Graphene Oxide-Linezolid Combination as Potential New Anti-Tuberculosis Treatment
Abstract
1. Introduction
2. Materials and Methods
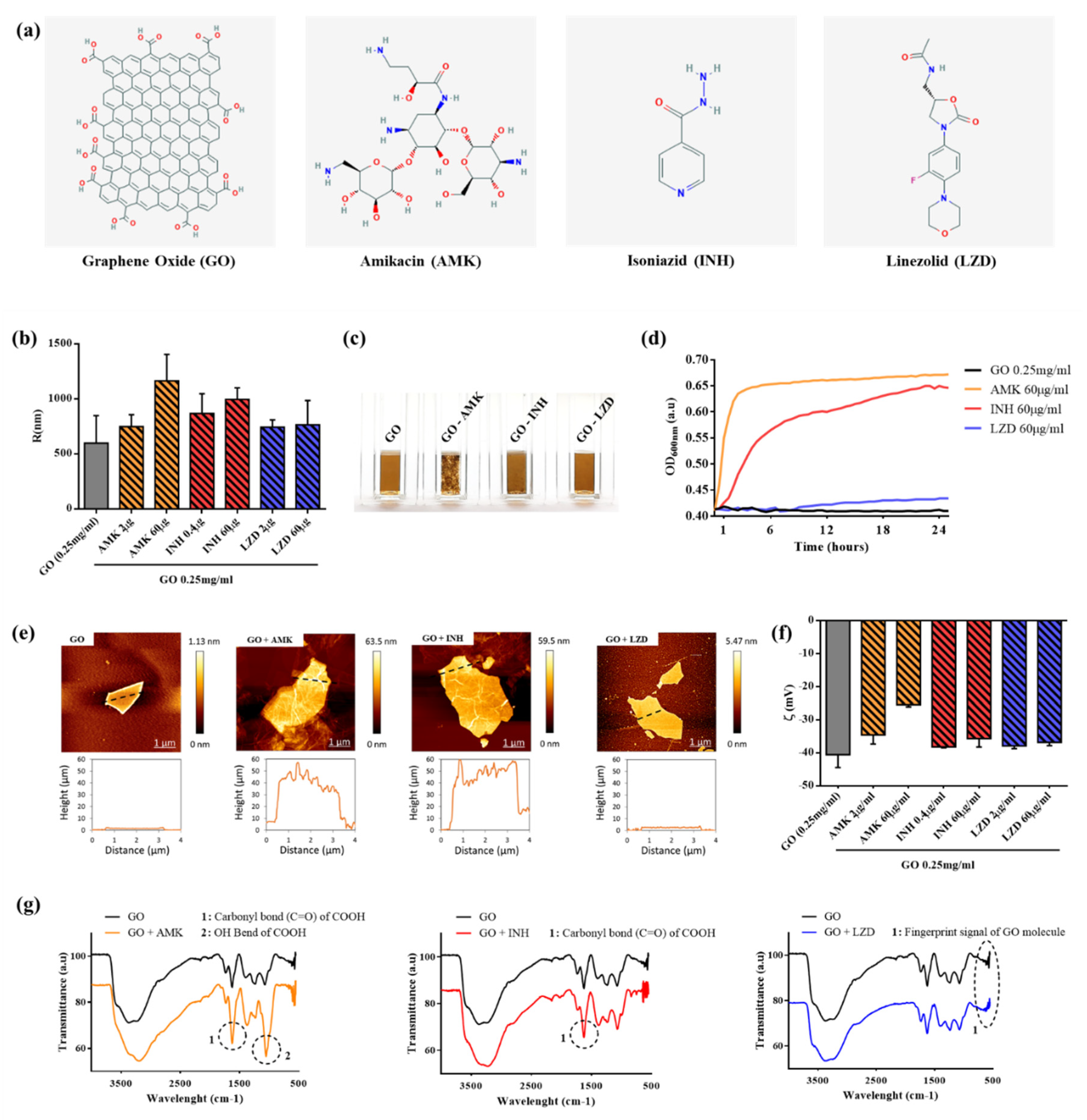
2.1. GO Characterization
2.2. Bacterial Manipulation
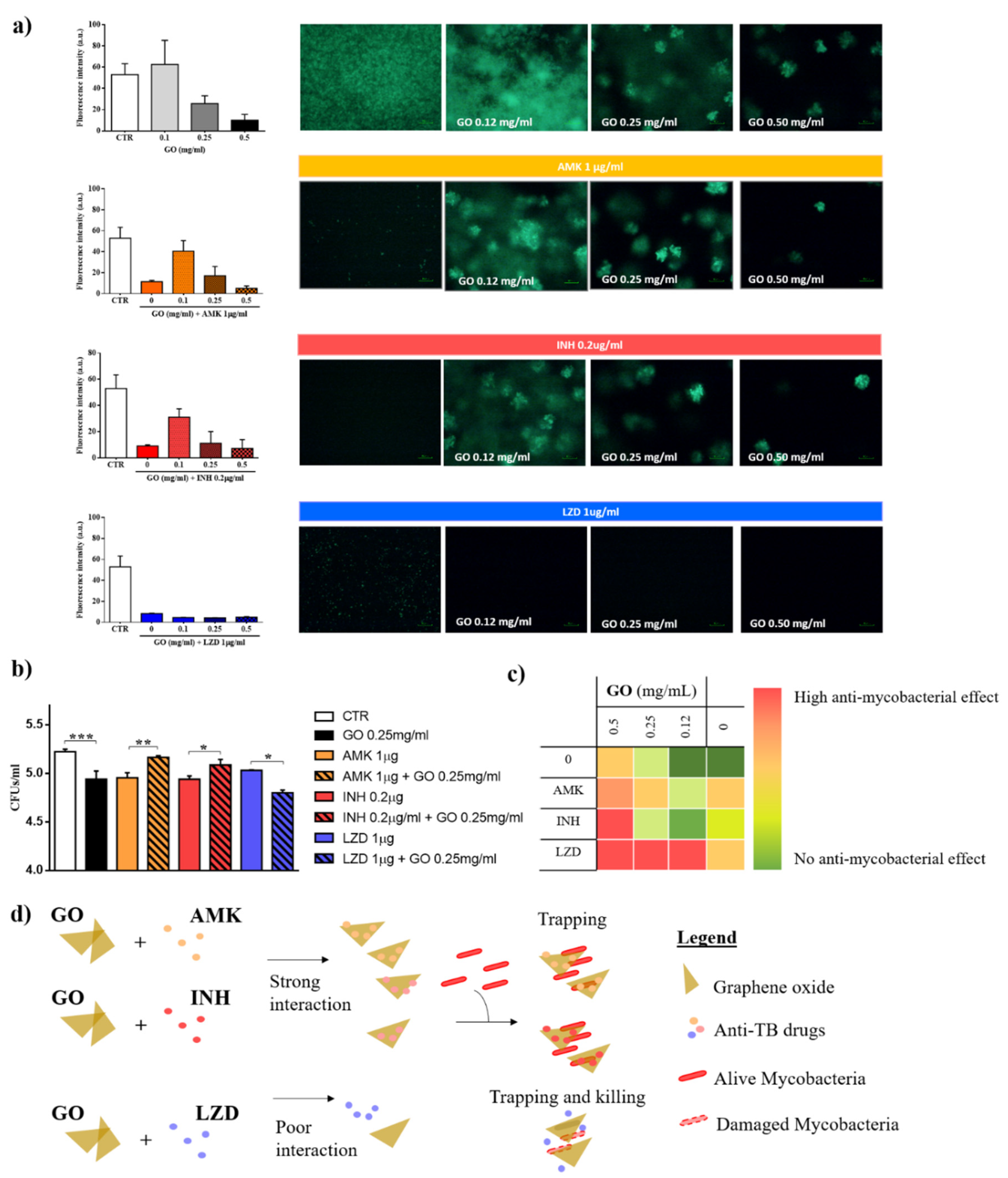
2.3. In Vitro Antimicrobial Assay
2.4. Cell Culture
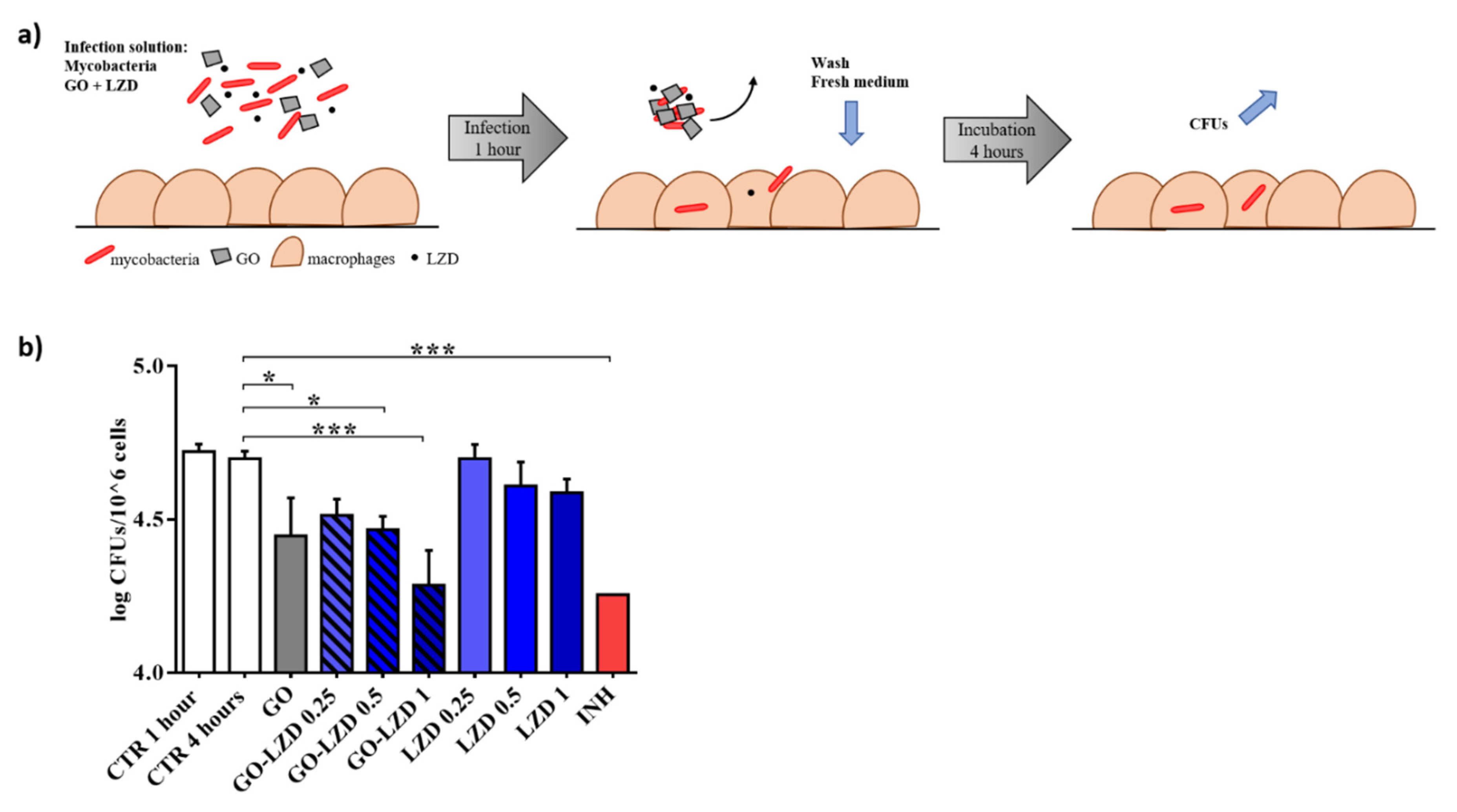
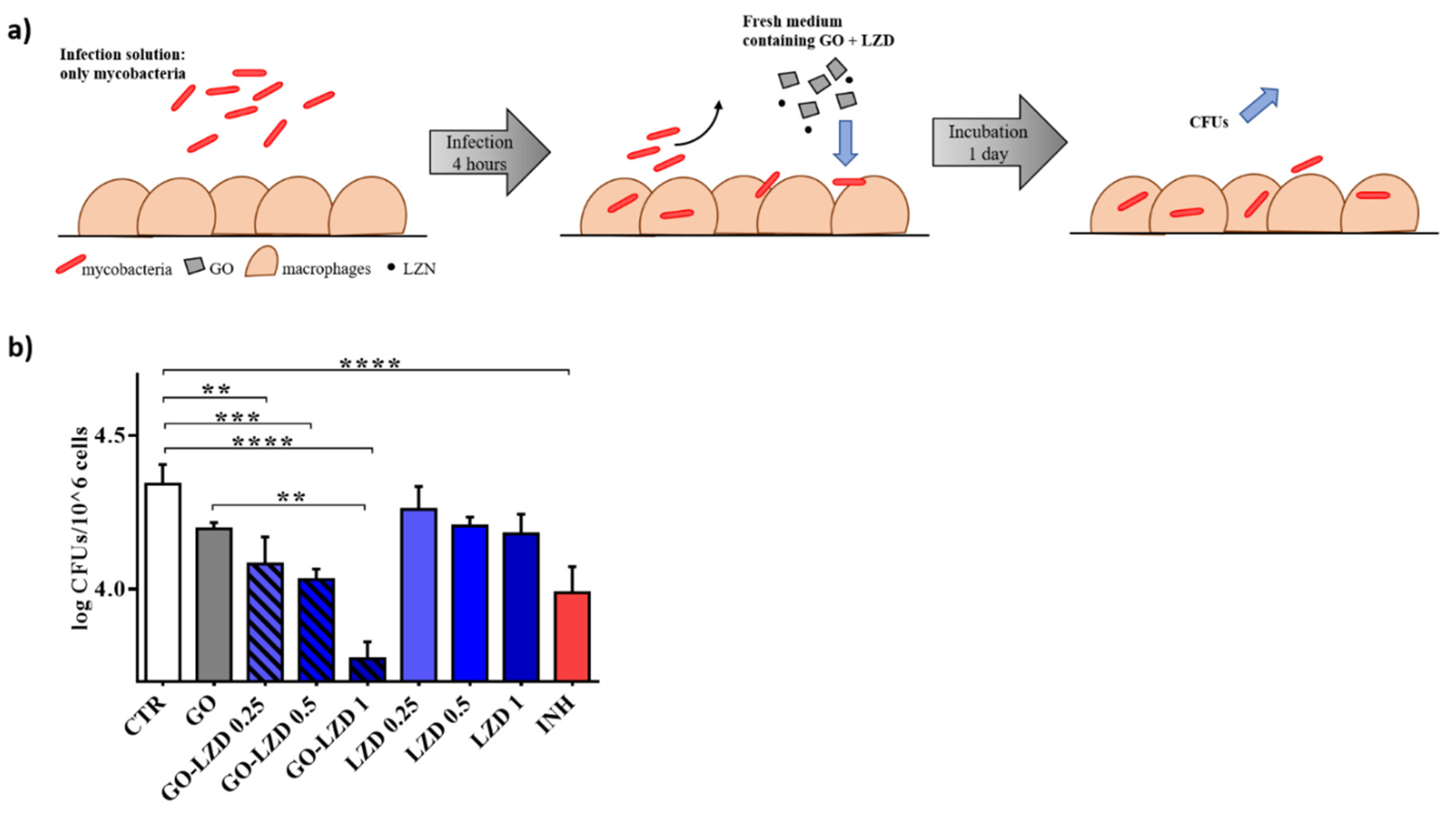
2.5. Mycobacterial Infection
2.6. LDH and ROS Detection
3. Results
3.1. Characterization of GO-Drugs Interaction
3.2. Graphene Oxide Has an Additive Effect with Linezolid, but not with Isoniazid and Amikacin
3.3. GO Co-Administration Enhances LZD Activity during the Infection of Macrophages
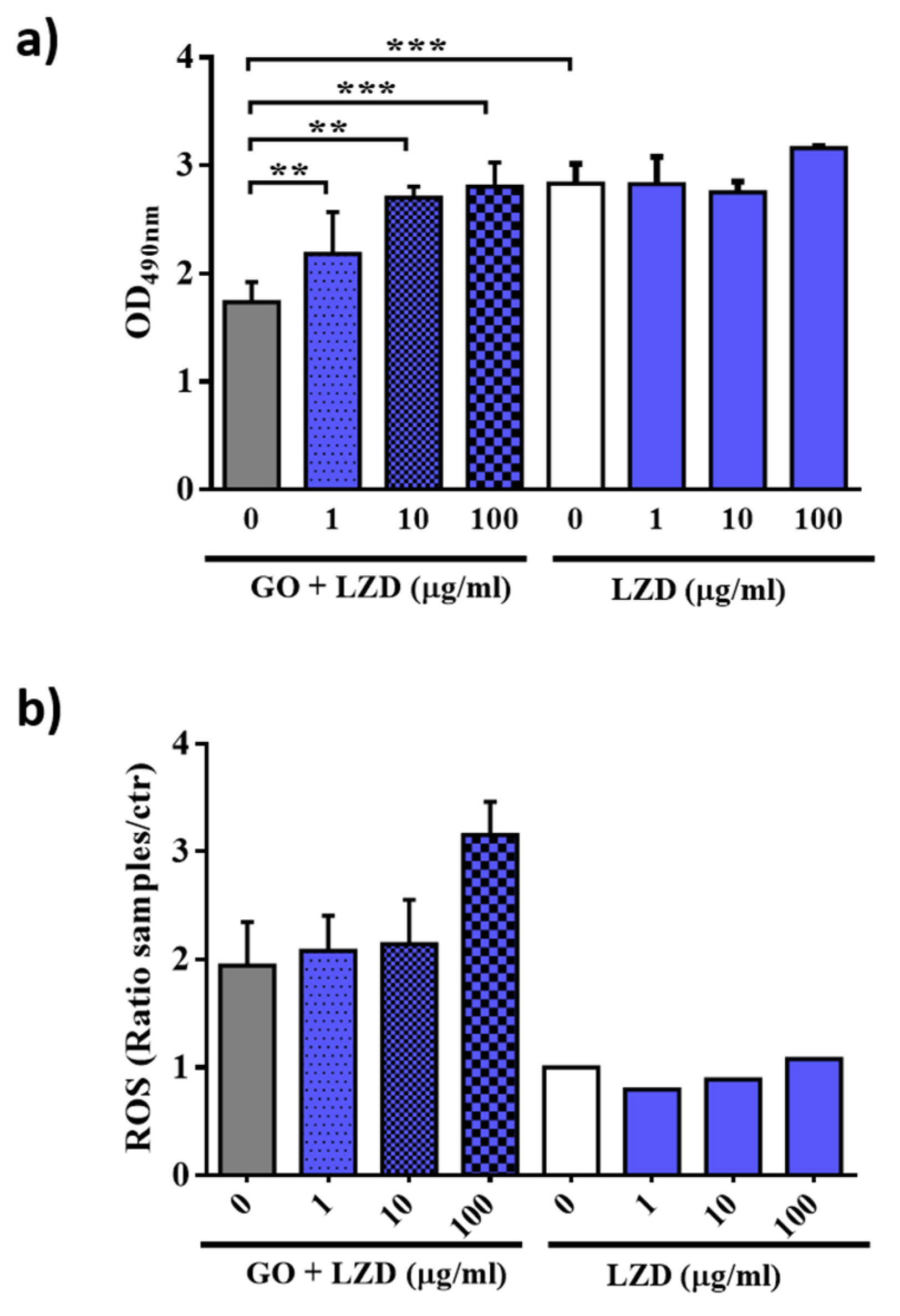
3.4. GO Affects Macrophages Permeability and ROS Production
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horizons 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Maleki, A.; De La Guardia, M.; Bani, M.S.; Chenab, K.K.; Pashazadeh-Panahi, P.; Baradaran, B.; Mokhtarzadeh, A.; Hamblin, M.R. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review. J. Adv. Res. 2019, 18, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Barba, M.; Di Pietro, L.; Gentilini, S.; Braidotti, M.C.; Ciancico, C.; Bugli, F.; Ciasca, G.; Larciprete, R.; Lattanzi, W.; et al. Reduction and shaping of graphene-oxide by laser-printing for controlled bone tissue regeneration and bacterial killing. 2D Mater. 2017, 5, 015027. [Google Scholar] [CrossRef]
- Palmieri, V.; Bugli, F.; Cacaci, M.; Perini, G.; De Maio, F.; Delogu, G.; Torelli, R.; Conti, C.; Sanguinetti, M.; De Spirito, M.; et al. Graphene oxide coatings prevent Candida albicans biofilm formation with a controlled release of curcumin-loaded nanocomposites. Nanomedicine 2018, 13, 2867–2879. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Papi, M.; Conti, C.; Ciasca, G.; Maulucci, G.; De Spirito, M. The future development of bacteria fighting medical devices: The role of graphene oxide. Expert Rev. Med Devices 2016, 13, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Lauriola, M.C.; Ciasca, G.; Conti, C.; De Spirito, M.; Papi, M. The graphene oxide contradictory effects against human pathogens. Nanotechnology 2017, 28, 152001. [Google Scholar] [CrossRef]
- Bugli, F.; Cacaci, M.; Palmieri, V.; Di Santo, R.; Torelli, R.; Ciasca, G.; Di Vito, M.; Vitali, A.; Conti, C.; Sanguinetti, M.; et al. Curcumin-loaded graphene oxide flakes as an effective antibacterial system against methicillin-resistant Staphylococcus aureus. Interface Focus 2018, 8, 20170059. [Google Scholar] [CrossRef]
- Palmieri, V.; Papi, M. Can graphene take part in the fight against COVID-19? Nano Today 2020, 33, 100883. [Google Scholar] [CrossRef]
- Palmieri, V.; Bugli, F.; Lauriola, M.C.; Cacaci, M.; Torelli, R.; Ciasca, G.; Conti, C.; Sanguinetti, M.; Papi, M.; De Spirito, M. Bacteria Meet Graphene: Modulation of Graphene Oxide Nanosheet Interaction with Human Pathogens for Effective Antimicrobial Therapy. ACS Biomater. Sci. Eng. 2017, 3, 619–627. [Google Scholar] [CrossRef]
- De Maio, F.; Palmieri, V.; Salustri, A.; Perini, G.; Sanguinetti, M.; De Spirito, M.; Delogu, G.; Papi, M. Graphene oxide prevents mycobacteria entry into macrophages through extracellular entrapment. Nanoscale Adv. 2019, 1, 1421–1431. [Google Scholar] [CrossRef]
- Delogu, G.; Sali, M.; Fadda, G. The biology of mycobacterium tuberculosis infection. Mediterr. J. Hematol. Infect. Dis. 2013, 5, e2013070. [Google Scholar] [CrossRef]
- Park, E.-J.; Lee, G.-H.; Han, B.S.; Lee, B.-S.; Lee, S.; Cho, M.-H.; Kim, J.-H.; Kim, D.-W. Toxic response of graphene nanoplatelets in vivo and in vitro. Arch. Toxicol. 2014, 89, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- De Maio, F.; Palmieri, V.; De Spirito, M.; Delogu, G.; Papi, M. Carbon nanomaterials: A new way against tuberculosis. Expert Rev. Med Devices 2019, 16, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Timmins, G.S.; Deretic, V. Mechanisms of action of isoniazid. Mol. Microbiol. 2006, 62, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment; WHO: Geneva, Switzerland, 2019.
- Rée, H. Treatment of tuberculosis: Guidelines for national programmes (2nd edition). Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 72. [Google Scholar] [CrossRef]
- Lee, M.; Lee, J.; Carroll, M.W.; Choi, H.; Min, S.; Song, T.; E Via, L.; Goldfeder, L.C.; Kang, E.; Jin, B.; et al. Linezolid for Treatment of Chronic Extensively Drug-Resistant Tuberculosis. New Engl. J. Med. 2012, 367, 1508–1518. [Google Scholar] [CrossRef]
- Palmieri, V.; Dalchiele, E.A.; Perini, G.; Motta, A.; De Spirito, M.; Zanoni, R.; Marrani, A.G.; Papi, M. Biocompatible N-acetyl cysteine reduces graphene oxide and persists at the surface as a green radical scavenger. Chem. Commun. 2019, 55, 4186–4189. [Google Scholar] [CrossRef]
- Patel, K.D.; Kim, T.-H.; Lee, E.-J.; Han, C.-M.; Lee, J.-Y.; Singh, R.K.; Kim, H.-W. Nanostructured Biointerfacing of Metals with Carbon Nanotube/Chitosan Hybrids by Electrodeposition for Cell Stimulation and Therapeutics Delivery. ACS Appl. Mater. Interfaces 2014, 6, 20214–20224. [Google Scholar] [CrossRef]
- De Maio, F.; Battah, B.; Palmieri, V.; Petrone, L.; Corrente, F.; Salustri, A.; Palucci, I.; Bellesi, S.; Papi, M.; Rubino, S.; et al. PE_PGRS3 of Mycobacterium tuberculosis is specifically expressed at low phosphate concentration, and its arginine-rich C-terminal domain mediates adhesion and persistence in host tissues when expressed in Mycobacterium smegmatis. Cell. Microbiol. 2018, 20, e12952. [Google Scholar] [CrossRef]
- Battah, B.; Chemi, G.; Butini, S.; Campiani, G.; Brogi, S.; Delogu, G.; Gemma, S. A Repurposing Approach for Uncovering the Anti-Tubercular Activity of FDA-Approved Drugs with Potential Multi-Targeting Profiles. Molecules 2019, 24, 4373. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676. [Google Scholar] [CrossRef] [PubMed]
- De Maio, F.; Maulucci, G.; Minerva, M.; Anoosheh, S.; Palucci, I.; Iantomasi, R.; Palmieri, V.; Camassa, S.; Sali, M.; Sanguinetti, M.; et al. Impact of Protein Domains on PE_PGRS30 Polar Localization in Mycobacteria. PLoS ONE 2014, 9, e112482. [Google Scholar] [CrossRef] [PubMed]
- Rasoulzadeh, M.; Namaziab, H. Carboxymethyl cellulose/graphene oxide bio-nanocomposite hydrogel beads as anticancer drug carrier agent. Carbohydr. Polym. 2017, 168, 320–326. [Google Scholar] [CrossRef]
- Huth, F.; Govyadinov, A.; Amarie, S.; Nuansing, W.; Keilmann, F.; Hillenbrand, R. Nano-FTIR Absorption Spectroscopy of Molecular Fingerprints at 20 nm Spatial Resolution. Nano Lett. 2012, 12, 3973–3978. [Google Scholar] [CrossRef]
- Lange, C.; Chesov, D.; Heyckendorf, J.; Leung, C.; Udwadia, Z.; Dheda, K. Drug-resistant tuberculosis: An update on disease burden, diagnosis and treatment. Respirology 2018, 23, 656–673. [Google Scholar] [CrossRef] [PubMed]
- Dheda, K.; Migliori, G.B. The global rise of extensively drug-resistant tuberculosis: Is the time to bring back sanatoria now overdue? Lancet 2012, 379, 773–775. [Google Scholar] [CrossRef]
- Hashemian, S.M.; Farhadi, T.; Ganjparvar, M. Linezolid: A review of its properties, function, and use in critical care. Drug Des. Dev. Ther. 2018, 12, 1759–1767. [Google Scholar] [CrossRef]
- Alcalá, L.; Ruiz-Serrano, M.J.; Turégano, C.P.-F.; De Viedma, D.G.; Díaz-Infantes, M.; Marín-Arriaza, M.; Bouza, E. In Vitro Activities of Linezolid against Clinical Isolates of Mycobacterium tuberculosis That Are Susceptible or Resistant to First-Line Antituberculous Drugs. Antimicrob. Agents Chemother. 2003, 47, 416–417. [Google Scholar] [CrossRef]
- Tato, M.; De La Pedrosa, E.G.-G.; Canton, R.; Gómez-García, I.; Fortun, J.; Martín-Dávila, P.; Baquero, F.; Gomez-Mampaso, E. In vitro activity of linezolid against Mycobacterium tuberculosis complex, including multidrug-resistant Mycobacterium bovis isolates. Int. J. Antimicrob. Agents 2006, 28, 75–78. [Google Scholar] [CrossRef]
- Zurenko, G.E.; Yagi, B.H.; Schaadt, R.D.; Allison, J.W.; Kilburn, J.O.; Glickman, S.E.; Hutchinson, D.K.; Barbachyn, M.R.; Brickner, S.J. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 1996, 40, 839–845. [Google Scholar] [CrossRef]
- Cynamon, M.H.; Klemens, S.P.; Sharpe, C.A.; Chase, S. Activities of Several Novel Oxazolidinones againstMycobacterium tuberculosis in a Murine Model. Antimicrob. Agents Chemother. 1999, 43, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Bruns, H.; Stenger, S. New insights into the interaction of Mycobacterium tuberculosis and human macrophages. Futur. Microbiol. 2014, 9, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Bienert, M.D.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta Biomembr. 2006, 1758, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Seaver, L.C.; Imlay, J.A. Hydrogen Peroxide Fluxes and Compartmentalization inside Growing Escherichia coli. J. Bacteriol. 2001, 183, 7182–7189. [Google Scholar] [CrossRef]
- Rey-Jurado, E.; Tudo, G.; Soy, D.; González-Martín, J. Activity and interactions of levofloxacin, linezolid, ethambutol and amikacin in three-drug combinations against Mycobacterium tuberculosis isolates in a human macrophage model. Int. J. Antimicrob. Agents 2013, 42, 524–530. [Google Scholar] [CrossRef]
- Queval, C.J.; Brosch, R.; Simeone, R. The Macrophage: A Disputed Fortress in the Battle against Mycobacterium tuberculosis. Front. Microbiol. 2017, 8, 2284. [Google Scholar] [CrossRef]
- Orme, I.M.; Basaraba, R.J. The formation of the granuloma in tuberculosis infection. Semin. Immunol. 2014, 26, 601–609. [Google Scholar] [CrossRef]
- Ehlers, S.; Schaible, U.E. The Granuloma in Tuberculosis: Dynamics of a Host–Pathogen Collusion. Front. Immunol. 2013, 3, 411. [Google Scholar] [CrossRef] [PubMed]
- Gengenbacher, M.; Kaufmann, S.H. Mycobacterium tuberculosis: Success through dormancy. FEMS Microbiol. Rev. 2012, 36, 514–532. [Google Scholar] [CrossRef]
- Eigler, S. Controlled Chemistry Approach to the Oxo-Functionalization of Graphene. Chem. A Eur. J. 2016, 22, 7012–7027. [Google Scholar] [CrossRef]
- Cao, J.; He, P.; Mohammed, M.A.; Zhao, X.; Young, R.J.; Derby, B.; Kinloch, I.A.; Dryfe, R.A.W. Two-Step Electrochemical Intercalation and Oxidation of Graphite for the Mass Production of Graphene Oxide. J. Am. Chem. Soc. 2017, 139, 17446–17456. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Maio, F.; Palmieri, V.; Santarelli, G.; Perini, G.; Salustri, A.; Palucci, I.; Sali, M.; Gervasoni, J.; Primiano, A.; Ciasca, G.; et al. Graphene Oxide-Linezolid Combination as Potential New Anti-Tuberculosis Treatment. Nanomaterials 2020, 10, 1431. https://doi.org/10.3390/nano10081431
De Maio F, Palmieri V, Santarelli G, Perini G, Salustri A, Palucci I, Sali M, Gervasoni J, Primiano A, Ciasca G, et al. Graphene Oxide-Linezolid Combination as Potential New Anti-Tuberculosis Treatment. Nanomaterials. 2020; 10(8):1431. https://doi.org/10.3390/nano10081431
Chicago/Turabian StyleDe Maio, Flavio, Valentina Palmieri, Giulia Santarelli, Giordano Perini, Alessandro Salustri, Ivana Palucci, Michela Sali, Jacopo Gervasoni, Aniello Primiano, Gabriele Ciasca, and et al. 2020. "Graphene Oxide-Linezolid Combination as Potential New Anti-Tuberculosis Treatment" Nanomaterials 10, no. 8: 1431. https://doi.org/10.3390/nano10081431
APA StyleDe Maio, F., Palmieri, V., Santarelli, G., Perini, G., Salustri, A., Palucci, I., Sali, M., Gervasoni, J., Primiano, A., Ciasca, G., Sanguinetti, M., De Spirito, M., Delogu, G., & Papi, M. (2020). Graphene Oxide-Linezolid Combination as Potential New Anti-Tuberculosis Treatment. Nanomaterials, 10(8), 1431. https://doi.org/10.3390/nano10081431








