factors to electrospun PLLA nano-fibers. J. Biomater. Sci. Polym. Ed. 2013, 24, 2006–2017. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Z.; Wang, H.; Wang, Y.; Carlson, M.A.; Teusink, M.J.; MacEwan, M.R.; Gu, L.; Xie, J. Expanded 3D nanofiber scaffolds: Cell penetration, neovascularization, and host response. Adv. Healthc. Mater. 2016, 5, 2993–3003. [Google Scholar] [CrossRef]
- Wickham, A.; Sjölander, D.; Bergström, G.; Wang, E.; Rajendran, V.; Hildesjö, C.; Skoglund, K.; Nilsson, K.P.R.; Aili, D. Near-Infrared Emitting and Pro-Angiogenic Electrospun Conjugated Polymer Scaffold for Optical Biomaterial Tracking. Adv. Funct. Mater. 2015, 25, 4274–4281. [Google Scholar] [CrossRef]
- Castellano, D.; Blanes, M.; Marco, B.; Cerrada, I.; Ruiz-Saurí, A.; Pelacho, B.; Arana, M.; Montero, J.A.; Cambra, V.; Prosper, F. A comparison of electrospun polymers reveals poly (3-hydroxybutyrate) fiber as a superior scaffold for cardiac repair. Stem Cells Dev. 2014, 23, 1479–1490. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.; Ke, Q.; Chang, J. An anisotropically and heterogeneously aligned patterned electrospun scaffold with tailored mechanical property and improved bioactivity for vascular tissue engineering. ACS Appl. Mater. Interfaces 2015, 7, 8706–8718. [Google Scholar] [CrossRef]
- Castellano, D.; Sanchis, A.; Blanes, M.; Pérez del Caz, M.D.; Ruiz-Saurí, A.; Piquer-Gil, M.; Pelacho, B.; Marco, B.; Garcia, N.; Ontoria-Oviedo, I. Electrospun poly (hydroxybutyrate) scaffolds promote engraftment of human skin equivalents via macrophage M2 polarization and angiogenesis. J. Tissue Eng. Regen. Med. 2018, 12, e983–e994. [Google Scholar] [CrossRef]
- Gombotz, W.R.; Pettit, D.K. Biodegradable polymers for protein and peptide drug delivery. Bioconjugate Chem. 1995, 6, 332–351. [Google Scholar] [CrossRef]
- Zamani, M.; Prabhakaran, M.P.; Ramakrishna, S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed. 2013, 8, 2997. [Google Scholar]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef]
- Li, Z.; Song, L.; Huang, X.; Wang, H.; Shao, H.; Xie, M.; Xu, Y.; Zhang, Y. Tough and VEGF-releasing scaffolds composed of artificial silk fibroin mats and a natural acellular matrix. Rsc Adv. 2015, 5, 16748–16758. [Google Scholar] [CrossRef]
- Liao, I.-C.; Leong, K.W. Efficacy of engineered FVIII-producing skeletal muscle enhanced by growth factor-releasing co-axial electrospun fibers. Biomaterials 2011, 32, 1669–1677. [Google Scholar] [CrossRef]
- Zigdon-Giladi, H.; Khutaba, A.; Elimelech, R.; Machtei, E.E.; Srouji, S. VEGF release from a polymeric nanofiber scaffold for improved angiogenesis. J. Biomed. Mater. Res. Part A 2017, 105, 2712–2721. [Google Scholar] [CrossRef]
- Jiang, Y.-C.; Wang, X.-F.; Xu, Y.-Y.; Qiao, Y.-H.; Guo, X.; Wang, D.-F.; Li, Q.; Turng, L.-S. Polycaprolactone nanofibers containing vascular endothelial growth factor-encapsulated gelatin particles enhance mesenchymal stem cell differentiation and angiogenesis of endothelial cells. Biomacromolecules 2018, 19, 3747–3753. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, X.; Han, F.; Zhao, J.; Zhao, Y.; Fan, Y.; Yuan, X. Dual-delivery of VEGF and PDGF by double-layered electrospun membranes for blood vessel regeneration. Biomaterials 2013, 34, 2202–2212. [Google Scholar] [CrossRef]
- Lai, H.-J.; Kuan, C.-H.; Wu, H.-C.; Tsai, J.-C.; Chen, T.-M.; Hsieh, D.-J.; Wang, T.-W. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta Biomater. 2014, 10, 4156–4166. [Google Scholar] [CrossRef]
- Tian, L.; Prabhakaran, M.P.; Ding, X.; Kai, D.; Ramakrishna, S. Emulsion electrospun vascular endothelial growth factor encapsulated poly (l-lactic acid-co-ε-caprolactone) nanofibers for sustained release in cardiac tissue engineering. J. Mater. Sci. 2012, 47, 3272–3281. [Google Scholar] [CrossRef]
- Nillesen, S.T.; Geutjes, P.J.; Wismans, R.; Schalkwijk, J.; Daamen, W.F.; van Kuppevelt, T.H. Increased angiogenesis and blood vessel maturation in acellular collagen–heparin scaffolds containing both FGF2 and VEGF. Biomaterials 2007, 28, 1123–1131. [Google Scholar] [CrossRef]
- Guo, X.; Elliott, C.G.; Li, Z.; Xu, Y.; Hamilton, D.W.; Guan, J. Creating 3D angiogenic growth factor gradients in fibrous constructs to guide fast angiogenesis. Biomacromolecules 2012, 13, 3262–3271. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, T.; Zhi, W.; Wei, L.; Weng, J.; Zhang, C.; Li, X. Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials 2011, 32, 4243–4254. [Google Scholar] [CrossRef]
- Zhu, X.H.; Tabata, Y.; Wang, C.-H.; Tong, Y.W. Delivery of basic fibroblast growth factor from gelatin microsphere scaffold for the growth of human umbilical vein endothelial cells. Tissue Eng. Part A 2008, 14, 1939–1947. [Google Scholar] [CrossRef]
- Jin, Q.; Wei, G.; Lin, Z.; Sugai, J.V.; Lynch, S.E.; Ma, P.X.; Giannobile, W.V. Nanofibrous scaffolds incorporating PDGF-BB microspheres induce chemokine expression and tissue neogenesis in vivo. PLoS ONE 2008, 3, e1729. [Google Scholar] [CrossRef]
- Roy, H.; Bhardwaj, S.; Ylä-Herttuala, S. Biology of vascular endothelial growth factors. FEBS Lett. 2006, 580, 2879–2887. [Google Scholar] [CrossRef]
- Senger, D.R.; Galli, S.J.; Dvorak, A.M.; Perruzzi, C.A.; Harvey, V.S.; Dvorak, H.F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983, 219, 983–985. [Google Scholar] [CrossRef]
- Bouïs, D.; Kusumanto, Y.; Meijer, C.; Mulder, N.H.; Hospers, G.A. A review on pro-and anti-angiogenic factors as targets of clinical intervention. Pharm. Res. 2006, 53, 89–103. [Google Scholar] [CrossRef]
- DeVolder, R.J.; Bae, H.; Lee, J.; Kong, H. Directed blood vessel growth using an angiogenic microfiber/microparticle composite patch. Adv. Mater. 2011, 23, 3139–3143. [Google Scholar] [CrossRef]
- Clapp, C.; Thebault, S.; Jeziorski, M.C.; Martinez De la Escalera, G. Peptide hormone regulation of angiogenesis. Physiol. Rev. 2009, 89, 1177–1215. [Google Scholar] [CrossRef]
- Satish, A.; Korrapati, P.S. Nanofiber-Mediated Sustained Delivery of Triiodothyronine: Role in Angiogenesis. AAPS PharmSciTech 2019, 20, 110. [Google Scholar] [CrossRef]
- Gasowska, K.; Naumnik, B.; Klejna, K.; Myśliwiec, M. The influence of unfractionated and low-molecular weight heparins on the properties of human umbilical vein endothelial cells (HUVEC). Folia Histochem. Cytobiol. 2009, 47, 17–23. [Google Scholar] [CrossRef][Green Version]
- Kuang, H.; Yang, S.; Wang, Y.; He, Y.; Ye, K.; Hu, J.; Shen, W.; Morsi, Y.; Lu, S.; Mo, X. Electrospun Bilayer Composite Vascular Graft with an Inner Layer Modified by Polyethylene Glycol and Haparin to Regenerate the Blood Vessel. J. Biomed. Nanotechnol. 2019, 15, 77–84. [Google Scholar] [CrossRef]
- Pitarresi, G.; Fiorica, C.; Palumbo, F.S.; Rigogliuso, S.; Ghersi, G.; Giammona, G. Heparin functionalized polyaspartamide/polyester scaffold for potential blood vessel regeneration. J. Biomed. Mater. Res. Part A 2014, 102, 1334–1341. [Google Scholar] [CrossRef]
- Ahangari, N.; Kargozar, S.; Ghayour-Mobarhan, M.; Baino, F.; Pasdar, A.; Sahebkar, A.; Ferns, G.A.; Kim, H.W.; Mozafari, M. Curcumin in tissue engineering: A traditional remedy for modern medicine. Biofactors 2019, 45, 135–151. [Google Scholar] [CrossRef]
- Hu, Y.; Cao, S.; Chen, J.; Zhao, Y.; He, F.; Li, Q.; Zou, L.; Shi, C. Biomimetic fabrication of Icariin loaded nano hydroxyapatite reinforced bioactive porous scaffolds for bone regeneration. Chem. Eng. J. 2020, 394, 124895. [Google Scholar] [CrossRef]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.-G.; Lee, B.-T. Curcumin incorporation into an oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system promotes wound healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Rubio-Elizalde, I.; Bernáldez-Sarabia, J.; Moreno-Ulloa, A.; Vilanova, C.; Juárez, P.; Licea-Navarro, A.; Castro-Ceseña, A.B. Scaffolds based on alginate-PEG methyl ether methacrylate-Moringa oleifera-Aloe vera for wound healing applications. Carbohydr. Polym. 2019, 206, 455–467. [Google Scholar] [CrossRef]
- Chung, B.-H.; Kim, J.-D.; Kim, C.-K.; Kim, J.H.; Won, M.-H.; Lee, H.-S.; Dong, M.-S.; Ha, K.-S.; Kwon, Y.-G.; Kim, Y.-M. Icariin stimulates angiogenesis by activating the MEK/ERK-and PI3K/Akt/eNOS-dependent signal pathways in human endothelial cells. Biochem. Biophys. Res. Commun. 2008, 376, 404–408. [Google Scholar] [CrossRef]
- Basak, S.; Srinivas, V.; Mallepogu, A.; Duttaroy, A.K. Curcumin stimulates angiogenesis through VEGF and expression of HLA-G in first-trimester human placental trophoblasts. Cell Biol. Int. 2020, 44, 1237–1251. [Google Scholar] [CrossRef]
- Hosseini, A.; Rasmi, Y.; Rahbarghazi, R.; Aramwit, P.; Daeihassani, B.; Saboory, E. Curcumin modulates the angiogenic potential of human endothelial cells via FAK/P-38 MAPK signaling pathway. Gene 2019, 688, 7–12. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Chen, J.-X. Effects of Curcumin on Vessel Formation Insight into the Pro- and Antiangiogenesis of Curcumin. Evid. Based Complementary Altern. Med. 2019, 2019, 1390795. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.; Du, L.; Jin, Y. Preparation of asiaticoside-loaded coaxially electrospinning nanofibers and their effect on deep partial-thickness burn injury. Biomed. Pharmacother. 2016, 83, 33–40. [Google Scholar] [CrossRef]
- Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Concheiro, A.; Silva, M.; Dominguez, F.; Sheikh, F.A.; Cantu, T.; Desai, R.; Garcia, V.L.; Macossay, J. Biodegradable electrospun nanofibers coated with platelet-rich plasma for cell adhesion and proliferation. Mater. Sci. Eng. C 2014, 40, 180–188. [Google Scholar] [CrossRef]
- Cheng, G.; Ma, X.; Li, J.; Cheng, Y.; Cao, Y.; Wang, Z.; Shi, X.; Du, Y.; Deng, H.; Li, Z. Incorporating platelet-rich plasma into coaxial electrospun nanofibers for bone tissue engineering. Int. J. Pharm. 2018, 547, 656–666. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, S.; Pan, Y.; Zhang, Q.; Wang, K.; Song, D.; Wang, X.; Feng, G.; Liu, R.; Xu, H. Functional modification of fibrous PCL scaffolds with fusion protein VEGF-HGFI enhanced cellularization and vascularization. Adv. Healthc. Mater. 2016, 5, 2376–2385. [Google Scholar] [CrossRef]
- John, J.V.; Choksi, M.; Chen, S.; Boda, S.K.; Su, Y.; McCarthy, A.; Teusink, M.J.; Reinhardt, R.A.; Xie, J. Tethering peptides onto biomimetic and injectable nanofiber microspheres to direct cellular response. Nanomed. Nanotechnol. Biol. Med. 2019, 22, 102081. [Google Scholar] [CrossRef]
- Deng, A.; Yang, Y.; Du, S.; Yang, S. Electrospinning of in situ crosslinked recombinant human collagen peptide/chitosan nanofibers for wound healing. Biomater. Sci. 2018, 6, 2197–2208. [Google Scholar] [CrossRef]
- Rameshbabu, A.P.; Datta, S.; Bankoti, K.; Subramani, E.; Chaudhury, K.; Lalzawmliana, V.; Nandi, S.K.; Dhara, S. Polycaprolactone nanofibers functionalized with placental derived extracellular matrix for stimulating wound healing activity. J. Mater. Chem. B 2018, 6, 6767–6780. [Google Scholar] [CrossRef]
- Zhou, Z.; Long, D.; Hsu, C.-C.; Liu, H.; Chen, L.; Slavin, B.; Lin, H.; Li, X.; Tang, J.; Yiu, S. Nanofiber-reinforced decellularized amniotic membrane improves limbal stem cell transplantation in a rabbit model of corneal epithelial defect. Acta Biomater. 2019, 97, 310–320. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mani, M.P.; Khudzari, A.Z.M. Electrospun combination of peppermint oil and copper sulphate with conducive physico-chemical properties for wound dressing applications. Polymers 2019, 11, 586. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mani, M.P. Electrospun polyurethane nanofibrous composite impregnated with metallic copper for wound-healing application. 3 Biotech 2018, 8, 327. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Samadikuchaksaraei, A.; Seifalian, A.M.; Urbanska, A.M.; Ghanbarian, H.; Hardy, J.G.; Omrani, M.D.; Mozafari, M.; Reis, R.L.; Kundu, S.C. Silk fibroin/amniotic membrane 3D bi-layered artificial skin. Biomed. Mater. 2018, 13, 035003. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive glasses: Sprouting angiogenesis in tissue engineering. Trends Biotechnol. 2018, 36, 430–444. [Google Scholar] [CrossRef]
- Malhotra, A.; Habibovic, P. Calcium phosphates and angiogenesis: Implications and advances for bone regeneration. Trends Biotechnol. 2016, 34, 983–992. [Google Scholar] [CrossRef]
- Zhai, W.; Lu, H.; Wu, C.; Chen, L.; Lin, X.; Naoki, K.; Chen, G.; Chang, J. Stimulatory effects of the ionic products from Ca–Mg–Si bioceramics on both osteogenesis and angiogenesis in vitro. Acta Biomater. 2013, 9, 8004–8014. [Google Scholar] [CrossRef]
- Xia, L.; Yin, Z.; Mao, L.; Wang, X.; Liu, J.; Jiang, X.; Zhang, Z.; Lin, K.; Chang, J.; Fang, B. Akermanite bioceramics promote osteogenesis, angiogenesis and suppress osteoclastogenesis for osteoporotic bone regeneration. Sci. Rep. 2016, 6, 22005. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Zhu, X.; Tang, Z.; Yang, X.; Tan, Y.; Fan, Y.; Zhang, X. Enhanced effect of β-tricalcium phosphate phase on neovascularization of porous calcium phosphate ceramics: In vitro and in vivo evidence. Acta Biomater. 2015, 11, 435–448. [Google Scholar] [CrossRef]
- Wang, J.; Qian, S.; Liu, X.; Xu, L.; Miao, X.; Xu, Z.; Cao, L.; Wang, H.; Jiang, X. M2 macrophages contribute to osteogenesis and angiogenesis on nanotubular TiO 2 surfaces. J. Mater. Chem. B 2017, 5, 3364–3376. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive glasses entering the mainstream. Drug Discov. Today 2018, 23, 1700–1704. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xiao, W.; Bal, B.S.; Rahaman, M.N. Effect of copper-doped silicate 13–93 bioactive glass scaffolds on the response of MC3T3-E1 cells in vitro and on bone regeneration and angiogenesis in rat calvarial defects in vivo. Mater. Sci. Eng. C 2016, 67, 440–452. [Google Scholar] [CrossRef]
- Kargozar, S.; Lotfibakhshaiesh, N.; Ai, J.; Samadikuchaksaraie, A.; Hill, R.G.; Shah, P.A.; Milan, P.B.; Mozafari, M.; Fathi, M.; Joghataei, M.T. Synthesis, physico-chemical and biological characterization of strontium and cobalt substituted bioactive glasses for bone tissue engineering. J. Non-Cryst. Solids 2016, 449, 133–140. [Google Scholar] [CrossRef]
- Weng, L.; Boda, S.K.; Teusink, M.J.; Shuler, F.D.; Li, X.; Xie, J. Binary doping of strontium and copper enhancing osteogenesis and angiogenesis of bioactive glass nanofibers while suppressing osteoclast activity. ACS Appl. Mater. Interfaces 2017, 9, 24484–24496. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Mozafari, M.; Hill, R.G.; Brouki Milan, P.; Taghi Joghataei, M.; Hamzehlou, S.; Baino, F. Synergistic combination of bioactive glasses and polymers for enhanced bone tissue regeneration. Mater. Today Proc. 2018, 5, 15532–15539. [Google Scholar] [CrossRef]
- Deliormanlı, A.M. Electrospun cerium and gallium-containing silicate based 13-93 bioactive glass fibers for biomedical applications. Ceram. Int. 2016, 42, 897–906. [Google Scholar] [CrossRef]
- Serio, F.; Miola, M.; Vernè, E.; Pisignano, D.; Boccaccini, A.R.; Liverani, L. Electrospun Filaments Embedding Bioactive Glass Particles with Ion Release and Enhanced Mineralization. Nanomaterials 2019, 9, 182. [Google Scholar] [CrossRef]
- Sachot, N.; Roguska, A.; Planell, J.A.; Lewandowska, M.; Engel, E.; Castaño, O. Fast-degrading PLA/ORMOGLASS fibrous composite scaffold leads to a calcium-rich angiogenic environment. Int. J. Nanomed. 2017, 12, 4901. [Google Scholar] [CrossRef]
- Shamosi, A.; Mehrabani, D.; Azami, M.; Ebrahimi-Barough, S.; Siavashi, V.; Ghanbari, H.; Sharifi, E.; Roozafzoon, R.; Ai, J. Differentiation of human endometrial stem cells into endothelial-like cells on gelatin/chitosan/bioglass nanofibrous scaffolds. Artif. Cells Nanomed. Biotechnol. 2017, 45, 163–173. [Google Scholar] [CrossRef]
- Oliveira, H.; Catros, S.; Boiziau, C.; Siadous, R.; Marti-Munoz, J.; Bareille, R.; Rey, S.; Castano, O.; Planell, J.; Amédée, J. The proangiogenic potential of a novel calcium releasing biomaterial: Impact on cell recruitment. Acta Biomater. 2016, 29, 435–445. [Google Scholar] [CrossRef]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013, 31, 594–605. [Google Scholar] [CrossRef]
- Song, Y.; Wu, H.; Gao, Y.; Li, J.; Lin, K.; Liu, B.; Lei, X.; Cheng, P.; Zhang, S.; Wang, Y. Zinc Silicate/Nano-Hydroxyapatite/Collagen Scaffolds Promote Angiogenesis and Bone Regeneration via the p38 MAPK Pathway in Activated Monocytes. ACS Appl. Mater. Interfaces 2020, 12, 16058–16075. [Google Scholar] [CrossRef]
- Wang, M.; Yu, Y.; Dai, K.; Ma, Z.; Liu, Y.; Wang, J.; Liu, C. Improved osteogenesis and angiogenesis of magnesium-doped calcium phosphate cement via macrophage immunomodulation. Biomater. Sci. 2016, 4, 1574–1583. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, Y.; Zhang, X.; Xia, W.; Xu, Y.; Lin, K. Injectable nano-structured silicon-containing hydroxyapatite microspheres with enhanced osteogenic differentiation and angiogenic factor expression. Ceram. Int. 2018, 44, 20457–20464. [Google Scholar] [CrossRef]
- Ye, H.; Zhu, J.; Deng, D.; Jin, S.; Li, J.; Man, Y. Enhanced osteogenesis and angiogenesis by PCL/chitosan/Sr-doped calcium phosphate electrospun nanocomposite membrane for guided bone regeneration. J. Biomater. Sci. Polym. Ed. 2019, 30, 1505–1522. [Google Scholar] [CrossRef]
- Kang, B.-J.; Kim, H.; Lee, S.K.; Kim, J.; Shen, Y.; Jung, S.; Kang, K.-S.; Im, S.G.; Lee, S.Y.; Choi, M.; et al. Umbilical-cord-blood-derived mesenchymal stem cells seeded onto fibronectin-immobilized polycaprolactone nanofiber improve cardiac function. Acta Biomater. 2014, 10, 3007–3017. [Google Scholar] [CrossRef]
- Mukherjee, S.; Darzi, S.; Paul, K.; Cousins, F.L.; Werkmeister, J.A.; Gargett, C.E. Electrospun Nanofiber Meshes With Endometrial MSCs Modulate Foreign Body Response by Increased Angiogenesis, Matrix Synthesis, and Anti-Inflammatory Gene Expression in Mice: Implication in Pelvic Floor. Front. Pharmacol. 2020, 11, 353. [Google Scholar] [CrossRef]
- Kook, Y.-M.; Kim, H.; Kim, S.; Heo, C.Y.; Park, M.H.; Lee, K.; Koh, W.-G. Promotion of vascular morphogenesis of endothelial cells co-cultured with human adipose-derived mesenchymal stem cells using polycaprolactone/gelatin nanofibrous scaffolds. Nanomaterials 2018, 8, 117. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.; Li, J.; Orschell, C.M.; March, K.L. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 2003, 107, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Kalka, C.; Masuda, H.; Takahashi, T.; Kalka-Moll, W.M.; Silver, M.; Kearney, M.; Li, T.; Isner, J.M.; Asahara, T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. USA 2000, 97, 3422–3427. [Google Scholar] [CrossRef] [PubMed]
- Coultas, L.; Chawengsaksophak, K.; Rossant, J. Endothelial cells and VEGF in vascular development. Nature 2005, 438, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 2014, 92, 387–397. [Google Scholar] [CrossRef]
- Grange, C.; Tapparo, M.; Collino, F.; Vitillo, L.; Damasco, C.; Deregibus, M.C.; Tetta, C.; Bussolati, B.; Camussi, G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011, 71, 5346–5356. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Hu, X.; Wang, Z.; Wu, S.; Yi, Y. Extracellular vesicles derived from human adipose-derived stem cells promote the exogenous angiogenesis of fat grafts via the let-7/AGO1/VEGF signalling pathway. Sci. Rep. 2020, 10, 5313. [Google Scholar] [CrossRef]
- Deregibus, M.C.; Cantaluppi, V.; Calogero, R.; Lo Iacono, M.; Tetta, C.; Biancone, L.; Bruno, S.; Bussolati, B.; Camussi, G. Endothelial progenitor cell–derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood J. Am. Soc. Hematol. 2007, 110, 2440–2448. [Google Scholar] [CrossRef]
- Braghirolli, D.; Helfer, V.; Chagastelles, P.; Dalberto, T.; Gamba, D.; Pranke, P. Electrospun scaffolds functionalized with heparin and vascular endothelial growth factor increase the proliferation of endothelial progenitor cells. Biomed. Mater. 2017, 12, 025003. [Google Scholar] [CrossRef]
- Dikici, S.; Claeyssens, F.; MacNeil, S. Pre-Seeding of Simple Electrospun Scaffolds with a Combination of Endothelial Cells and Fibroblasts Strongly Promotes Angiogenesis. Tissue Eng. Regen. Med. 2020, 17, 445–458. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Y.; Wang, J.; Zhang, C.; Yan, H.; Zhu, M.; Wang, K.; Li, C.; Xu, Q.; Kong, D. Effect of resveratrol on modulation of endothelial cells and macrophages for rapid vascular regeneration from electrospun poly (ε-caprolactone) scaffolds. ACS Appl. Mater. Interfaces 2017, 9, 19541–19551. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lin, R.Z.; Qi, H.; Yang, Y.; Bae, H.; Melero-Martin, J.M.; Khademhosseini, A. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Kang, E.Y.; Byeon, J.; Jung, S.-h.; Hwang, C. Embossed membranes with vascular patterns guide vascularization in a 3D tissue model. Polymers 2019, 11, 792. [Google Scholar] [CrossRef] [PubMed]
- Pill, K.; Hofmann, S.; Redl, H.; Holnthoner, W. Vascularization mediated by mesenchymal stem cells from bone marrow and adipose tissue: A comparison. Cell Regen. 2015, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Gao, P.-L.; Wang, K.; Wang, J.-Y.; Zhong, Y.; Luo, Y. Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: A new dimension in cell-material interaction. Biomaterials 2017, 141, 74–85. [Google Scholar] [CrossRef]
- Chen, J.; Zhan, Y.; Wang, Y.; Han, D.; Tao, B.; Luo, Z.; Ma, S.; Wang, Q.; Li, X.; Fan, L. Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats. Acta Biomater. 2018, 80, 154–168. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Nuzzo, M.; Yang, X.; Yang, Y.; Zhang, X. Layer-by-layer nanofiber-enabled engineering of biomimetic periosteum for bone repair and reconstruction. Biomaterials 2018, 182, 279–288. [Google Scholar] [CrossRef]
- Srouji, S.; Kizhner, T.; Suss-Tobi, E.; Livne, E.; Zussman, E. 3-D Nanofibrous electrospun multilayered construct is an alternative ECM mimicking scaffold. J. Mater. Sci. Mater. Med. 2008, 19, 1249–1255. [Google Scholar] [CrossRef]
- Rufaihah, A.J.; Huang, N.F.; Jamé, S.; Lee, J.C.; Nguyen, H.N.; Byers, B.; De, A.; Okogbaa, J.; Rollins, M.; Reijo-Pera, R. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler. Thromb. Vasc. Biol. 2011, 31, e72–e79. [Google Scholar] [CrossRef]
- Li, J.; Huang, N.F.; Zou, J.; Laurent, T.J.; Lee, J.C.; Okogbaa, J.; Cooke, J.P.; Ding, S. Conversion of human fibroblasts to functional endothelial cells by defined factors. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1366–1375. [Google Scholar] [CrossRef]
- Lai, W.-H.; Ho, J.C.; Chan, Y.-C.; Ng, J.H.; Au, K.-W.; Wong, L.-Y.; Siu, C.-W.; Tse, H.-F. Attenuation of hind-limb ischemia in mice with endothelial-like cells derived from different sources of human stem cells. PLoS ONE 2013, 8, e57876. [Google Scholar] [CrossRef]
- Tan, R.P.; Chan, A.H.; Lennartsson, K.; Miravet, M.M.; Lee, B.S.; Rnjak-Kovacina, J.; Clayton, Z.E.; Cooke, J.P.; Ng, M.K.; Patel, S. Integration of induced pluripotent stem cell-derived endothelial cells with polycaprolactone/gelatin-based electrospun scaffolds for enhanced therapeutic angiogenesis. Stem Cell Res. Ther. 2018, 9, 70. [Google Scholar] [CrossRef]
- Sheikh, I.; Tchekanov, G.; Krum, D.; Hare, J.; Djelmami-Hani, M.; Maddikunta, R.; Mortada, M.E.; Karakozov, P.; Baibekov, I.; Hauck, J. Effect of electrical stimulation on arteriogenesis and angiogenesis after bilateral femoral artery excision in the rabbit hind-limb ischemia model. Vasc. Endovasc. Surg. 2005, 39, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Hang, J.; Kong, L.; Gu, J.; Adair, T. VEGF gene expression is upregulated in electrically stimulated rat skeletal muscle. Am. J. Physiol. -Heart Circ. Physiol. 1995, 269, H1827–H1831. [Google Scholar] [CrossRef]
- McLean, N.A.; Verge, V.M. Dynamic impact of brief electrical nerve stimulation on the neural immune axis—Polarization of macrophages toward a pro-repair phenotype in demyelinated peripheral nerve. Glia 2016, 64, 1546–1561. [Google Scholar] [CrossRef] [PubMed]
- Ud-Din, S.; Sebastian, A.; Giddings, P.; Colthurst, J.; Whiteside, S.; Morris, J.; Nuccitelli, R.; Pullar, C.; Baguneid, M.; Bayat, A. Angiogenesis is induced and wound size is reduced by electrical stimulation in an acute wound healing model in human skin. PLoS ONE 2015, 10, e0124502. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Syed, F.; Perry, D.; Balamurugan, V.; Colthurst, J.; Chaudhry, I.H.; Bayat, A. Acceleration of cutaneous healing by electrical stimulation: Degenerate electrical waveform down-regulates inflammation, up-regulates angiogenesis and advances remodeling in temporal punch biopsies in a human volunteer study. Wound Repair Regen. 2011, 19, 693–708. [Google Scholar] [CrossRef]
- Bai, H.; Forrester, J.V.; Zhao, M. DC electric stimulation upregulates angiogenic factors in endothelial cells through activation of VEGF receptors. Cytokine 2011, 55, 110–115. [Google Scholar] [CrossRef]
- Zhao, M.; Bai, H.; Wang, E.; Forrester, J.V.; McCaig, C.D. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J. Cell Sci. 2004, 117, 397–405. [Google Scholar] [CrossRef]
- Kim, I.S.; Song, J.K.; Song, Y.M.; Cho, T.H.; Lee, T.H.; Lim, S.S.; Kim, S.J.; Hwang, S.J. Novel effect of biphasic electric current on in vitro osteogenesis and cytokine production in human mesenchymal stromal cells. Tissue Eng. Part A 2009, 15, 2411–2422. [Google Scholar] [CrossRef]
- Beugels, J.; Molin, D.G.M.; Ophelders, D.R.M.G.; Rutten, T.; Kessels, L.; Kloosterboer, N.; Grzymala, A.A.P.d.; Kramer, B.W.W.; van der Hulst, R.R.W.J.; Wolfs, T.G.A.M. Electrical stimulation promotes the angiogenic potential of adipose-derived stem cells. Sci. Rep. 2019, 9, 12076. [Google Scholar] [CrossRef]
- Rackauskas, G.; Saygili, E.; Rana, O.R.; Saygili, E.; Gemein, C.; Laucevicius, A.; Aidietis, A.; Marinskis, G.; Serpytis, P.; Plisiene, J. Subthreshold high-frequency electrical field stimulation induces VEGF expression in cardiomyocytes. Cell Transplant. 2015, 24, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Song, J.K.; Zhang, Y.L.; Lee, T.H.; Cho, T.H.; Song, Y.M.; Kim, S.J.; Hwang, S.J. Biphasic electric current stimulates proliferation and induces VEGF production in osteoblasts. BBA-Mol. Cell Res. 2006, 1763, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J. Colloid Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef]
- Mihardja, S.S.; Sievers, R.E.; Lee, R.J. The effect of polypyrrole on arteriogenesis in an acute rat infarct model. Biomaterials 2008, 29, 4205–4210. [Google Scholar] [CrossRef]
- Sekuła, M.; Domalik-Pyzik, P.; Morawska-Chochół, A.; Bobis-Wozowicz, S.; Karnas, E.; Noga, S.; Boruczkowski, D.; Adamiak, M.; Madeja, Z.; Chłopek, J. Polylactide-and polycaprolactone-based substrates enhance angiogenic potential of human umbilical cord-derived mesenchymal stem cells in vitro-implications for cardiovascular repair. Mater. Sci. Eng. C 2017, 77, 521–533. [Google Scholar] [CrossRef]
- Streeter, B.W.; Xue, J.; Xia, Y.; Davis, M.E. Electrospun Nanofiber-Based Patches for the Delivery of Cardiac Progenitor Cells. ACS Appl. Mater. Interfaces 2019, 11, 18242–18253. [Google Scholar] [CrossRef]
- Wu, J.; Huang, C.; Liu, W.; Yin, A.; Chen, W.; He, C.; Wang, H.; Liu, S.; Fan, C.; Bowlin, G.L. Cell infiltration and vascularization in porous nanoyarn scaffolds prepared by dynamic liquid electrospinning. J. Biomed. Nanotechnol. 2014, 10, 603–614. [Google Scholar] [CrossRef]
- Said, S.S.; Pickering, J.G.; Mequanint, K. Controlled delivery of fibroblast growth factor-9 from biodegradable poly (ester amide) fibers for building functional neovasculature. Pharm. Res. 2014, 31, 3335–3347. [Google Scholar] [CrossRef]
- Said, S.S.; O’Neil, C.; Yin, H.; Nong, Z.; Pickering, J.G.; Mequanint, K. Concurrent and sustained delivery of FGF2 and FGF9 from electrospun poly (ester amide) fibrous mats for therapeutic angiogenesis. Tissue Eng. Part A 2016, 22, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.K.; Lam, C.R.I.; Wen, F.; Chong, S.K.M.; Tan, N.S.; Jerry, C.; Pal, M.; Tan, L.P. Novel method to improve vascularization of tissue engineered constructs with biodegradable fibers. Biofabrication 2016, 8, 015004. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Xia, T.; Wang, H.; Wei, L.; Luo, X.; Li, X. Multiple release of polyplexes of plasmids VEGF and bFGF from electrospun fibrous scaffolds towards regeneration of mature blood vessels. Acta Biomater. 2012, 8, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, L.; Touroo, J.; Reed, R.; Boland, E.; Hoying, J.B.; Williams, S.K. Vascularization and cellular isolation potential of a novel electrospun cell delivery vehicle. J. Biomed. Mater. Res. Part A 2014, 102, 2208–2219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Z.; Sun, B.; Zhang, M.; Ou, L.; Che, Y.; Zhang, J.; Kong, D. Functionalization of electrospun poly (ε-caprolactone) scaffold with heparin and vascular endothelial growth factors for potential application as vascular grafts. J. Bioact. Compat. Polym. 2013, 28, 154–166. [Google Scholar] [CrossRef]
- Shafiq, M.; Jung, Y.; Kim, S.H. In situ vascular regeneration using substance P-immobilised poly (L-lactide-co-ε-caprolactone) scaffolds: Stem cell recruitment, angiogenesis, and tissue regeneration. Eur. Cell Mater. 2015, 30, 282–302. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ge, L.; Wang, H.; Cheng, Y.; Gorantla, S.; Poluektova, L.Y.; Gombart, A.F.; Xie, J. Eluted 25-hydroxyvitamin D3 from radially aligned nanofiber scaffolds enhances cathelicidin production while reducing inflammatory response in human immune system-engrafted mice. Acta Biomater. 2019, 97, 187–199. [Google Scholar] [CrossRef]
- Shan, Y.-H.; Peng, L.-H.; Liu, X.; Chen, X.; Xiong, J.; Gao, J.-Q. Silk fibroin/gelatin electrospun nanofibrous dressing functionalized with astragaloside IV induces healing and anti-scar effects on burn wound. Int. J. Pharm. 2015, 479, 291–301. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Chen, S.; Tang, D.; Jiang, L.; Kong, D.; Wang, S. Electrospun poly-ε-caprolactone scaffold modified with catalytic nitric oxide generation and heparin for small-diameter vascular graft. RSC Adv. 2017, 7, 18775–18784. [Google Scholar] [CrossRef]
- Govindarajan, D.; Lakra, R.; Korapatti, P.S.; Ramasamy, J.; Kiran, M.S. Nanoscaled Biodegradable Metal–Polymeric Three-Dimensional Framework for Endothelial Cell Patterning and Sustained Angiogenesis. ACS Biomater. Sci. Eng. 2019, 5, 2519–2531. [Google Scholar] [CrossRef]
- Kuttappan, S.; Mathew, D.; Jo, J.-i.; Tanaka, R.; Menon, D.; Ishimoto, T.; Nakano, T.; Nair, S.V.; Nair, M.B.; Tabata, Y. Dual release of growth factor from nanocomposite fibrous scaffold promotes vascularisation and bone regeneration in rat critical sized calvarial defect. Acta Biomater. 2018, 78, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Kaigler, D.; Rice, K.G.; Krebsbach, P.H.; Mooney, D.J. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J. Bone Miner. Res. 2005, 20, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Lü, L.; Deegan, A.; Musa, F.; Xu, T.; Yang, Y. The effects of biomimetically conjugated VEGF on osteogenesis and angiogenesis of MSCs (human and rat) and HUVECs co-culture models. Colloids Surfaces B Biointer. 2018, 167, 550–559. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, Y.J.; Cho, H.-J.; Kim, D.W.; Shin, H. The incorporation of bFGF mediated by heparin into PCL/gelatin composite fiber meshes for guided bone regeneration. Drug Deliv. Transl. Res. 2015, 5, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Yin, C.; Tu, H.; Jiang, S.; Wang, Q.; Zhou, X.; Xing, X.; Xie, C.; Shi, X.; Du, Y. Controlled Co-delivery of Growth Factors through Layer-by-Layer Assembly of Core–Shell Nanofibers for Improving Bone Regeneration. ACS Nano 2019, 13, 6372–6382. [Google Scholar] [CrossRef]
- Oliveira, H.; Catros, S.; Castano, O.; Rey, S.; Siadous, R.; Clift, D.; Marti-Munoz, J.; Batista, M.; Bareille, R.; Planell, J. The proangiogenic potential of a novel calcium releasing composite biomaterial: Orthotopic in vivo evaluation. Acta Biomater. 2017, 54, 377–385. [Google Scholar] [CrossRef]
- Patel, J.J.; Modes, J.E.; Flanagan, C.L.; Krebsbach, P.H.; Edwards, S.P.; Hollister, S.J. Dual delivery of epo and bmp2 from a novel modular poly-ɛ-caprolactone construct to increase the bone formation in prefabricated bone flaps. Tissue Eng. Part C Methods 2015, 21, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Boda, S.K.; Wang, H.; Teusink, M.J.; Shuler, F.D.; Xie, J. Novel 3D hybrid nanofiber aerogels coupled with BMP-2 peptides for cranial bone regeneration. Adv. Healthc. Mater. 2018, 7, 1701415. [Google Scholar] [CrossRef]
- Ikeda, K.; Takeshita, S. Factors and mechanisms involved in the coupling from bone resorption to formation: How osteoclasts talk to osteoblasts. J. Bone Metab. 2014, 21, 163–167. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, W.; Zhao, X.; Wen, S.; Sun, Y.; Han, J.; Zhang, H. Bone remodeling-inspired dual delivery electrospun nanofibers for promoting bone regeneration. Nanoscale 2019, 11, 60–71. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Tandara, A.A.; Mustoe, T.A. Oxygen in wound healing—More than a nutrient. World J. Surg. 2004, 28, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Tchemtchoua, V.T.; Atanasova, G.; Aqil, A.; Filée, P.; Garbacki, N.; Vanhooteghem, O.; Deroanne, C.; Noël, A.; Jérome, C.; Nusgens, B. Development of a chitosan nanofibrillar scaffold for skin repair and regeneration. Biomacromolecules 2011, 12, 3194–3204. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.I.; Dias, A.M.; Carvalho, E.; de Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.C.; Chan, J.S.K.; Goh, C.Q.; Gounko, N.V.; Luo, B.; Wang, X.; Foo, S.; Wong, M.T.C.; Choong, C.; Kersten, S. Angiopoietin-like 4 stimulates STAT3-mediated iNOS expression and enhances angiogenesis to accelerate wound healing in diabetic mice. Mol. Ther. 2014, 22, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, L.; Wicks, J.; Ling, C.; Zhao, X.; Yan, Y.; Qi, J.; Cui, W.; Deng, L. Quickly promoting angiogenesis by using a DFO-loaded photo-crosslinked gelatin hydrogel for diabetic skin regeneration. J. Mater. Chem. B 2016, 4, 3770–3781. [Google Scholar] [CrossRef]
- Zhang, Q.; Oh, J.-H.; Park, C.H.; Baek, J.-H.; Ryoo, H.-M.; Woo, K.M. Effects of dimethyloxalylglycine-embedded poly (ε-caprolactone) fiber meshes on wound healing in diabetic rats. ACS Appl. Mater. Interfaces 2017, 9, 7950–7963. [Google Scholar] [CrossRef]
- Senturk, B.; Mercan, S.; Delibasi, T.; Guler, M.O.; Tekinay, A.B. Angiogenic peptide nanofibers improve wound healing in STZ-induced diabetic rats. ACS Biomater. Sci. Eng. 2016, 2, 1180–1189. [Google Scholar] [CrossRef]
- Ren, X.; Han, Y.; Wang, J.; Jiang, Y.; Yi, Z.; Xu, H.; Ke, Q. An aligned porous electrospun fibrous membrane with controlled drug delivery–an efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta Biomater. 2018, 70, 140–153. [Google Scholar] [CrossRef]
- Xie, Z.; Paras, C.B.; Weng, H.; Punnakitikashem, P.; Su, L.-C.; Vu, K.; Tang, L.; Yang, J.; Nguyen, K.T. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater. 2013, 9, 9351–9359. [Google Scholar] [CrossRef]
- Agarwal, A.; Nelson, T.B.; Kierski, P.R.; Schurr, M.J.; Murphy, C.J.; Czuprynski, C.J.; McAnulty, J.F.; Abbott, N.L. Polymeric multilayers that localize the release of chlorhexidine from biologic wound dressings. Biomaterials 2012, 33, 6783–6792. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Kim, B.-S.; Kim, S.R.; Hammond, P.T.; Irvine, D.J. Layer-by-layer-assembled multilayer films for transcutaneous drug and vaccine delivery. ACS Nano 2009, 3, 3719–3729. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Ghosh, C.; Hwang, S.-G.; Chanunpanich, N.; Park, J.S. Porous core/sheath composite nanofibers fabricated by coaxial electrospinning as a potential mat for drug release system. Int. J. Pharm. 2012, 439, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lv, F.; Li, T.; Han, Y.; Yi, Z.; Liu, M.; Chang, J.; Wu, C. Electrospun micropatterned nanocomposites incorporated with Cu2S nanoflowers for skin tumor therapy and wound healing. ACS Nano 2017, 11, 11337–11349. [Google Scholar] [CrossRef]
- Lundborg, G. Nerve Injury and Repair: Regeneration, Reconstruction, and Cortical Remodeling; Elsevier/Churchill Livingstone: Philadelphia, PA, USA, 2005. [Google Scholar]
- Hu, J.; Zhu, Q.-T.; Liu, X.-L.; Xu, Y.-b.; Zhu, J.-K. Repair of extended peripheral nerve lesions in rhesus monkeys using acellular allogenic nerve grafts implanted with autologous mesenchymal stem cells. Exp. Neurol. 2007, 204, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Inai, R.; Kotaki, M.; Ramakrishna, S. Aligned biodegradable nanofibrous structure: A potential scaffold for blood vessel engineering. Biomaterials 2004, 25, 877–886. [Google Scholar] [CrossRef]
- Wang, H.B.; Mullins, M.E.; Cregg, J.M.; Hurtado, A.; Oudega, M.; Trombley, M.T.; Gilbert, R.J. Creation of highly aligned electrospun poly-L-lactic acid fibers for nerve regeneration applications. J. Neural Eng. 2008, 6, 016001. [Google Scholar] [CrossRef]
- Schnell, E.; Klinkhammer, K.; Balzer, S.; Brook, G.; Klee, D.; Dalton, P.; Mey, J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-ε-caprolactone and a collagen/poly-ε-caprolactone blend. Biomaterials 2007, 28, 3012–3025. [Google Scholar] [CrossRef]
- Chew, S.Y.; Mi, R.; Hoke, A.; Leong, K.W. Aligned protein–polymer composite fibers enhance nerve regeneration: A potential tissue-engineering platform. Adv. Funct. Mater. 2007, 17, 1288–1296. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, J.; Shao, H.; Song, L.; Zhang, Y. Dual-factor loaded functional silk fibroin scaffolds for peripheral nerve regeneration with the aid of neovascularization. RSC Adv. 2016, 6, 7683–7691. [Google Scholar] [CrossRef]
- Colello, R.J.; Chow, W.N.; Bigbee, J.W.; Lin, C.; Dalton, D.; Brown, D.; Jha, B.S.; Mathern, B.E.; Lee, K.D.; Simpson, D.G. The incorporation of growth factor and chondroitinase ABC into an electrospun scaffold to promote axon regrowth following spinal cord injury. J. Tissue Eng. Regen. Med. 2016, 10, 656–668. [Google Scholar] [CrossRef] [PubMed]
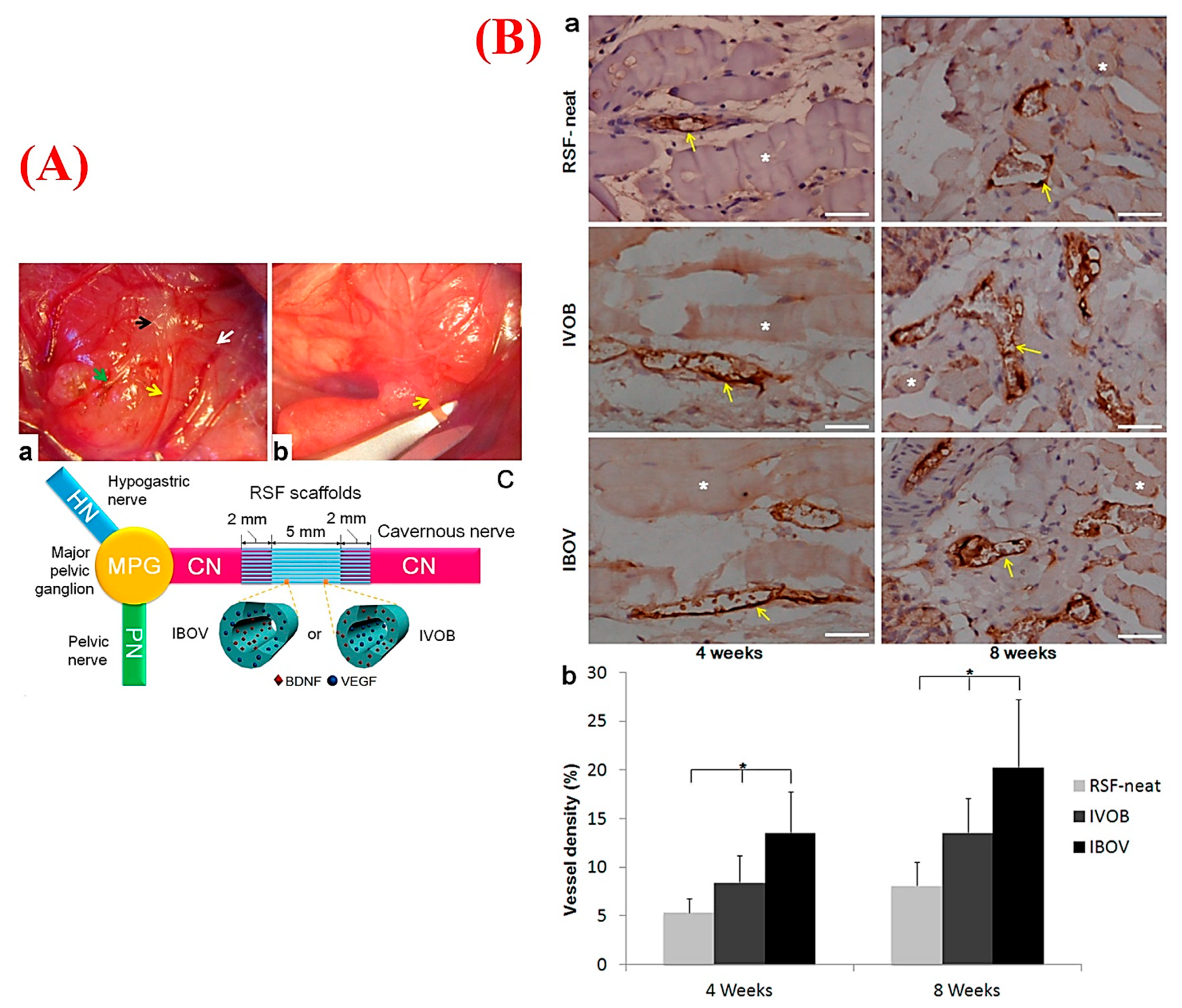
- Zhang, Y.; Huang, J.; Huang, L.; Liu, Q.; Shao, H.; Hu, X.; Song, L. Silk fibroin-based scaffolds with controlled delivery order of VEGF and BDNF for cavernous nerve regeneration. ACS Biomater. Sci. Eng. 2016, 2, 2018–2025. [Google Scholar] [CrossRef]
- Xia, B.; Lv, Y. Dual-delivery of VEGF and NGF by emulsion electrospun nanofibrous scaffold for peripheral nerve regeneration. Mater. Sci. Eng. C 2018, 82, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Casavitri, C.; Suhaeri, M.; Wang, P.-Y.; Lee, J.H.; Koh, W.-G.; Park, K. A Fibrous Hybrid Patch Couples Cell-Derived Matrix and Poly (l-lactide-co-caprolactone) for Endothelial Cells Delivery and Skin Wound Repair. ACS Biomater. Sci. Eng. 2018, 5, 900–910. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, M.; Bao, F.; Xing, M.; Wang, E.; Xu, Q.; Huan, Z.; Guo, F.; Chang, J. Multifunctional Zn doped hollow mesoporous silica/polycaprolactone electrospun membranes with enhanced hair follicle regeneration and antibacterial activity for wound healing. Nanoscale 2019, 11, 6315–6333. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Luo, G.; He, W.; Xu, K.; Xu, R.; Lei, Q.; Tan, J.; Wu, J.; Xing, M. In-situ-generated vasoactive intestinal peptide loaded microspheres in mussel-inspired polycaprolactone nanosheets creating spatiotemporal releasing microenvironment to promote wound healing and angiogenesis. ACS Appl. Mater. Interfaces 2016, 8, 7411–7421. [Google Scholar] [CrossRef]
- Samadian, H.; Salehi, M.; Farzamfar, S.; Vaez, A.; Ehterami, A.; Sahrapeyma, H.; Goodarzi, A.; Ghorbani, S. In vitro and in vivo evaluation of electrospun cellulose acetate/gelatin/hydroxyapatite nanocomposite mats for wound dressing applications. Artif. Cell. Nanomed. Biotechnol. 2018, 46, 964–974. [Google Scholar] [CrossRef]
- Meka, S.R.K.; Kumar Verma, S.; Agarwal, V.; Chatterjee, K. In Situ Silication of Polymer Nanofibers to Engineer Multi-Biofunctional Composites. ChemistrySelect 2018, 3, 3762–3773. [Google Scholar] [CrossRef]
- Jeon, J.-K.; Seo, H.; Park, J.; Son, S.J.; Kim, Y.R.; Kim, E.S.; Park, J.W.; Jung, W.-G.; Jeon, H.; Kim, Y.-C. Conceptual Study for Tissue-Regenerative Biodegradable Magnesium Implant Integrated with Nitric Oxide-Releasing Nanofibers. Met. Mater. Int. 2019, 25, 1098–1107. [Google Scholar] [CrossRef]
- Yun, H.-M.; Kang, S.-K.; Singh, R.K.; Lee, J.-H.; Lee, H.-H.; Park, K.-R.; Yi, J.-K.; Lee, D.-W.; Kim, H.-W.; Kim, E.-C. Magnetic nanofiber scaffold-induced stimulation of odontogenesis and pro-angiogenesis of human dental pulp cells through Wnt/MAPK/NF-κB pathways. Dent. Mater. 2016, 32, 1301–1311. [Google Scholar] [CrossRef]
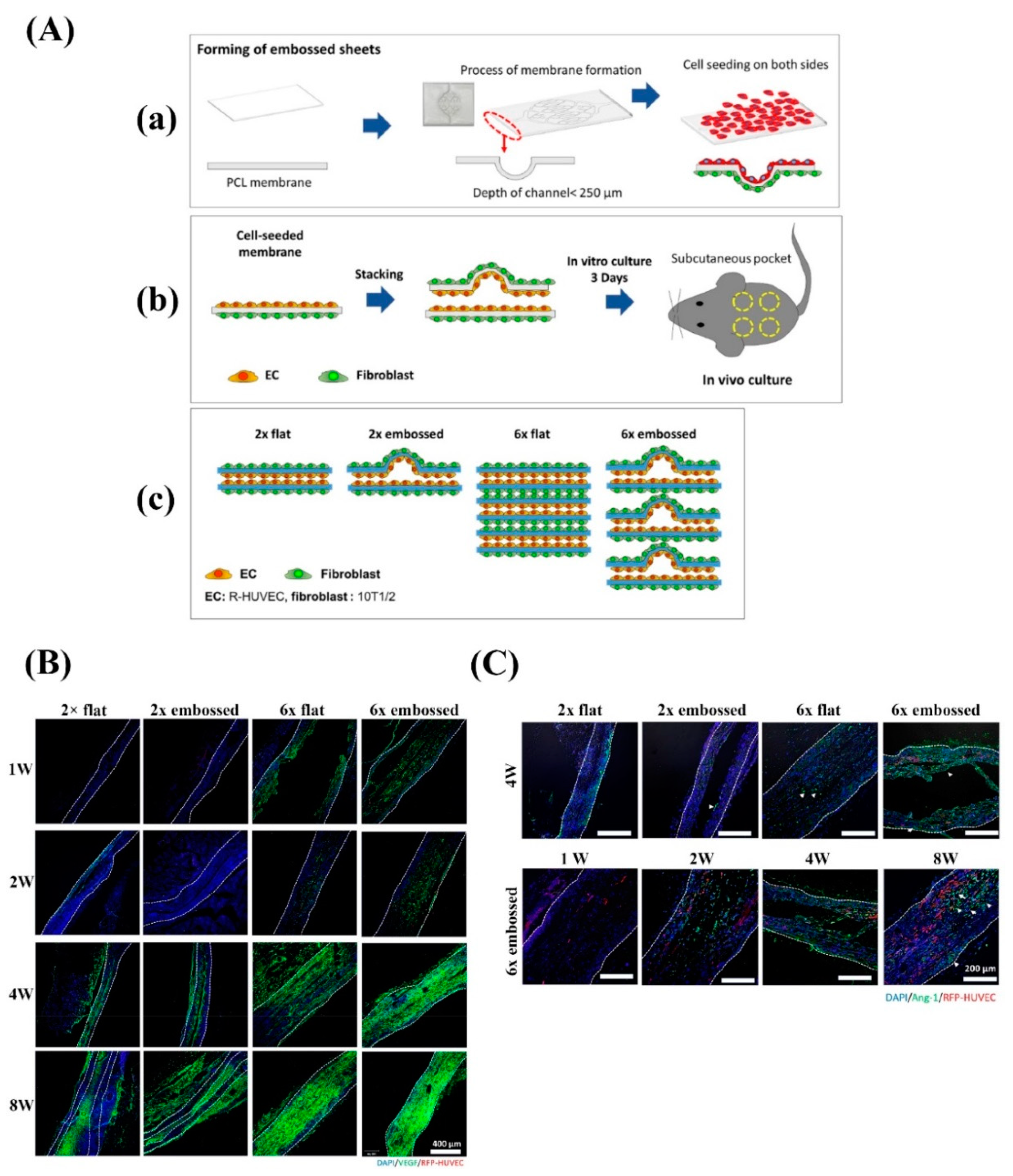
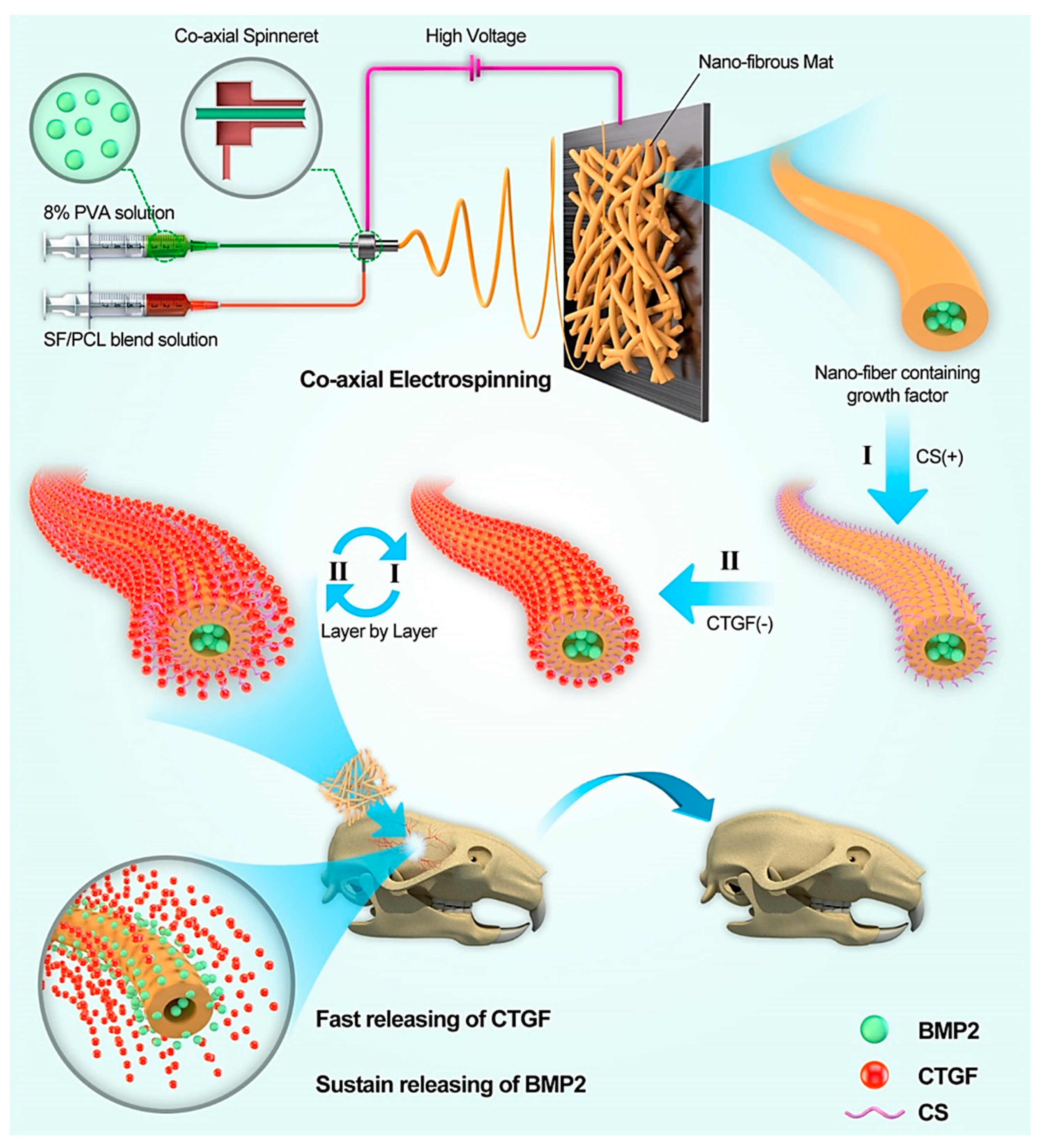

| Bioactive Molecule | Function |
|---|---|
| VEGF family | Stimulating angiogenesis, permeability, and leukocyte adhesion |
| Angiopoetin1 (Ang1) and Tie2 | Stabilizing vessels and inhibiting permeability |
| Platelet-derived growth factor-BB (PDGF-BB) | Recruiting of smooth muscle cells (SMCs) |
| Transforming growth factor-β (TGF-β1) | Stimulating extracellular matrix (ECM) production |
| Fibroblast growth factor (FGF) and hepatocyte growth factor (HGF) | Stimulating angio/arteriogenesis |
| Matrix metalloproteinase (MMPs) | Matrix remodeling, release and activation of growth factors |
| Plasminogen activator inhibitor-1 (PAI-1) | Stabilizing nascent vessels |
| Nitric oxide synthase (NOS) | Promoting angiogenesis and vasodilation |
| Parameter | Possible Effect on Angiogenesis | Ref(s) |
|---|---|---|
| Porosity | A minimum porosity of 30 to 40 µm is required for metabolite exchange and endothelial cell (EC) entrance | [22,23] |
| Pore size | Pores greater than 300 µm are required for new blood vessel formation of the constructs | [24,25] |
| Fiber orientation | Aligned nanofibers promote neovascularization | [26] |
| Heparin-functionalized nanofibers | Promoting angiogenesis through binding to angiogenic growth factors such as VEGF, HGF, and FGF-2 | [27] |
| Polymer degradation | Slower polymer degradation leads to greater cell mobilization and angiogenesis owing to less acidic environment formation (however, electrospun nanofibrous mats are not mentioned) | [28] |
| Scaffold stiffness | Greater surface stiffness leads to higher EC spreading and a larger number and greater length of new sprout formation (however, nanofibers are not mentioned) | [29] |
| Parameters | Effects on Nanofiber Properties | Ref. |
|---|---|---|
| Polymer parameters | ||
| Polymer concentration | Higher polymer concentration leads to increased fiber diameter and higher pore size and porosity | [21] |
| Solution viscosity | Increased viscosity results in increased nanofiber uniformity and diameter and reduced beaded morphology | [80] |
| Molecular weight of polymer | Increased molecular weight leads to higher nanofiber diameter and less bead formation | [81] |
| Surface tension | Less surface tension leads to proper jet initiation | [82] |
| Conductivity | Higher solution conductivity leads to decreased fiber diameter | [83] |
| Electrospinning parameters | ||
| Applied voltage | Initially decreases nanofiber diameter and then increases after a point | [84] |
| Needle tip to collector distance | Too short and too long distances lead to bead formation | [85] |
| Flow rate | Higher flow rate leads to bead formation, decreased flow rate leads to a decrease in fiber diameter | [86] |
| Temperature | Higher temperature leads to decreased fiber diameter | [87] |
| Humidity | Increase in humidity leads to circular pores on the fibers | [88] |
| Polymer(s) | Fiber Diameter | Biomedical Application | Remark(s) | Ref. |
|---|---|---|---|---|
| Expanded 3D PCL nanofiber | Not mentioned | Neovascularization after subcutaneous implantation | Promoted cell infiltration and tissue integration Enhanced regenerative response owing to increased expression of CD68, CCR7, and CD163 markers | [120] |
| Poly [2-bis-(3 octyloxyphenyl) quinoxaline-5,8-diyl-alt-thiophene-2,5-diyl] (TQ1) | Not mentioned | Angiogenesis | A semiconducting luminescent polymer that could be visualized in situ up to 90 days after subcutaneous implantation using fluorescence imaging Limited inflammation and formation of small capillaries around the fibers | [121] |
| PLA | 657 +/− 101 nm for random and 568+/− 81 nm for aligned nanofibers | Angiogenesis and neurogenesis | Radially aligned nanofibers supported neuronal migration Long-term viability and integration of newly generated neurons | [111] |
| PHB, PCL, PLA, and PA (polyamide) | Not mentioned | Angiogenesis and cardiac repair | Among the scaffolds used, PHB had the most biocompatibility, biodegradability, angiogenic and potential, as well as expression of pro-inflammatory cytokines including interleukin (IL)-1β, IL-4, IL-6, IL-10, IL-13, and IFN-γ after epicardial implantation | [122] |
| PCL/collagen/PEO | 250 nm +/− 73 nm | Angiogenesis | Lower sprouting vessels in the aligned scaffold, but earlier vascularization in the center of the construct compared with nonwoven scaffolds using microCT scan images | [30] |
| PDLLA/PCL/gelatin | 500–700 nm | Angiogenesis | Anisotropically and heterogeneously aligned scaffold Excellent mechanical properties and bioactivity | [123] |
| PHB | 1603 +/− 73 nm | Angiogenesis and skin reconstruction | Good biocompatibility Advanced properties compared with PCL for skin regeneration Polarization of macrophages to the M2 phenotype | [124] |
| Polymer | Pro-Angiogenic Factor | Method of Integration | Remarks | Ref(s) |
|---|---|---|---|---|
| Cells | ||||
| PLA/PCL | hUC-MSCs | Seeding cells on the scaffold | PCL significantly increased the angiogenic potential of hUC-MSCs with no additional factors Increased migratory and pro-angiogenic potential | [228] |
| Polydioxan-one (PDO) | Bone marrow-derived macrophages | Seeding cells on the scaffold | Higher concentrations of polymer led to a larger fiber diameter, pore size, and porosity Increased secretion of pro-angiogenic cytokines including VEGF, bFGF, and TGF-β for the scaffolds with larger pore sizes Pore size of the scaffold is more critical than fiber diameter in macrophage polarization | [21] |
| PCL/gelatin | Adipose tissue-derived mesenchymal stem cells (ADSCs) | Seeding and coculturing of ADSCs and HUVECs on the scaffolds | Greater sprouting of endothelial cells, formation of a mature blood vessel-like network, and enhanced expression of tight junction proteins | [191] |
| PCL/gelatin/fi-bronectin | Cardiac progenitor cells (CPC) | Seeding cells on the patches | Electrospun scaffolds maintained durable CPC viability Reduced fibrotic gene expression in rat cardiac fibroblasts Tube formation of HUVECs by media collected from the nanofibrous patches demonstrating the pro-angiogenic potential of the patch | [229] |
| PLCL/colla-gen nanoyarn fibers | Pig iliac endothelial cells (PIECs) and MC3T3-E1 pre-osteoblastic cells | Seeding cells on nanoyarn scaffold | Formation of complex capillary-like structures after 7 days More cell infiltration into this morphology of electrospun scaffolds compared with conventional nanofibrous electrospun mats | [230] |
| Growth factors | ||||
| Gelatin | bFGF | Physical immobilization | Proliferation rate of HUVECs was proportional to the loading concentration of bFGF (0–100 ng/mL) The gradient growth factor distribution effects on vessel direction | [33] |
| Pullulan/dex-tran nanofibers loaded with fucoidan | VEGF | Physical immobilization | Increased fucoidan content led to increased retention of VEGF bioactivity and angiogenic response up to 14 days Promoted cellular infiltration and complete biodegradation of the construct up to 7 days after subcutaneous implantation in mice | [133] |
| Poly(estera-mide) PEA | FGF2, FGF9 | Emulsion electrospinning | Sustained release and preserved bioactivity of FGF2 and FGF9 over 28 days Enhanced tubular formation | [35,231,232] |
| PLGA | Collagen containing VEGF | Surface coating | Improved pre-vascularization of the construct after seeding HUVECs Anastomose formation between the implanted construct and mice vasculature confirmed by immunostaining of CD31 and von Willebrand factor | [233] |
| Others | ||||
| PEG | bFGF and VEGF-encoding plasmids | Coaxial electrospinning | Improved cell viability and attachment and extracellular secretion of collagen Ⅳ and laminin Alleviated inflammation reaction and enhanced microvessel generation | [234] |
| Nylon | Insulin | Outer porous layer supports cellular infiltration and vascularization Inner low porosity layer supports cellular isolation Localized cell transplantation | [235] | |
| PCL | Heparin and VEGF | Immersing in heparin and VEGF solution, respectively | Stimulated neovascularization with minimum immunological rejection | [236] |
| PLCL | Substance P | Sustained release of substance P up to 30 days Improved host cell infiltration, blood vessel formation, and MSC recruitment in vivo Existence of laminin-positive blood vessels and von Willebrand factor cells | [237] | |
| PCL | Vitamin D3 | Blending | Promoted cellular infiltration and neovascularization Reduced inflammation and infection | [238] |
| Silk fibroin/gelatin | Astragaloside IV | Accelerated wound healing and prevented scar formation by stimulating wound closure and increasing angiogenesis in partial-thickness burn wounds | [239] | |
| PCL | Collagenase | Surface immobilization | Collagenase (0.01 mg/mL) promoted smooth muscle cell (SMC) migration Enhanced capillary formation after subcutaneous implantation | [240] |
| Gelatin/PLGA | Cobalt and PEGylated curcumin | Core-shell electrospinning | Promoted EC patterning and enhanced VEGF production | [241] |
| PLLA/chitosan nanofibers coated with polydopa-mine | Icariin and deferoxamine | Surface modification | Synergistic effects on osteogenesis and angiogenesis | [43] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarnezhad, S.; Baino, F.; Kim, H.-W.; Webster, T.J.; Kargozar, S. Electrospun Nanofibers for Improved Angiogenesis: Promises for Tissue Engineering Applications. Nanomaterials 2020, 10, 1609. https://doi.org/10.3390/nano10081609
Nazarnezhad S, Baino F, Kim H-W, Webster TJ, Kargozar S. Electrospun Nanofibers for Improved Angiogenesis: Promises for Tissue Engineering Applications. Nanomaterials. 2020; 10(8):1609. https://doi.org/10.3390/nano10081609
Chicago/Turabian StyleNazarnezhad, Simin, Francesco Baino, Hae-Won Kim, Thomas J. Webster, and Saeid Kargozar. 2020. "Electrospun Nanofibers for Improved Angiogenesis: Promises for Tissue Engineering Applications" Nanomaterials 10, no. 8: 1609. https://doi.org/10.3390/nano10081609
APA StyleNazarnezhad, S., Baino, F., Kim, H.-W., Webster, T. J., & Kargozar, S. (2020). Electrospun Nanofibers for Improved Angiogenesis: Promises for Tissue Engineering Applications. Nanomaterials, 10(8), 1609. https://doi.org/10.3390/nano10081609