Effects of Annealing Temperature on the Crystal Structure, Morphology, and Optical Properties of Peroxo-Titanate Nanotubes Prepared by Peroxo-Titanium Complex Ion
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Materials
2.2. Characterization
3. Results and Discussion
3.1. Thermal Properties of Materials
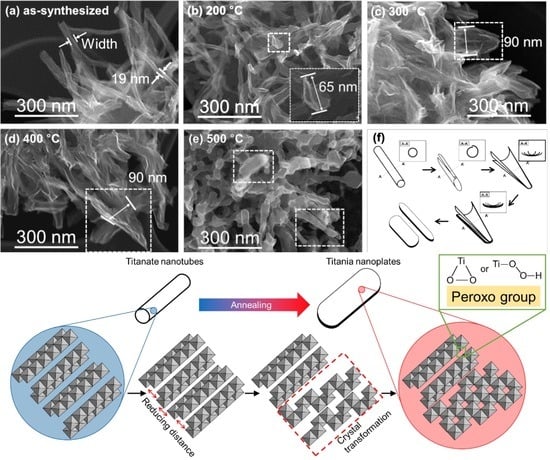
3.2. Morphology of Materials
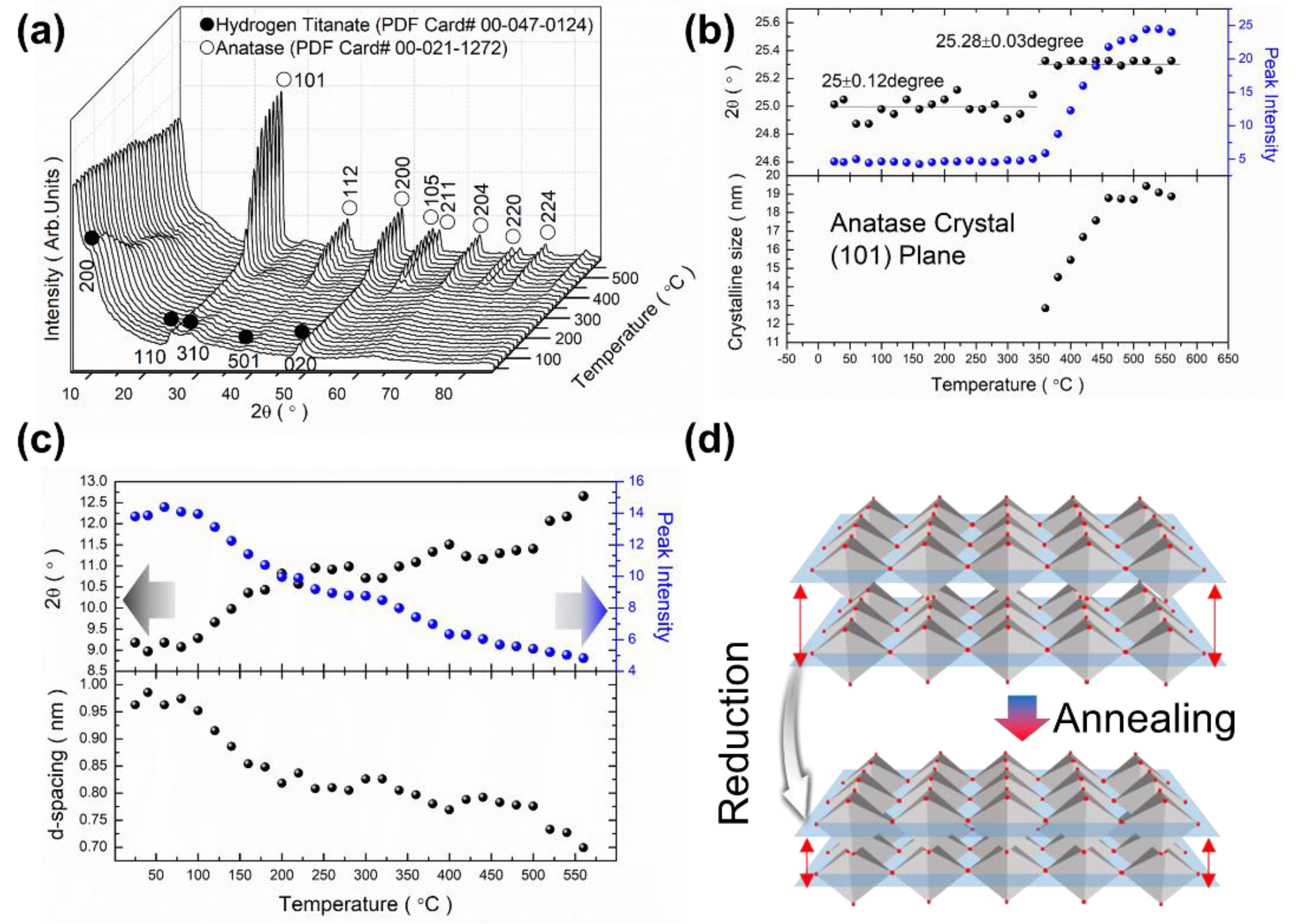
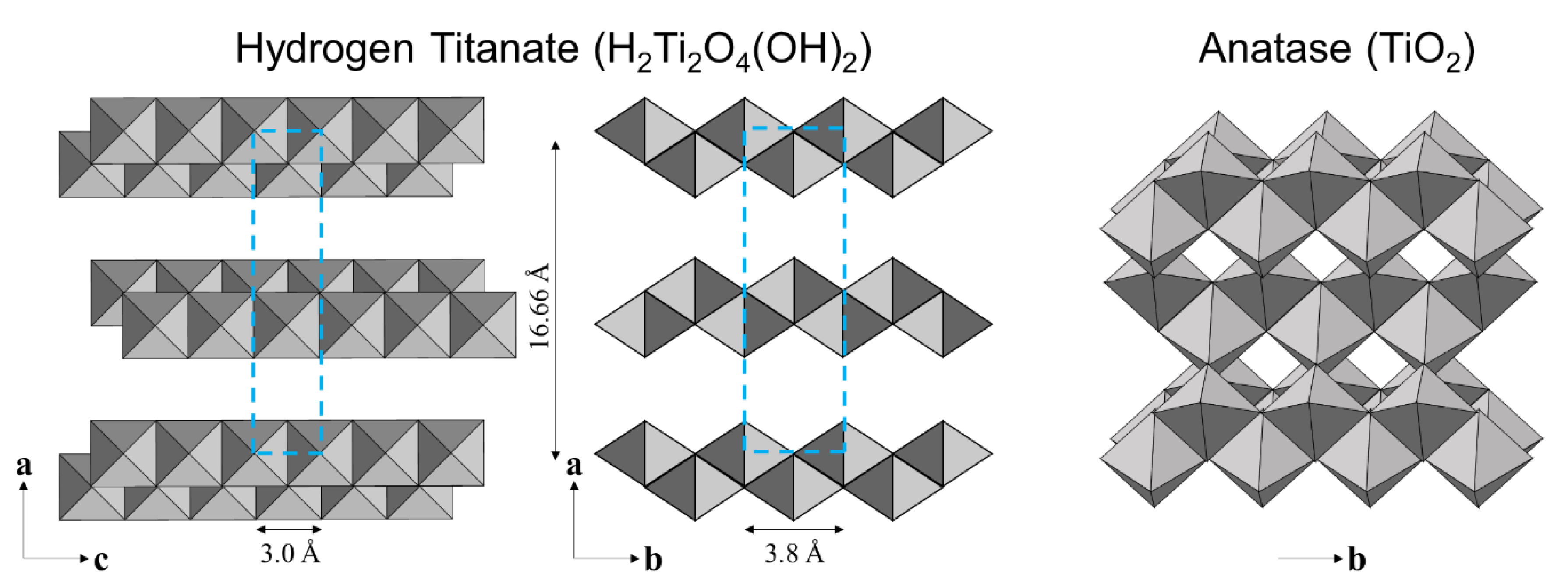
3.3. Crystallographic Characteristics of Materials
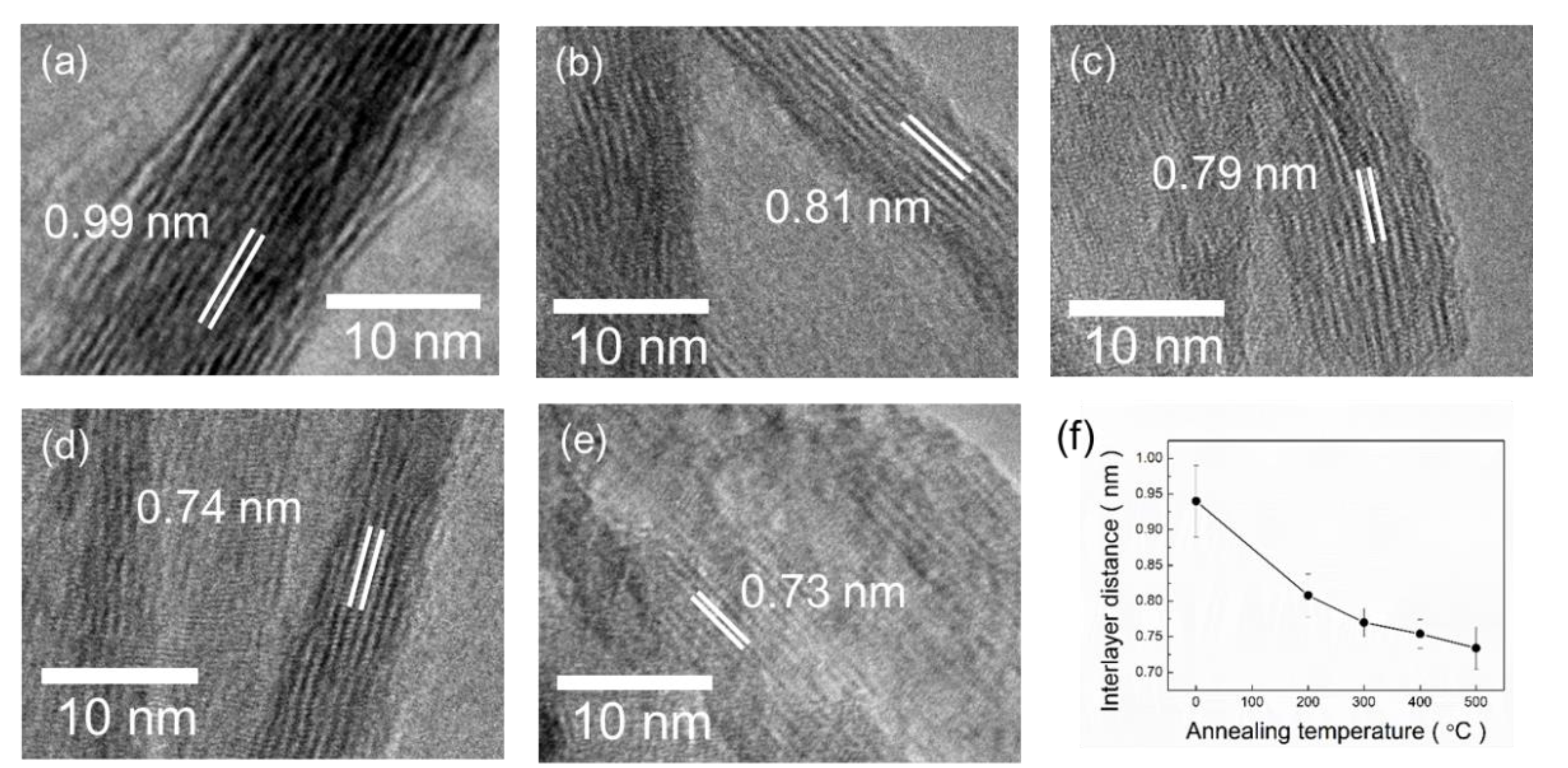
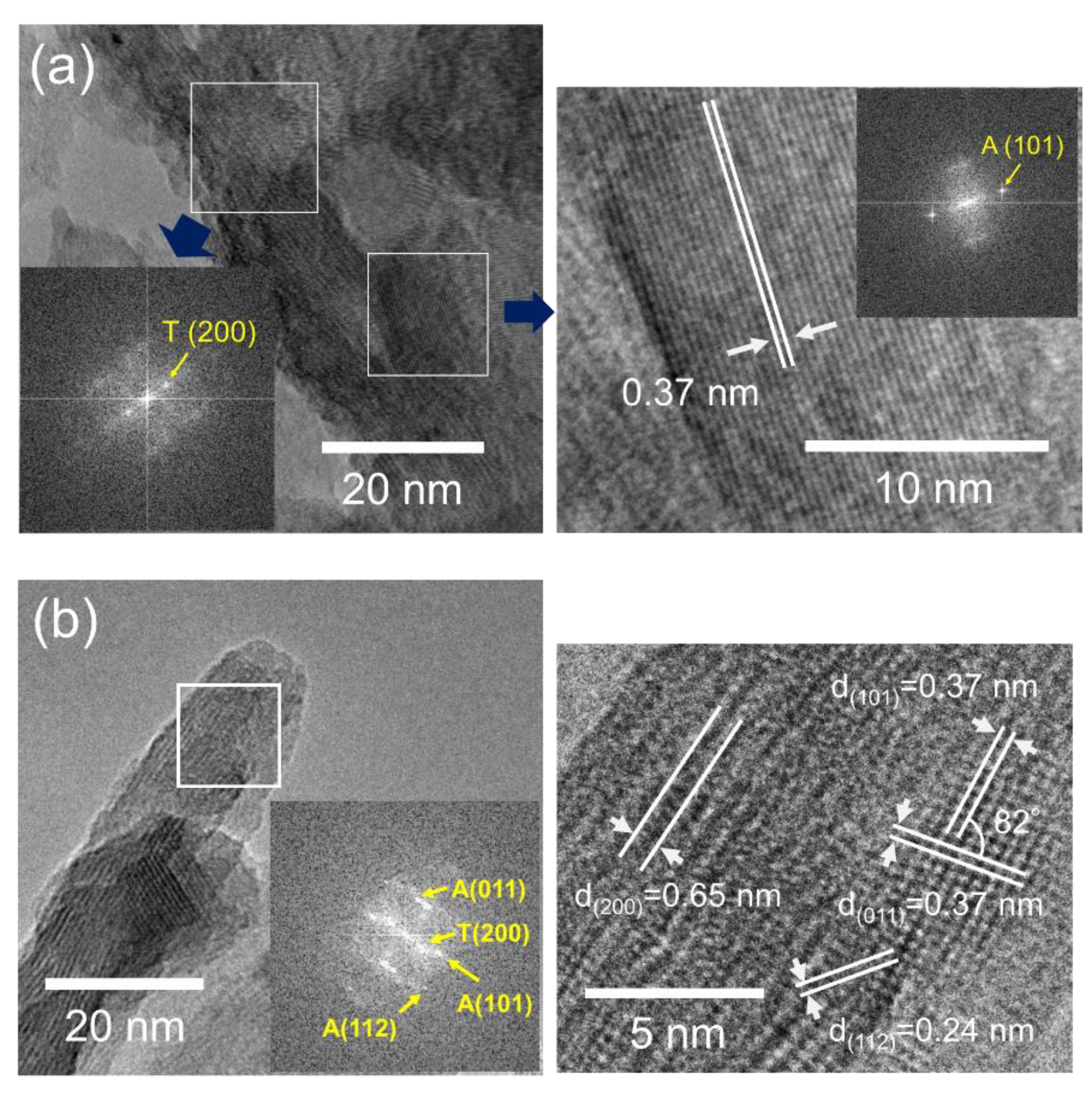
3.4. Transmission Electron Microscopy
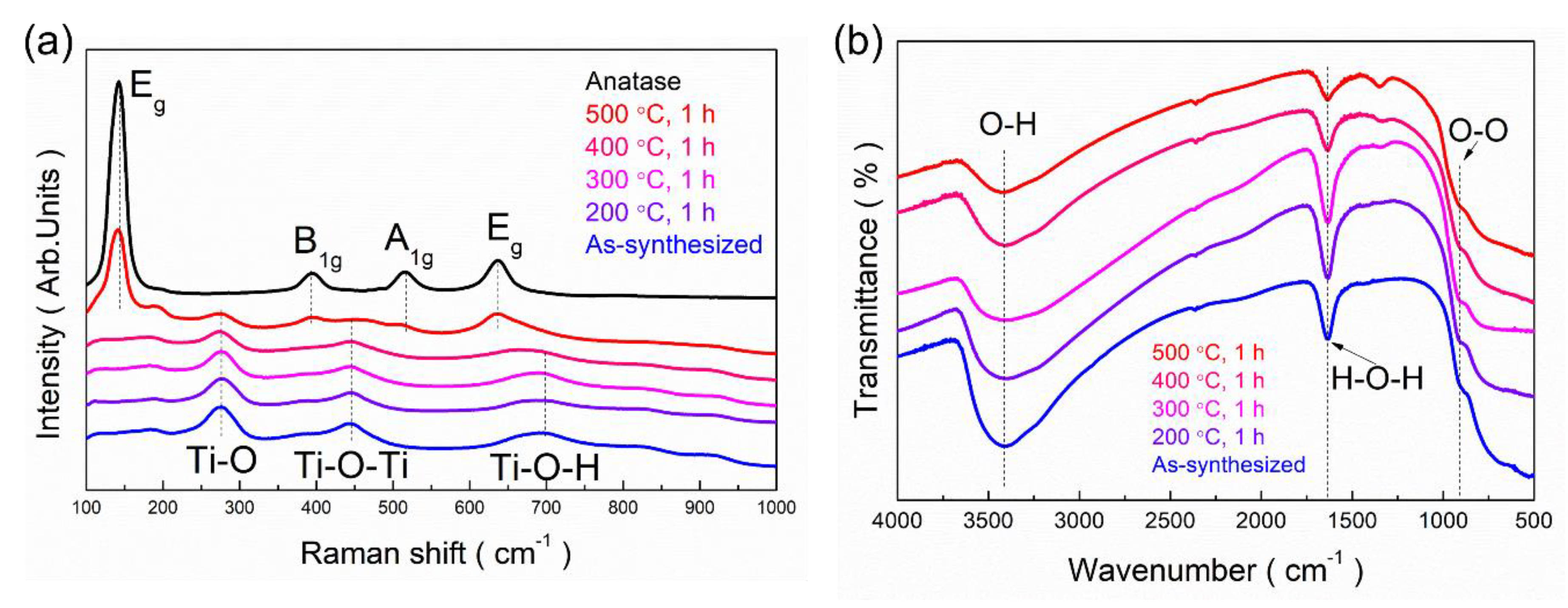
3.5. Bonding Characteristics
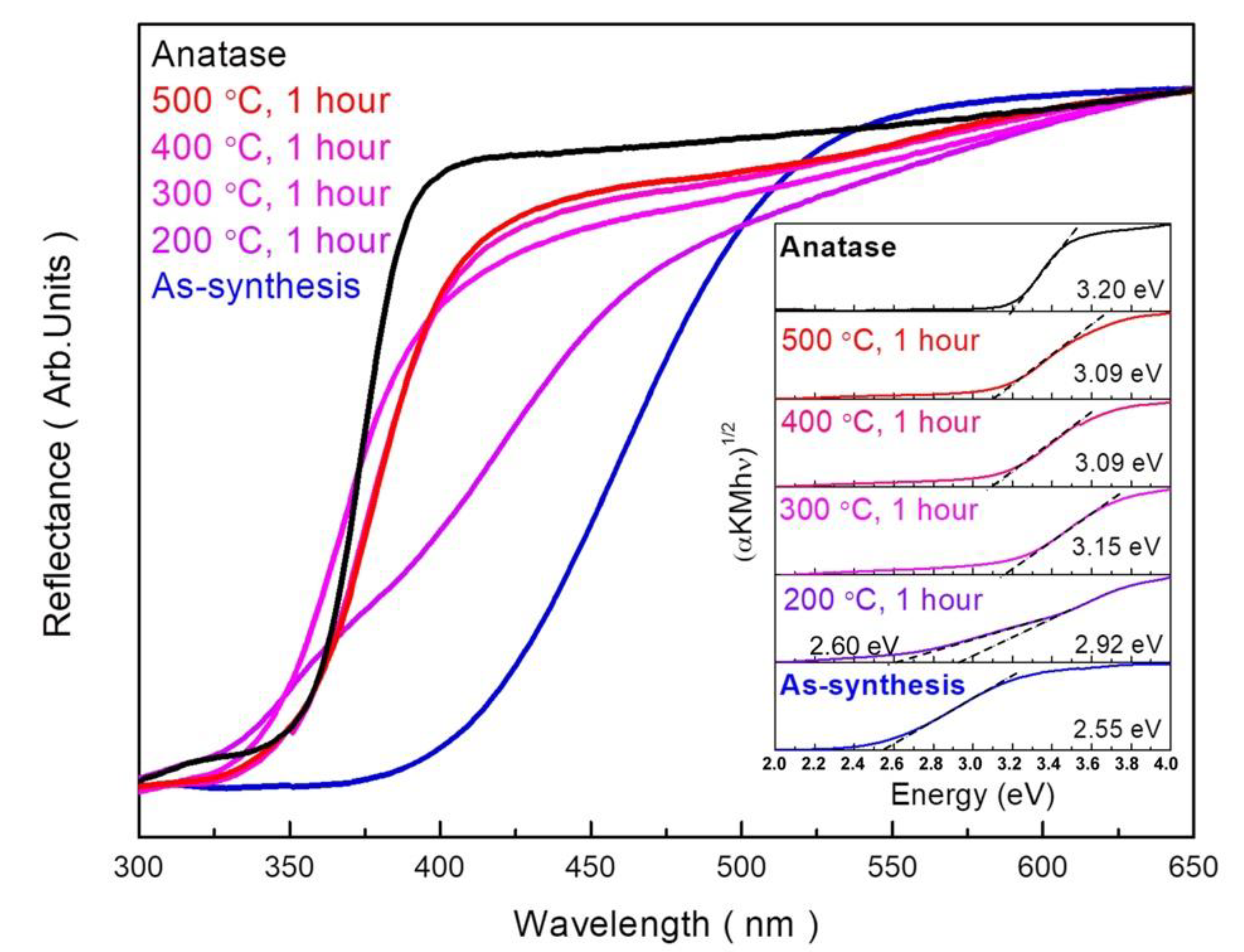
3.6. Optical Properties of Materials
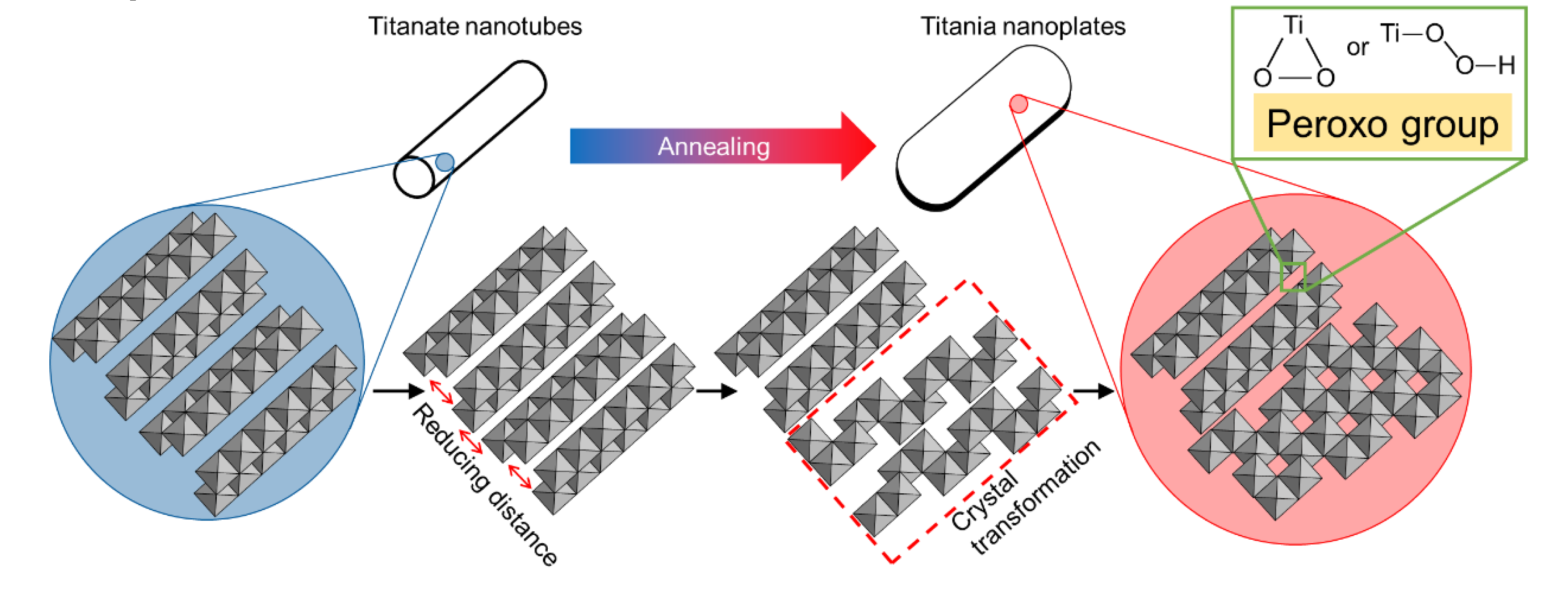
3.7. Formation Mechanism of Peroxo-Modified Anatase Crystal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joshi, U.A.; Yoon, S.; Balk, S.; Lee, J.S. Surfactant-free hydrothermal synthesis of highly tetragonal barium titanate nanowires: A structural investigation. J. Phys. Chem. B 2006, 110, 12249–12256. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, X.; Li, Y. Large-scale synthesis of sodium and potassium titanate nanobelts. Inorg. Chem. 2002, 41, 4996–4998. [Google Scholar] [CrossRef]
- Kolen’ko, Y.V.; Kovnir, K.A.; Gavrilov, A.I.; Garshev, A.V.; Frantti, J.; Lebedev, O.I.; Churagulov, B.R.; Van Tendeloo, G.; Yoshimura, M. Hydrothermal synthesis and characterization of nanorods of various titanates and titanium dioxide. J. Phys. Chem. B 2006, 110, 4030–4038. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, T.; Sekino, T.; Hoson, A.; Hiramatsu, M.; Niihara, K. Formation of Titanium Oxide Nanotube. Langmuir 2002, 14, 3160–3163. [Google Scholar] [CrossRef]
- Xiao, N.; Li, Z.; Liu, J.; Gao, Y. Effects of calcination temperature on the morphology, structure and photocatalytic activity of titanate nanotube thin films. Thin Solid Films 2010, 519, 541–548. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, F.C. Protonated titanates and TiO2 nanostructured materials: Synthesis, properties, and applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Hur, S.G.; Choy, J.-H.; Choi, W.; Hwang, S.-J.; Park, H.; Kim, T.W. Heterostructured Visible-Light-Active Photocatalyst of Chromia-Nanoparticle-Layered Titanate. Adv. Funct. Mater. 2007, 17, 307–314. [Google Scholar] [CrossRef]
- Tang, Y.; Lai, Y.; Gong, D.; Goh, K.H.; Lim, T.T.; Dong, Z.; Chen, Z. Ultrafast synthesis of layered titanate microspherulite particles by electrochemical spark discharge spallation. Chem. A Eur. J. 2010, 16, 7704–7708. [Google Scholar] [CrossRef]
- Wang, J.M.; Niu, H.Y.; Wei, F.S.; Shi, Y.L.; Cai, Y.Q. Adsorption behavior of arsenic onto protonated titanate nanotubes prepared via hydrothermal method. Microporous Mesoporous Mater. 2009, 122, 28–35. [Google Scholar] [CrossRef]
- Nikolskaia, A.; Vildanova, M.; Kozlov, S.; Tsvetkov, N.; Larina, L.; Shevaleevskiy, O. Charge Transfer in Mixed-Phase TiO2 Photoelectrodes for Perovskite Solar Cells. Sustainability 2020, 12, 788. [Google Scholar] [CrossRef]
- Etacheri, V.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. Oxygen rich titania: A dopant free, high temperature stable, and visible-light active anatase photocatalyst. Adv. Funct. Mater. 2011, 21, 3744–3752. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Stetsyshyn, Y. Stability of Halloysite, Imogolite, and Boron Nitride Nanotubes in Solvent Media. Appl. Sci. 2018, 8, 1068. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Su, B.L. Titanium oxide nanotubes, nanofibers and nanowires. Colloids Surf. A Physicochem. Eng. Asp. 2004, 241, 173–183. [Google Scholar] [CrossRef]
- Seo, H.K.; Kim, G.S.; Ansari, S.G.; Kim, Y.S.; Shin, H.S.; Shim, K.H.; Suh, E.K. A study on the structure/phase transformation of titanate nanotubes synthesized at various hydrothermal temperatures. Sol. Energy Mater. Sol. Cells 2008, 92, 1533–1539. [Google Scholar] [CrossRef]
- Gang, L.I.; Zhongqing, L.I.U.; Xin, Y.A.N.; Zhao, Z. Preparation of Titania Nanotube Arrays by Hydrothermal Reaction in Combination with Anodic Aluminum Oxide Template Attached to Aluminum Substrate. Chin. J. Catal. 2008, 29, 680–682. [Google Scholar]
- Hernandez, B.A.; Chang, K.S.; Fisher, E.R.; Dorhout, P.K. Sol-gel template synthesis and characterization of BaTiO3 and PbTiO3 nanotubes. Chem. Mater. 2002, 14, 480–482. [Google Scholar] [CrossRef]
- Kasuga, T. Formation of titanium oxide nanotubes using chemical treatments and their characteristic properties. Thin Solid Films 2006, 496, 141–145. [Google Scholar] [CrossRef]
- Wang, W.; Varghese, O.K.; Paulose, M.; Grimes, C.A.; Wang, Q.; Dickey, E.C. A study on the growth and structure of titania nanotubes. J. Mater. Res. 2004, 19, 417–422. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, H.; Liu, H.; Wang, Y.; An, T.; Cai, W.; Yang, H.; Yao, X.; Zhu, G.; Webb, R.; et al. Vapor-Phase Hydrothermal Growth of Novel Segmentally Configured Nanotubular Crystal Structure. Small 2013, 9, 3043–3050. [Google Scholar] [CrossRef]
- Kobayashi, M.; Petrykin, V.V.; Kakihana, M.; Tomita, K.; Yoshimura, M. One-step synthesis of TiO2(B) nanoparticles from a water-soluble titanium complex. Chem. Mater. 2007, 19, 5373–5376. [Google Scholar] [CrossRef]
- Sutradhar, N.; Sinhamahapatra, A.; Kumar Pahari, S.; Bajaj, H.C.; Baran Panda, A. Room temperature synthesis of protonated layered titanate sheets using peroxo titanium carbonate complex solution. Chem. Commun. 2011, 47, 7731–7733. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Jeong, R.H.; Kim, D.I.; Yu, J.H.; Nam, S.H.; Boo, J.H. Facile synthesis of amorphous Ti-peroxo complex for photocatalytic activity under visible-light irradiation. J. Clean. Prod. 2019, 239, 118013. [Google Scholar] [CrossRef]
- Kong, X.; Zeng, C.; Wang, X.; Huang, J.; Li, C.; Fei, J.; Li, J.; Feng, Q. Ti-O-O coordination bond caused visible light photocatalytic property of layered titanium oxide. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, C.; Zhao, J. Mechanism of photodecomposition of H2O2 on TiO2 surfaces under visible light irradiation. Langmuir 2001, 17, 4118–4122. [Google Scholar] [CrossRef]
- Tighineanu, A.; Ruff, T.; Albu, S.; Hahn, R.; Schmuki, P. Conductivity of TiO2 nanotubes: Influence of annealing time and temperature. Chem. Phys. Lett. 2010, 494, 260–263. [Google Scholar] [CrossRef]
- Park, H.; Joo, S.; Choi, J.-P.; Kim, W.-B. The Preparation of Dye-Sensitized Solar Cell Paste Used the Peroxo Titanium Complex and Characteristics by Annealing Temperature. J. Korean Powder Metall. Inst. 2015, 22, 396–402. [Google Scholar] [CrossRef]
- Kalaivani, T.; Anilkumar, P. Role of Temperature on the Phase Modification of TiO2 Nanoparticles Synthesized by the Precipitation Method. Silicon 2018, 10, 1679–1686. [Google Scholar] [CrossRef]
- Galatsis, K.; Li, Y.X.; Wlodarski, W.; Comini, E.; Faglia, G.; Sberveglieri, G. Semiconductor MoO3-TiO2 thin film gas sensors. Sens. Actuators B Chem. 2001, 77, 472–477. [Google Scholar] [CrossRef]
- Cortés-Jácome, M.A.; Ferrat-Torres, G.; Ortiz, L.F.F.; Angeles-Chávez, C.; López-Salinas, E.; Escobar, J.; Mosqueira, M.L.; Toledo-Antonio, J.A. In situ thermo-Raman study of titanium oxide nanotubes. Catal. Today 2007, 126, 248–255. [Google Scholar] [CrossRef]
- Yoshida, R.; Suzuki, Y.; Yoshikawa, S. Effects of synthetic conditions and heat-treatment on the structure of partially ion-exchanged titanate nanotubes. Mater. Chem. Phys. 2005, 91, 409–416. [Google Scholar] [CrossRef]
- Razali, M.H.; Mohd Noor, A.F.; Mohamed, A.R.; Sreekantan, S. Morphological and structural studies of titanate and titania nanostructured materials obtained after heat treatments of hydrothermally produced layered titanate. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Sekino, T. Synthesis and Applications of Titanium Oxide Nanotubes. Top. Appl. Phys. 2010, 117, 17–32. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, Z.; Zhang, J.; Guo, X.; Yang, J.; Li, W.; Wang, X.; Zhang, Z. Effect of annealing temperature on morphology, structure and photocatalytic behavior of nanotubed H2Ti2O4(OH)2. J. Mol. Catal. A Chem. 2004, 217, 203–210. [Google Scholar] [CrossRef]
- Tada, M.; Tomita, K.; Petrykin, V.; Kakihana, M. Preparation and characterization of citratoperoxotitanate barium compound for BaTiO3 synthesis. Solid State Ion. 2002, 151, 293–297. [Google Scholar] [CrossRef]
- Mitchell, D.R.G. DiffTools: Electron diffraction software tools for DigitalMicrographTM. Microsc. Res. Tech. 2008, 71, 588–593. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Rane, K.S.; Mhalsiker, R.; Yin, S.; Sato, T.; Cho, K.; Dunbar, E.; Biswas, P. Visible light-sensitive yellow TiO2-xNx and Fe-N co-doped Ti1-yFeyO2-xNx anatase photocatalysts. J. Solid State Chem. 2006, 179, 3033–3044. [Google Scholar] [CrossRef]
- Kong, L.; Wang, C.; Zheng, H.; Zhang, X.; Liu, Y. Defect-Induced Yellow Color in Nb-Doped TiO2 and Its Impact on Visible-Light Photocatalysis. J. Phys. Chem. C 2015, 119, 16623–16632. [Google Scholar] [CrossRef]
- Ferreira, O.P.; Souza Filho, A.G.; Mendes Filho, J.; Alves, O.L. Unveiling the structure and composition of titanium oxide nanotubes through ion exchange chemical reactions and thermal decomposition processes. J. Braz. Chem. Soc. 2006, 17, 393–402. [Google Scholar] [CrossRef]
- Qian, L.; Du, Z.L.; Yang, S.Y.; Jin, Z.S. Raman study of titania nanotube by soft chemical process. J. Mol. Struct. 2005, 749, 103–107. [Google Scholar] [CrossRef]
- Li, J.; Tang, Z.; Zhang, Z. Preparation and novel lithium intercalation properties of titanium oxide nanotubes. Electrochem. Solid State Lett. 2005, 8, 316–319. [Google Scholar] [CrossRef]
- Tsai, C.C.; Teng, H. Structural features of nanotubes synthesized from NaOH treatment on TiO2 with different post-treatments. Chem. Mater. 2006, 18, 367–373. [Google Scholar] [CrossRef]
- Yang, J.; Jin, Z.; Wang, X.; Li, W.; Zhang, J.; Zhang, S.; Guo, X.; Zhang, Z. Study on composition, structure and formation process of nanotube Na2Ti2O4(OH)2. J. Chem. Soc. Dalt. Trans. 2003, 4, 3898–3901. [Google Scholar] [CrossRef]
- Sugita, M.; Tsuji, M.; Abe, M. Synthetic Inorganic Ion-Exchange Materials. LVIII. Hydrothermal Synthesis of a New Layered Lithium Titanate and Its Alkali Ion Exchange. Bull. Chem. Soc. Jpn. 1990, 63, 1978–1984. [Google Scholar] [CrossRef]
- Ma, R.; Fukuda, K.; Sasaki, T.; Osada, M.; Bando, Y. Structural features of titanate nanotubes/nanobelts revealed by raman, X-ray absorption fine structure and electron diffraction characterizations. J. Phys. Chem. B 2005, 109, 6210–6214. [Google Scholar] [CrossRef]
- De Mendonça, V.R.; Lopes, O.F.; Avansi, W.; Arenal, R.; Ribeiro, C. Insights into formation of anatase TiO2 nanoparticles from peroxo titanium complex degradation under microwave-assisted hydrothermal treatment. Ceram. Int. 2019, 45, 22998–23006. [Google Scholar] [CrossRef]
- Savinkina, E.V.; Obolenskaya, L.N.; Kuzmicheva, G.M.; Kabachkov, E.N.; Gainanova, A.A.; Zubavichus, Y.V.; Murzin, V.Y.; Sadovskaya, N.V. Introduction of peroxo groups into titania: Preparation, characterization and properties of the new peroxo-containing phase. CrystEngComm 2015, 17, 7113–7123. [Google Scholar] [CrossRef]
- Nian, J.N.; Teng, H. Hydrothermal synthesis of single-crystalline anatase TiO2 nanorods with nanotubes as the precursor. J. Phys. Chem. B 2006, 110, 4193–4198. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, L.-M.; Chen, Q.; Du, G.H.; Dawson, G.; Zhou, W.Z. Formation Mechanism of H2Ti3O7 Nanotubes. Phys. Rev. Lett. 2003, 91, 256103. [Google Scholar] [CrossRef]
- Pohorecki, R.; Bridgwater, J.; Gani, M.; Gallegos, C. Chemical Engineering and Chemical Process Technology-Volume VII: Rheology-Part II; EOLSS Publications: Oxford, UK, 2010. [Google Scholar]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Goto, T.; Cho, S.; Lee, S.W.; Kakihana, M.; Sekino, T. Effects of Annealing Temperature on the Crystal Structure, Morphology, and Optical Properties of Peroxo-Titanate Nanotubes Prepared by Peroxo-Titanium Complex Ion. Nanomaterials 2020, 10, 1331. https://doi.org/10.3390/nano10071331
Park H, Goto T, Cho S, Lee SW, Kakihana M, Sekino T. Effects of Annealing Temperature on the Crystal Structure, Morphology, and Optical Properties of Peroxo-Titanate Nanotubes Prepared by Peroxo-Titanium Complex Ion. Nanomaterials. 2020; 10(7):1331. https://doi.org/10.3390/nano10071331
Chicago/Turabian StylePark, Hyunsu, Tomoyo Goto, Sunghun Cho, Soo Wohn Lee, Masato Kakihana, and Tohru Sekino. 2020. "Effects of Annealing Temperature on the Crystal Structure, Morphology, and Optical Properties of Peroxo-Titanate Nanotubes Prepared by Peroxo-Titanium Complex Ion" Nanomaterials 10, no. 7: 1331. https://doi.org/10.3390/nano10071331
APA StylePark, H., Goto, T., Cho, S., Lee, S. W., Kakihana, M., & Sekino, T. (2020). Effects of Annealing Temperature on the Crystal Structure, Morphology, and Optical Properties of Peroxo-Titanate Nanotubes Prepared by Peroxo-Titanium Complex Ion. Nanomaterials, 10(7), 1331. https://doi.org/10.3390/nano10071331