Simultaneous Analysis of Hydroquinone, Arbutin, and Ascorbyl Glucoside Using a Nanocomposite of Ag@AgCl Nanoparticles, Ag2S Nanoparticles, Multiwall Carbon Nanotubes, and Chitosan
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Synthesis of Chitosan Stabilized Ag2S NPs
2.3. Synthesis of the Ag@AgCl/Ag2S/CNTs on the Glassy Carbon Electrode
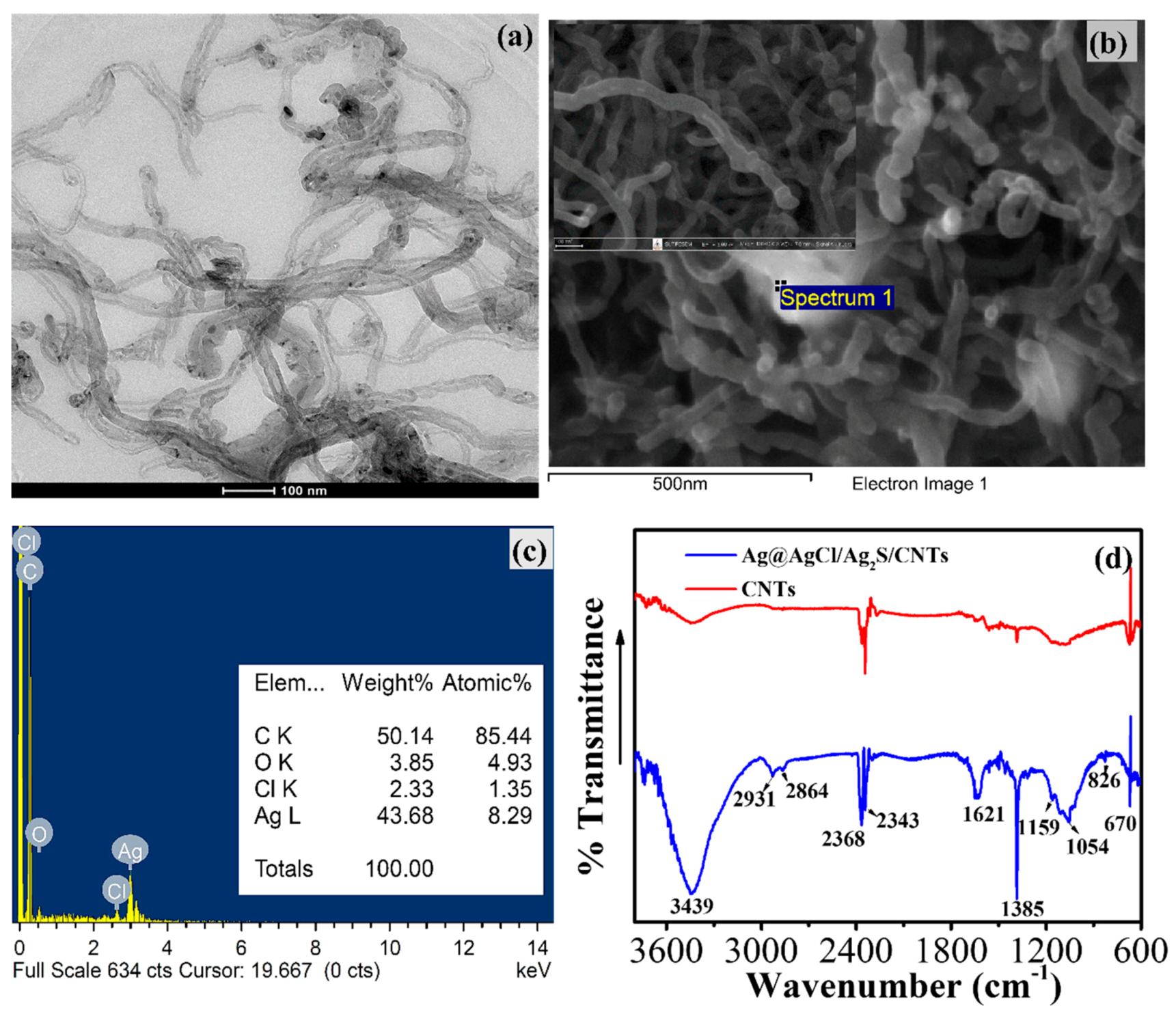
2.4. Characterization
2.5. Sample Preparation
2.6. Electrochemical Measurements
2.7. High-Performance Liquid Chromatography Measurements
3. Results and Discussion
3.1. Silver Sulfide Stabilized by Chitosan
3.2. Morphological Characterization of the Modified GCE
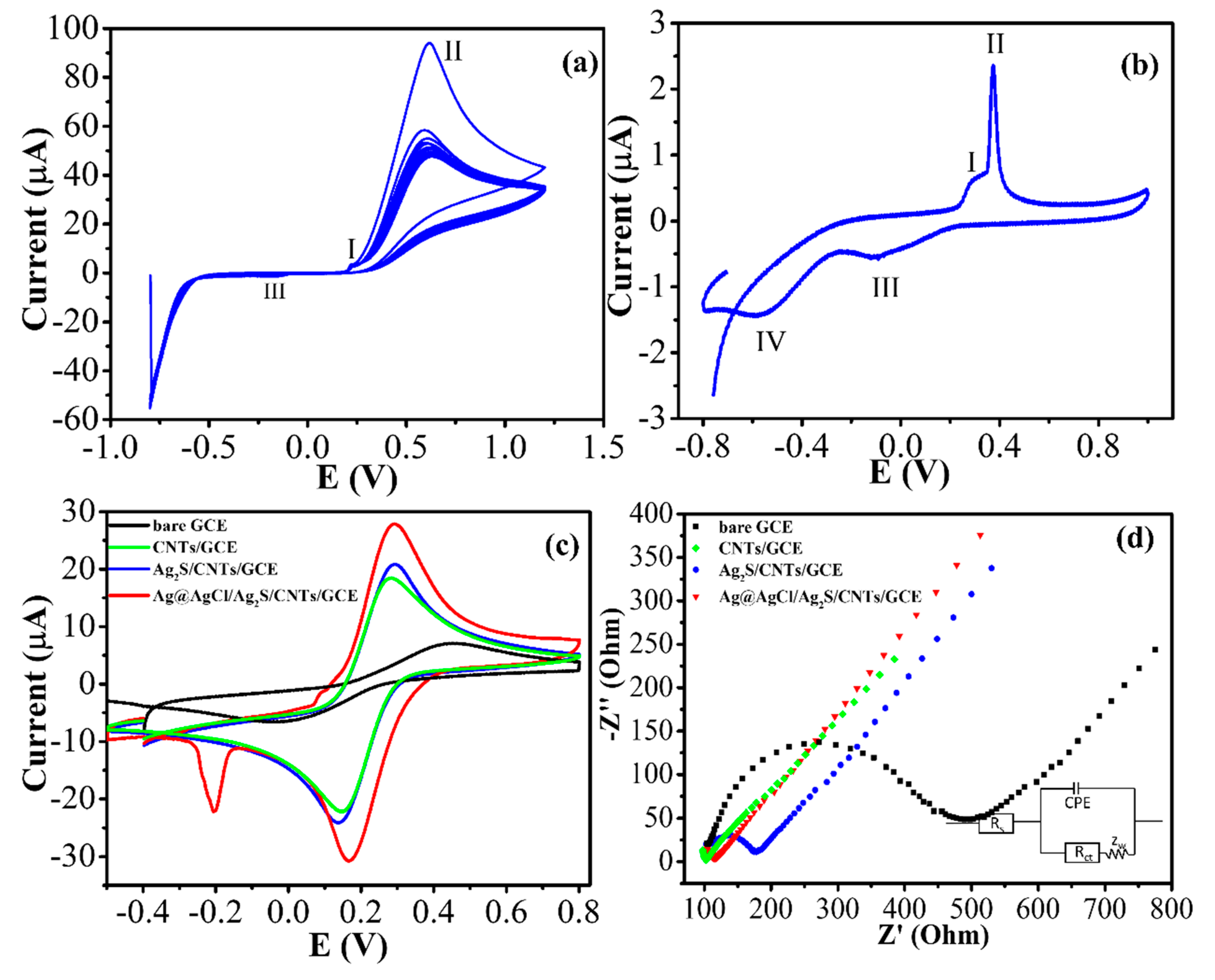
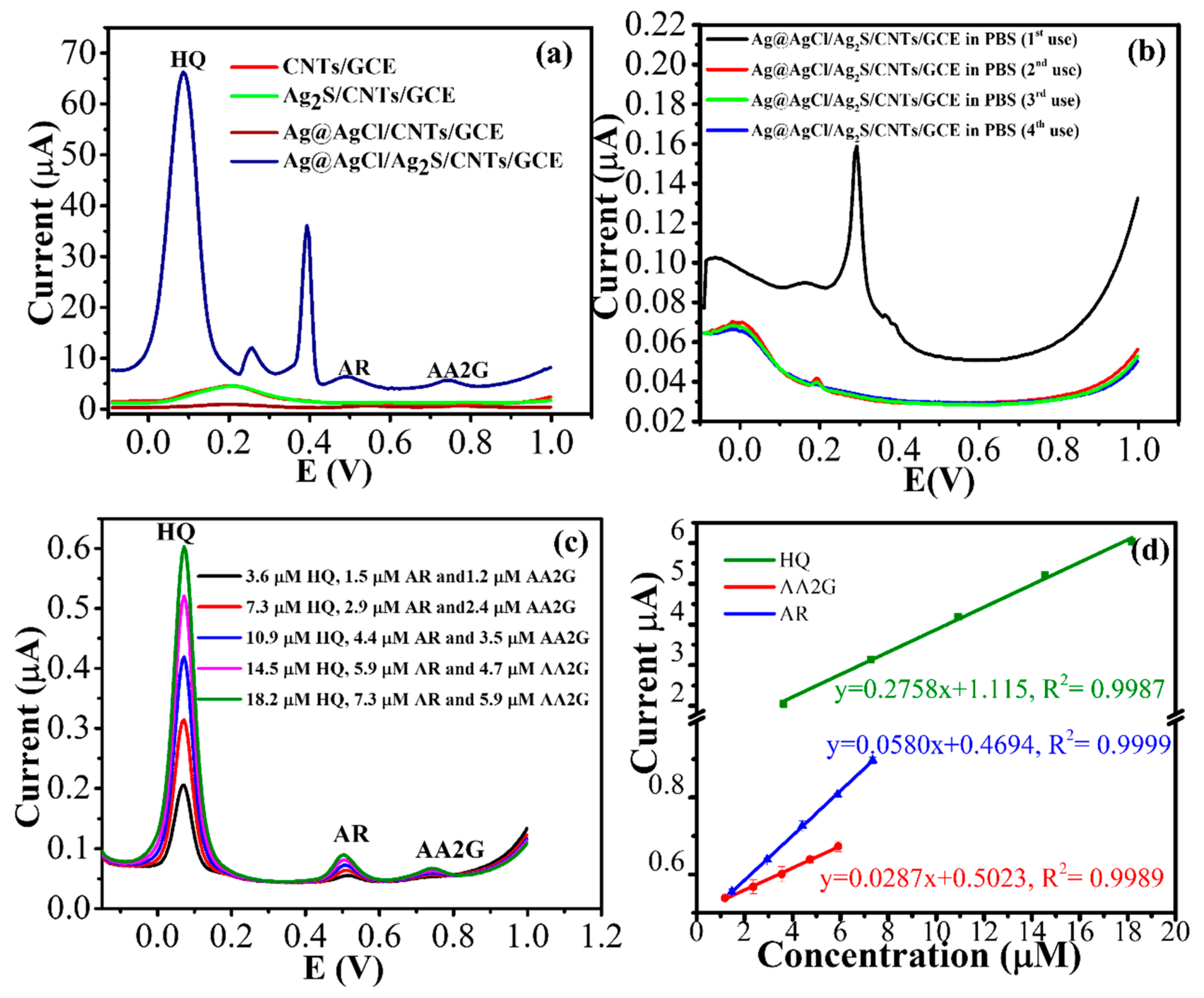
3.3. Electrochemical Properties of the Modified Electrode
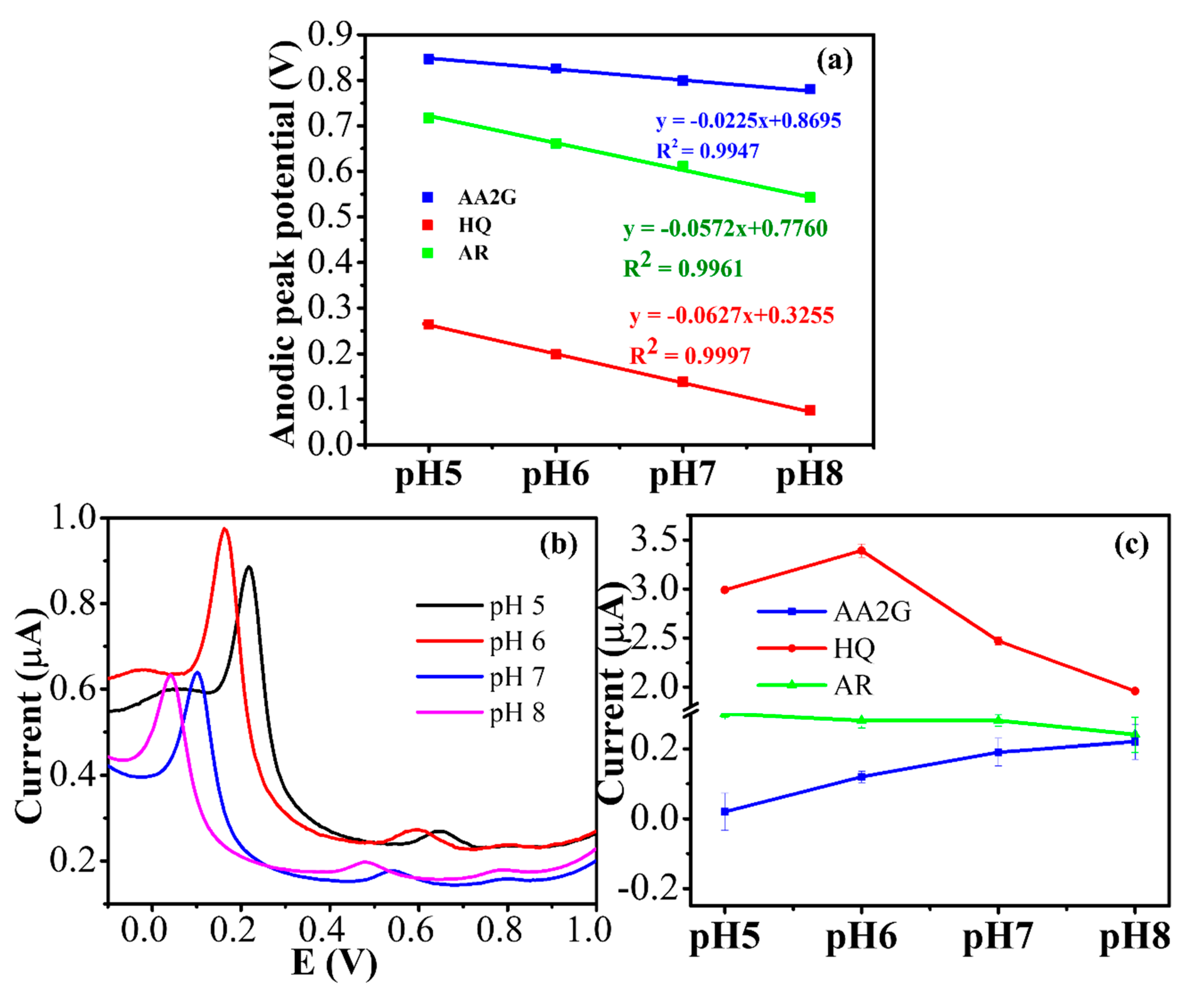
3.4. Effect of pH
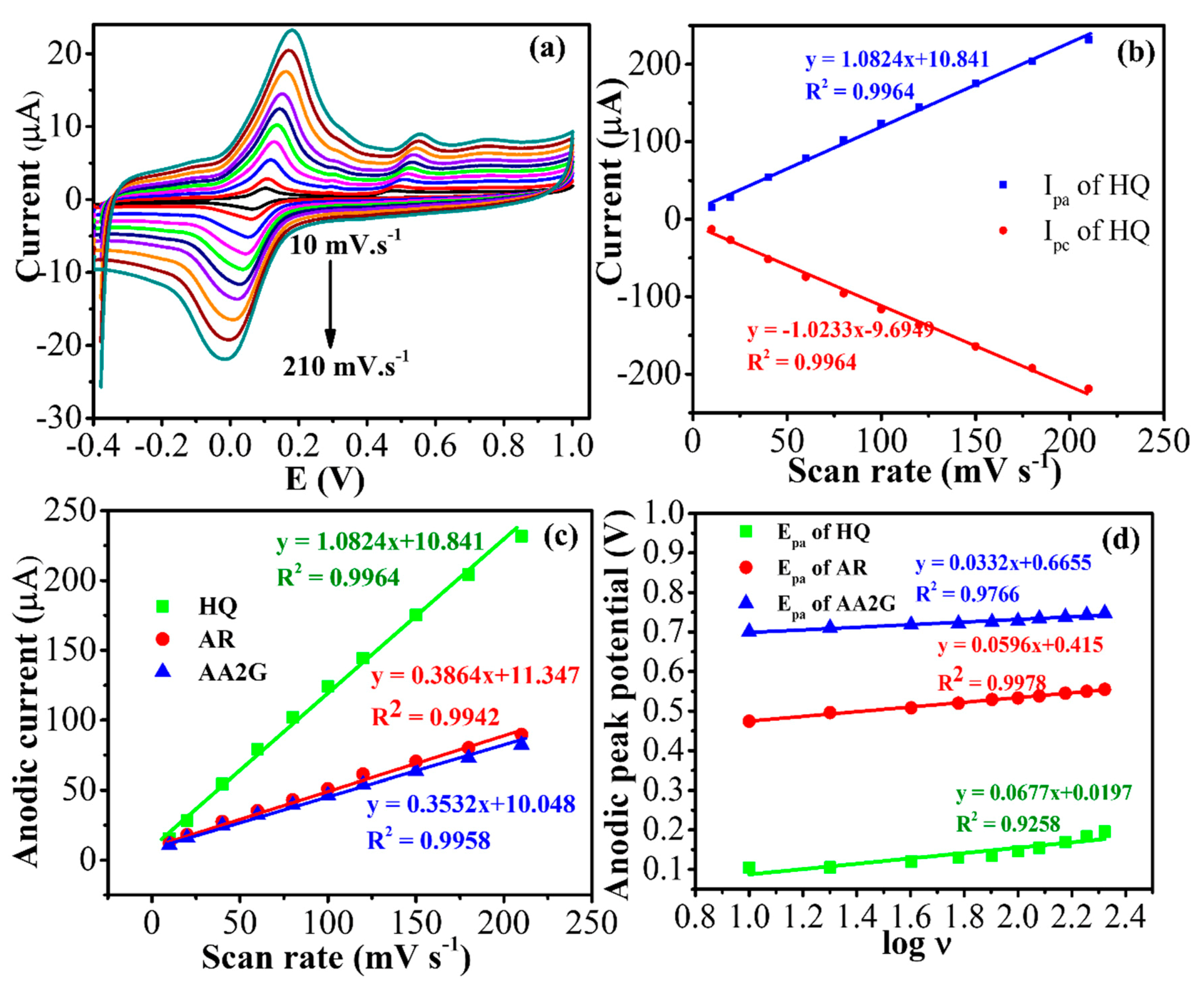
3.5. Electrocatalysis of HQ, AR, and AA2G at the Ag@AgCl/Ag2S/CNTs/GCE
3.6. Analytical Performance of the Modified Electrode
3.7. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kooyers, T.; Westerhof, W. Toxicology and health risks of hydroquinone in skin lightening formulations. J. Eur. Acad. Dermatol. Venereol. 2005, 20, 777–780. [Google Scholar] [CrossRef]
- Bang, S.-H.; Han, S.-J.; Kim, D.-H. Hydrolysis of arbutin to hydroquinone by human skin bacteria and its effect on antioxidant activity. J. Cosmet. Dermatol. 2008, 7, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-S.; Kim, H.-T.; Kim, M.-G.; Oh, M.-S.; Hong, S.-R.; Yoon, M.-H.; Cho, S.-M.; Shin, H.-C.; Shim, J.-H.; Ramadan, A.; et al. Simultaneous determination of water-soluble whitening ingredients and adenosine in different cosmetic formulations by high-performance liquid chromatography coupled with photodiode array detection. Int. J. Cosmet. Sci. 2016, 38, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Harsini, M.; Untari, U.; Fitriany, E.; Farida, A.N.; Fahmi, M.Z.; Sakti, S.C.W.; Pari, G. Voltammetric analysis of hydroquinone in skin whitening cosmetic using ferrocene modified carbon paste electrode. Rasayan J. Chem. 2019, 12, 2296–2305. [Google Scholar] [CrossRef]
- Shih, Y.; Zen, J.-M. Flow injection analysis of ascorbyl glucoside in cosmetics by a disposable printed-circuit board waste modified electrode. J. Chin. Chem. Soc. 2006, 53, 857–862. [Google Scholar] [CrossRef]
- Wu, F.; Huang, T.; Hu, Y.; Yang, X.; Ouyang, Y.; Xie, Q. Differential pulse voltammetric simultaneous determination of ascorbic acid, dopamine and uric acid on a glassy carbon electrode modified with electroreduced graphene oxide and imidazolium groups. Microchim. Acta 2016, 183, 2539–2546. [Google Scholar] [CrossRef]
- Mohammed Modawe Alshik Edris, N.; Abdullah, J.; Kamaruzaman, S.; Sulaiman, Y. Voltammetric determination of hydroquinone, catechol, and resorcinol by using a glassy carbon electrode modified with electrochemically reduced graphene oxide-poly (Eriochrome black T) and gold nanoparticles. Microchim. Acta 2019, 186, 261. [Google Scholar] [CrossRef]
- Xue, J.; Liu, J.; Liu, Y.; Li, H.; Wang, Y.; Sun, D.; Wang, W.; Huang, L.; Tang, J. Recent advances in synthetic methods and applications of Ag2S-based heterostructure photocatalysts. J. Mater. Chem. C 2019, 7, 3988–4003. [Google Scholar] [CrossRef]
- Sadovnikov, S.I.; Gusev, A.I. Structure and properties of Ag2S/Ag semiconductor/metal heteronanostructure. J. Nanotechnol. Mater. Sci. 2016, 3, 1–10. [Google Scholar] [CrossRef][Green Version]
- Schürch, D.; Currao, A.; Sarkar, S.; Hodes, G.; Calzaferri, G. The Silver chloride photoanode in photoelectrochemical water splitting. J. Phys. Chem. B 2002, 106, 12764–12775. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, B.; Pareek, V.; Wang, S.; Li, X.; Liu, L.; Liu, S. Sustainable synthesis of highly efficient sunlight-driven Ag embedded AgCl photocatalysts. RSC Adv. 2015, 5, 80488–80495. [Google Scholar] [CrossRef]
- Kryukov, A.I.; Stroyuk, A.L.; Zin’chuk, N.N.; Korzhak, A.V.; Kuchmii, S.Y. Optical and catalytic properties of Ag2S nanoparticles. J. Mol. Catal. A Chem. 2004, 221, 209–221. [Google Scholar] [CrossRef]
- Geng, Z.; Wang, X.; Guo, X.; Zhang, Z.; Chen, Y.; Wang, Y. Electrodeposition of chitosan based on coordination with metal ions in situ-generated by electrochemical oxidation. J. Mater. Chem. B 2016, 4, 3331–3338. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Ahmad, R.; Mousa, H.M.; Kim, I.-G.; Kim, J.I.; Neupane, M.P.; Park, C.H.; Kim, C.S. High-performance glucose biosensor based on chitosan-glucose oxidase immobilized polypyrrole/Nafion/functionalized multi-walled carbon nanotubes bio-nanohybrid film. J. Colloid Interface Sci. 2016, 482, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Sadovnikov, S.I.; Kuznetsova, Y.V.; Rempel, A.A. Ag2S silver sulfide nanoparticles and colloidal solutions: Synthesis and properties. Nano Struct. Nano Objects 2016, 7, 81–91. [Google Scholar] [CrossRef]
- Gao, F.; Lu, Q.; Zhao, D. Controllable assembly of ordered semiconductor Ag2S nanostructures. Nano Lett. 2003, 3, 85–88. [Google Scholar] [CrossRef]
- Her, S.-C.; Lai, C.-Y. Dynamic behavior of nanocomposites reinforced with multi-walled carbon nanotubes (MWCNTs). Materials 2013, 6, 2274–2284. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Cinteza, L.O.; Scomoroscenco, C.; Voicu, S.N.; Nistor, C.L.; Nitu, S.G.; Trica, B.; Jecu, M.L.; Petcu, C. Chitosan-stabilized Ag nanoparticles with superior biocompatibility and their synergistic antibacterial effect in mixtures with essential oils. Nanomaterials 2018, 8, 826. [Google Scholar] [CrossRef]
- Butwong, N.; Khajonklin, J.; Thongbor, A.; Luong, J.H.T. Electrochemical sensing of histamine using a glassy carbon electrode modified with multiwalled carbon nanotubes decorated with Ag-Ag2O nanoparticles. Microchim. Acta 2019, 186, 714. [Google Scholar] [CrossRef]
- Shaban, M.; Kholidy, I.; Ahmed, G.M.; Negem, M.; Abd El-Salam, H.M. Cyclic voltammetry growth and characterization of Sn–Ag alloys of different nanomorphologies and compositions for efficient hydrogen evolution in alkaline solutions. RSC Adv. 2019, 9, 22389–22400. [Google Scholar] [CrossRef]
- Larpant, N.; Pham, A.D.; Shafaat, A.; Gonzalez-Martinez, J.F.; Sotres, J.; Sjöholm, J.; Laiwattanapaisal, W.; Faridbod, F.; Ganjali, M.R.; Arnebrant, T.; et al. Sensing by wireless reading Ag/AgCl redox conversion on RFID tag: Universal, battery-less biosensor design. Sci. Rep. 2019, 9, 12948. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.-S.; Jia, M.-M.; Fan, J.-Q.; Qin, F.-M.; Yu, J.-Q. Silver nanoparticle impregnated porous carbon as a nonenzymatic hydrogen peroxide sensor. Int. J. Electrochem. Sci. 2016, 11, 5781–5791. [Google Scholar] [CrossRef]
- Sidambaram, P.; Colleran, J. Nanomole silver detection in chloride-free phosphate buffer using platinum and gold micro- and nanoelectrodes. J. Electrochem. Soc. 2019, 166, B532–B541. [Google Scholar] [CrossRef]
- Qin, X.; Wang, H.; Miao, Z.; Wang, X.; Fang, Y.; Chen, Q.; Shao, X. Synthesis of silver nanowires and their applications in the electrochemical detection of halide. Talanta 2011, 84, 673–678. [Google Scholar] [CrossRef]
- Jurczakowski, R.; Hitz, C.; Lasia, A. Impedance of porous gold electrodes in the presence of electroactive species. J. Electroanal. Chem. 2005, 582, 85–96. [Google Scholar] [CrossRef]
- Ngai, K.S.; Tan, W.T.; Zainal, Z.; Zawawi, R.B.M.; Zidan, M. Electrochemical oxidation of ascorbic acid mediated by single walled carbon nanotube/tungsten oxide nanoparticles modified glassy carbon electrode. Int. J. Electrochem. Sci. 2012, 7, 4210–4222. [Google Scholar]
- Zeng, B.; Wei, S.; Xiao, F.; Zhao, F. Voltammetric behavior and determination of rutin at a single-walled carbon nanotubes modified gold electrode. Sens. Actuators B Chem. 2006, 115, 240–246. [Google Scholar] [CrossRef]
- Song, D.; Xia, J.; Zhang, F.; Bi, S.; Xiang, W.; Wang, Z.; Xia, L.; Xia, Y.; Li, Y.; Xia, L. Multiwall carbon nanotubes-poly(diallyldimethylammonium chloride)-graphene hybrid composite film for simultaneous determination of catechol and hydroquinone. Sens. Actuators B Chem. 2015, 206, 111–118. [Google Scholar] [CrossRef]
- Mo, F.; Tian, X.; Tian, D.; Wang, Z. Synthesis and evaluation of chitosan-vitamin C complex. Indian J. Pharm. Sci. 2009, 71, 371. [Google Scholar] [CrossRef] [PubMed]
- Sharp, M.; Petersson, M.; Edström, K.A. Comparison of the charge transfer kinetics between platinum and ferrocene in solution and in the surface attached state. J. Electroanal. Chem. Interf. Electrochem. 1980, 109, 271–288. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Zhao, L.; Rong, L.; Lan, S.; Sun, H.; Zhang, H.; Yang, B. Hydroquinone-assisted synthesis of branched Au–Ag nanoparticles with polydopamine coating as highly efficient photothermal agents. ACS Appl. Mater. Inter. 2015, 7, 11613–11623. [Google Scholar] [CrossRef] [PubMed]
- Shahamirifard, S.A.; Ghaedi, M. A new electrochemical sensor for simultaneous determination of arbutin and vitamin C based on hydroxyapatite-ZnO-Pd nanoparticles modified carbon paste electrode. Biosens. Bioelectron. 2019, 141, 111474. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zhang, J.; Xiao, Y.; Zhao, Z.; Zhang, M.; Wang, L.; Zhang, X.; Zhou, C. High-efficiency adsorption of and competition between phenol and hydroquinone in aqueous solution on highly cationic amino-poly(vinylamine)-functionalized GO-(o-MWCNTs) magnetic nanohybrids. Chem. Eng. 2020, 389, 124223. [Google Scholar] [CrossRef]
- Vittal, R.; Gomathi, H.; Kim, K.-J. Beneficial role of surfactants in electrochemistry and in the modification of electrodes. Adv. Colloid Interface Sci. 2006, 119, 55–68. [Google Scholar] [CrossRef] [PubMed]
| Electrode | Analyte | LOD (µM) | Potential (V) | Linear Range (μM) | Method | References |
|---|---|---|---|---|---|---|
| Carbon/ferrocene paste | HQ | 0.06 | 0.213 | 0.20–10 | LSV | [4] |
| AR | - | 0.660 | ||||
| Cu PCB/SPE 1 | AA2G | 0.73 | 0.6 | 7.39–473 | CV | [5] |
| ERGO-pEBT/AuNPs 2 | HQ | 0.015 | 0.166 | 0.52–31.4 | DPV | [7] |
| CC 4 | 0.008 | 0.277 | 1.44–31.2 | |||
| RC 5 | 0.039 | 0.660 | 3.8–72.2 | |||
| HAP-ZnO-Pd NPs/CPE 3 | AR | 0.086 | 0.50 | 0.12–56 | DPV | [33] |
| VC 6 | 0.019 | 0.7 | 0.12–55.36 | |||
| Ag@AgCl/Ag2S/CNTs/GCE | HQ | 0.4 | 0.05 | 0.91–27.2 | DPV | This work |
| AR | 0.1 | 0.5 | 0.73–14.7 | |||
| AA2G | 0.25 | 0.7 | 1.18–11.8 |
| Concentration Found (mg⋅L−1) HPLC | Concentration Found (mg⋅L−1) DPV | |
|---|---|---|
| HQ | ND | ND |
| AR | 2.1 (±0.3) × 104 | 2.1 (±0.1) × 104 |
| AA2G | 12.5 (±1.5) | 40.2 (±0.7) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butwong, N.; Kunawong, T.; Luong, J.H.T. Simultaneous Analysis of Hydroquinone, Arbutin, and Ascorbyl Glucoside Using a Nanocomposite of Ag@AgCl Nanoparticles, Ag2S Nanoparticles, Multiwall Carbon Nanotubes, and Chitosan. Nanomaterials 2020, 10, 1583. https://doi.org/10.3390/nano10081583
Butwong N, Kunawong T, Luong JHT. Simultaneous Analysis of Hydroquinone, Arbutin, and Ascorbyl Glucoside Using a Nanocomposite of Ag@AgCl Nanoparticles, Ag2S Nanoparticles, Multiwall Carbon Nanotubes, and Chitosan. Nanomaterials. 2020; 10(8):1583. https://doi.org/10.3390/nano10081583
Chicago/Turabian StyleButwong, Nutthaya, Thidarat Kunawong, and John H. T. Luong. 2020. "Simultaneous Analysis of Hydroquinone, Arbutin, and Ascorbyl Glucoside Using a Nanocomposite of Ag@AgCl Nanoparticles, Ag2S Nanoparticles, Multiwall Carbon Nanotubes, and Chitosan" Nanomaterials 10, no. 8: 1583. https://doi.org/10.3390/nano10081583
APA StyleButwong, N., Kunawong, T., & Luong, J. H. T. (2020). Simultaneous Analysis of Hydroquinone, Arbutin, and Ascorbyl Glucoside Using a Nanocomposite of Ag@AgCl Nanoparticles, Ag2S Nanoparticles, Multiwall Carbon Nanotubes, and Chitosan. Nanomaterials, 10(8), 1583. https://doi.org/10.3390/nano10081583






