Potential of TiO2 with Various Au Nanoparticles for Catalyzing Mesotrione Removal from Wastewaters under Sunlight
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Solutions and Catalysts
2.2. Synthesis of Au–S–CH2–CH2–OH and Au–S–CH2–CH2–OH–FNP Nanoparticles
2.3. Characterization of Nanoparticles
2.4. Photodegradation Procedure
2.5. Analytical Procedure
3. Results and Discussion
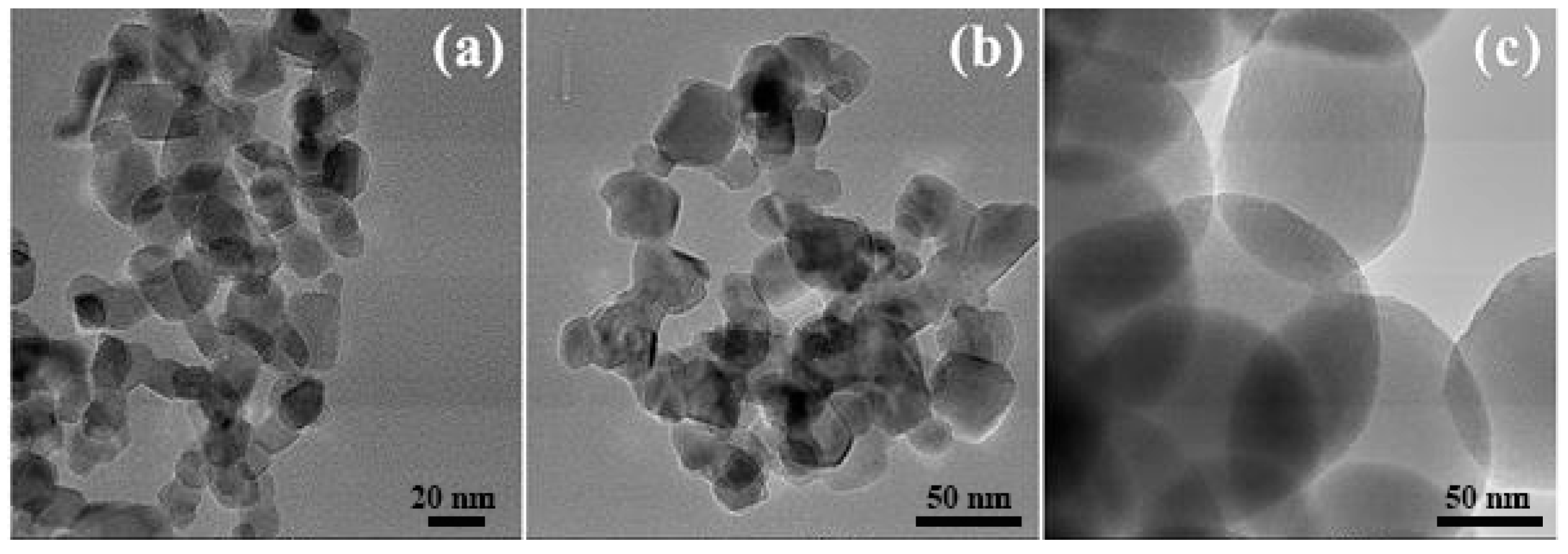
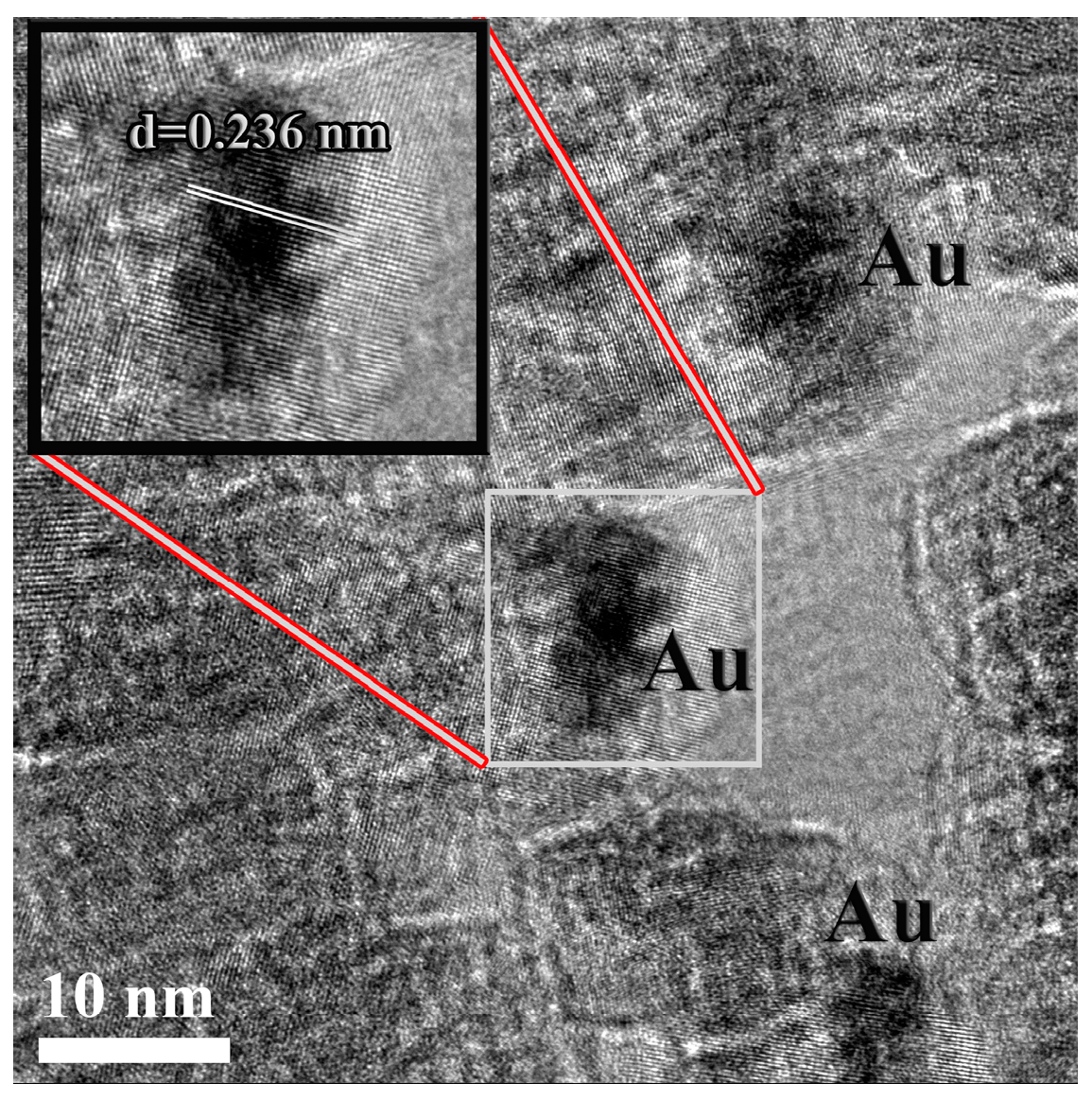
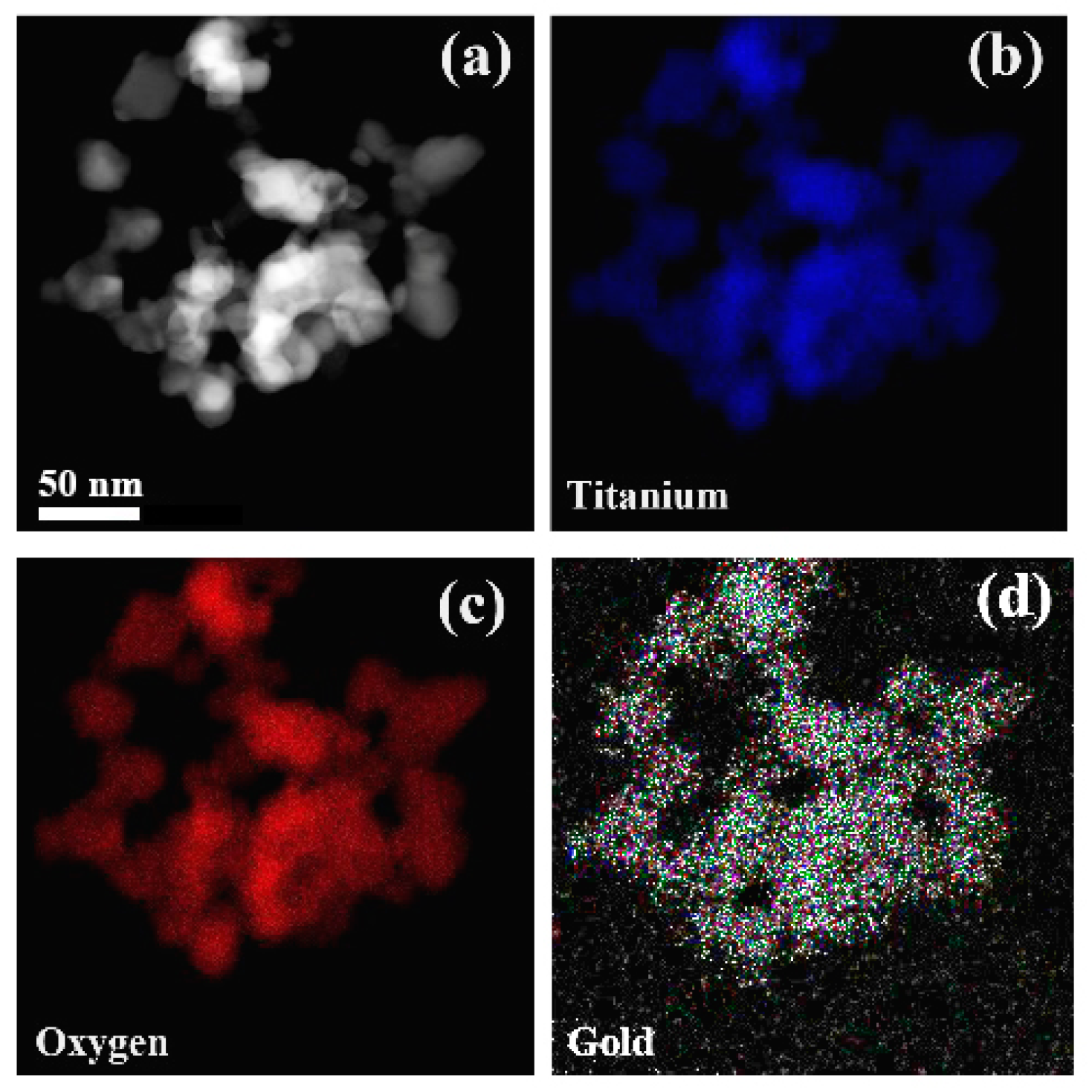
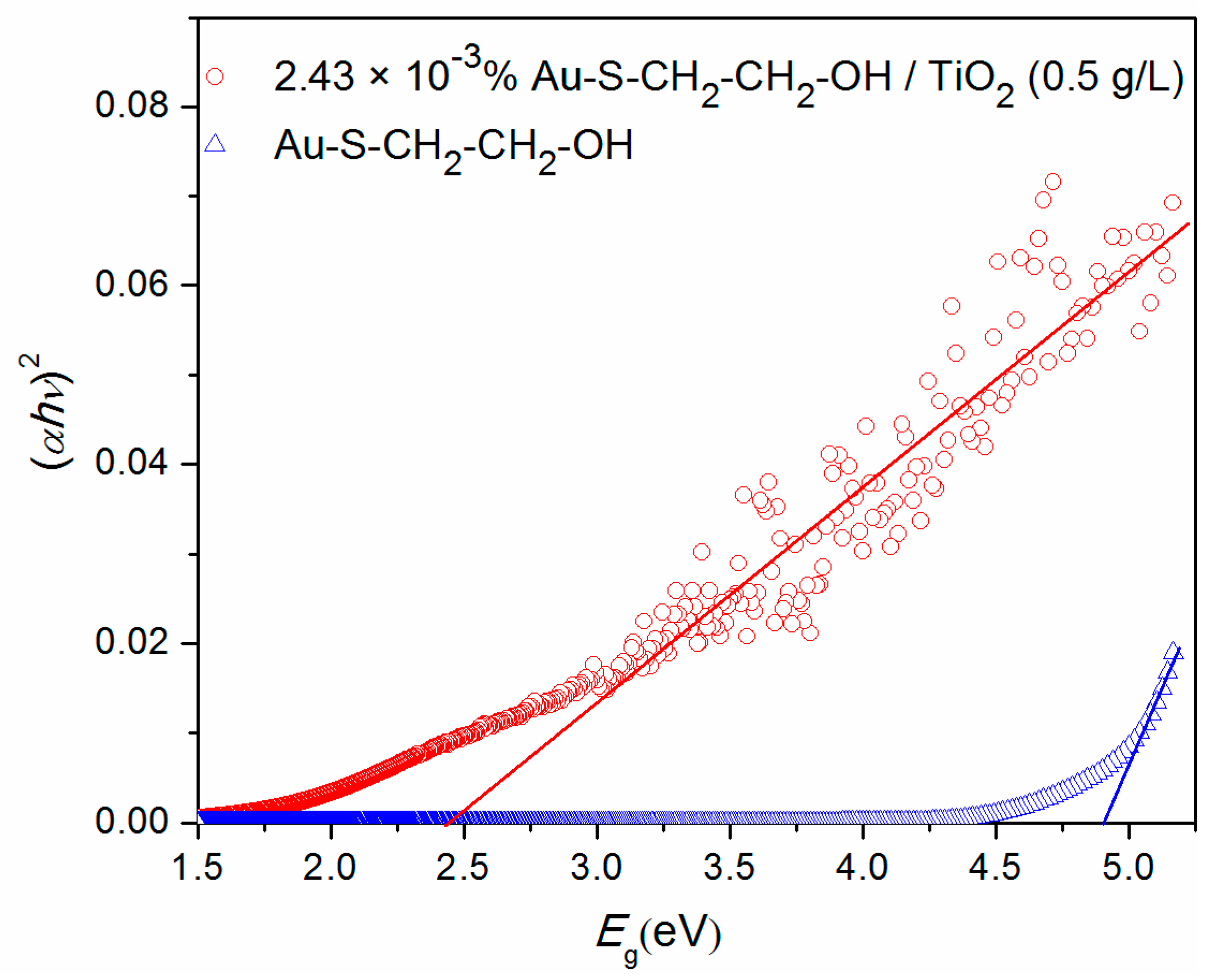
3.1. Characterization
3.2. Photolytic and Photocatalytic Degradation
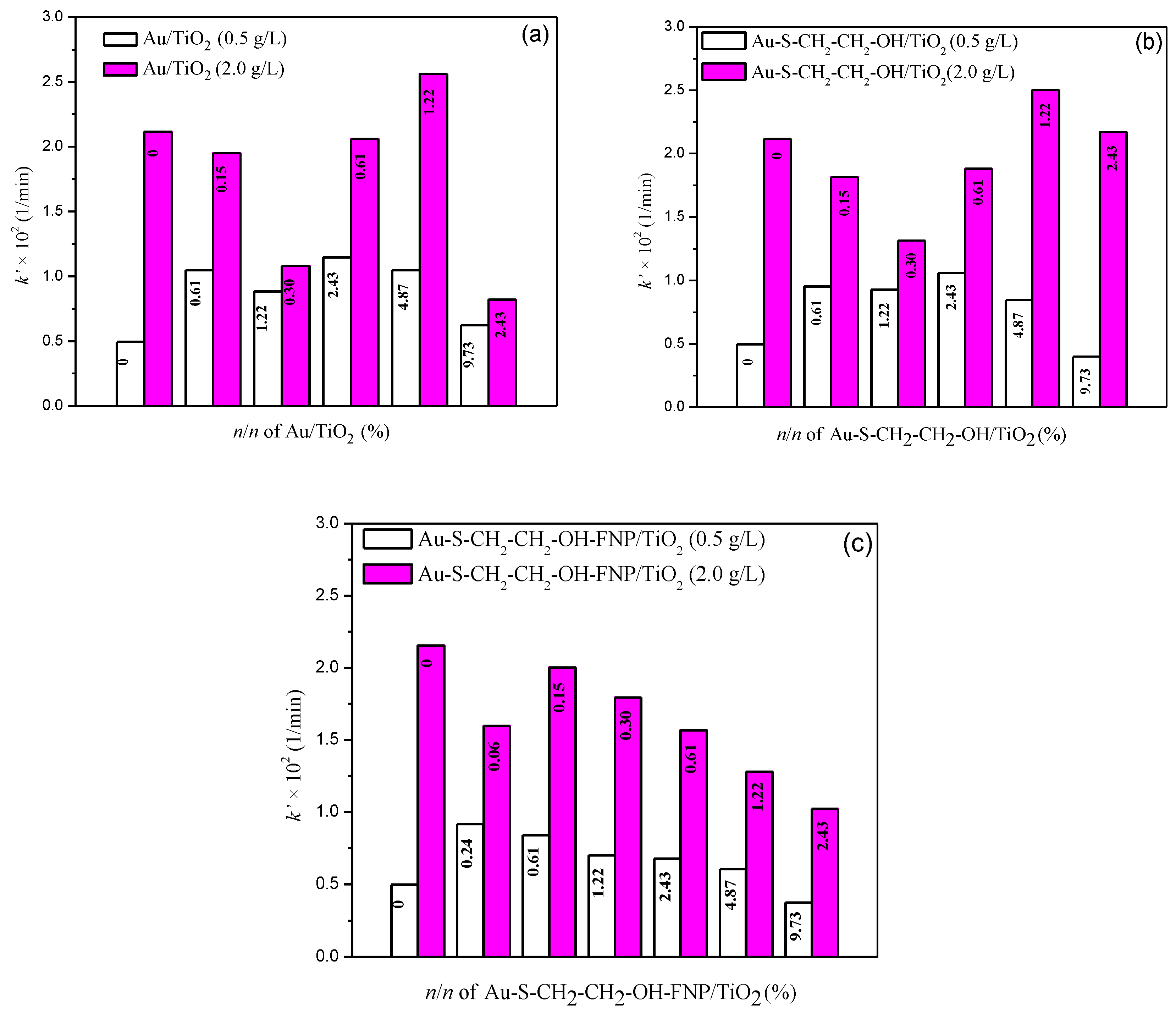
3.2.1. Activity of TiO2 (0.5 g/L) without and with Different Au Nanoparticles
3.2.2. Activity of TiO2 (2.0 g/L) with/without Different Au Nanoparticles
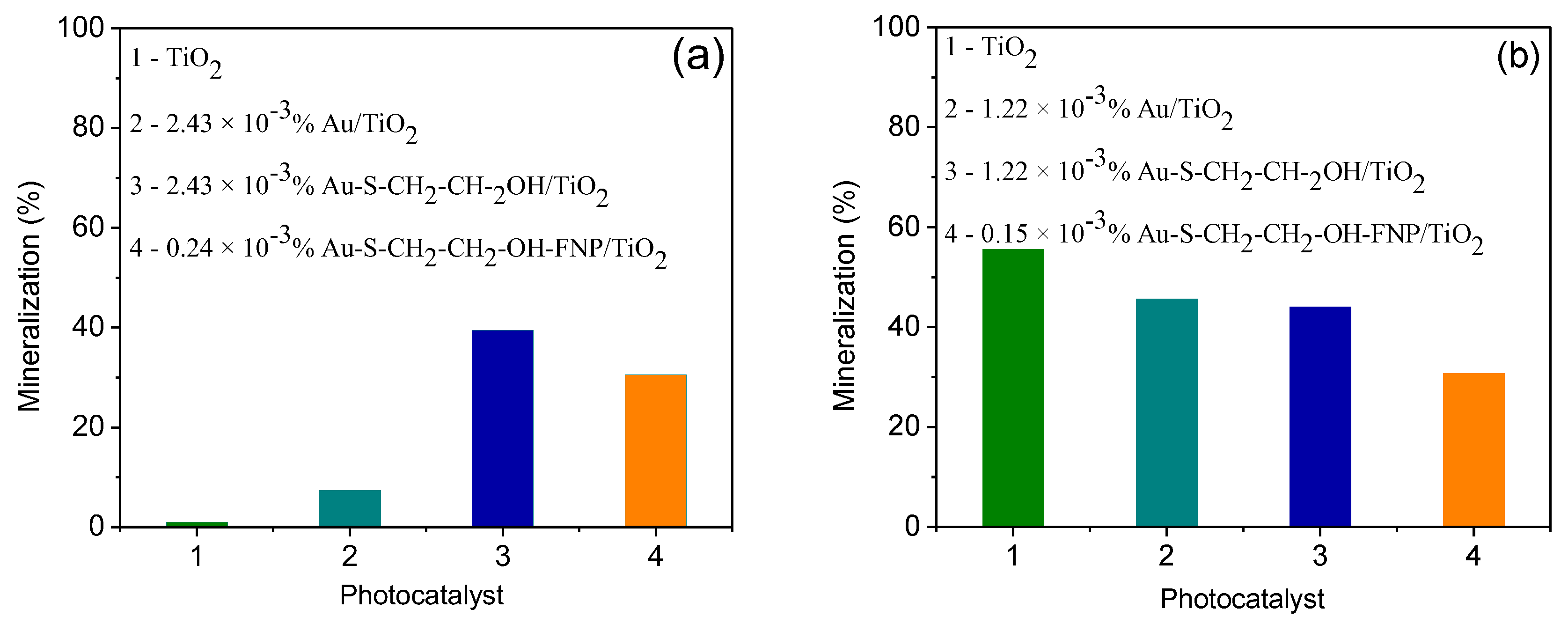
3.3. Evaluation of Mineralization
3.4. Effect of Hydroxyl Radicals and Holes Scavengers
3.5. LC–ESI–MS Identification of Mesotrione Degradation Intermediates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mitchell, G.; Bartlett, D.W.; Fraser, T.E.M.; Hawkes, T.R.; Holt, D.C.; Townson, J.K.; Wichert, R.A. Mesotrione: A new selective herbicide for use in maize. Pest Manag. Sci. 2001, 57, 120–128. [Google Scholar]
- Mendes, K.F.; Reis, M.R.; Inoue, M.H.; Pimpinato, R.F.; Tornisielo, V.L. Sorption and desorption of mesotrione alone and mixed with S-metolachlor + terbuthylazine in Brazilian soils. Geoderma 2016, 280, 22–28. [Google Scholar]
- Moro, C.V.; Bricheux, G.; Portelli, C.; Bohatier, J. Comparative effects of the herbicides chlortoluron and mesotrione on freshwater microalgae. Environ. Toxicol. Chem. 2012, 31, 778–786. [Google Scholar]
- Bonnet, J.L.; Bonnemoy, F.; Dusser, M.; Bohatier, J. Toxicity assessment of the herbicides sulcotrione and mesotrione toward two reference environmental microorganisms: Tetrahymena pyriformis Vibrio fischeri. Arch. Environ. Contam. Toxicol. 2008, 55, 576–583. [Google Scholar]
- Du, P.; Wu, X.; Xu, J.; Dong, F.; Liu, X.; Wei, D.; Zheng, Y. Determination and dissipation of mesotrione and its metabolites in rice using UPLC and triple-quadrupole tandem mass spectrometry. Food Chem. 2017, 229, 260–267. [Google Scholar]
- Su, W.; Hao, H.; Wu, R.; Xu, H.; Xue, F.; Lu, C. Degradation of mesotrione affected by environmental conditions. Bull. Environ. Contam. Toxicol. 2017, 98, 212–217. [Google Scholar]
- Stoob, K.; Singer, H.P.; Goetz, C.W.; Ruff, M.; Mueller, S.R. Fully automated online solid phase extraction coupled directly to liquid chromatography–tandem mass spectrometry: Quantification of sulfonamide antibiotics, neutral and acidic pesticides at low concentrations in surface waters. J. Chromatogr. A 2005, 1097, 138–147. [Google Scholar]
- Di, J.; Xia, J.; Ge, Y.; Li, H.; Ji, H.; Xu, H.; Zhang, Q.; Li, H.; Li, M. Novel visible-light-driven CQDs/Bi2WO6 hybrid materials with enhanced photocatalytic activity toward organic pollutants degradation and mechanism insight. Appl. Catal. B Environ. 2015, 168–169, 51–61. [Google Scholar]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar]
- Li, K.; Zeng, Z.; Yan, L.; Huo, M.; Guo, Y.; Luo, S.; Luo, X. Fabrication of C/X-TiO2@C3N4NTs (X = N, F, Cl) composites by using phenolic organic pollutants as raw materials and their visible-light photocatalytic performance in different photocatalytic systems. Appl. Catal. B Environ. 2016, 187, 269–280. [Google Scholar]
- Šojić Merkolov, D.V.; Lazarević, M.J.; Despotović, V.N.; Banić, N.D.; Finčur, N.L.; Maletić, S.P.; Abramović, B.F. The effects of inorganic anions and organic matter on mesotrione (Callisto®) removal from environmental waters. J. Serb. Chem. Soc. 2017, 82, 343–355. [Google Scholar]
- Thiruvenkatachari, R.; Vigneswaran, S.; Moon, I.S. A review on UV/TiO2 photocatalytic oxidation process. Korean J. Chem. Eng. 2008, 25, 64–72. [Google Scholar]
- Luo, X.; Deng, F.; Min, L.; Luo, S.; Guo, B.; Zeng, G.; Au, C. Facile one-step synthesis of inorganic-framework molecularly imprinted TiO2/WO3 nanocomposite and its molecular recognitive photocatalytic degradation of target contaminant. Environ. Sci. Technol. 2013, 47, 7404–7412. [Google Scholar]
- Qiu, B.; Xing, M.; Zhang, J. Mesoporous TiO2 nanocrystals grown in situ on graphene aerogels for high photocatalysis and lithium-ion batteries. J. Am. Chem. Soc. 2014, 136, 5852–5855. [Google Scholar]
- Chen, J.-J.; Wang, W.-K.; Li, W.-W.; Pei, D.-N.; Yu, H.-Q. Roles of crystal surface in Pt-loaded titania for photocatalytic conversion of organic pollutants: A first-principle theoretical calculation. ACS Appl. Mater. Interfaces 2015, 7, 12671–12678. [Google Scholar]
- Šojić, D.; Despotović, V.; Abramović, B.; Todorova, N.; Giannakopoulou, T.; Trapalis, C. Photocatalytic degradation of mecoprop and clopyralid in aqueous suspensions of nanostructured N-doped TiO2. Molecules 2010, 15, 2994–3009. [Google Scholar]
- Cao, Y.-C.; Fu, Z.; Wei, W.; Zou, L.; Mi, T.; He, D.; Yan, C.; Liu, X.; Zhu, Y.; Chen, L.; et al. Reduced graphene oxide supported titanium dioxide nanomaterials for the photocatalysis with long cycling life. App. Surf. Sci. 2015, 355, 1289–1294. [Google Scholar]
- Kowalska, E.; Abe, R.; Ohtani, B. Visible light-induced photocatalytic reaction of gold-modified titanium(IV) oxide particles: Action spectrum analysis. Chem. Commun. 2009, 2, 241–243. [Google Scholar]
- Tanaka, A.; Sakaguchi, S.; Hashimoto, K.; Kominami, H. Preparation of Au/TiO2 exhibiting strong surface plasmon resonance effective for photoinduced hydrogen formation from organic and inorganic compounds under irradiation of visible light. Catal. Sci. Technol. 2012, 2, 907–909. [Google Scholar]
- Wang, G.; Wang, X.; Liu, J.; Sun, X. Mesoporous Au/TiO2 nanocomposite microspheres for visible-light photocatalysis. Chem. Eur. J. 2012, 18, 5361–5366. [Google Scholar]
- Subramanian, V.; Wolf, E.; Kamat, P.V. Semiconductor-metal composite nanostructures. To what extent do metal nanoparticles improve the photocatalytic activity of TiO2 films? J. Phys. Chem. B 2001, 105, 11439–11446. [Google Scholar]
- Bannat, I.; Wessels, K.; Oekermann, T.; Rathousky, J.; Bahnemann, D.; Wark, M. Improving the photocatalytic performance of mesoporoustitania films by modification with gold nanostructures. Chem. Mater. 2009, 21, 1645–1653. [Google Scholar]
- Shi, J.; Chen, J.; Li, G.; An, T.; Yamashita, H. Fabrication of Au/TiO2nanowires@carbon fiber paper ternary composite for visible-light photocatalytic degradation of gaseous styrene. Catal. Today 2017, 281, 621–629. [Google Scholar]
- Rajabi, H.R.; Farsi, M. Study of capping agent effect on the structural, optical and photocatalytic properties of zinc sulfide quantum dots. Mater. Sci. Semicond. Process. 2016, 48, 14–22. [Google Scholar]
- Wohltjen, H.; Snow, A.W. Colloidal metal-insulator-metal ensemble chemiresistor sensor. Anal. Chem. 1998, 70, 2856–2859. [Google Scholar]
- McConnell, W.P.; Novak, J.P.; Brousseau, L.C.; Fuierer, R.R.; Tenent, R.C.; Feldheim, D.L. Electronic and optical properties of chemically modified metal nanoparticles and molecularly bridged nanoparticle arrays. J. Phys. Chem. B 2000, 104, 8925–8930. [Google Scholar]
- Gu, T.; Whitesell, J.K.; Fox, M.A. Energy transfer from a surface-bound arene to the gold core in ω-fluorenyl-alkane-1-thiolate monolayer-protected gold clusters. Chem. Mater. 2003, 15, 1358–1366. [Google Scholar]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar]
- Zheng, N.; Stucky, G.D. A general synthetic strategy for oxide-supported metal nanoparticle catalysts. J. Am. Chem. Soc. 2006, 128, 14278–14280. [Google Scholar]
- Grainge, D.W.; Castner, D.G. Nanobiomaterials and nanoanalysis: Opportunities for improving the science to benefit biomedical technologies. Adv. Mater. 2008, 20, 867–877. [Google Scholar]
- Shibu, E.S.; HabeebMuhammed, M.A.; Tsukuda, T.; Pradeep, T. Ligand exchange of Au25SG18 leading to functionalized gold clusters: Spectroscopy, kinetics, and luminescence. J. Phys. Chem. C 2008, 112, 12168–12176. [Google Scholar]
- Wu, Z.; Suhan, J.; Jin, R. One-pot synthesis of atomically monodisperse, thiol-functionalized Au25 nanoclusters. J. Mater. Chem. 2009, 19, 622–626. [Google Scholar]
- Shon, Y.-S.; Choo, H. [60]Fullerene-linked gold nanoparticles: Synthesis and layer-by-layer growth on a solid surface. Chem. Commun. 2002, 21, 2560–2561. [Google Scholar]
- Lu, F.; Xiao, S.; Li, Y.; Song, Y.; Liu, H.; Li, H.; Zhuang, J.; Liu, Y.; Gan, L.; Zhu, D. Fullerene-functionalized gold core–shell nanoparticles: Preparation and optical limiting properties. Inorg. Chem. Commun. 2004, 7, 960–962. [Google Scholar]
- Geng, M.; Zhang, Y.; Huang, Q.; Zhang, B.; Li, Q.; Li, W.; Li, J. Functionalization of C60 with gold nanoparticles. Carbon 2010, 48, 3570–3574. [Google Scholar]
- Shih, S.-M.; Su, W.-F.; Lin, Y.-J.; Wu, C.-S.; Chen, C.-D. Two-dimensional arrays of self-assembled gold and sulfur-containing fullerene nanoparticles. Langmuir 2002, 18, 3332–3335. [Google Scholar]
- Sudeep, P.K.; Ipe, B.I.; Thomas, K.G.; George, M.V.; Barazzouk, S.; Hotchandani, S.; Kamat, P.V. Fullerene-functionalized gold nanoparticles. A self-assembled photoactive antenna-metal nanocore assembly. Nano Lett. 2002, 2, 29–35. [Google Scholar]
- Dinh, T.; Shon, Y.-S. Direct Assembly of Photoresponsive C60−Gold Nanoparticle Hybrid Films. ACS Appl. Mater. Interfaces 2009, 1, 2699–2702. [Google Scholar]
- Djordjević, A.; Vojinović-Miloradov, M.; Petranović, N.; Devečerski, A.; Lazar, D.; Ribar, B. Catalytic preparation and characterization of C60Br24. Fuller. Sci. Technol. 1998, 6, 689–694. [Google Scholar]
- Mirkov, S.M.; Djordjevic, A.N.; Andric, N.L.; Andric, S.A.; Kostic, T.S.; Bogdanovic, G.M.; Vojinovic-Miloradov, M.B.; Kovacevic, R.Z. Nitric oxide scavenging activity of polyhydroxylatedfullerenol, C60(OH)24. Nitric Oxide 2004, 11, 201–207. [Google Scholar]
- Miki, N.; Abdelmoula, K.; Nihoul, G.; Marlière, C.; Renard, D. Expansion of ultrathin cobalt films in (Au/Co) multilayers measured by diffraction methods. Thin Solid Films 1993, 224, 14–21. [Google Scholar]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar]
- Kubelka, P.; Munk, F. An article on optics of paint layers. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Kubelka, P. New contributions to the optics of intensely light-scattering materials. Part I. J. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar]
- Djordjevic, A.; Šojić Merkulov, D.; Lazarević, M.; Borišev, I.; Medić, I.; Pavlović, V.; Miljević, B.; Abramović, B. Enhancement of nano titanium dioxide coatings by fullerene and polyhydroxy fullerene in the photocatalytic degradation of the herbicide mesotrione. Chemosphere 2018, 196, 145–152. [Google Scholar]
- Doudrick, K.; Monzón, O.; Mangonon, A.; Hristovski, K.; Westerhoff, P. Nitrate reduction in water using commercial titanium dioxide photocatalysts (P25, P90, and Hombikat UV100). J. Environ. Eng. 2012, 138, 852–861. [Google Scholar]
- Alonso-Tellez, A.; Masson, R.; Robert, D.; Keller, N.; Keller, V. Comparison of Hombikat UV100 and P25 TiO2 performance in gas-phase photocatalytic oxidation reactions. J. Photochem. Photobiol. A Chem. 2012, 250, 58–65. [Google Scholar]
- Ahmed, S.; Rasul, M.G.; Brown, R.; Hashib, M.A. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (●OH/●O‒) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar]
- Abramović, B.; Despotović, V.; Šojić, D.; Finčur, N. Mechanism of clomazone photocatalytic degradation: Hydroxyl radical, electron and hole scavengers. Reac. Kinet. Mech. Cat. 2015, 115, 67–79. [Google Scholar]
- Finčur, N.L.; Krstić, J.B.; Šibul, F.S.; Šojić, D.V.; Despotović, V.N.; Banić, N.D.; Agbaba, J.R.; Abramović, B.F. Removal of alprazolam from aqueous solutions by heterogeneous photocatalysis: Influencing factors, intermediates, and products. Chem. Eng. J. 2017, 307, 1105–1115. [Google Scholar]
- Xiaoju, Y.; Ruiling, B.; Shuili, Y.; Qiongfang, L.; Qingfeng, J. The roles of hydroxyl radicals, photo-generated holes and oxygen in the photocatalytic degradation of humic acid. Russ. J. Phys. Chem. A 2012, 86, 1479–1485. [Google Scholar]
- Jović, M.; Manojlović, D.; Stanković, D.; Dojčinović, B.; Obradović, B.; Gašić, U.; Roglić, G. Degradation of triketone herbicides, mesotrione and sulcotrione, using advanced oxidation processes. J. Hazard. Mater. 2013, 260, 1092–1099. [Google Scholar]
- Bensalah, N.; Khodary, A.; Abdel-Wahab, A. Kinetic and mechanistic investigations of mesotrione degradation in aqueous medium by Fenton process. J. Hazard. Mater. 2011, 189, 479–485. [Google Scholar]
- Barchanska, H.; Kluza, A.; Krajczewska, K.; Maj, J. Degradation study of mesotrione and other triketone herbicides on soils and sediments. J. Soils. Sediments 2016, 16, 125–133. [Google Scholar]
- Šojić, D.V.; Orčić, D.Z.; Četojević-Simin, D.D.; Despotović, V.N.; Abramović, B.F. Kinetics and the mechanism of the photocatalytic degradation of mesotrione in aqueous suspension and toxicity of its degradation mixtures. J. Mol. Catal. A Chem. 2014, 392, 67–75. [Google Scholar]

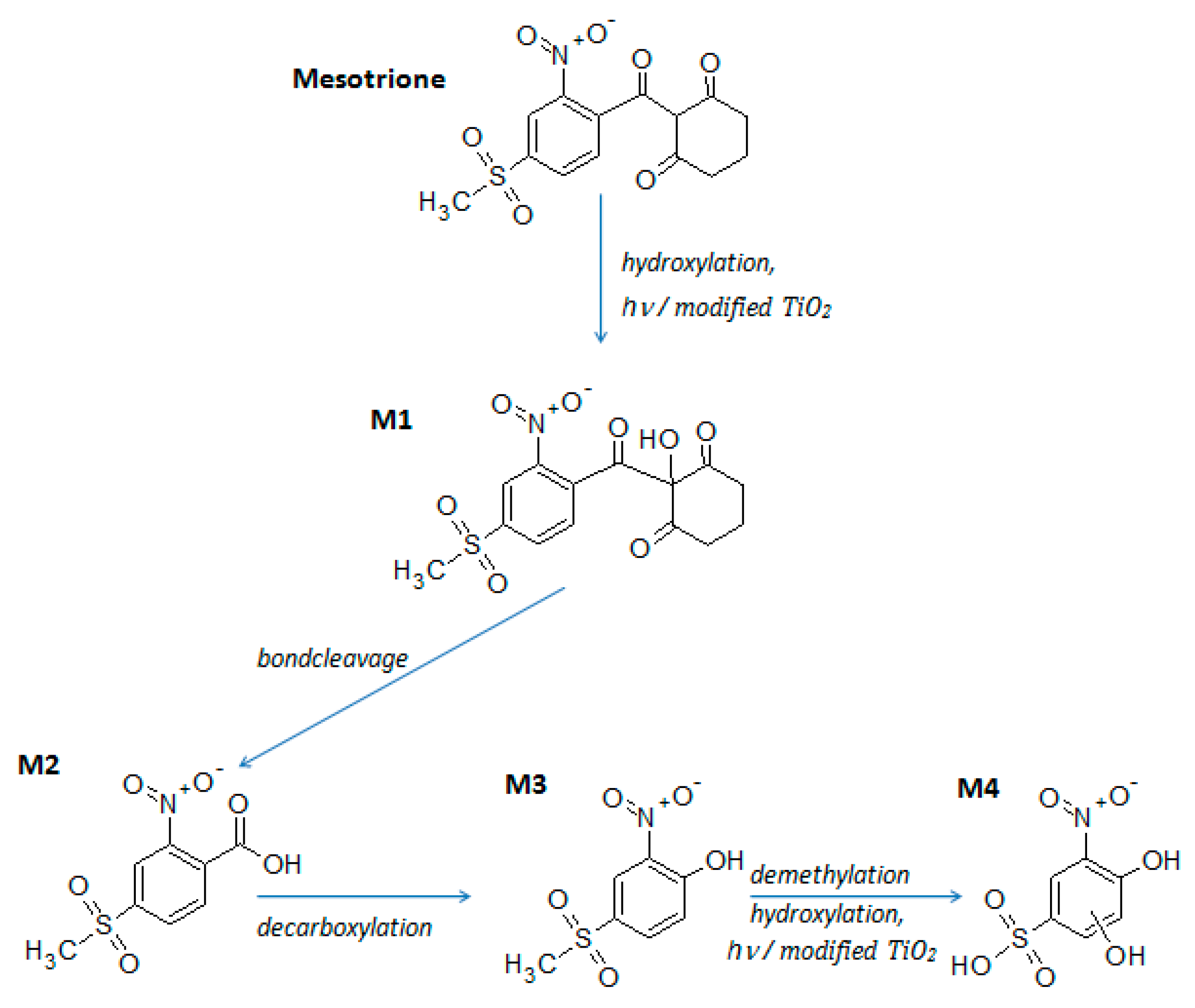
| Peak label | tr (min) | m/z ([M–H]‒) | M (Da) |
|---|---|---|---|
| Mesotrione | 6.10 | 338.2 (100), 291.2 (34), 339.2 (17) | 339.3 |
| M1 | 2.61 | 354.3 (100), 113.2 (93), 97.1 (25) | 355.3 |
| M2 | 1.75 | 244.2 (100), 200.2 (86), 62.2 (28) | 245.2 |
| M3 | 2.81 | 216.2 (100), 213.2 (45), 91.2 (30), 212.3 (9) | 217.2 |
| M4 | 2.10 | 234.2 (100), 91.2 (50), 157.1 (48), 214.2 (41), 62.2 (21) | 235.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šojić Merkulov, D.; Lazarević, M.; Djordjevic, A.; Náfrádi, M.; Alapi, T.; Putnik, P.; Rakočević, Z.; Novaković, M.; Miljević, B.; Bognár, S.; et al. Potential of TiO2 with Various Au Nanoparticles for Catalyzing Mesotrione Removal from Wastewaters under Sunlight. Nanomaterials 2020, 10, 1591. https://doi.org/10.3390/nano10081591
Šojić Merkulov D, Lazarević M, Djordjevic A, Náfrádi M, Alapi T, Putnik P, Rakočević Z, Novaković M, Miljević B, Bognár S, et al. Potential of TiO2 with Various Au Nanoparticles for Catalyzing Mesotrione Removal from Wastewaters under Sunlight. Nanomaterials. 2020; 10(8):1591. https://doi.org/10.3390/nano10081591
Chicago/Turabian StyleŠojić Merkulov, Daniela, Marina Lazarević, Aleksandar Djordjevic, Máté Náfrádi, Tünde Alapi, Predrag Putnik, Zlatko Rakočević, Mirjana Novaković, Bojan Miljević, Szabolcs Bognár, and et al. 2020. "Potential of TiO2 with Various Au Nanoparticles for Catalyzing Mesotrione Removal from Wastewaters under Sunlight" Nanomaterials 10, no. 8: 1591. https://doi.org/10.3390/nano10081591
APA StyleŠojić Merkulov, D., Lazarević, M., Djordjevic, A., Náfrádi, M., Alapi, T., Putnik, P., Rakočević, Z., Novaković, M., Miljević, B., Bognár, S., & Abramović, B. (2020). Potential of TiO2 with Various Au Nanoparticles for Catalyzing Mesotrione Removal from Wastewaters under Sunlight. Nanomaterials, 10(8), 1591. https://doi.org/10.3390/nano10081591











