Surface Functionalization of Biomedical Ti-6Al-7Nb Alloy by Liquid Metal Dealloying
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
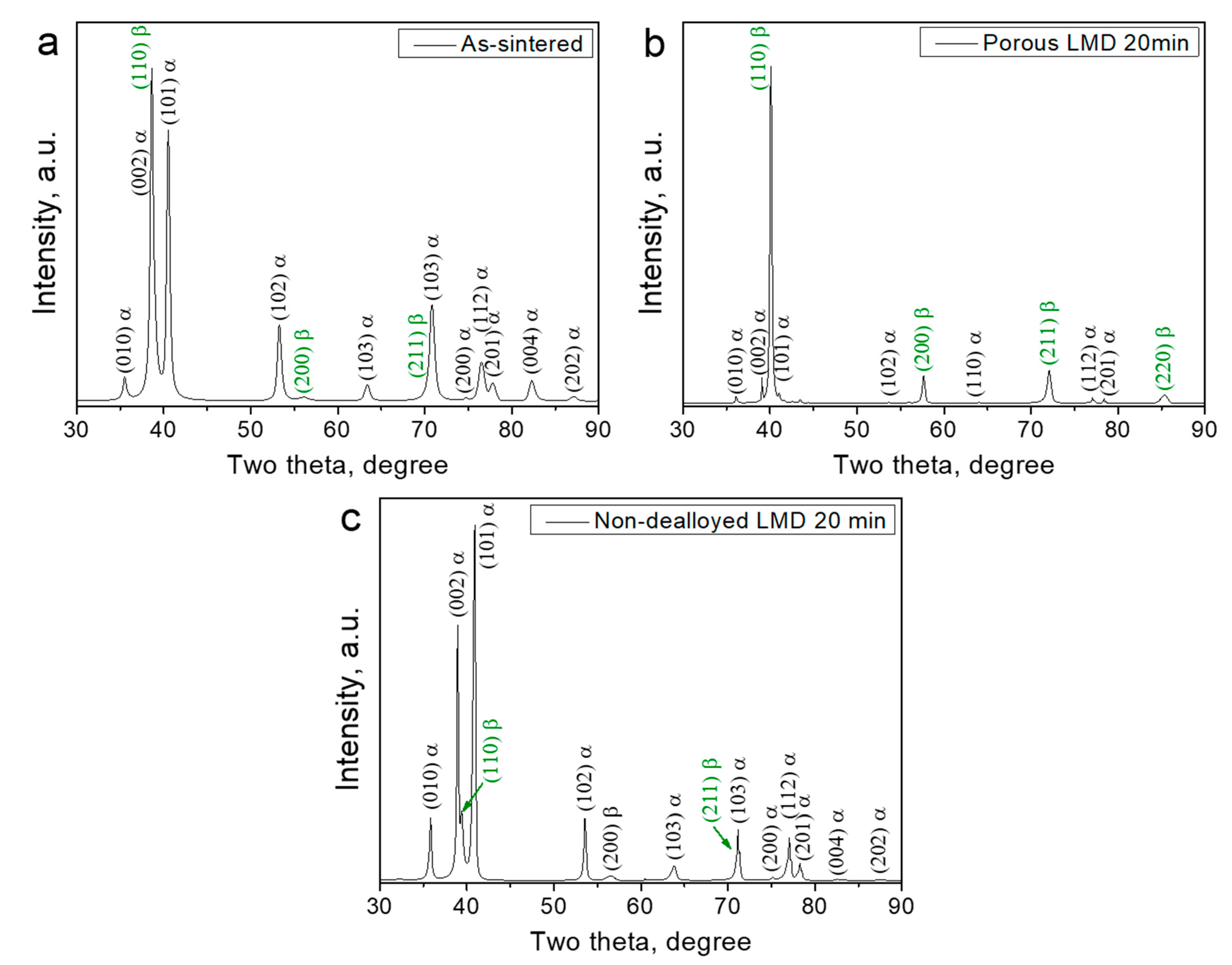
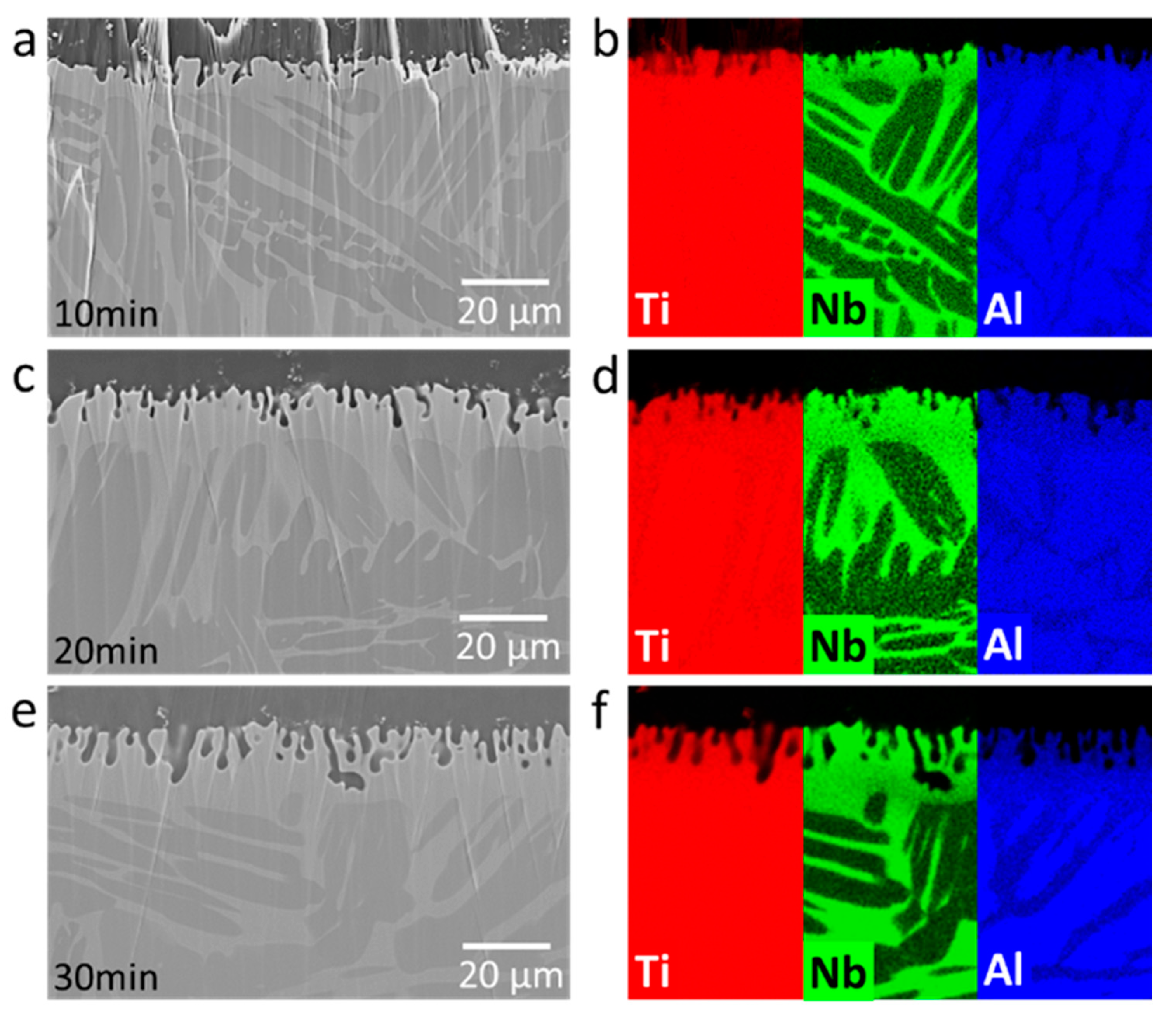
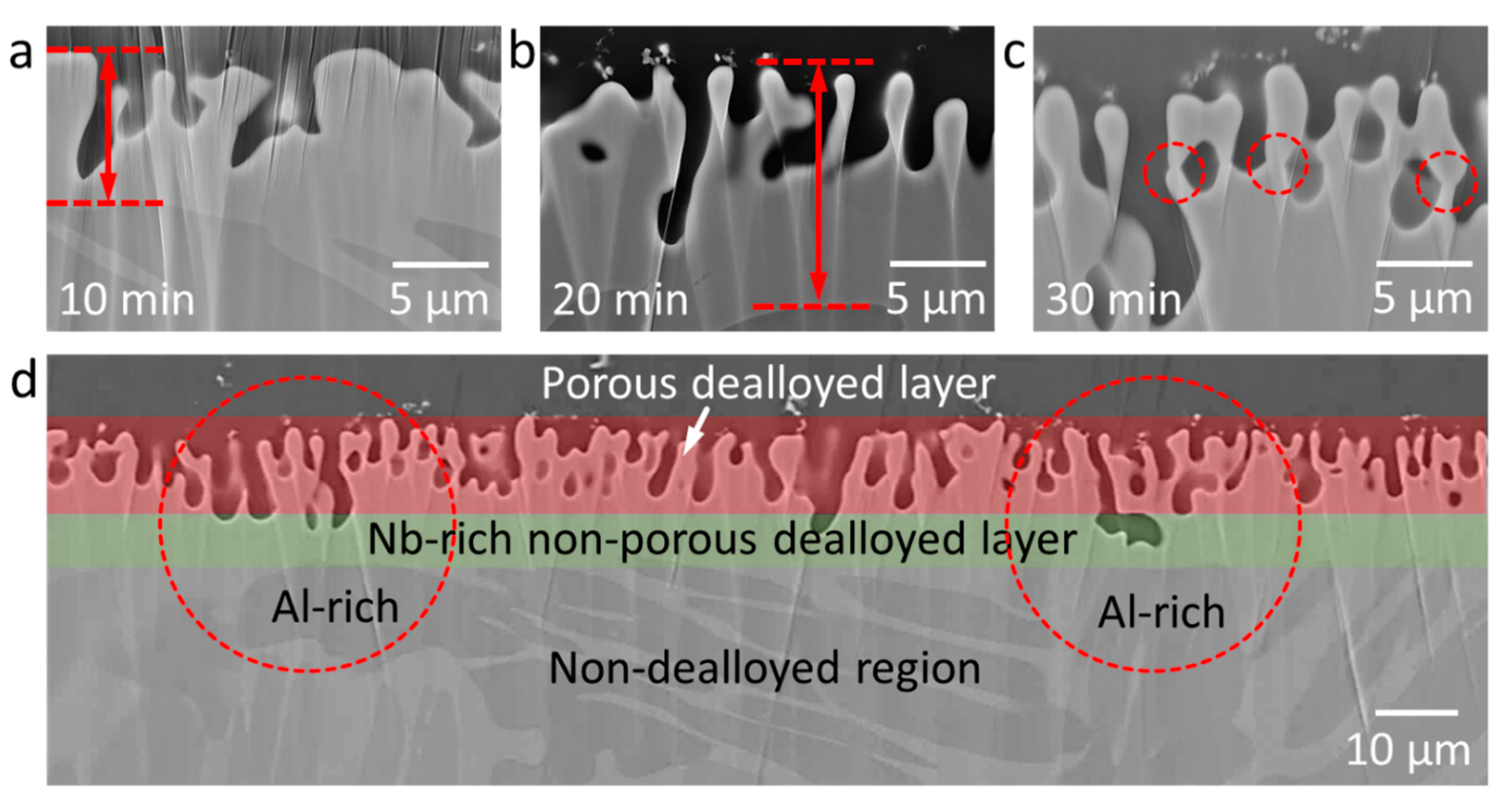
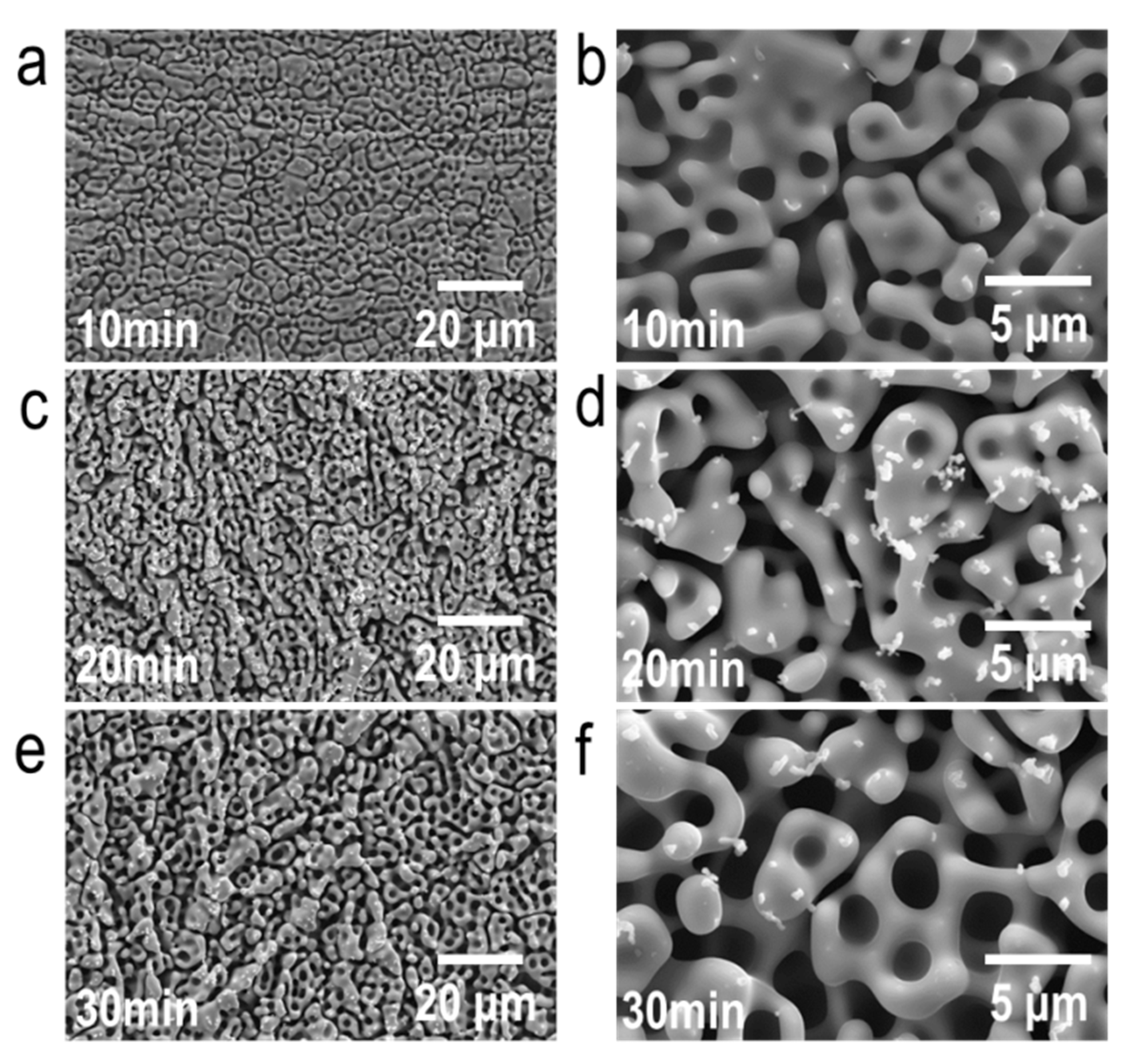
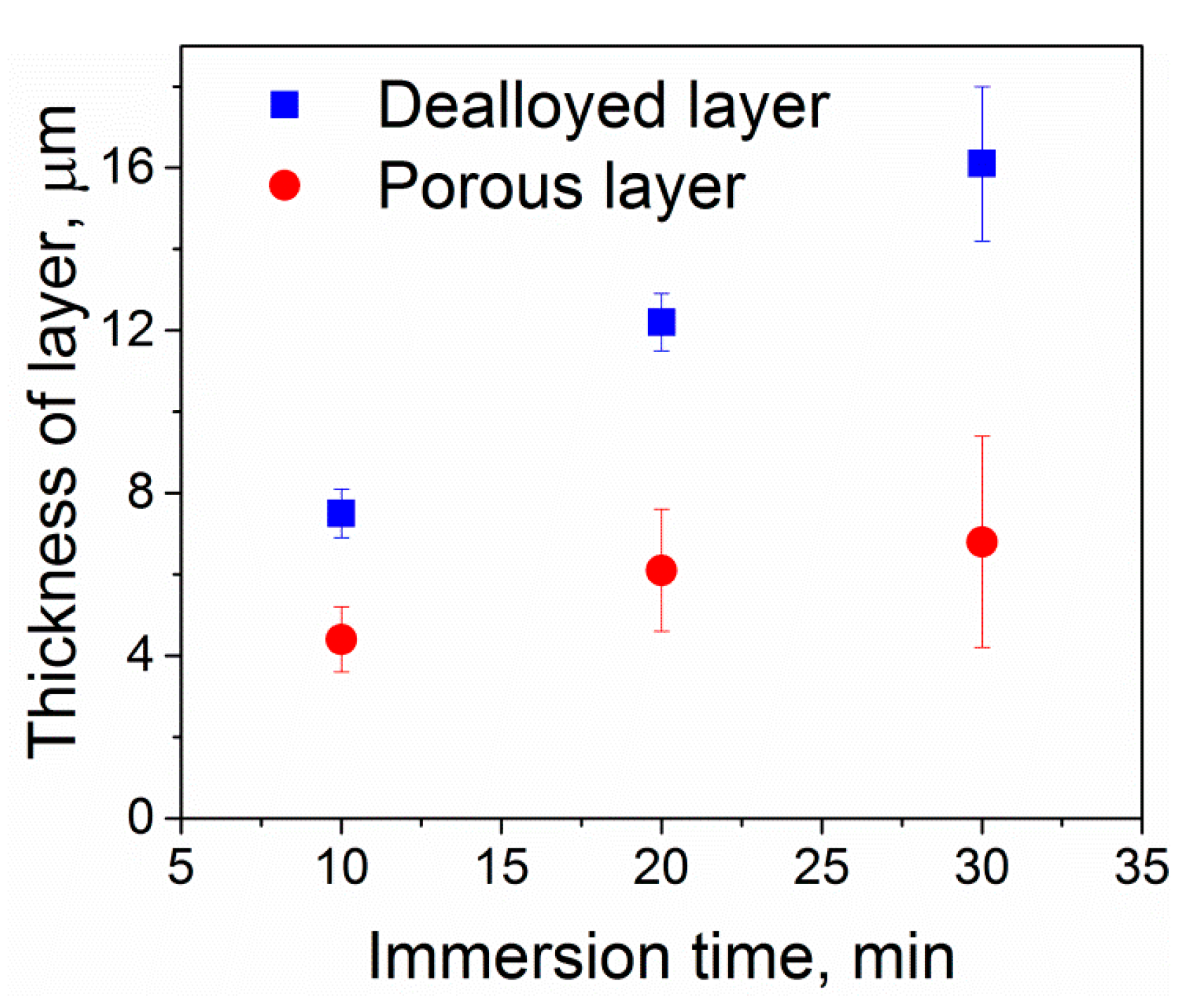
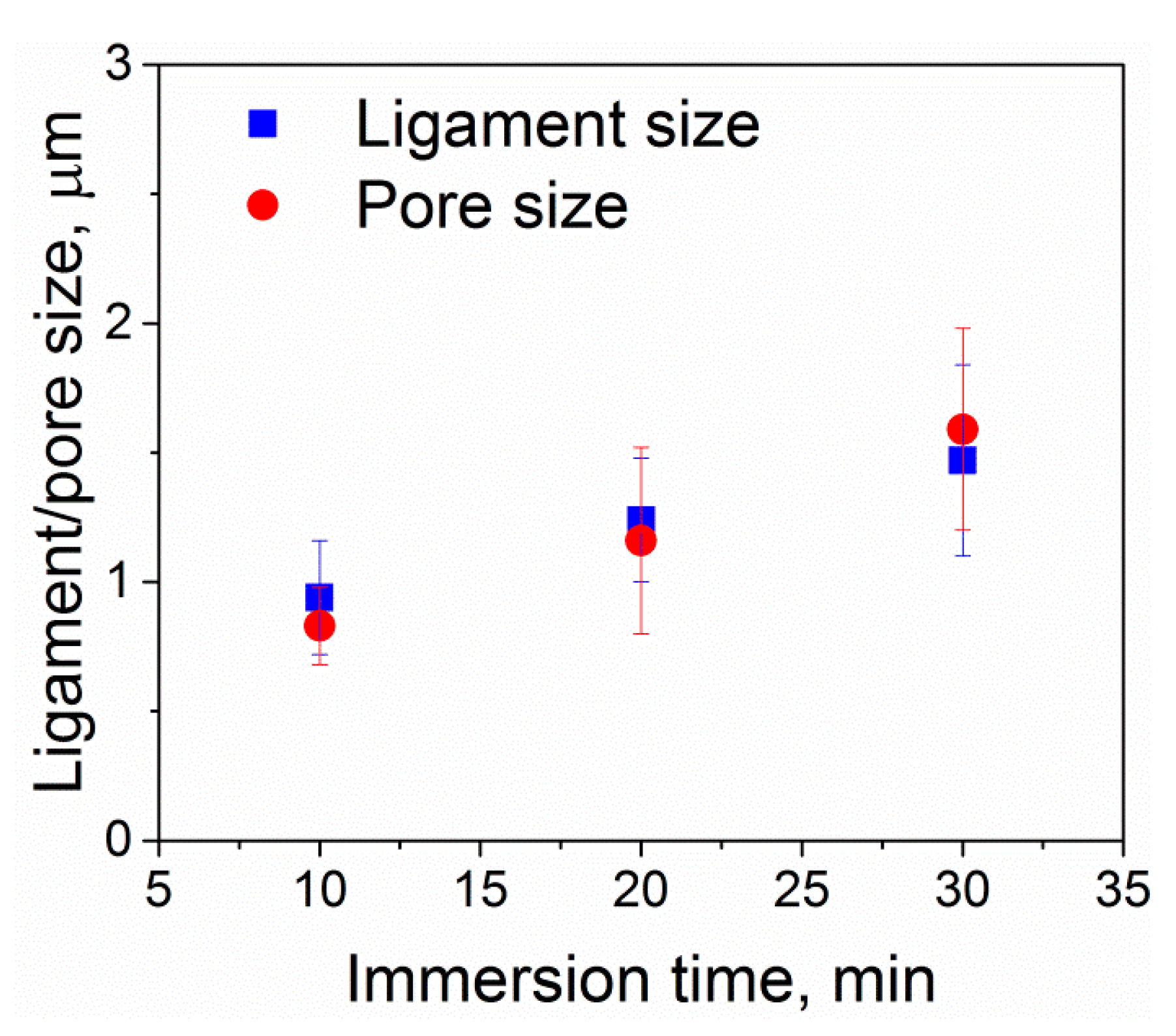
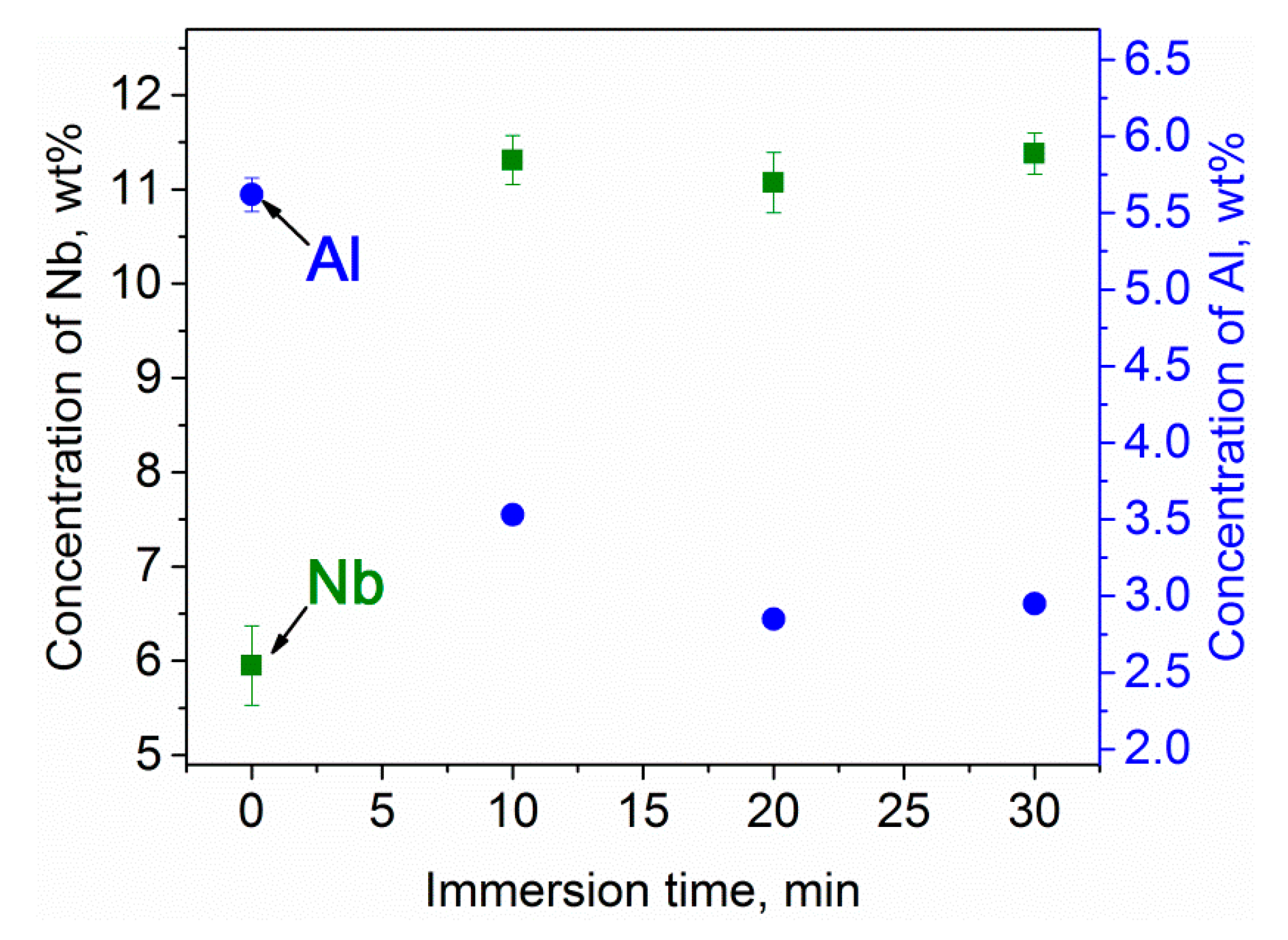
3.1. Structural Investigations
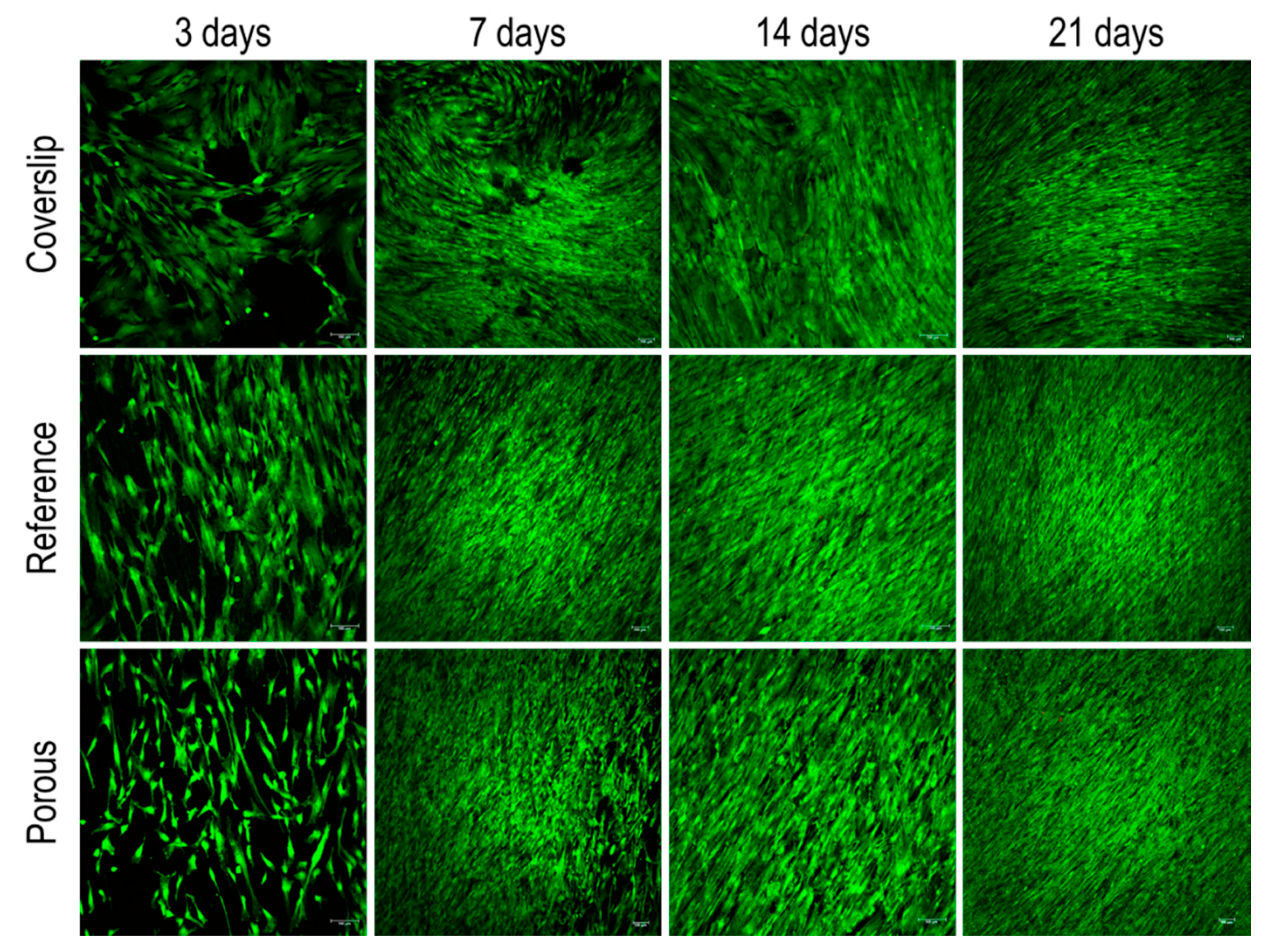
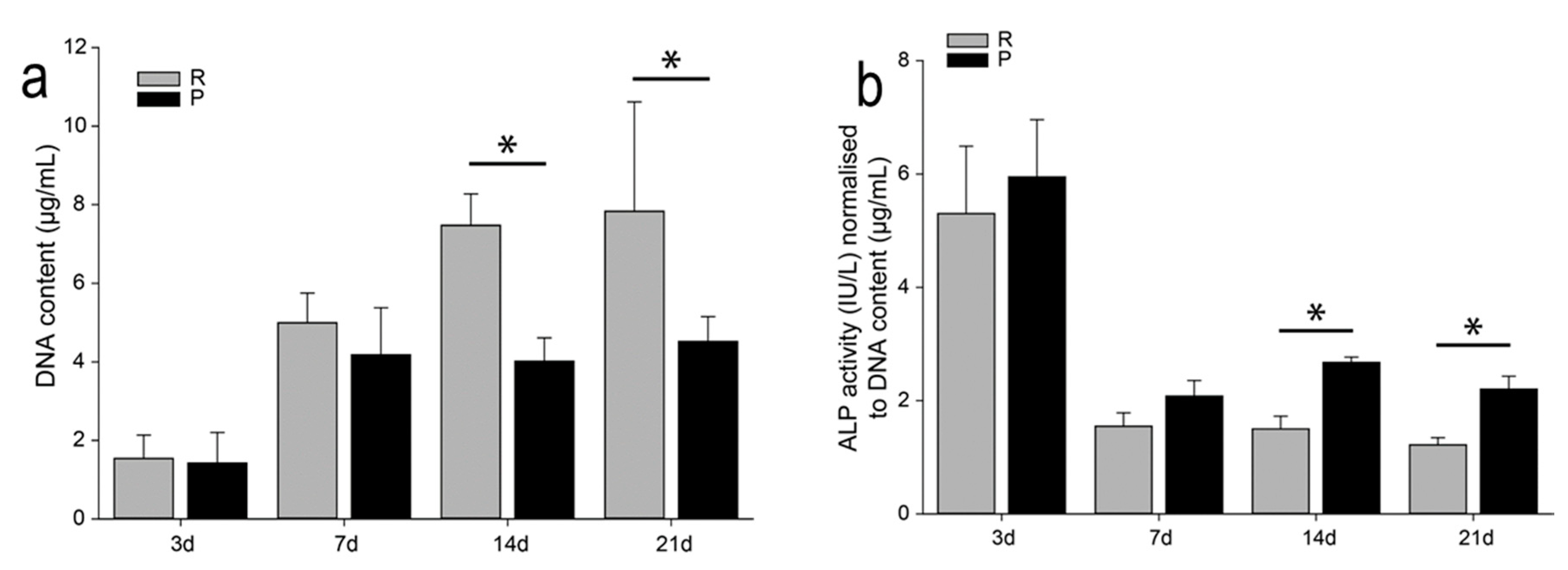
3.2. Cytocompatibility Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Okulov, I.V.; Wendrock, H.; Volegov, A.S.; Attar, H.; Kühn, U.; Skrotzki, W.; Eckert, J. High strength beta titanium alloys: New design approach. Mater. Sci. Eng. A 2015, 628, 297–302. [Google Scholar] [CrossRef]
- Okulov, I.V.; Bönisch, M.; Okulov, A.V.; Volegov, A.S.; Attar, H.; Ehtemam-Haghighi, S.; Calin, M.; Wang, Z.; Hohenwarter, A.; Kaban, I.; et al. Phase formation, microstructure and deformation behavior of heavily alloyed TiNb- and TiV-based titanium alloys. Mater. Sci. Eng. A 2018, 733, 80–86. [Google Scholar] [CrossRef]
- Niinomi, M.; Hattori, T.; Morikawa, K.; Kasuga, T.; Suzuki, A.; Fukui, H.; Niwa, S. Development of Low Rigidity Beta-type Titanium Alloy for Biomedical Applications. Mater. Trans. 2002, 43, 2970–2977. [Google Scholar] [CrossRef]
- Okulov, I.V.; Volegov, A.S.; Attar, H.; Bönisch, M.; Calin, M.; Eckert, J. Composition optimization of low modulus and high-strength TiNb-based alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2017, 65, 866–871. [Google Scholar] [CrossRef]
- Okulov, I.V.; Kühn, U.; Marr, T.; Freudenberger, J.; Soldatov, I.V.; Schultz, L.; Oertel, C.-G.; Skrotzki, W.; Eckert, J. Microstructure and mechanical properties of new composite structured Ti–V–Al–Cu–Ni alloys for spring applications. Mater. Sci. Eng. A 2014, 603, 76–83. [Google Scholar] [CrossRef]
- Okulov, I.V.; Bönisch, M.; Volegov, A.S.; Shakur, H.S.; Wendrock, H.; Gemming, T.; Eckert, J. Micro-to-nano-scale deformation mechanism of a Ti-based dendritic-ultrafine eutectic alloy exhibiting large tensile ductility. Mater. Sci. Eng. A 2017, 682, 673–678. [Google Scholar] [CrossRef]
- Prashanth, K.G.; Zhuravleva, K.; Okulov, I.; Calin, M.; Eckert, J.; Gebert, A. Mechanical and Corrosion Behavior of New Generation Ti-45Nb Porous Alloys Implant Devices. Technologies 2016, 4, 33. [Google Scholar] [CrossRef]
- Okulov, I.V.; Okulov, A.V.; Soldatov, I.V.; Luthringer, B.; Willumeit-Römer, R.; Wada, T.; Kato, H.; Weissmüller, J.; Markmann, J. Open porous dealloying-based biomaterials as a novel biomaterial platform. Mater. Sci. Eng. C 2018, 83, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Okulov, I.V.; Weissmüller, J.; Markmann, J. Dealloying-based interpenetrating-phase nanocomposites matching the elastic behavior of human bone. Sci. Rep. 2017, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Okulov, I.V.; Geslin, P.-A.; Soldatov, I.V.; Ovri, H.; Joo, S.-H.; Kato, H. Anomalously low modulus of the interpenetrating-phase composite of Fe and Mg obtained by liquid metal dealloying. Scr. Mater. 2019, 163, 133–136. [Google Scholar] [CrossRef]
- Varanasi, V.G.; Velten, M.F.; Odatsu, T.; Ilyas, A.; Iqbal, S.M.; Aswath, P.B. Chapter 9—Surface Modifications and Surface Characterization of Biomaterials Used in Bone Healing. In Materials for Bone Disorders; Bose, S., Bandyopadhyay, A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 405–452. [Google Scholar] [CrossRef]
- Kujala, S.; Ryhänen, J.; Danilov, A.; Tuukkanen, J. Effect of porosity on the osteointegration and bone ingrowth of a weight-bearing nickel–titanium bone graft substitute. Biomaterials 2003, 24, 4691–4697. [Google Scholar] [CrossRef]
- Lewallen, E.A.; Riester, S.M.; Bonin, C.A.; Kremers, H.M.; Dudakovic, A.; Kakar, S.; Cohen, R.C. Biological Strategies for Improved Osseointegration and Osteoinduction of Porous Metal Orthopedic Implants. Tissue Eng. Part B Rev. 2015, 21, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-C.; Chen, L.-Y.; Wang, L. Surface Modification of Titanium and Titanium Alloys: Technologies, Developments, and Future Interests. Adv. Eng. Mater. 2020, 22, 1901258. [Google Scholar] [CrossRef]
- Mediaswanti, K.; Wen, C.; Ivanova, E.P.; Berndt, C.C.; Malherbe, F.; Thi, V.; Pham, H.; Wang, J. A Review on Bioactive Porous Metallic Biomaterials. J. Biomimetics Biomater. Tissue Eng. 2013, 18, 1–8. [Google Scholar] [CrossRef]
- Agliardi, E.L.; Romeo, D.; Wenger, A.; Gastaldi, G.; Gherlone, E. Immediate rehabilitation of the posterior maxilla with extensive sinus pneumatization with one axial and one trans-sinus tilted implant: A 3-year clinical report and a classification. J. Prosthet. Dent. 2015, 113, 163–168. [Google Scholar] [CrossRef]
- Alves, S.F.; Wassall, T. In vitro evaluation of osteoblastic cell adhesion on machined osseointegrated implants. Braz. Oral Res. 2009, 23, 131–136. [Google Scholar] [CrossRef]
- Heiden, M.; Johnson, D.; Stanciu, L. Surface modifications through dealloying of Fe-Mn and Fe-Mn-Zn alloys developed to create tailorable, nanoporous, bioresorbable surfaces. Acta Mater. 2016, 103, 115–127. [Google Scholar] [CrossRef]
- Sasaki, K.; Osamu, S.; Takahashi, N. Interface oral health science 2014: Innovative research on biosis-abiosis intelligent interface. In Interface Oral Health Science 2016: Innovative Research on Biosis–Abiosis Intelligent Interface; Springer: Tokyo, Japan, 2015; pp. 1–351. [Google Scholar] [CrossRef]
- Fukuzumi, Y.; Wada, T.; Kato, H. Surface Improvement for Biocompatibility of Ti-6Al-4V by Dealloying in Metallic Melt. In Interface Oral Health Science 2014; Sasaki, K., Suzuki, O., Takahashi, N., Eds.; Springer: Tokyo, Japan, 2015; pp. 93–101. [Google Scholar]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef]
- Wada, T.; Yubuta, K.; Inoue, A.; Kato, H. Dealloying by metallic melt. Mater. Lett. 2011, 65, 1076–1078. [Google Scholar] [CrossRef]
- Geslin, P.; Mccue, I.; Erlebacher, J.; Karma, A. Topology-generating interfacial pattern formation during liquid metal dealloying. Nat. Commun. 2015, 6, 1–19. [Google Scholar] [CrossRef] [PubMed]
- McCue, I.; Gaskey, B.; Geslin, P.A.; Karma, A.; Erlebacher, J. Kinetics and morphological evolution of liquid metal dealloying. Acta Mater. 2016, 115, 10–23. [Google Scholar] [CrossRef]
- Mameka, N.; Wang, K.; Markmann, J.; Lilleodden, E.T.; Weissmüller, J. Nanoporous Gold—Testing Macro-scale Samples to Probe Small-scale Mechanical Behavior. Mater. Res. Lett. 2015, 3831, 1–10. [Google Scholar] [CrossRef]
- Shi, S.; Markmann, J.; Weissmüller, J. Actuation by hydrogen electrosorption in hierarchical nanoporous palladium. Philos. Mag. 2017, 97, 1571–1587. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, X.-G.; Wang, S.-G.; Jin, H.-J. Nanoporous Aluminum by Galvanic Replacement: Dealloying and Inward-Growth Plating. J. Electrochem. Soc. 2018, 165, C492–C496. [Google Scholar] [CrossRef]
- Joo, S.-H.; Wada, T.; Kato, H. Development of porous FeCo by liquid metal dealloying: Evolution of porous morphology and effect of interaction between ligaments and melt. Mater. Des. 2019, 180, 107908. [Google Scholar] [CrossRef]
- Joo, S.-H.; Kato, H. Transformation mechanisms and governing orientation relationships through selective dissolution of Ni via liquid metal dealloying from (FeCo)xNi100−x precursors. Mater. Des. 2020, 185, 108271. [Google Scholar] [CrossRef]
- Mokhtari, M.; Wada, T.; le Bourlot, C.; Duchet-Rumeau, J.; Kato, H.; Maire, E.; Mary, N. Corrosion resistance of porous ferritic stainless steel produced by liquid metal dealloying of Incoloy 800. Corros. Sci. 2020, 108468. [Google Scholar] [CrossRef]
- Mokhtari, M.; Wada, T.; le Bourlot, C.; Mary, N.; Duchet-Rumeau, J.; Kato, H.; Maire, E. Low cost high specific surface architectured nanoporous metal with corrosion resistance produced by liquid metal dealloying from commercial nickel superalloy. Scr. Mater. 2019, 163, 5–8. [Google Scholar] [CrossRef]
- Xiang, Y.-H.; Liu, L.-Z.; Shao, J.-C.; Jin, H.-J. A universal scaling relationship between the strength and Young’s modulus of dealloyed porous Fe0.80Cr0.20. Acta Mater. 2020, 186, 105–115. [Google Scholar] [CrossRef]
- Joo, S.-H.; Kato, H. Effect of dealloying rate on transformation behavior during liquid metal dealloying. J. Alloys Compd. 2020, 831, 154733. [Google Scholar] [CrossRef]
- Wada, T.; Setyawan, A.D.; Yubuta, K.; Kato, H. Nano- to submicro-porous β-Ti alloy prepared from dealloying in a metallic melt. Scr. Mater. 2011, 65, 532–535. [Google Scholar] [CrossRef]
- Okulov, A.V.; Volegov, A.S.; Weissmüller, J.; Markmann, J.; Okulov, I.V. Dealloying-based metal-polymer composites for biomedical applications. Scr. Mater. 2018, 146, 290–294. [Google Scholar] [CrossRef]
- Okulov, I.V.; Okulov, A.V.; Volegov, A.S.; Markmann, J. Tuning microstructure and mechanical properties of open porous TiNb and TiFe alloys by optimization of dealloying parameters. Scr. Mater. 2018, 154, 68–72. [Google Scholar] [CrossRef]
- Song, T.; Tang, H.P.; Li, Y.; Qian, M. Liquid metal dealloying of titanium-tantalum (Ti-Ta) alloy to fabricate ultrafine Ta ligament structures: A comparative study in molten copper (Cu) and Cu-based alloys. Corros. Sci. 2020, 169, 108600. [Google Scholar] [CrossRef]
- Zeng, L.; You, C.; Cai, X.; Wang, C.; Zhang, X.; Liang, T. Preparation of nanoporous CoCr alloy by dealloying CrCoNi medium entropy alloys. J. Mater. Res. Technol. 2020, 9, 6909–6915. [Google Scholar] [CrossRef]
- Wada, T.; Ichitsubo, T.; Yubuta, K.; Segawa, H.; Yoshida, H.; Kato, H. Bulk-nanoporous-silicon negative electrode with extremely high cyclability for lithium-ion batteries prepared using a top-down process. Nano Lett. 2014, 14, 4505–4510. [Google Scholar] [CrossRef]
- Yu, S.G.; Yubuta, K.; Wada, T.; Kato, H. Three-dimensional bicontinuous porous graphite generated in low temperature metallic liquid. Carbon 2016, 96, 403–410. [Google Scholar] [CrossRef]
- Shao, J.-C.; Jin, H.-J. From liquid metal dealloying to liquid metal expulsion. J. Mater. Sci. 2020, 55, 8337–8345. [Google Scholar] [CrossRef]
- Okulov, I.V.; Lamaka, S.V.; Wada, T.; Yubuta, K.; Zheludkevich, M.L.; Weissmüller, J.; Markmann, J.; Kato, H. Nanoporous magnesium. Nano Res. 2018, 11, 6428–6435. [Google Scholar] [CrossRef]
- Joo, S.-H.; Bae, J.W.; Park, W.-Y.; Shimada, Y.; Wada, T.; Kim, H.S.; Takeuchi, A.; Konno, T.J.; Kato, H.; Okulov, I.V. Beating Thermal Coarsening in Nanoporous Materials via High-Entropy Design. Adv. Mater. 2020, 32, 1906160. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Capparé, P.; Gherlone, E. Comparison of magnesium-enriched hydroxyapatite and porcine bone in human extraction socket healing: A histologic and histomorphometric evaluation. Int. J. Oral Maxillofac. Implant. 2011, 26, 1057–1062. [Google Scholar]
- Kobayashi, E.; Wang, T.J.; Yoneyama, T.; Hamanaka, H. Mechanical properties and corrosion resistance of Ti–6Al–7Nb alloy dental castings. J. Mater. Sci. Mater. Med. 1998, 9, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Semlitsch, M.F.; Weber, H.; Streicher, R.M.; Schön, R. Joint replacement components made of hot-forged and surface-treated Ti-6Al-7Nb alloy. Biomaterials 1992, 13, 781–788. [Google Scholar] [CrossRef]
- Joseph, L.A.; Israel, O.K.; Edet, E.J. Comparative Evaluation of Metal Ions Release From Titanium and Ti-6Al-7Nb Into Bio-Fluids. Dent. Res. J. 2009, 6, 7–11. [Google Scholar]
- Kim, H.-M.; Miyaji, F.; Kokubo, T.; Nakamura, T. Preparation of bioactive Ti and its alloys via simple chemical surface treatment. J. Biomed. Mater. Res. 1996, 32, 409–417. [Google Scholar] [CrossRef]
- Spriano, S.; Bosetti, M.; Bronzoni, M.; Vernè, E.; Maina, G.; Bergo, V.; Cannas, M. Surface properties and cell response of low metal ion release Ti-6Al-7Nb alloy after multi-step chemical and thermal treatments. Biomaterials 2005, 26, 1219–1229. [Google Scholar] [CrossRef]
- Hidalgo, A.A.; Ebel, T.; Limberg, W.; Pyczak, F. Influence of Oxygen on the Fatigue Behaviour of Ti-6Al-7Nb Alloy. Key Eng. Mater. 2016, 704, 44–52. [Google Scholar] [CrossRef]
- Umbilical, H.; Perivascular, C.; Cells, H.; Sarugaser, R.; Lickorish, D.; Baksh, D.; Hosseini, M.M.; Davies, J.E. Human umbilical cord perivascular (HUCPV) cells: A source of mesenchymal progenitors. Stem Cells. 2005, 23, 220–229. [Google Scholar] [CrossRef]
- Gierlotka, W.; Lothongkum, G.; Lohwongwatana, B.; Puncreoburt, C. Atomic mobility in Titanium grade 5 (Ti6Al4V). J. Min. Metall. Sect. B Metall. 2019, 55, 65–77. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Takeuchi, A.; Inoue, A. Metallic Glasses By Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application To Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- EGolub, E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Van den Heuvel, S. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression AU—Ruijtenberg, Suzan. Cell Cycle. 2016, 15, 196–212. [Google Scholar] [CrossRef]
| Dealloying Time (min) | Ti (wt.%) | Nb (wt.%) | Al (wt.%) |
|---|---|---|---|
| 0 (initial chemical composition) | 88.43 ± 0.08 | 5.95 ± 0.42 | 5.62 ± 0.11 |
| 10 | 85.16 ± 0.21 | 11.31 ± 0.26 | 3.53 ± 0.06 |
| 20 | 86.08 ± 0.17 | 11.07 ± 0.32 | 2.85 ± 0.04 |
| 30 | 85.67 ± 0.19 | 11.38 ± 0.22 | 2.95 ± 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okulov, I.V.; Joo, S.-H.; Okulov, A.V.; Volegov, A.S.; Luthringer, B.; Willumeit-Römer, R.; Zhang, L.; Mädler, L.; Eckert, J.; Kato, H. Surface Functionalization of Biomedical Ti-6Al-7Nb Alloy by Liquid Metal Dealloying. Nanomaterials 2020, 10, 1479. https://doi.org/10.3390/nano10081479
Okulov IV, Joo S-H, Okulov AV, Volegov AS, Luthringer B, Willumeit-Römer R, Zhang L, Mädler L, Eckert J, Kato H. Surface Functionalization of Biomedical Ti-6Al-7Nb Alloy by Liquid Metal Dealloying. Nanomaterials. 2020; 10(8):1479. https://doi.org/10.3390/nano10081479
Chicago/Turabian StyleOkulov, Ilya Vladimirovich, Soo-Hyun Joo, Artem Vladimirovich Okulov, Alexey Sergeevich Volegov, Bérengère Luthringer, Regine Willumeit-Römer, Laichang Zhang, Lutz Mädler, Jürgen Eckert, and Hidemi Kato. 2020. "Surface Functionalization of Biomedical Ti-6Al-7Nb Alloy by Liquid Metal Dealloying" Nanomaterials 10, no. 8: 1479. https://doi.org/10.3390/nano10081479
APA StyleOkulov, I. V., Joo, S.-H., Okulov, A. V., Volegov, A. S., Luthringer, B., Willumeit-Römer, R., Zhang, L., Mädler, L., Eckert, J., & Kato, H. (2020). Surface Functionalization of Biomedical Ti-6Al-7Nb Alloy by Liquid Metal Dealloying. Nanomaterials, 10(8), 1479. https://doi.org/10.3390/nano10081479











