Effect of Hematite Doping with Aliovalent Impurities on the Electrochemical Performance of α-Fe2O3@rGO-Based Anodes in Sodium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Graphene Oxide and Nanocomposites
- (i)
- The hematite doping with aliovalent impurities (Ti or Mn) and of;
- (ii)
- The nominal rGO content of the nanocomposites (50 or 30 wt %);
2.3. Nanocomposite Characterization
2.4. Electrochemical Measurement
3. Results and Discussion
3.1. Nanocomposite Physicochemical Properties
3.2. Electrochemical Behavior
4. Conclusions
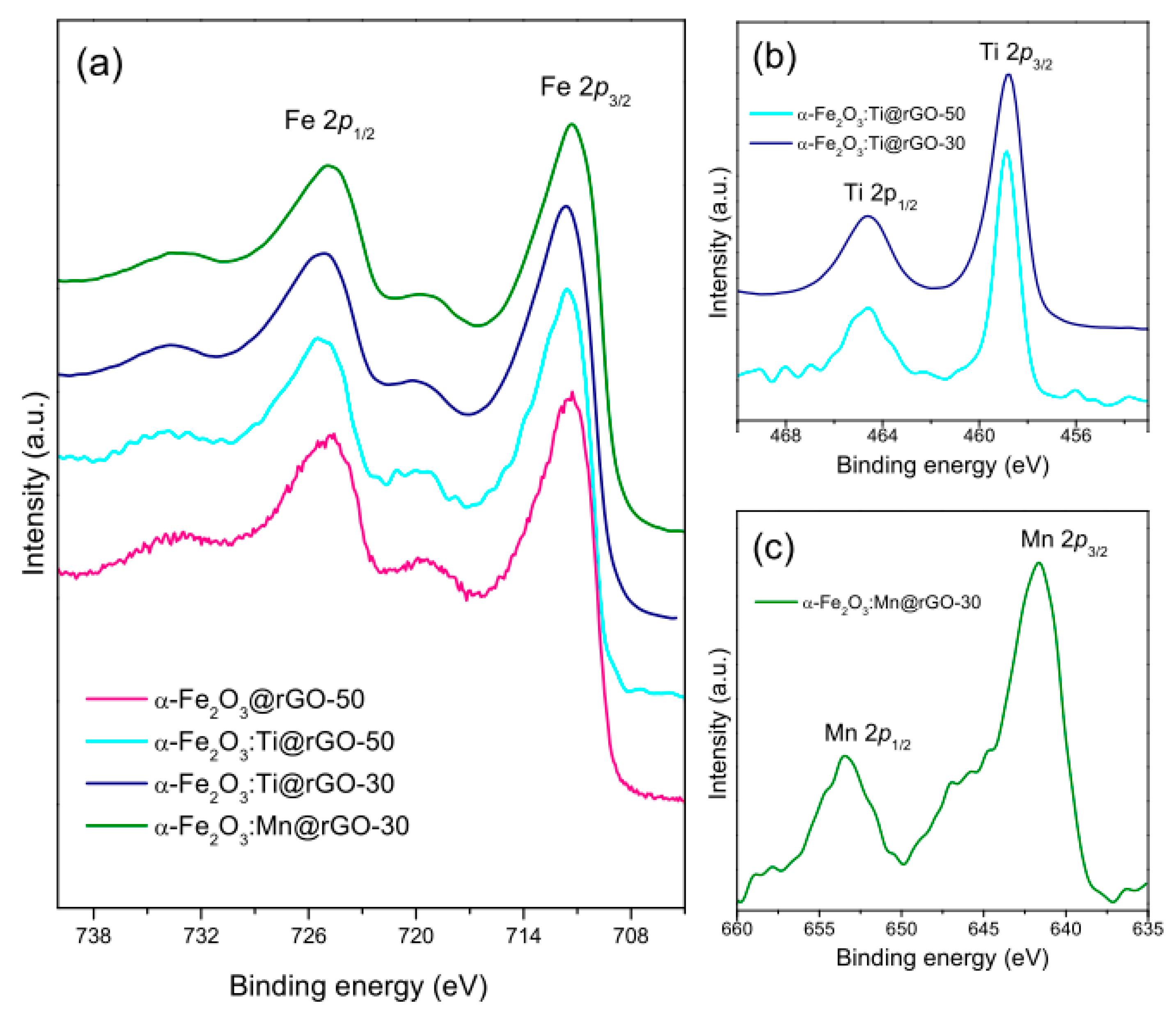
- Titanium was incorporated in the hematite lattice as Ti4+ ions with electron transfer to surrounding Fe atoms (n-doping), whereas the type (p- or n-) of doping by manganese could not clearly be assessed due to is multivalent nature;
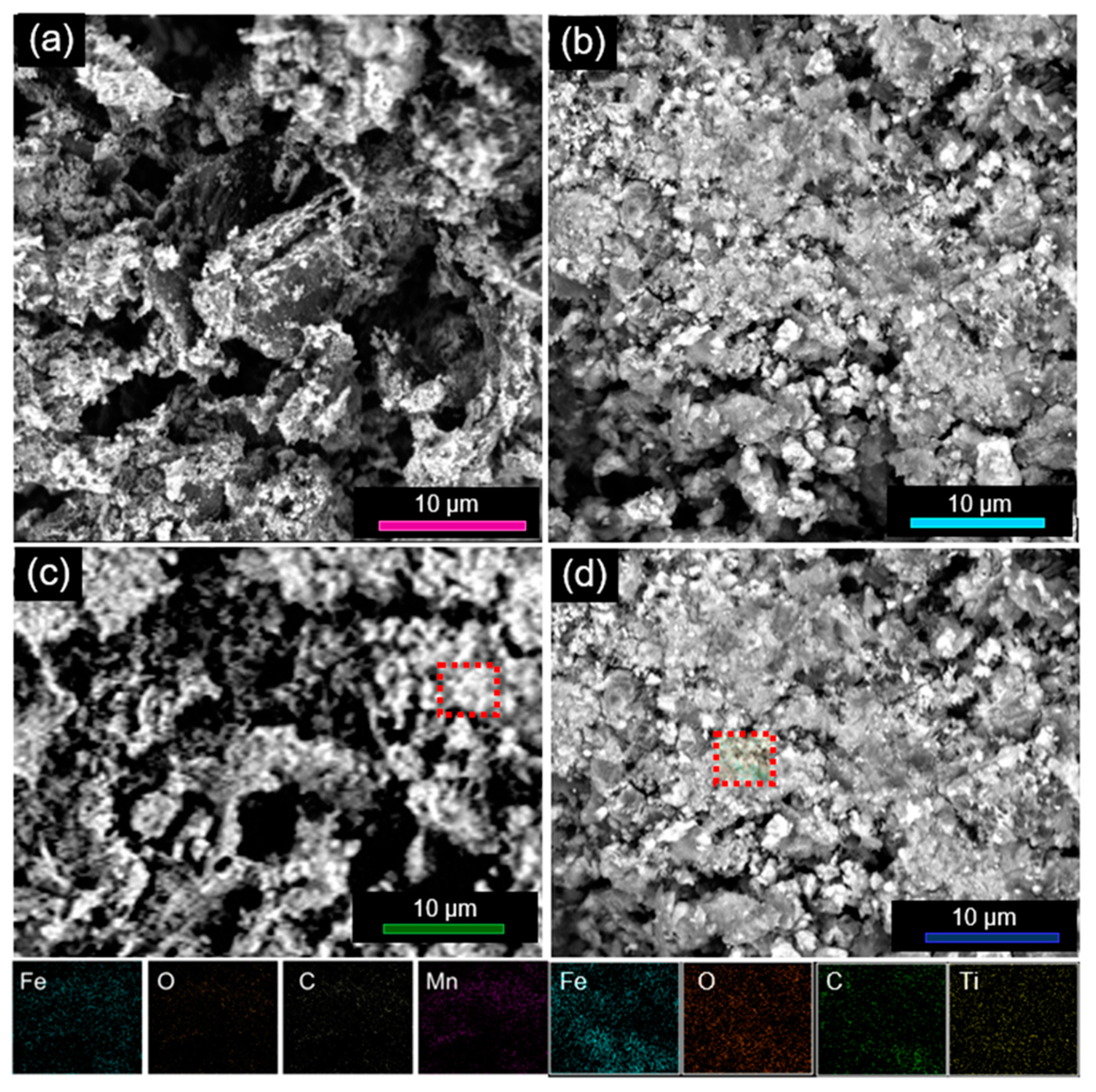
- The doping did not influence the crystalline phase and morphology of the iron oxide nanoparticles anchored on the rGO sheets;
- Conversely, it remarkably improved the electrochemical performance with respect to the anode based on the composite α-Fe2O3@rGO;
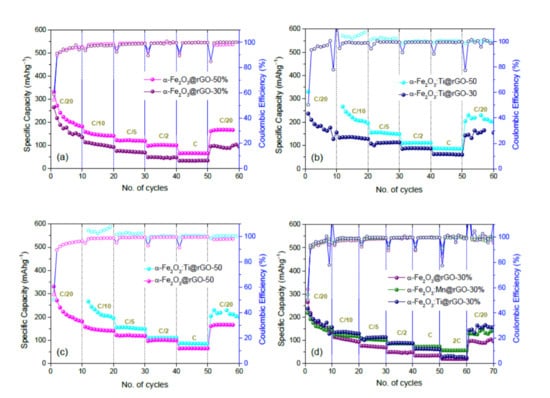
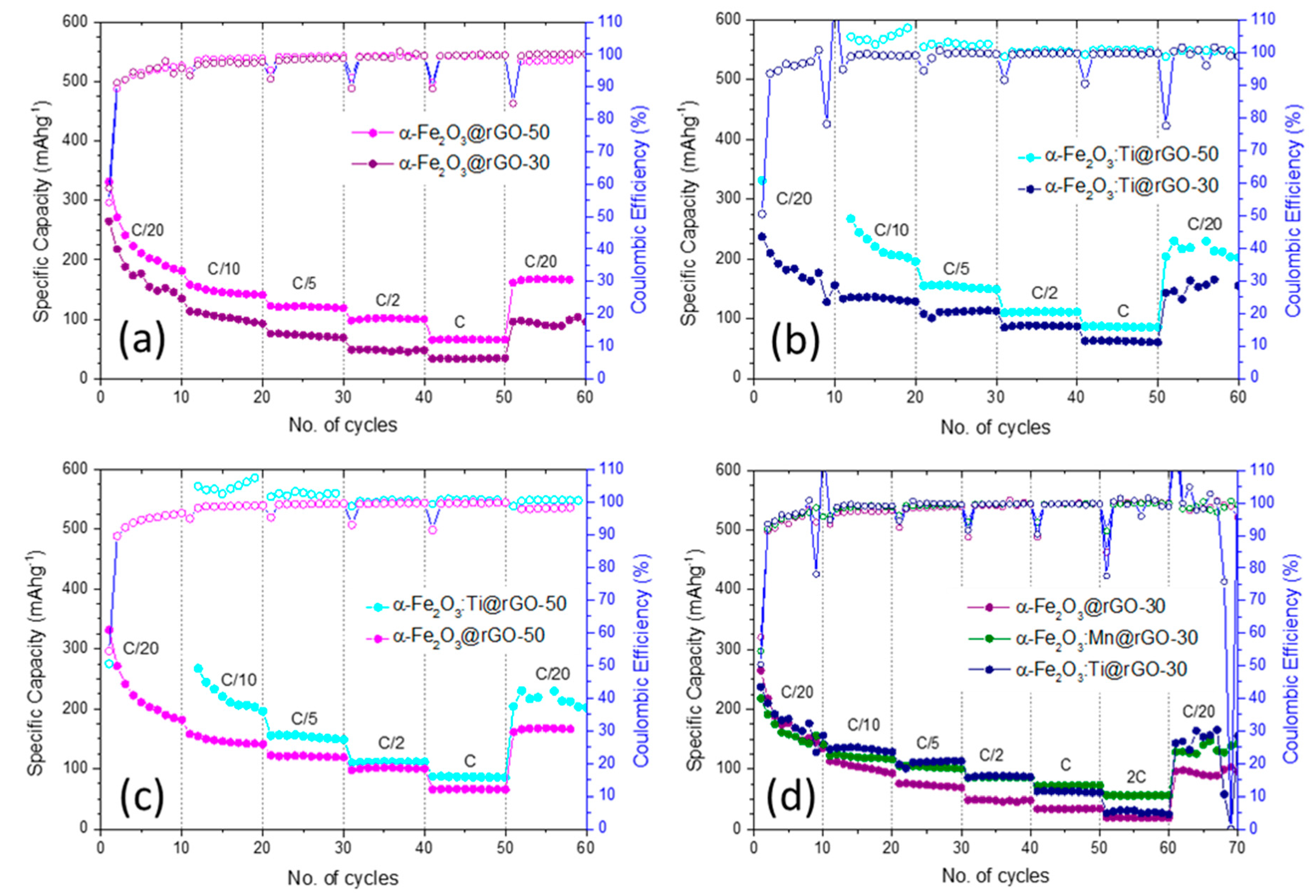
- For fixed rGO content, the α-Fe2O3:Ti@rGO-based anodes exhibited better rate capability at lower rates, whereas α-Fe2O3:Mn@rGO-based anodes show enhanced stability at higher rates, still retaining 56 mAhg−1 at a rate of 2 C;
- Increasing the rGO content of the nanocomposites from 30 to 50 wt % was beneficial to a specific capacity at any rate.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, K.; Gratzel, M. Themed issue: Nanomaterials for energy conversion and storage. J. Mater. Chem. 2012, 22, 24190–24194. [Google Scholar] [CrossRef]
- Lott, M.; Kim, S.; Tam, C.; Elzinga, D.; Heinen, S.; Munuera, L.; Remme, U. Technology Roadmap: Energy Storage; International Energy Agency: Paris, France, 2014. [Google Scholar]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.-M. Nanomaterials for Rechargeable Lithium Batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Marom, R.; Amalraj, S.F.; Leifer, N.; Jacob, D.; Aurbach, D. A review of advanced and practical lithium battery materials. J. Mater. Chem. 2011, 21, 9938–9954. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef]
- Tarascon, J.-M. Is lithium the new gold? Nat. Chem. 2010, 2, 510. [Google Scholar] [CrossRef] [PubMed]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Kundu, D.; Talaie, E.; Duffort, V.; Nazar, L.F. The Emerging Chemistry of Sodium Ion Batteries for Electrochemical Energy Storage. Angew. Chem. Int. Ed. 2015, 54, 3431–3448. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, J.M.; Prakash, A.S. Advancement of technology towards developing Na-ion batteries. J. Power Sources 2018, 378, 268–300. [Google Scholar] [CrossRef]
- Park, S.; Khan, Z.; Shin, T.J.; Kim, Y.; Ko, H. Rechargeable Na/Ni batteries based on the Ni(OH)2/NiOOH redox couple with high energy density and good cycling performance. J. Mater. Chem. A 2019, 7, 1564–1573. [Google Scholar] [CrossRef]
- Opra, D.; Gnedenkov, S.; Sokolov, A.; Podgorbunsky, A.; Ustinov, A.; Mayorov, V.; Kuryavyi, V.; Sinebryukhov, S. Vanadium-doped TiO2-B/anatase mesoporous nanotubes with improved rate and cycle performance for rechargeable lithium and sodium batteries. J. Mater. Sci. Technol. 2020, 54, 181–189. [Google Scholar] [CrossRef]
- Adelhelm, P.; Hartmann, P.; Bender, C.L.; Busche, M.; Eufinger, C.; Janek, J. From lithium to sodium: Cell chemistry of room temperature sodium–air and sodium–sulfur batteries. Beilstein J. Nanotechnol. 2015, 6, 1016–1055. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.; Sun, Y. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Wang, P.-F.; You, Y.; Yin, Y.-X.; Guo, Y.-G. Layered Oxide Cathodes for Sodium-Ion Batteries: Phase Transition, Air Stability, and Performance. Adv. Energy Mater. 2017, 8, 1701912. [Google Scholar] [CrossRef]
- Park, H.; Yoo, J.-K.; Ko, W.; Lee, Y.; Park, I.; Myung, S.; Kim, J. Monoclinic Fe2(SO4)3: A new Fe-based cathode material with superior electrochemical performances for Na-ion batteries. J. Power Sources 2019, 434, 226750. [Google Scholar] [CrossRef]
- Han, M.H.; Gonzalo, E.; Singh, G.; Lu, J. A comprehensive review of sodium layered oxides: Powerful cathodes for Na-ion batteries. Energy Environ. Sci. 2015, 8, 81–102. [Google Scholar] [CrossRef]
- Vitoriano, N.O.; Drewett, N.E.; Gonzalo, E.; Rojo, T. High performance manganese-based layered oxide cathodes: Overcoming the challenges of sodium ion batteries. Energy Environ. Sci. 2017, 10, 1051–1074. [Google Scholar] [CrossRef]
- Mason, C.W.; Gocheva, I.; Hoster, H.E.; Yu, D.Y. Iron (III) sulfate: A stable, cost effective electrode material for sodium ion batteries. Chem. Commun. 2014, 50, 2249–2251. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.L.; Makahnouk, W.R.M.; Makimura, Y.; Toghill, K.; Nazar, L.F. A multifunctional 3.5 V iron-based phosphate cathode for rechargeable batteries. Nat. Mater. 2007, 6, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fang, Y.; Xiao, L.; Qian, J.; Cao, Y.; Ai, X.; Yang, H. Graphene-Scaffolded Na3V2(PO4)3 Microsphere Cathode with High Rate Capability and Cycling Stability for Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 7177–7184. [Google Scholar] [CrossRef]
- Jin, T.; Liu, Y.; Li, Y.; Cao, K.; Wang, X.; Jiao, L. Electrospun NaVPO4 F/C Nanofibers as Self-Standing Cathode Material for Ultralong Cycle Life Na-Ion Batteries. Adv. Energy Mater. 2017, 7, 1700087. [Google Scholar] [CrossRef]
- Guan, W.; Pan, B.; Zhou, P.; Mi, J.; Zhang, D.; Xu, J.; Jiang, Y. A High Capacity, Good Safety and Low Cost Na2FeSiO4-Based Cathode for Rechargeable Sodium-Ion Battery. ACS Appl. Mater. Interfaces 2017, 9, 22369–22377. [Google Scholar] [CrossRef]
- Song, H.J.; Kim, D.-S.; Kim, J.-C.; Hong, S.-H.; Kim, D.-W. An approach to flexible Na-ion batteries with exceptional rate capability and long lifespan using Na2FeP2O7 nanoparticles on porous carbon cloth. J. Mater. Chem. A 2017, 5, 5502–5510. [Google Scholar] [CrossRef]
- Chung, S.-C.; Ming, J.; Lander, L.; Lu, J.; Yamada, A. Rhombohedral NASICON-type NaxFe2(SO4)3 for sodium-ion battery: Comparison with the phosphate and alluaudite phases. J. Mater. Chem. A 2018, 6, 3919–3925. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B. Prussian blue: A new framework of electrode materials for sodium batteries. Chem. Commun. 2012, 48, 6544–6546. [Google Scholar] [CrossRef]
- Qian, J.; Wu, C.; Cao, Y.; Ma, Z.-F.; Huangb, Y.; Ai, X.; Yang, H. Prussian Blue Cathode Materials for Sodium-Ion Batteries and Other Ion Batteries. Adv. Energy Mater. 2018, 8, 1702619. [Google Scholar] [CrossRef]
- Guntlin, C.; Zünd, T.; Kravchyk, K.V.; Wörle, M.; Bodnarchuk, M.I.; Kovalenko, M.V. Nanocrystalline FeF 3 and MF 2 (M = Fe, Co, and Mn) from metal trifluoroacetates and their Li(Na)-ion storage properties. J. Mater. Chem. A 2017, 5, 7383–7393. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, M.; Lei, Y. Organic materials for rechargeable sodium-ion batteries. Mater. Today 2018, 21, 60–78. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. The Mechanisms of Lithium and Sodium Insertion in Carbon Materials. J. Electrochem. Soc. 2001, 148, A803. [Google Scholar] [CrossRef]
- Valvo, M.; Lindgren, F.; Lafont, U.; Björefors, F.; Edström, K. Towards more sustainable negative electrodes in Na-ion batteries via nanostructured iron oxide. J. Power Sources 2014, 245, 967–978. [Google Scholar] [CrossRef]
- Thomas, P.; Billaud, D. Electrochemical insertion of sodium into hard carbons. Electrochimica Acta 2002, 47, 3303–3307. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L.V.; Yang, Z.; Liu, J. Sodium Ion Insertion in Hollow Carbon Nanowires for Battery Applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar] [CrossRef]
- Tang, K.; Fu, L.; White, R.J.; Yu, L.; Titirici, M.-M.; Antonietti, M.; Maier, J. Hollow Carbon Nanospheres with Superior Rate Capability for Sodium-Based Batteries. Adv. Energy Mater. 2012, 2, 873–877. [Google Scholar] [CrossRef]
- Luo, W.; Schardt, J.; Bommier, C.; Wang, B.; Razink, J.; Simonsen, J.; Ji, X. Carbon nanofibers derived from cellulose nanofibers as a long-life anode material for rechargeable sodium-ion batteries. J. Mater. Chem. A 2013, 1, 10662. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Luo, Y.; Qiu, W.; Liu, P.; Tong, Y. A review of carbon materials and their composites with alloy metals for sodium ion battery anodes. Carbon 2016, 98, 162–178. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, Y.-G. Highly Disordered Carbon as a Superior Anode Material for Room-Temperature Sodium-Ion Batteries. ChemElectroChem 2013, 1, 83–86. [Google Scholar] [CrossRef]
- Xiao, L.; Cao, Y.; Henderson, W.A.; Sushko, M.L.; Shao, Y.; Xiao, J.; Wang, W.; Engelhard, M.H.; Nie, Z.; Liu, J. Hard carbon nanoparticles as high-capacity, high-stability anodic materials for Na-ion batteries. Nano Energy 2016, 19, 279–288. [Google Scholar] [CrossRef]
- Hou, H.; Qiu, X.; Wei, W.; Zhang, Y.; Ji, X. Carbon Anode Materials for Advanced Sodium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602898. [Google Scholar] [CrossRef]
- Chevrier, V.L.; Ceder, G. Challenges for Na-ion Negative Electrodes. J. Electrochem. Soc. 2011, 158, A1011. [Google Scholar] [CrossRef]
- Wang, J.W.; Liu, X.H.; Mao, S.X.; Huang, J.Y. Microstructural Evolution of Tin Nanoparticles during in Situ Sodium Insertion and Extraction. Nano Lett. 2012, 12, 5897–5902. [Google Scholar] [CrossRef]
- Baggetto, L.; Keum, J.K.; Browning, J.F.; Veith, G. Germanium as negative electrode material for sodium-ion batteries. Electrochem. Commun. 2013, 34, 41–44. [Google Scholar] [CrossRef]
- Darwiche, A.; Marino, C.; Sougrati, M.-T.; Fraisse, B.; Stievano, L.; Monconduit, L. Better Cycling Performances of Bulk Sb in Na-Ion Batteries Compared to Li-Ion Systems: An Unexpected Electrochemical Mechanism. J. Am. Chem. Soc. 2012, 134, 20805–20811. [Google Scholar] [CrossRef]
- Qian, J.; Wu, X.; Cao, Y.; Ai, X.; Yang, H. High Capacity and Rate Capability of Amorphous Phosphorus for Sodium Ion Batteries. Angew. Chem. Int. Ed. 2013, 52, 4633–4636. [Google Scholar] [CrossRef]
- Kim, Y.; Ha, K.-H.; Oh, S.M.; Lee, K.T. High-Capacity Anode Materials for Sodium-Ion Batteries. Chem. A Eur. J. 2014, 20, 11980–11992. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, K.; Hu, Z.; Duan, W.; Cheng, F.; Chen, J. Porous CuO nanowires as the anode of rechargeable Na-ion batteries. Nano Res. 2013, 7, 199–208. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, W.; Li, N.; Yan, N.; Hu, B.; Sun, Z.; Pan, L. Metal–organic framework derived porous CuO/Cu2O composite hollow octahedrons as high performance anode materials for sodium ion batteries. Chem. Commun. 2015, 51, 16413–16416. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Chen, Y.-M.; Liu, K.; Yu, Z.; Liang, W.; Bhaway, S.M.; Gao, M.; Zhu, Y. Metal organic frameworks derived hierarchical hollow NiO/Ni/Graphene composites for lithium and sodium storage. ACS Nano 2016, 10, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Jaffer, S.; Yu, H. Transition metal oxides for sodium-ion batteries. Energy Storage Mater. 2016, 5, 116–131. [Google Scholar] [CrossRef]
- Li, Z.; Ding, J.; Mitlin, D. Tin and Tin Compounds for Sodium Ion Battery Anodes: Phase Transformations and Performance. Accounts Chem. Res. 2015, 48, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Ma, C.; Alvarado, J.; Kidera, T.; Dimov, N.; Meng, Y.S.; Okada, S. Electrochemical properties of tin oxide anodes for sodium-ion batteries. J. Power Sources 2015, 284, 287–295. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, M.; Zhang, D.; Yuan, T.; Sun, W.; Xu, B.B.; Yan, M. Transition metal oxides for high performance sodium ion battery anodes. Nano Energy 2014, 5, 60–66. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Wang, W.; Choi, D.; Nie, Z.; Yu, J.; Saraf, L.V.; Yang, Z.; Liu, J. Reversible Sodium Ion Insertion in Single Crystalline Manganese Oxide Nanowires with Long Cycle Life. Adv. Mater. 2011, 23, 3155–3160. [Google Scholar] [CrossRef]
- Wen, J.-W.; Zhang, D.-W.; Zang, Y.; Sun, X.; Cheng, B.; Ding, C.-X.; Yu, Y.; Chen, C. Li and Na storage behavior of bowl-like hollow Co3O4 microspheres as an anode material for lithium-ion and sodium-ion batteries. Electrochimica Acta 2014, 132, 193–199. [Google Scholar] [CrossRef]
- Santangelo, S.; Fiore, M.; Pantò, F.; Stelitano, S.; Marelli, M.; Frontera, P.; Antonucci, P.; Longoni, G.; Ruffo, R. Electro-spun Co3O4 anode material for Na-ion rechargeable batteries. Solid State Ionics 2017, 309, 41–47. [Google Scholar] [CrossRef]
- Li, L.; Wang, Q.; Zhang, X.; Fang, L.; Li, X.; Zhang, W. Unique three-dimensional Co3O4@N-CNFs derived from ZIFs and bacterial cellulose as advanced anode for sodium-ion batteries. Appl. Surf. Sci. 2020, 508, 145295. [Google Scholar] [CrossRef]
- Fu, Y.; Wei, Q.; Wang, X.; Zhang, G.; Shu, H.; Yang, X.; Tavares, A.C.; Sun, S. A facile synthesis of Fe3O4 nanoparticles/graphene for high-performance lithium/sodium-ion batteries. RSC Adv. 2016, 6, 16624–16633. [Google Scholar] [CrossRef]
- Xu, Z.-L.; Yao, S.; Cui, J.; Zhou, L.; Kim, J.-K. Atomic scale, amorphous FeOx/carbon nanofiber anodes for Li-ion and Na-ion batteries. Energy Storage Mater. 2017, 8, 10–19. [Google Scholar] [CrossRef]
- Fiore, M.; Longoni, G.; Santangelo, S.; Pantò, F.; Stelitano, S.; Frontera, P.; Antonucci, P.; Ruffo, R.; Michele, F. Electrochemical characterization of highly abundant, low cost iron (III) oxide as anode material for sodium-ion rechargeable batteries. Electrochim. Acta 2018, 269, 367–377. [Google Scholar] [CrossRef]
- Huang, B.; Tai, K.; Zhang, M.; Xiao, Y.; Dillon, S.J. Comparative Study of Li and Na Electrochemical Reactions with Iron Oxide Nanowires. Electrochim. Acta 2014, 118, 143–149. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Wu, C.; Guan, L. A general strategy for synthesis of metal oxide nanoparticles attached on carbon nanomaterials. Nanoscale Res. Lett. 2011, 6, 71. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, Z.; Xu, Z.J. Yolk–shell Fe2O3⊙C composites anchored on MWNTs with enhanced lithium and sodium storage. Nanoscale 2015, 7, 9520–9525. [Google Scholar] [CrossRef]
- Zhang, N.; Han, X.; Liu, Y.; Hu, X.; Zhao, Q.; Chen, J. 3D Porous γ-Fe2O3@C Nanocomposite as High-Performance Anode Material of Na-Ion Batteries. Adv. Energy Mater. 2014, 5, 1401123. [Google Scholar] [CrossRef]
- Li, H.; Xu, L.; Sitinamaluwa, H.; Wasalathilake, K.C.; Yan, C. Coating Fe2O3 with graphene oxide for high-performance sodium-ion battery anode. Compos. Commun. 2016, 1, 48–53. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tosi, M.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef]
- Dong, X.; Xing, Z.; Zheng, G.; Gao, X.; Hong, H.; Ju, Z.; Zhuang, Q. MoS2/N-doped graphene aerogles composite anode for high performance sodium/potassium ion batteries. Electrochim. Acta 2020, 339, 135932. [Google Scholar] [CrossRef]
- Jian, Z.; Zhao, B.; Liu, P.; Li, F.; Zheng, M.; Chen, M.; Shi, Y.; Zhou, H. Fe2O3 nanocrystals anchored onto graphene nanosheets as the anode material for low-cost sodium-ion batteries. Chem. Commun. 2014, 50, 1215–1217. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Huang, Z.X.; Cheng, C.; Wang, Y.; Liu, B.; Yang, H.Y. Seed-assisted growth of ?-Fe2O3 nanorod arrays on reduced graphene oxide: A superior anode for high-performance Li-ion and Na-ion batteries. J. Mater. Chem. A 2016, 4, 11800–11811. [Google Scholar] [CrossRef]
- Liu, X.; Chen, T.; Chu, H.; Niu, L.; Sun, Z.; Pan, L.; Sun, C.Q. Fe2O3-reduced graphene oxide composites synthesized via microwave-assisted method for sodium ion batteries. Electrochim. Acta 2015, 166, 12–16. [Google Scholar] [CrossRef]
- Modafferi, V.; Fiore, M.; Fazio, E.; Patanè, S.; Triolo, C.; Santangelo, S.; Ruffo, R.; Neri, F.; Musolino, M.G. Synthesis and characterization of Fe2O3/reduced graphene oxide nanocomposite as a high-performance anode material for sodium-ion batteries. Model. Meas. Control. B 2018, 87, 129–134. [Google Scholar] [CrossRef]
- Modafferi, V.; Santangelo, S.; Fiore, M.; Fazio, E.; Triolo, C.; Patanè, S.; Ruffo, R.; Musolino, M.G. Transition Metal Oxides on Reduced Graphene Oxide Nanocomposites: Evaluation of Physicochemical Properties. J. Nanomater. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Saraf, M.; Natarajan, K.; Mobin, S.M. Microwave assisted fabrication of a nanostructured reduced graphene oxide (rGO)/Fe2O3 composite as a promising next generation energy storage material. RSC Adv. 2017, 7, 309–317. [Google Scholar] [CrossRef]
- Meng, F.; Li, J.; Cushing, S.K.; Bright, J.; Zhi, M.; Rowley, J.D.; Hong, Z.; Manivannan, A.; Bristow, A.D.; Wu, N. Photocatalytic Water Oxidation by Hematite/Reduced Graphene Oxide Composites. ACS Catal. 2013, 3, 746–751. [Google Scholar] [CrossRef]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Jiang, W.; Nadeau, G.; Zaghib, K.; Kinoshita, K. Thermal analysis of the oxidation of natural graphite—effect of particle size. Thermochim. Acta 2000, 351, 85–93. [Google Scholar] [CrossRef]
- Wu, Q.-H.; Qu, B.; Tang, J.; Wang, C.; Wang, D.; Li, Y.Y.; Ren, J.-G. An Alumina-Coated Fe3O4-Reduced Graphene Oxide Composite Electrode as a Stable Anode for Lithium-ion Battery. Electrochimica Acta 2015, 156, 147–153. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Rebuttini, V.; Fazio, E.; Santangelo, S.; Neri, F.; Caputo, G.; Martin, C.; Brousse, T.; Favier, F.; Pinna, N. Chemical Modification of Graphene Oxide through Diazonium Chemistry and Its Influence on the Structure-Property Relationships of Graphene Oxide-Iron Oxide Nanocomposites. Chem. A Eur. J. 2015, 21, 12465–12474. [Google Scholar] [CrossRef]
- Ramya, S.S.; Mahadevan, C.K. Preparation and structural, optical, magnetic, and electrical characterization of Mn2+/Co2+/Cu2+ doped hematite nanocrystals. J. Solid State Chem. 2014, 211, 37–50. [Google Scholar] [CrossRef]
- Subramanian, A.; Gracia-Espino, E.; Annamalai, A.; Lee, H.H.; Lee, S.Y.; Choi, S.H.; Jang, J.S. Effect of tetravalent dopants on hematite nanostructure for enhanced photoelectrochemical water splitting. Appl. Surf. Sci. 2018, 427, 1203–1212. [Google Scholar] [CrossRef]
- Varshney, D.; Yogi, A. Structural and Electrical conductivity of Mn doped Hematite (α-Fe2O3) phase. J. Mol. Struct. 2011, 995, 157–162. [Google Scholar] [CrossRef]
- Nikam, R.; Rayaprol, S.; Mukherjee, S.; Kaushik, S.; Goyal, P.; Babu, P.; Radha, S.; Siruguri, V. Structure and magnetic properties of Mn doped α-Fe2O3. Phys. B Condens. Matter 2019, 574, 411663. [Google Scholar] [CrossRef]
- KnowledgeDoor. Available online: http://www.knowledgedoor.com/2/elements_handbook/shannon-prewitt_effective_ionic_radius_part2.html#iron (accessed on 31 July 2020).
- Zhou, Z.; Huo, P.; Guo, L.; Prezhdo, O.V. Understanding Hematite Doping with Group IV Elements: A DFT+U Study. J. Phys. Chem. C 2015, 119, 26303–26310. [Google Scholar] [CrossRef]
- Cesar, I.; Sivula, K.; Kay, A.; Zbořil, R.; Grätzel, M. Influence of Feature Size, Film Thickness, and Silicon Doping on the Performance of Nanostructured Hematite Photoanodes for Solar Water Splitting. J. Phys. Chem. C 2008, 113, 772–782. [Google Scholar] [CrossRef]
- Chernyshova, I.; Hochella, M.F., Jr.; Madden, A.S. Size-dependent structural transformations of hematite nanoparticles. 1. Phase transition. Phys. Chem. Chem. Phys. 2007, 9, 1736. [Google Scholar] [CrossRef]
- Bersani, D.; Lottici, P.P.; Montenero, A. Micro-Raman investigation of iron oxide films and powders produced by sol-gel syntheses. J. Raman Spectrosc. 1999, 30, 355–360. [Google Scholar] [CrossRef]
- De Faria, D.; Lopes, F. Heated goethite and natural hematite: Can Raman spectroscopy be used to differentiate them? Vib. Spectrosc. 2007, 45, 117–121. [Google Scholar] [CrossRef]
- Akhavan, O.; Azimirad, R. Photocatalytic property of Fe2O3 nanograin chains coated by TiO2 nanolayer in visible light irradiation. Appl. Catal. A Gen. 2009, 369, 77–82. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Z.; Zheng, M.; Wang, T.; Yang, J.; Yuan, F.; Lu, X.; Liu, L.; Sun, D. Amorphous Fe2O3 nanoshells coated on carbonized bacterial cellulose nanofibers as a flexible anode for high-performance lithium ion batteries. J. Power Sources 2016, 307, 649–656. [Google Scholar] [CrossRef]
- Wang, X.; Tian, W.; Liu, D.; Zhi, C.; Bando, Y.; Golberg, D. Unusual formation of α-Fe2O3 hexagonal nanoplatelets in N-doped sandwiched graphene chamber for high-performance lithium-ions batteries. Nano Energy 2013, 2, 257–267. [Google Scholar] [CrossRef]
- Lee, M.H.; Park, J.H.; Han, H.S.; Song, H.J.; Cho, I.-S.; Noh, J.H.; Hong, K.S. Nanostructured Ti-doped hematite (α-Fe2O3) photoanodes for efficient photoelectrochemical water oxidation. Int. J. Hydrogen Energy 2014, 39, 17501–17507. [Google Scholar] [CrossRef]
- Spadaro, L.; Palella, A.; Arena, F. Copper-Iron-Zinc-Cerium oxide compositions as most suitable catalytic materials for the synthesis of green fuels via CO2 hydrogenation. Catal. Today 2020. [Google Scholar] [CrossRef]
- Lian, X.; Yang, X.; Liu, S.; Xu, Y.; Jiang, C.; Chen, J.; Wang, R. Enhanced photoelectrochemical performance of Ti-doped hematite thin films prepared by the sol–gel method. Appl. Surf. Sci. 2012, 258, 2307–2311. [Google Scholar] [CrossRef]
- Cui, H.J.; Cai, J.K.; Shi, J.W.; Yuan, B.; Ai, C.L.; Fu, M.L. Fabrication of 3D porous Mn doped α-Fe2O3 nanostructures for the removal of heavy metals from wastewater. RSC Adv. 2014, 4, 10176–10179. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, P.; Liu, J.; Lin, Y.; Cai, Y.; Ye, Y.; Liang, C. Facet-Dependent Selective Adsorption of Mn-Doped α-Fe2O3 Nanocrystals toward Heavy-Metal Ions. Chem. Mater. 2017, 29, 10198–10205. [Google Scholar] [CrossRef]
- XPS Reference Pages. Available online: http://www.xpsfitting.com/ (accessed on 20 January 2020).
- Zhao, S.; Wang, H.; Xiao, L.; Guo, N.; Zhao, D.; Yao, K.; Chen, N. High strain rate sensitivity of hardness in quinary Ti-Zr-Hf-Cu-Ni high entropy metallic glass thin films. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 94, 100–105. [Google Scholar] [CrossRef]
- Mai, L.; Wang, D.; Zhang, S.; Xie, Y.; Huang, C.; Zhang, Z. Synthesis and bactericidal ability of Ag/TiO2 composite films deposited on titanium plate. Appl. Surf. Sci. 2010, 257, 974–978. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Shen, X.; Peng, P.; Xiong, L.; Yu, Y. Octahedral Cu2O-modified TiO2 nanotube arrays for efficient photocatalytic reduction of CO2. Chin. J. Catal. 2015, 36, 2229–2236. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Kempiński, M.; Nowaczyk, G.; Jancelewicz, M.; Pavlenko, M.; Zaleski, K.; Jurga, S. Structural and XPS studies of PSi/TiO2 nanocomposites prepared by ALD and Ag-assisted chemical etching. Appl. Surf. Sci. 2015, 347, 777–783. [Google Scholar] [CrossRef]
- Huda, M.N.; Walsh, A.; Yan, Y.; Wei, S.-H.; Al-Jassim, M.M. Electronic, structural, and magnetic effects of 3d transition metals in hematite. J. Appl. Phys. 2010, 107, 123712. [Google Scholar] [CrossRef]
- Gu, X.; Yue, J.; Chen, L.; Liu, S.; Xu, H.; Yang, J.; Qian, Y.; Zhao, X. Coaxial MnO/N-doped carbon nanorods for advanced lithium-ion battery anodes. J. Mater. Chem. A 2015, 3, 1037–1041. [Google Scholar] [CrossRef]
- Raj, H.; Sil, A.; Pulagara, N.V. MnO anchored reduced graphene oxide nanocomposite for high energy applications of Li-ion batteries: The insight of charge-discharge process. Ceram. Int. 2019, 45, 14829–14841. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Gao, X.; Wang, B.; Liu, H.; Wu, H.; Liu, H.K.; Dou, S.X. Nitrogen-Doped Graphene Ribbon Assembled Core-Sheath MnO@Graphene Scrolls as Hierarchically Ordered 3D Porous Electrodes for Fast and Durable Lithium Storage. Adv. Funct. Mater. 2016, 26, 7754–7765. [Google Scholar] [CrossRef]
- Fazio, B.; Spadaro, L.; Trunfio, G.; Negro, J.; Arena, F. Raman scattering of MnOx-CeOx composite catalysts: Structural aspects and laser-heating effects. J. Raman Spectrosc. 2011, 42, 1583–1588. [Google Scholar] [CrossRef]
- Arena, F.; Di Chio, R.; Espro, C.; Palella, A.; Spadaro, L. A definitive assessment of the CO oxidation pattern of a nanocomposite MnCeOx catalyst. React. Chem. Eng. 2018, 3, 293–300. [Google Scholar] [CrossRef]
- Thermo scientific XPS. Available online: https://xpssimplified.com/elements/manganese.php (accessed on 31 July 2020).
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.; Lau, L.W.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Arena, F.; Di Chio, R.; Espro, C.; Fazio, B.; Palella, A.; Spadaro, L. A New Class of MnCeOx Materials for the Catalytic Gas Exhausts Emission Control: A Study of the CO Model Compound Oxidation. Top. Catal. 2018, 62, 259–265. [Google Scholar] [CrossRef]
- Gurudayal; Chiam, S.Y.; Kumar, M.H.; Bassi, P.S.; Seng, H.L.; Barber, J.; Wong, L.H. Improving the Efficiency of Hematite Nanorods for Photoelectrochemical Water Splitting by Doping with Manganese. ACS Appl. Mater. Interfaces 2014, 6, 5852–5859. [Google Scholar] [CrossRef] [PubMed]
- Gil Cha, H.; Noh, H.S.; Kang, M.J.; Kang, Y.S. Photocatalysis: Progress using manganese-doped hematite nanocrystals. New J. Chem. 2013, 37, 4004–4009. [Google Scholar] [CrossRef]

| Samples Code | rGO Content (wt %) | α-Fe2O3(:D) Content (wt %) | OC/C | d (nm) a | ||
|---|---|---|---|---|---|---|
| Nominal | Measured (TGA) | Measured (TGA) | Measured (XPS) | XPS | XRPD | |
| α-Fe2O3@rGO-50 | 50 | 46.7 | 53.3 | 59.4 | 0.319 | 27.2 |
| α-Fe2O3:Ti@rGO-50 | 50 | 48.3 | 51.7 | 55.8 | 0.323 | 17.3 |
| α-Fe2O3@rGO-30 | 30 | 33.5 | 66.5 | 28.7 | ||
| α-Fe2O3:Ti@rGO-30 | 30 | 41.2 | 58.8 | 68.7 | 0.285 | 17.8 |
| α-Fe2O3:Mn@rGO-30 | 30 | 38.9 | 61.1 | 66.5 | 0.091 | 29.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modafferi, V.; Triolo, C.; Fiore, M.; Palella, A.; Spadaro, L.; Pianta, N.; Ruffo, R.; Patanè, S.; Santangelo, S.; Musolino, M.G. Effect of Hematite Doping with Aliovalent Impurities on the Electrochemical Performance of α-Fe2O3@rGO-Based Anodes in Sodium-Ion Batteries. Nanomaterials 2020, 10, 1588. https://doi.org/10.3390/nano10081588
Modafferi V, Triolo C, Fiore M, Palella A, Spadaro L, Pianta N, Ruffo R, Patanè S, Santangelo S, Musolino MG. Effect of Hematite Doping with Aliovalent Impurities on the Electrochemical Performance of α-Fe2O3@rGO-Based Anodes in Sodium-Ion Batteries. Nanomaterials. 2020; 10(8):1588. https://doi.org/10.3390/nano10081588
Chicago/Turabian StyleModafferi, Vincenza, Claudia Triolo, Michele Fiore, Alessandra Palella, Lorenzo Spadaro, Nicolò Pianta, Riccardo Ruffo, Salvatore Patanè, Saveria Santangelo, and Maria Grazia Musolino. 2020. "Effect of Hematite Doping with Aliovalent Impurities on the Electrochemical Performance of α-Fe2O3@rGO-Based Anodes in Sodium-Ion Batteries" Nanomaterials 10, no. 8: 1588. https://doi.org/10.3390/nano10081588
APA StyleModafferi, V., Triolo, C., Fiore, M., Palella, A., Spadaro, L., Pianta, N., Ruffo, R., Patanè, S., Santangelo, S., & Musolino, M. G. (2020). Effect of Hematite Doping with Aliovalent Impurities on the Electrochemical Performance of α-Fe2O3@rGO-Based Anodes in Sodium-Ion Batteries. Nanomaterials, 10(8), 1588. https://doi.org/10.3390/nano10081588