Surface Stabilization Affects Toxicity of Silver Nanoparticles in Human Peripheral Blood Mononuclear Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of hPBMC
2.2. Synthesis and Characterization of AgNPs
2.3. Treatment of hPBMC with AgNPs
2.4. Flow Cytometry Experiments
2.4.1. Evaluation of Cell Death and AgNPs Uptake
2.4.2. Evaluation of Oxidative Stress Response
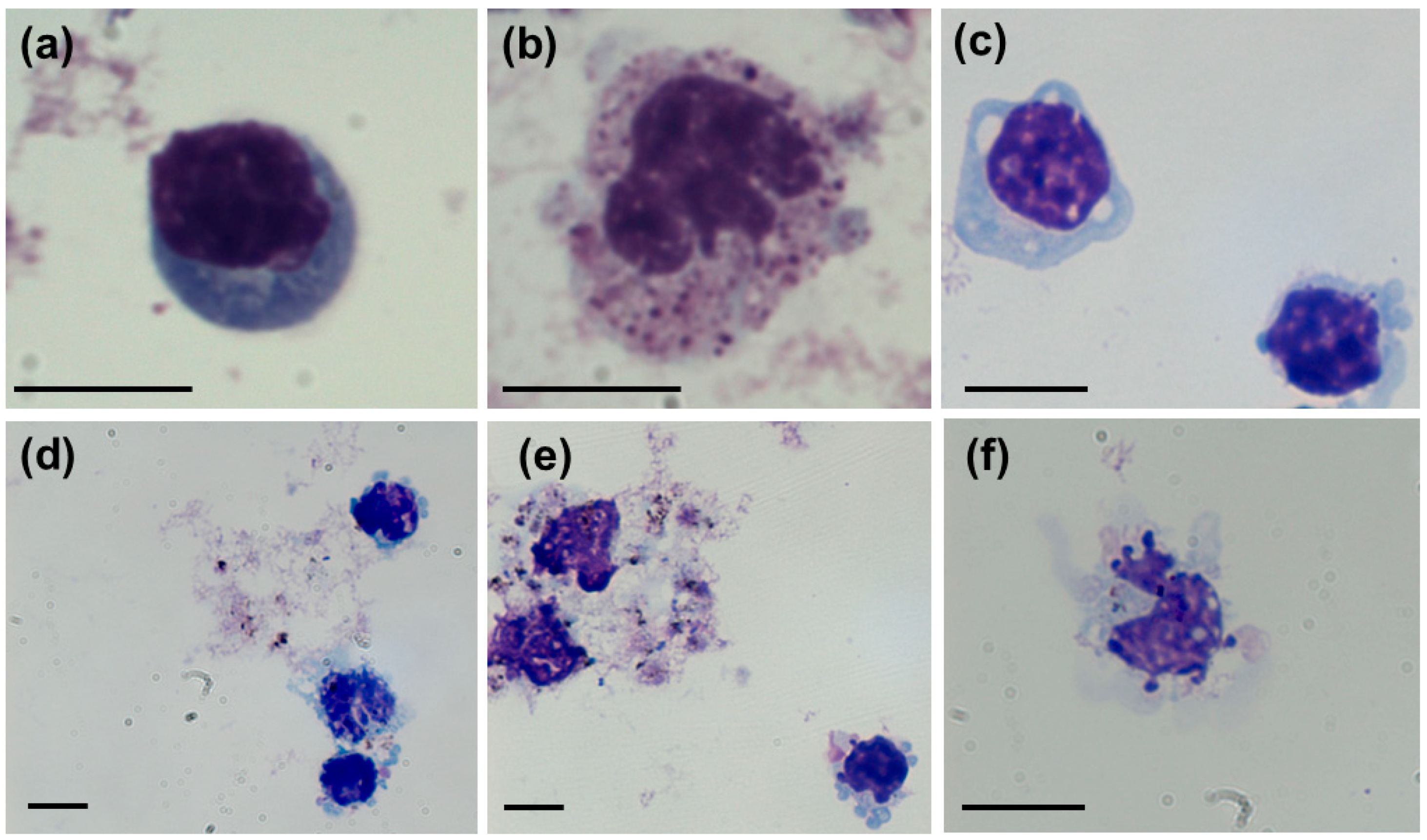
2.5. Visualization of Treated hPBMC
2.6. Comet Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characteristics and Stability of AgNPs
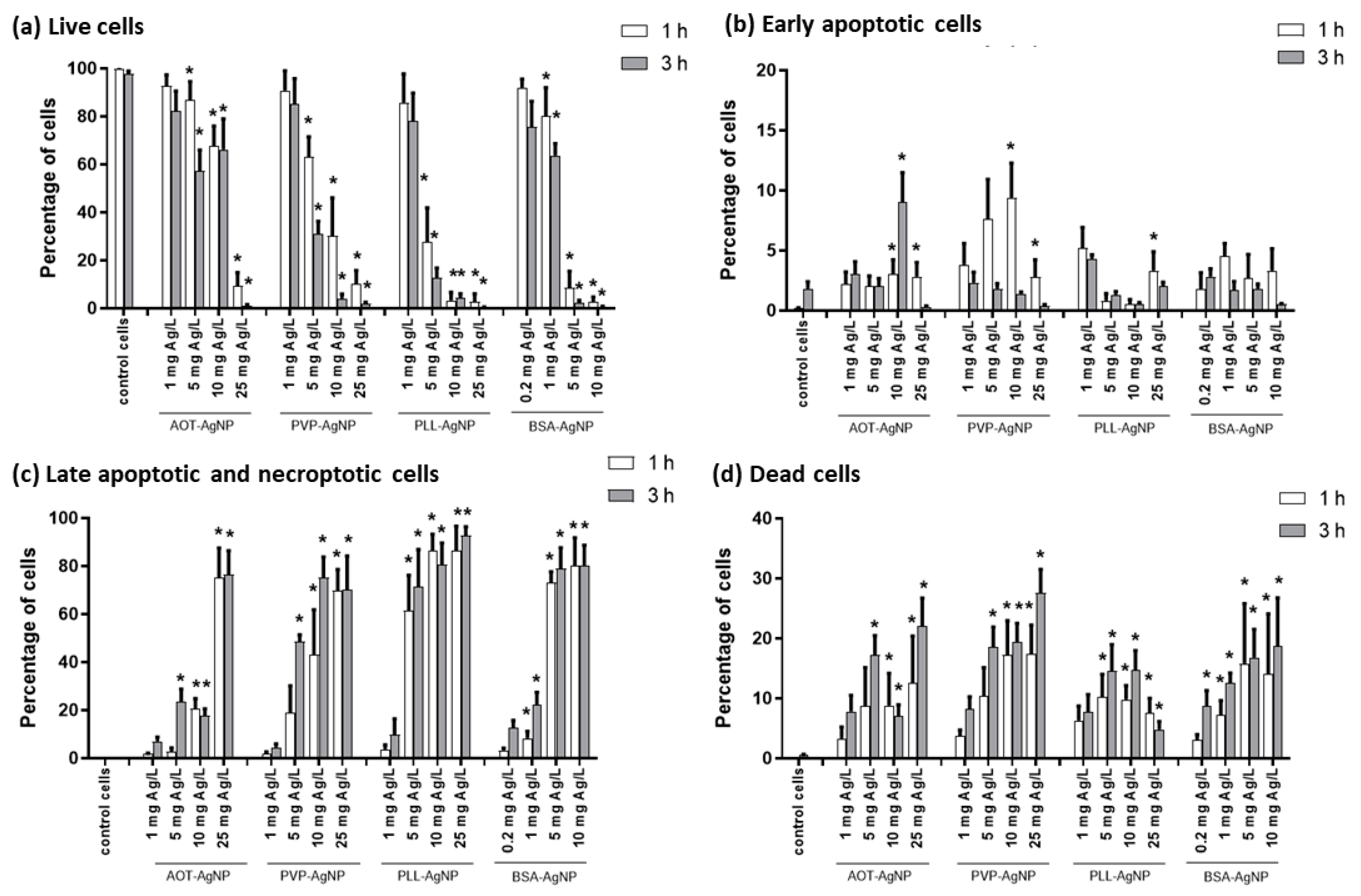
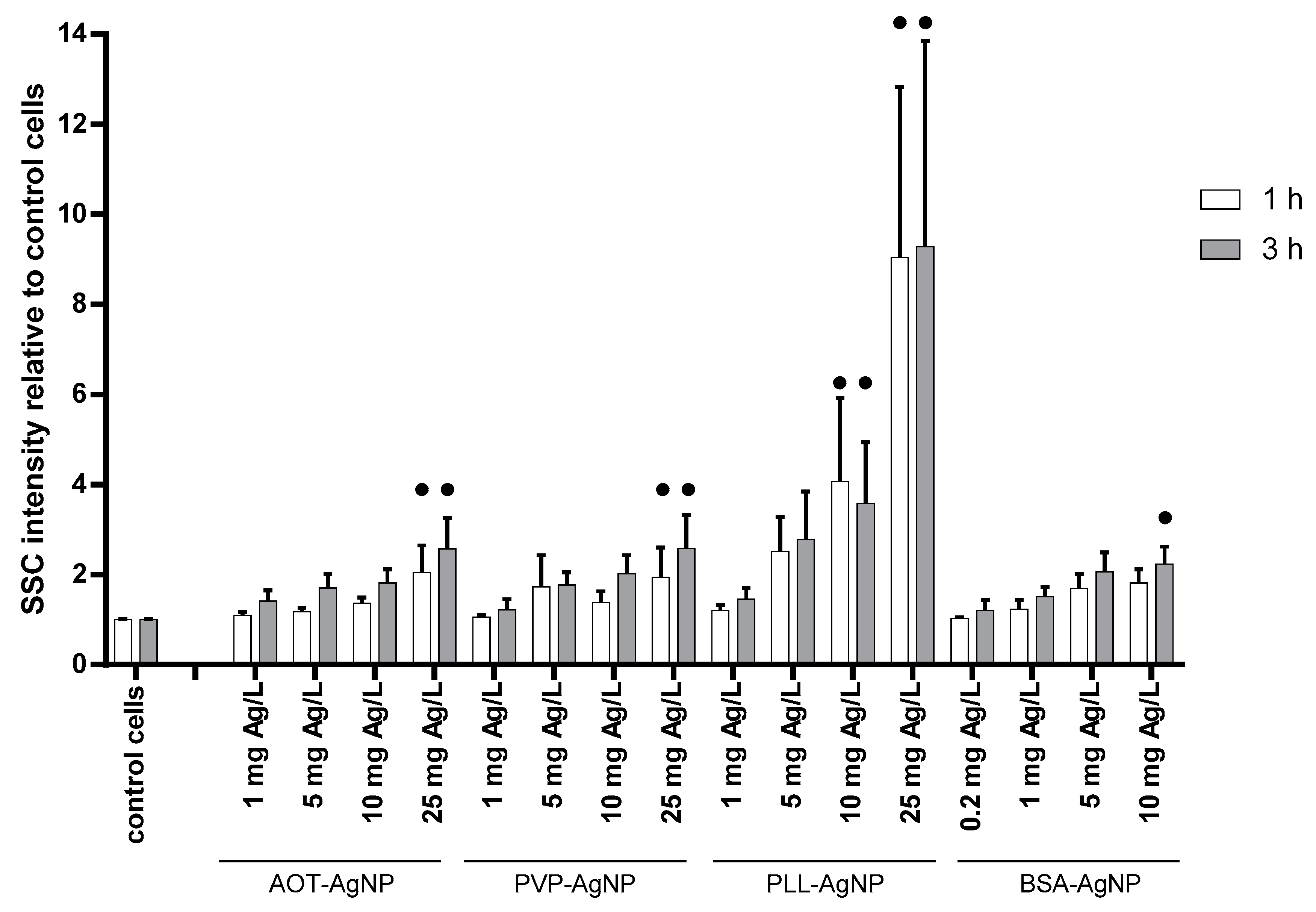
3.2. Cytotoxicity Effects and Cellular Uptake of AgNPs
3.3. Genotoxicity of AgNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mackevica, A.; Revilla, P.; Brinch, A.; Hansen, S.F. Current uses of nanomaterials in biocidal products and treated articles in the EU. Environ. Sci. Nano 2016, 3, 1195–1205. [Google Scholar] [CrossRef]
- Rosenberg, M.; Ilic, K.; Juganson, K.; Ivask, A.; Ahonen, M.; Vrček, I.V.; Kahru, A. Potential ecotoxicological effects of antimicrobial surface coatings: A literature survey backed up by analysis of market reports. PeerJ 2019, 2019, e6315. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Sato, M.; Sato, Y.; Ando, N.; Takayama, T.; Fujita, M.; Ishihara, M. Synthesis and application of silver nanoparticles (Ag nps) for the prevention of infection in healthcare workers. Int. J. Mol. Sci. 2019, 20, 3620. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.; Middelberg, A.P.J. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef]
- Lu, L.; Sun, R.W.Y.; Chen, R.; Hui, C.K.; Ho, C.M.; Luk, J.M.; Lau, G.K.K.; Che, C.M. Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 2008, 13, 252–262. [Google Scholar]
- Sun, L.; Singh, A.K.; Vig, K.; Pillai, S.R.; Singh, S.R. Silver nanoparticles inhibit replication of respiratory syncytial virus. J. Biomed. Nanotechnol. 2008, 4, 149–158. [Google Scholar]
- Baram-Pinto, D.; Shukla, S.; Perkas, N.; Gedanken, A.; Sarid, R. Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjug. Chem. 2009, 20, 1497–1502. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Olesen, P.; Hougaard, M.; Dang, D.A.; Hoffmann, H.J.; Autrup, H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol. Lett. 2009, 190, 156–162. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- de Lima, R.; Seabra, A.B.; Durán, N. Silver nanoparticles: A brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J. Appl. Toxicol. 2012, 32, 867–879. [Google Scholar] [CrossRef] [PubMed]
- The International Organization for Standardization. For ISO 10993, Part 4: Selection of Tests for Interactions with Blood. In Biological Evaluation of Medical Devices; The International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Blok, S.L.J.; Engels, G.E.; van Oeveren, W. In vitro hemocompatibility testing: The importance of fresh blood. Biointerphases 2016, 11, 029802. [Google Scholar] [CrossRef]
- Huang, H.; Lai, W.; Cui, M.; Liang, L.; Lin, Y.; Fang, Q.; Liu, Y.; Xie, L. An Evaluation of Blood Compatibility of Silver Nanoparticles. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ahonen, M.; Kahru, A.; Ivask, A.; Kasemets, K.; Kõljalg, S.; Mantecca, P.; Vrček, I.V.; Keinänen-Toivola, M.M.; Crijns, F. Proactive approach for safe use of antimicrobial coatings in healthcare settings: Opinion of the cost action network AMiCI. Int. J. Environ. Res. Public Health 2017, 14, 366. [Google Scholar] [CrossRef]
- Jurašin, D.D.; Ćurlin, M.; Capjak, I.; Crnković, T.; Lovrić, M.; Babič, M.; Horák, D.; Vrček, I.V.; Gajović, S. Surface coating affects behavior of metallic nanoparticles in a biological environment. Beilstein J. Nanotechnol. 2016, 7, 246–262. [Google Scholar] [CrossRef]
- Barbir, R.; Goessler, W.; Ćurlin, M.; Micek, V.; Milić, M.; Vuković, B.; Milić, M.; Ljubojević, M.; Domazet Jurašin, D.; Vinković Vrček, I. Protein Corona Modulates Distribution and Toxicological Effects of Silver Nanoparticles In Vivo. Part. Part. Syst. Charact. 2019, 36, 1–12. [Google Scholar] [CrossRef]
- Elblbesy, M.A. Hemocompatibility of Albumin Nanoparticles as a Drug Delivery System—An ‘In Vitro’ Study. J. Biomater. Nanobiotechnol. 2016, 07, 64–71. [Google Scholar] [CrossRef]
- Pietkiewicz, S.; Schmidt, J.H.; Lavrik, I.N. Quantification of apoptosis and necroptosis at the single cell level by a combination of Imaging Flow Cytometry with classical Annexin V/propidium iodide staining. J. Immunol. Methods 2015, 423, 99–103. [Google Scholar] [CrossRef]
- Zucker, R.M.; Daniel, K.M. Detection of TiO2 Nanoparticles in Cells by Flow Cytometry. In Nanoparticles in Biology and Medicine: Methods and Protocols; Soloviev, M., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 497–509. [Google Scholar]
- Zucker, R.M.; Daniel, K.M.; Massaro, E.J.; Karafas, S.J.; Degn, L.L.; Boyes, W.K. Detection of silver nanoparticles in cells by flow cytometry using light scatter and far-red fluorescence. Cytometry A 2013, 83, 962–972. [Google Scholar] [CrossRef]
- Pem, B.; Pongrac, I.M.; Ulm, L.; Pavičić, I.; Vrček, V.; Jurašin, D.D.; Ljubojević, M.; Krivohlavek, A.; Vrček, I.V. Interaction of Small Biothiols with Silver and Gold Nanoparticles. Beilstein Arch. 2019, 2019, 15. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Vrček, I.V.; Žuntar, I.; Petlevski, R.; Pavičić, I.; Dutour Sikirić, M.; Ćurlin, M.; Goessler, W. Comparison of in vitro toxicity of silver ions and silver nanoparticles on human hepatoma cells. Environ. Toxicol. 2016, 31, 679–692. [Google Scholar] [CrossRef]
- Milić, M.; Leitinger, G.; Pavičić, I.; Zebić Avdičević, M.; Dobrović, S.; Goessler, W.; Vinković Vrček, I. Cellular uptake and toxicity effects of silver nanoparticles in mammalian kidney cells. J. Appl. Toxicol. 2015, 35, 581–592. [Google Scholar] [CrossRef]
- Capjak, I.; Goreta, S.Š.; Jurašin, D.D.; Vrček, I.V. How protein coronas determine the fate of engineered nanoparticles in biological environment. Arh. Hig. Rada Toksikol. 2017, 68, 245–253. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Shurin, M.; Shvedova, A.A. Current understanding of interactions between nanoparticles and the immune system. Toxicol. Appl. Pharmacol. 2016, 299, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Zhornik, E.V.; Baranova, L.A.; Drozd, E.S.; Sudas, M.S.; Chau, N.H.; Buu, N.Q.; Dung, T.T.N.; Chizhik, S.A.; Volotovski, I.D. Silver nanoparticles induce lipid peroxidation and morphological changes in human lymphocytes surface. Cell Biophys. 2014, 59, 380–386. [Google Scholar] [CrossRef]
- Joksić, G.; Stašić, J.; Filipović, J.; Šobot, A.V.; Trtica, M. Size of silver nanoparticles determines proliferation ability of human circulating lymphocytes in vitro. Toxicol. Lett. 2016, 247, 29–34. [Google Scholar] [CrossRef]
- Li, W.T.; Chang, H.W.; Yang, W.C.; Lo, C.; Wang, L.Y.; Pang, V.F.; Chen, M.H.; Jeng, C.R. Immunotoxicity of Silver Nanoparticles (AgNPs) on the Leukocytes of Common Bottlenose Dolphins (Tursiops truncatus). Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Paino, I.M.M.; Zucolotto, V. Poly(vinyl alcohol)-coated silver nanoparticles: Activation of neutrophils and nanotoxicology effects in human hepatocarcinoma and mononuclear cells. Environ. Toxicol. Pharmacol. 2015, 39, 614–621. [Google Scholar] [CrossRef]
- Orta-García, S.T.; Plascencia-Villa, G.; Ochoa-Martínez, A.C.; Ruiz-Vera, T.; Pérez-Vázquez, F.J.; Velázquez-Salazar, J.J.; Yacamán, M.J.; Navarro-Contreras, H.R.; Pérez-Maldonado, I.N. Analysis of cytotoxic effects of silver nanoclusters on human peripheral blood mononuclear cells “in vitro”. J. Appl. Toxicol. 2015, 35, 1189–1199. [Google Scholar] [CrossRef]
- Kubo, A.L.; Capjak, I.; Vrček, I.V.; Bondarenko, O.M.; Kurvet, I.; Vija, H.; Ivask, A.; Kasemets, K.; Kahru, A. Antimicrobial potency of differently coated 10 and 50 nm silver nanoparticles against clinically relevant bacteria Escherichia coli and Staphylococcus aureus. Colloids Surf. B Biointerfaces 2018, 170, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Vinković Vrček, I.; Pavičić, I.; Crnković, T.; Jurašin, D.; Babič, M.; Horák, D.; Lovrić, M.; Ferhatović, L.; Ćurlin, M.; Gajović, S. Does surface coating of metallic nanoparticles modulate their interference with in vitro assays? RSC Adv. 2015, 5, 70787–70807. [Google Scholar] [CrossRef]
- Sabella, S.; Carney, R.P.; Brunetti, V.; Malvindi, M.A.; Al-Juffali, N.; Vecchio, G.; Janes, S.M.; Bakr, O.M.; Cingolani, R.; Stellacci, F.; et al. A general mechanism for intracellular toxicity of metal-containing nanoparticles. Nanoscale 2014, 6, 7052–7061. [Google Scholar] [CrossRef] [PubMed]
- Greulich, C.; Diendorf, J.; Geßmann, J.; Simon, T.; Habijan, T.; Eggeler, G.; Schildhauer, T.A.; Epple, M.; Köller, M. Cell type-specific responses of peripheral blood mononuclear cells to silver nanoparticles. Acta Biomater. 2011, 7, 3505–3514. [Google Scholar] [CrossRef] [PubMed]
- Brkić Ahmed, L.; Milić, M.; Pongrac, I.M.; Marjanović, A.M.; Mlinarić, H.; Pavičić, I.; Gajović, S.; Vinković Vrček, I. Impact of surface functionalization on the uptake mechanism and toxicity effects of silver nanoparticles in HepG2 cells. Food Chem. Toxicol. 2017, 107, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lenhart, J.J.; Walker, H.W. Dissolution-Accompanied Aggregation Kinetics of Silver Nanoparticles. Langmuir 2010, 26, 16690–16698. [Google Scholar] [CrossRef]
- Vergallo, C.; Panzarini, E.; Izzo, D.; Carata, E.; Mariano, S.; Buccolieri, A.; Serra, A.; Manno, D.; Dini, L. Cytotoxicity of β-d-glucose coated silver nanoparticles on human lymphocytes. AIP Conf. Proc. 2014, 1603, 78–85. [Google Scholar] [CrossRef]
- Garden, O.A.; Reynolds, P.R.; Yates, J.; Larkman, D.J.; Marelli-Berg, F.M.; Haskard, D.O.; Edwards, A.D.; George, A.J.T. A rapid method for labelling CD4+ T cells with ultrasmall paramagnetic iron oxide nanoparticles for magnetic resonance imaging that preserves proliferative, regulatory and migratory behaviour in vitro. J. Immunol. Methods 2006, 314, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Parboosing, R.; Kruger, H.G.; Maguire, G.E.M.; Govender, T. Intracellular localization of gold nanoparticles with targeted delivery in MT-4 lymphocytes. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Devanabanda, M.; Latheef, S.A.; Madduri, R. Immunotoxic effects of gold and silver nanoparticles: Inhibition of mitogen-induced proliferative responses and viability of human and murine lymphocytes in vitro. J. Immunotoxicol. 2016, 13, 897–902. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Garg, S.; He, D.; Pham, A.N.; Waite, T.D. Superoxide-Mediated Formation and Charging of Silver Nanoparticles. Environ. Sci. Technol. 2011, 45, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Collin, F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Maurer, L.L.; Meyer, J.N. A systematic review of evidence for silver nanoparticle-induced mitochondrial toxicity. Environ. Sci. Nano 2016, 3, 311–322. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Choi, C. Mitochondrial network determines intracellular ROS dynamics and sensitivity to oxidative stress through switching inter-mitochondrial messengers. PLoS ONE 2011, 6, e23211. [Google Scholar] [CrossRef]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (δψm) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef]
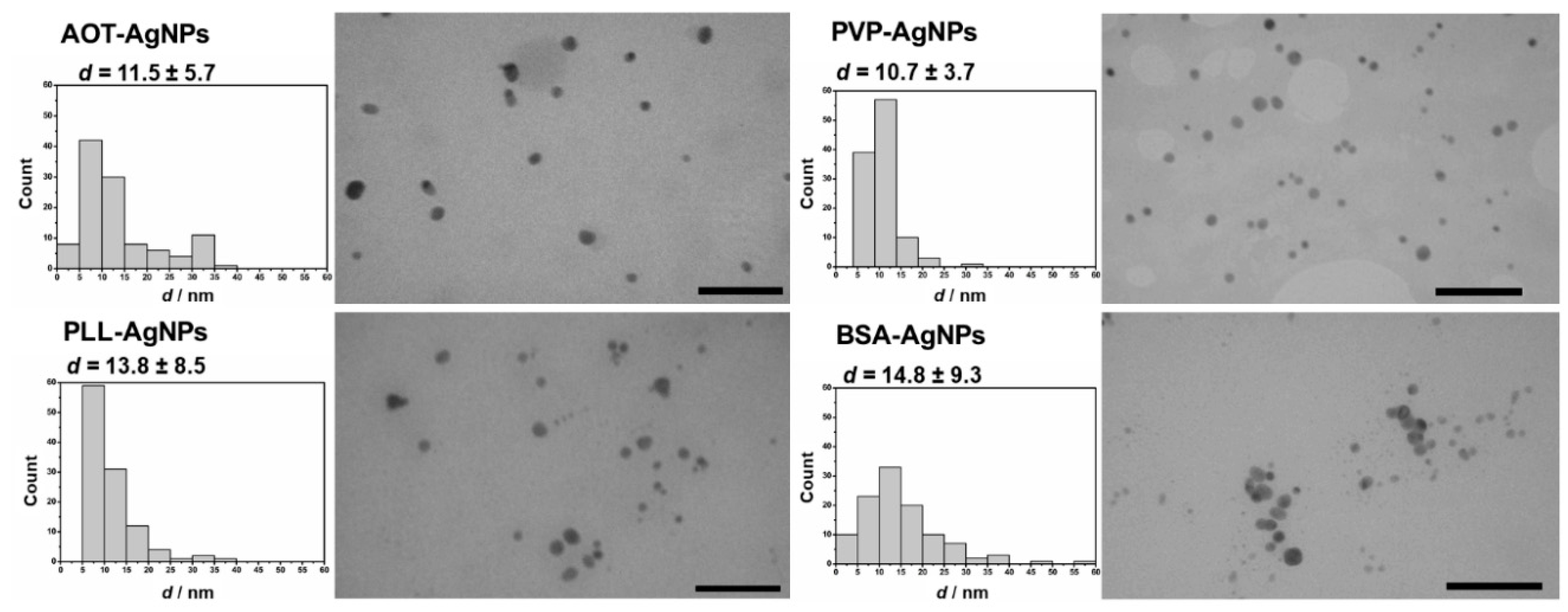
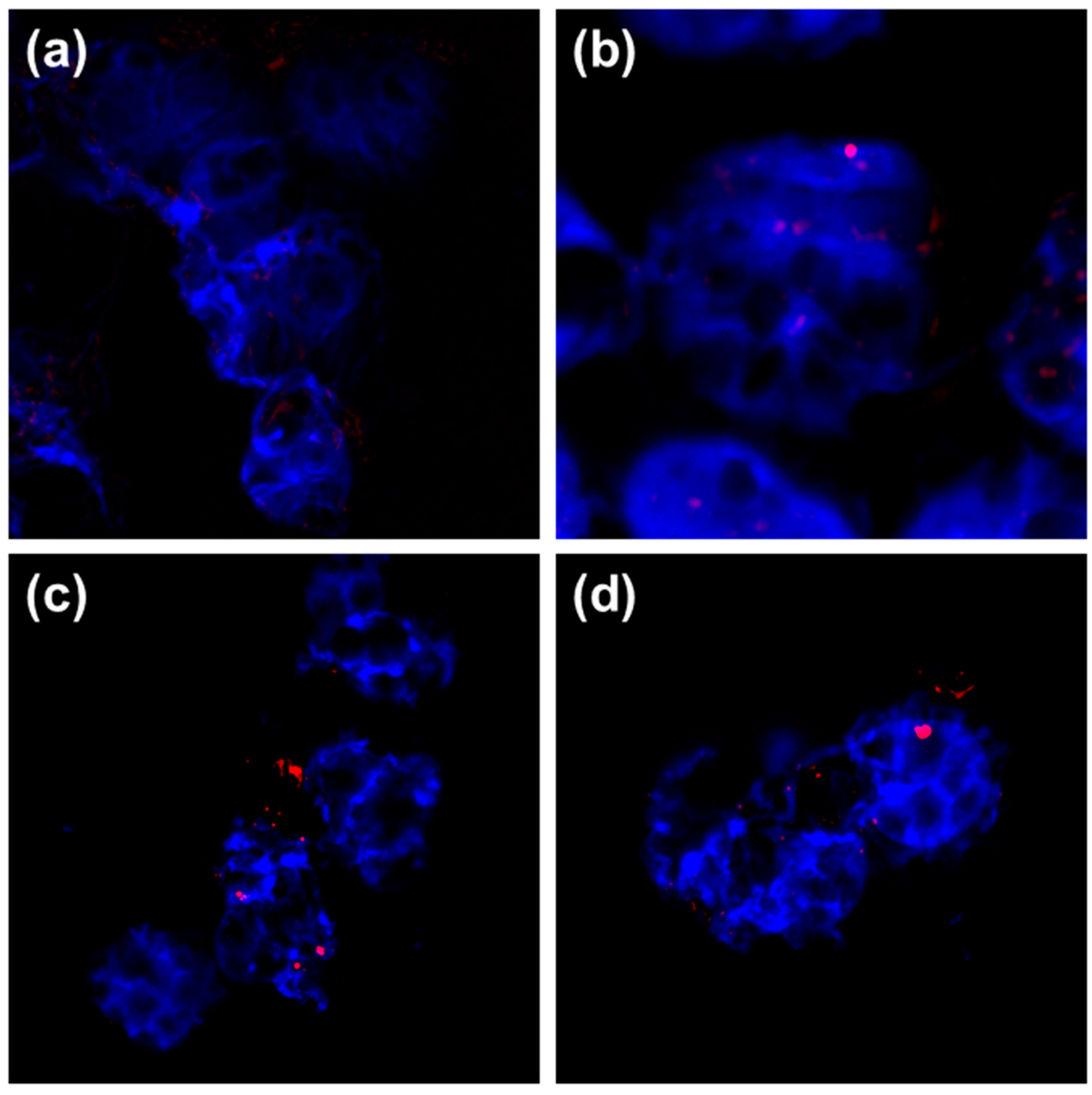
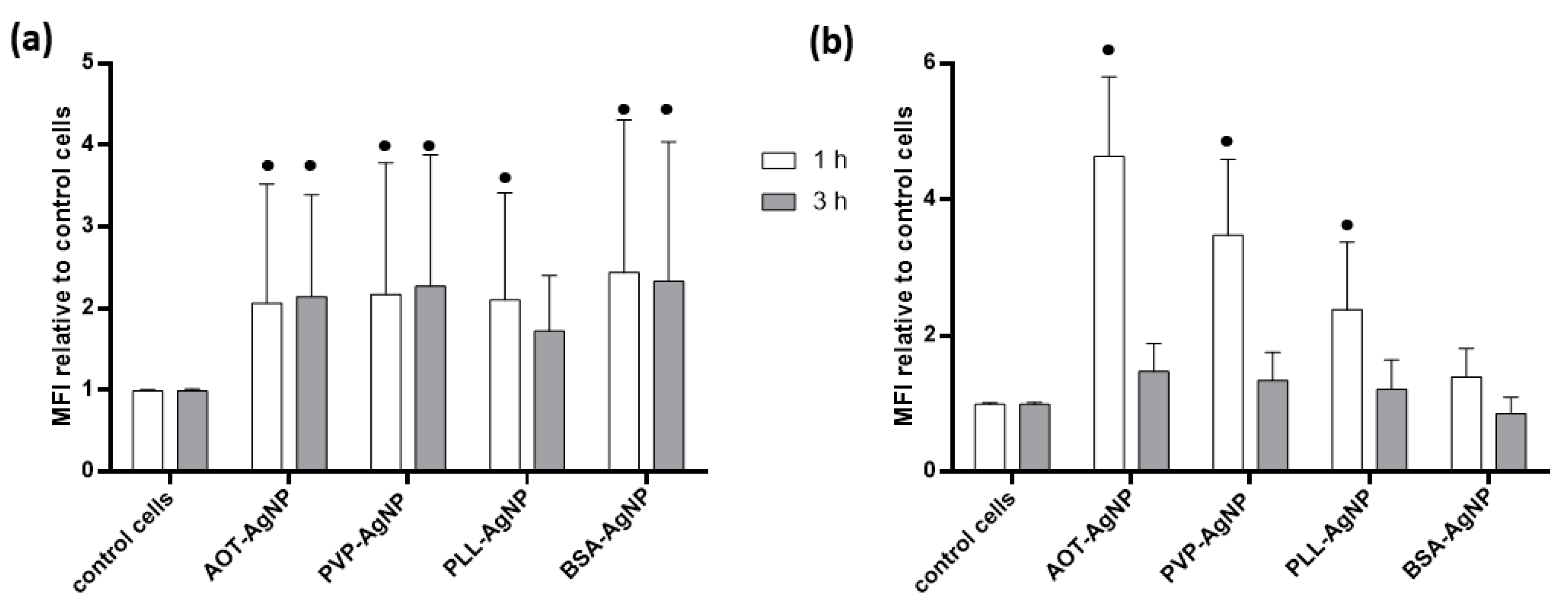
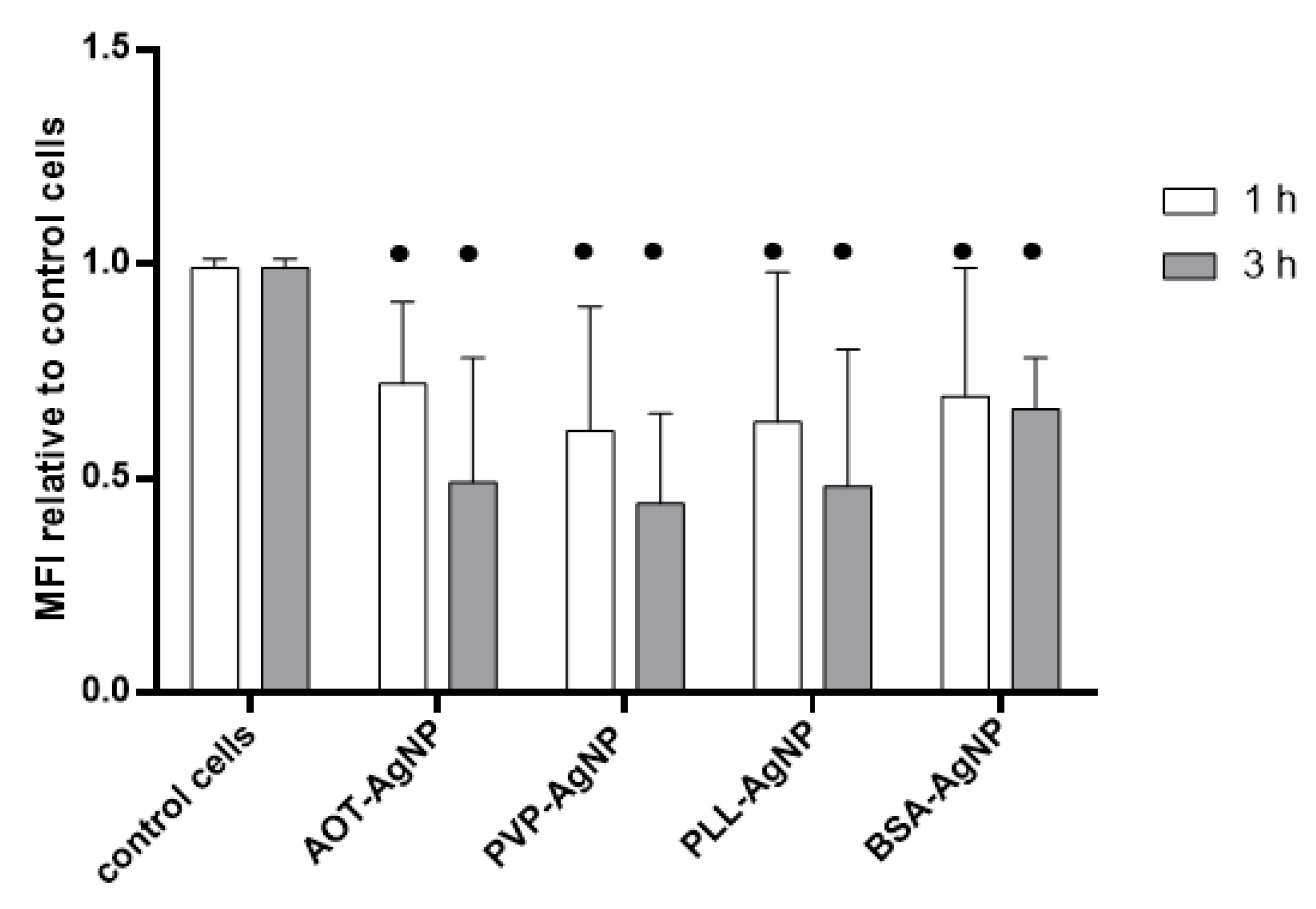
| NPs Type | dH (nm) | % Mean Volume | ζ Potential (mV) | % Ag+ |
|---|---|---|---|---|
| AOT-AgNPs | 14.9 ± 4.5 | 92.2 | −39.8 ± 3.8 | 0.4 |
| 28.4 ± 6.7 | 7.8 | |||
| PLL-AgNPs | 5.8 ± 1.6 | 94.8 | −19.3 ± 2.6 | 0.4 |
| 29.8 ± 6.1 | 5.2 | |||
| PVP-AgNPs | 8.8 ± 0.9 | 95.7 | 40.6 ± 1.5 | 1.1 |
| 52.6 ± 16.3 | 4.3 | |||
| BSA-AgNPs | 14.1 ± 3.3 | 86.3 | −10.6 ± 3.2 | 0.8 |
| 58.4 ± 18.1 | 13.7 |
| NPs Type | PBS | PBS + hPBMC | |||
|---|---|---|---|---|---|
| dH, nm (% Mean Volume) | ζ Potential (mV) | % Ag+ | μg Proteins/mg Ag | % Ag+ | |
| AOT-AgNPs | 32.4 ± 7.9 (15%) 328.3 ± 28.7 (85%) | −19.1 ± 4.2 | 0.3 | 796 ± 58 | 0.1 |
| PVP-AgNPs | 12.7 ± 8.1 (8%) 408.9 ± 67.7 (92%) | −13.7 ± 3.3 | 0.1 | 714 ± 43 | 0.3 |
| PLL-AgNPs | 12.8 ± 6.3 (18%) 512.5 ± 91.4 (82%) | −14.1 ± 2.5 | 0.8 | 803 ± 62 | 0.4 |
| BSA-AgNPs | 11.8 ± 4.6 (2%) 198.7 ± 56.4 (98%) | −15.4 ± 2.8 | 0.7 | 842 ± 74 | 0.2 |
| AgNPs Type | Dose, mg Ag/L | TL (µm) | TI (%DNA in Tail) | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Range | Mean ± SD | Median | Range | ||
| Control | 0 | 16.80 ± 4.27 | 16.25 | 8.75–31.25 | 0.30 ± 0.61 | 0.07 | 0.00–8.51 |
| AOT-AgNPs | 0.2 | 19.07 ± 5.00 * | 17.29 | 10.83–36.67 | 0.49 ± 0.55 * | 0.31 | 0.00–3.03 |
| 1 | 18.45 ± 4.19 * | 17.50 | 11.67–40.83 | 0.70 ± 0.94 * | 0.38 | 0.00–5.84 | |
| BSA-AgNPs | 0.2 | 17.55 ± 4.01 | 16.67 | 10.83–34.17 | 0.51 ± 0.58 * | 0.30 | 0.00–2.97 |
| 1 | 19.19 ± 4.74 * | 17.92 | 10.42–34.58 | 0.88 ± 0.91 * | 0.61 | 0.00–6.33 | |
| PLL-AgNPs | 0.2 | 18.20 ± 3.90 * | 16.67 | 12.08–30.83 | 0.31 ± 0.33 | 0.20 | 0.00–1.91 |
| 1 | 18.70 ± 4.54 * | 17.50 | 12.08–34.58 | 0.61 ± 0.73 * | 0.39 | 0.00–4.50 | |
| PVP-AgNPs | 0.2 | 17.06 ± 3.66 | 16.25 | 10.00–30.00 | 0.26 ± 0.35 | 0.09 | 0.00–2.39 |
| 1 | 18.40 ± 4.50 * | 17.08 | 12.08–31.17 | 0.49 ± 0.58 * | 0.27 | 0.00–3.32 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuković, B.; Milić, M.; Dobrošević, B.; Milić, M.; Ilić, K.; Pavičić, I.; Šerić, V.; Vrček, I.V. Surface Stabilization Affects Toxicity of Silver Nanoparticles in Human Peripheral Blood Mononuclear Cells. Nanomaterials 2020, 10, 1390. https://doi.org/10.3390/nano10071390
Vuković B, Milić M, Dobrošević B, Milić M, Ilić K, Pavičić I, Šerić V, Vrček IV. Surface Stabilization Affects Toxicity of Silver Nanoparticles in Human Peripheral Blood Mononuclear Cells. Nanomaterials. 2020; 10(7):1390. https://doi.org/10.3390/nano10071390
Chicago/Turabian StyleVuković, Barbara, Marija Milić, Blaženka Dobrošević, Mirta Milić, Krunoslav Ilić, Ivan Pavičić, Vatroslav Šerić, and Ivana Vinković Vrček. 2020. "Surface Stabilization Affects Toxicity of Silver Nanoparticles in Human Peripheral Blood Mononuclear Cells" Nanomaterials 10, no. 7: 1390. https://doi.org/10.3390/nano10071390
APA StyleVuković, B., Milić, M., Dobrošević, B., Milić, M., Ilić, K., Pavičić, I., Šerić, V., & Vrček, I. V. (2020). Surface Stabilization Affects Toxicity of Silver Nanoparticles in Human Peripheral Blood Mononuclear Cells. Nanomaterials, 10(7), 1390. https://doi.org/10.3390/nano10071390






