In Vitro Biological Impact of Nanocellulose Fibers on Human Gut Bacteria and Gastrointestinal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. NFC Materials
2.2. NFC Exposure Suspensions
2.3. Material Characterization
2.3.1. Surface Functional Group Density
2.3.2. Zeta Potential
2.3.3. Transmission Electron Microscopy
2.3.4. Degree of Crystallinity
2.4. Caco-2 Study
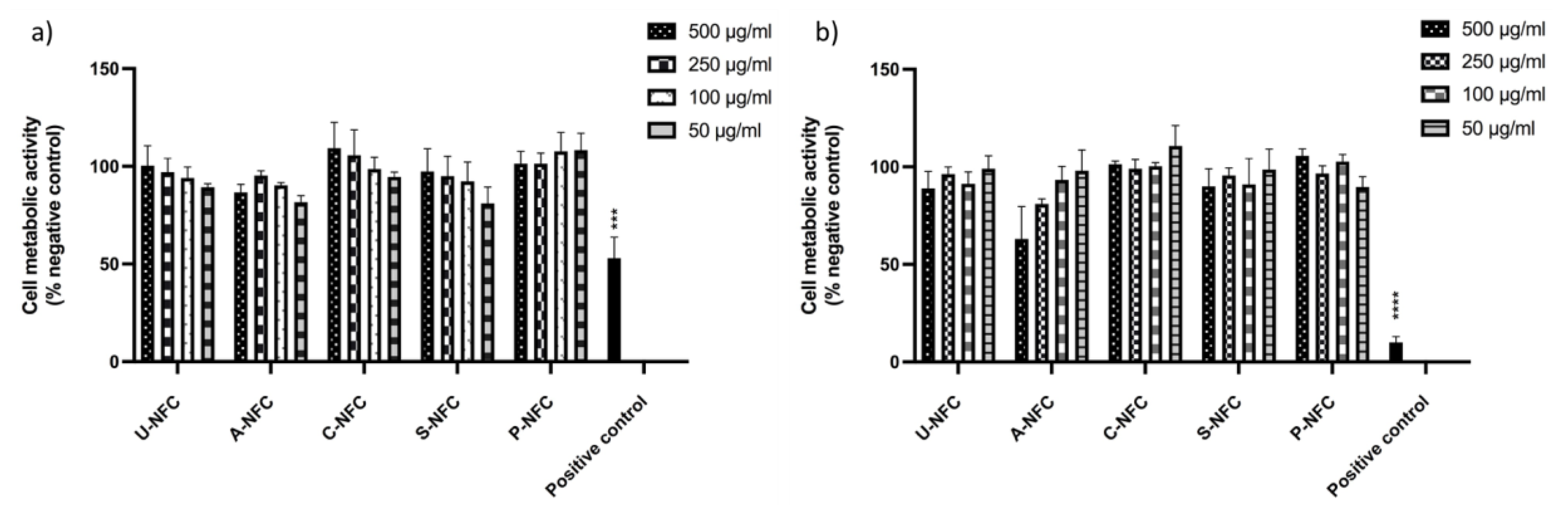
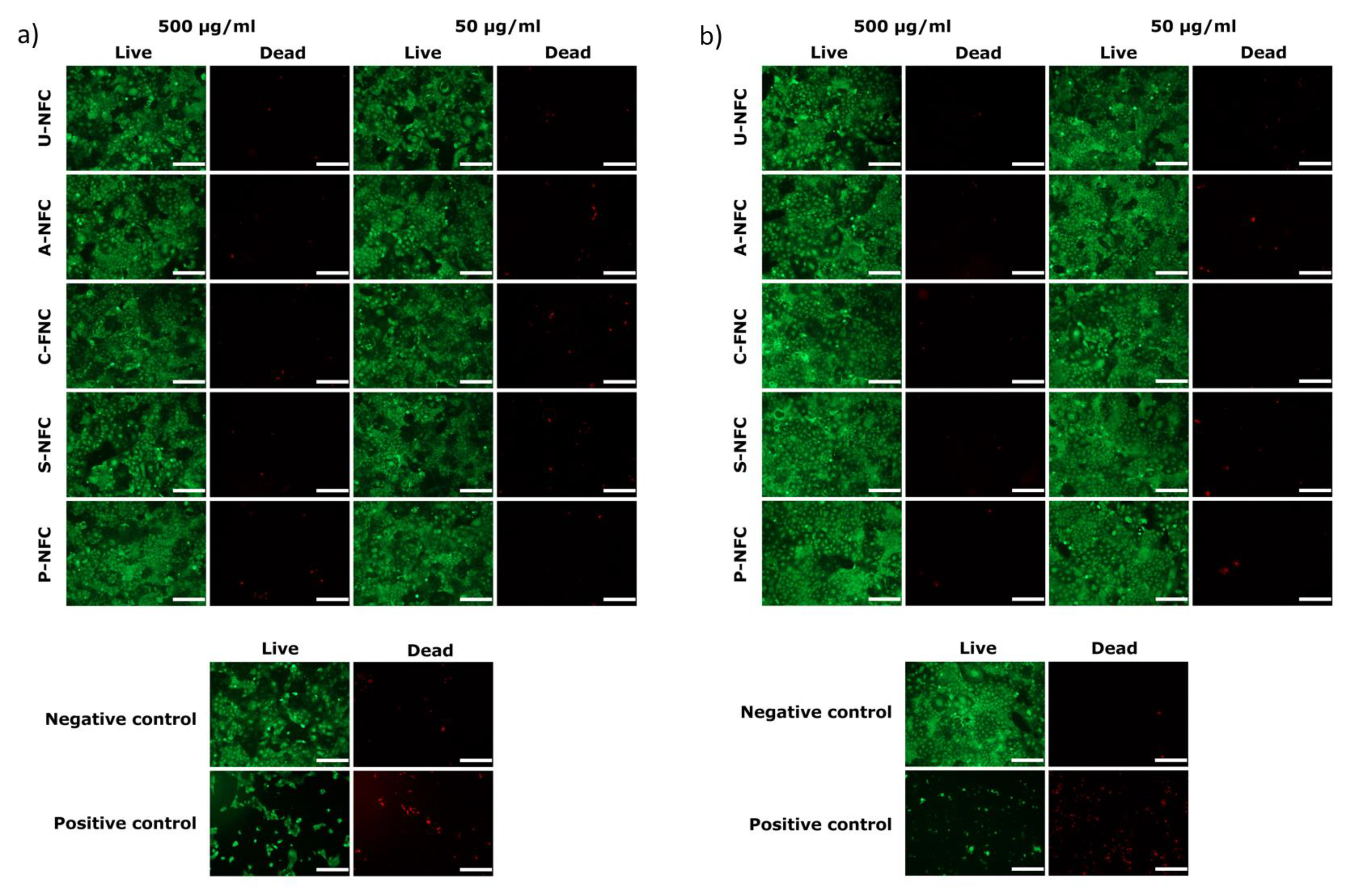
Cytotoxicity Assessment
2.5. Bacteria Study
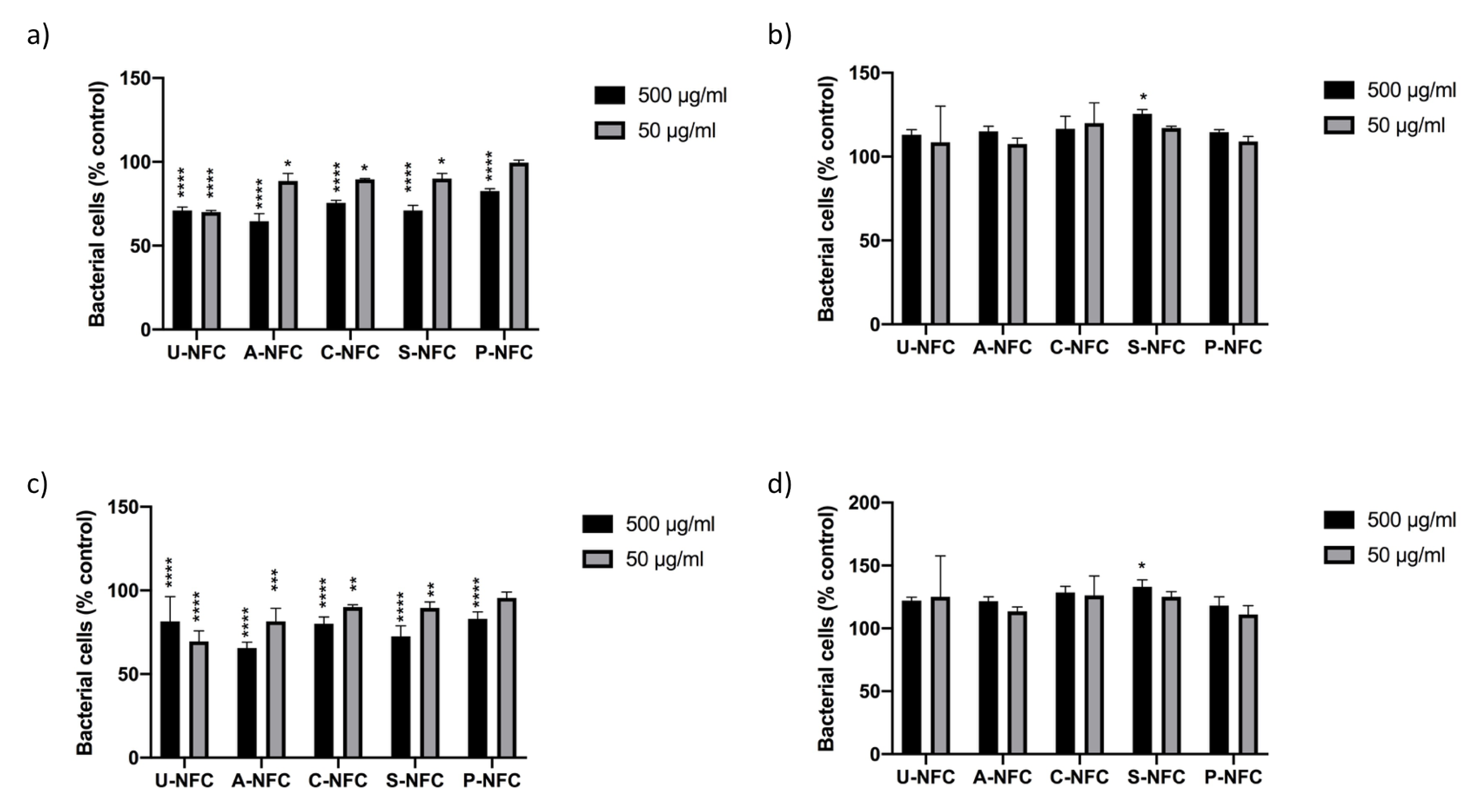
2.5.1. Measurement of Bacterial Growth in the Presence of NFC
2.5.2. Colony Forming Units (CFUs) Assay
2.6. Statistical Analysis
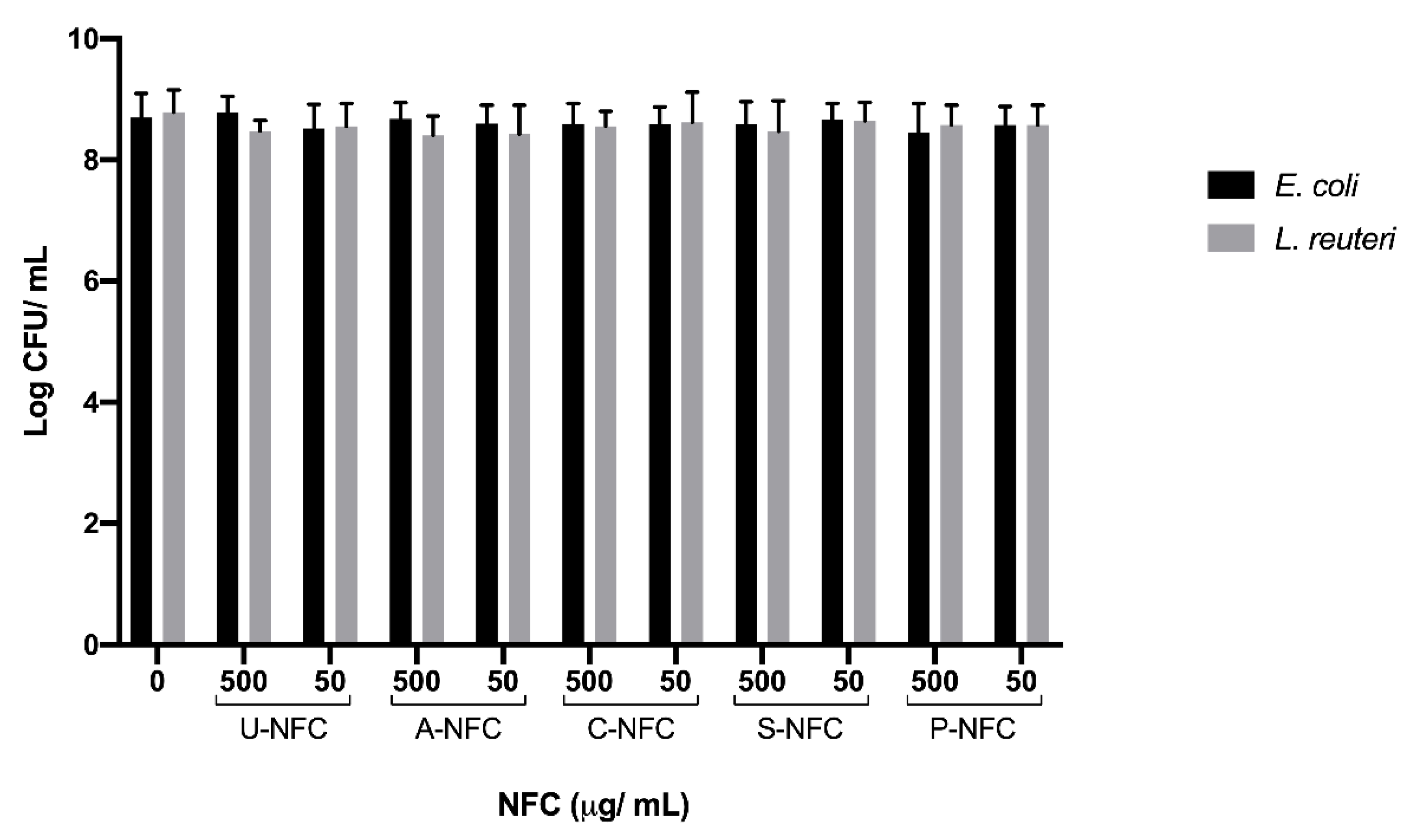
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiyah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Mondal, S. Preparation, properties and applications of nanocellulosic materials. Carbohyd. Polym. 2017, 163, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Heo, S.; Foo, M.; Chew, I.M.; Yoo, C. An insight into nanocellulose as soft condensed matter: Challenge and future prospective toward environmental sustainability. Sci. Total Environ. 2019, 650 Pt 1, 1309–1326. [Google Scholar] [CrossRef]
- Klemm, D.; Schumann, D.; Kramer, F.; Nadine, H.; Hornung, M.; Schmauder, H.; Marsch, S. Nanocelluloses as innovative polymers in research applications. Adv. Polym. Sci. 2006, 205, 49–96. [Google Scholar]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new composites materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. Engl. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef]
- Catalan, J.; Norppa, H. Safety Aspects of Bio-Based Nanomaterials. Bioengineering 2017, 4, 94. [Google Scholar] [CrossRef]
- Endes, C.; Camarero-Espinosa, S.; Mueller, S.; Foster, E.J.; Petri-Fink, A.; Rothen-Rutishauser, B.; Weder, C.; Clift, M.J. A critical review of the current knowledge regarding the biological impact of nanocellulose. J. Nanobiotechnol. 2016, 14, 78. [Google Scholar] [CrossRef]
- Shatkin, J.A.; Kim, B. Cellulose nanomaterials: Life cycle risk assessment, and environmental health and safety roadmap. Environ. Sci.-Nano 2015, 2, 477–499. [Google Scholar] [CrossRef]
- Gomez, C.; Serpa, A.; Velasquez-Cock, J.; Ganan, P.; Castro, C.; Velez, L.; Zuluaga, R. Vegetable nanocellulose in food science: A review. Food Hydrocolloid. 2016, 57, 178–186. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Saurabh, C.K.; Hossain, M.S.; Adnan, A.S.; Dungani, R.; Paridah, M.T.; Islam Sarker, M.Z.; Nurul Fazita, M.R.; Syakir, M.I.; et al. A review on nanocellulosic fibres as new material for sustainable packaging: Process and applications. Renew. Sustain. Energy Rev. 2016, 64, 823–836. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, S.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Impacts of Gut Bacteria on Human Health and Diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Pietroiusti, A.; Magrini, A.; Campagnolo, L. New frontiers in nanotoxicology: Gut microbiota/microbiome-mediated effects of engineered nanomaterials. Toxicol. Appl. Pharm. 2016, 299, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Bergin, I.L.; Witzmann, F.A. Nanoparticle toxicity by the gastrointestinal route: Evidence and knowledge gaps. Int. J. Biomed. Nanosci. Nanotechnol. 2013, 3. [Google Scholar] [CrossRef]
- Rol, F.; Belgacem, M.N.; Gandini, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Prog. Polym. Sci. 2019, 88, 241–264. [Google Scholar] [CrossRef]
- Pääkko, M.; Ankerfors, M.; Kosonen, H.; Nykanen, A.; Ahola, S.; Osterberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef]
- Hua, K.; Carlsson, D.O.; Ålander, E.; Lindström, T.; Strømme, M.; Mihranyan, A.; Ferraz, N. Translational study between structure and biological response of nanocellulose from varying sources. RSC Adv. 2014, 4, 2892–2903. [Google Scholar] [CrossRef]
- Naderi, A.; Lindstrøm, T.; Weise, C.F.; Flodberg, G.; Sundstrom, J.; Junel, K.; Erlandsson, J.; Runebjork, A. Phosphorylated nanofibrillated cellulose: Production and properties. Nord. Pulp. Pap. Res. J. 2016, 31, 20–29. [Google Scholar] [CrossRef]
- Naderi, A.; Koschella, A.; Heinze, T.; Shih, K.C.; Nieh, M.P.; Pfeifer, A.; Chang, C.C.; Erlandsson, J. Sulfoethylated nanofibrillated cellulose: Production and properties. Carbohydr. Polym. 2017, 169, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.; Ålander, E.; Lindström, T.; Mihranyan, A.; Strømme, M.; Ferraz, N. Surface chemistry of nanocellulose fibers directs monocyte/macrophage response. Biomacromolecules 2015, 16, 2787–2795. [Google Scholar] [CrossRef] [PubMed]
- White, B.; Banerjee, S.; O’Brien, S.; Turro, N.J.; Herman, I.P. Zeta-potential measurements of surfactant-wrapped individual single-walled carbon nanotubes. J. Phys. Chem. C 2007, 111, 13684–13690. [Google Scholar] [CrossRef]
- Usov, I.; Nyström, G.; Adamcik, J.; Handschin, S.; Schutz, C.; Fall, A.; Bergström, L.; Mezzenga, R. Understanding nanocellulose chirality and structure-properties relationship at the single fibril level. Nat. Commun. 2015, 6, 7564. [Google Scholar] [CrossRef]
- Ahvenainen, P.; Kontro, I.; Svedstrom, K. Comparison of sample crystallinity determination methods by X-ray diffraction for challenging cellulose I materials. Cellulose 2016, 23, 1073–1086. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Aberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef]
- Lopes, V.R.; Sanchez-Martinez, C.; Strømme, M.; Ferraz, N. In vitro biological responses to nanofibrillated cellulose by human dermal, lung and immune cells: Surface chemistry aspect. Part. Fibre Toxicol. 2017, 14, 1. [Google Scholar] [CrossRef]
- Wang, H.F.; Du, L.J.; Song, Z.M.; Chen, X.X. Progress in the characterization and safety evaluation of engineered inorganic nanomaterials in food. Nanomedicine 2013, 8, 2007–2025. [Google Scholar] [CrossRef]
- Ström, C.; Öhgren, C.; Ankefors, M. Nanocellulose as an Additive in Food-Stuff; Innventia: Stockholm, Sweden, 2013. [Google Scholar]
- Aaen, R.; Brodin, F.W.; Simon, S.; Heggset, E.B.; Syverud, K. Oil-in-Water Emulsions Stabilized by Cellulose Nanofibrils-The Effects of Ionic Strength and pH. Nanomaterials 2019, 9, 259. [Google Scholar] [CrossRef]
- Aaen, R.; Simon, S.; Brodin, F.W.; Syverud, K. The potential of TEMPO-oxidized cellulose nanofibrils as rheology modifiers in food systems. Cellulose 2019, 26, 5483–5496. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Sohal, I.S.; Lorente, L.R.; Molina, R.M.; Pyrgiotakis, G.; Stevanovic, A.; Zhang, R.J.; McClements, D.J.; Geitner, N.K.; Bousfield, D.W.; et al. Reducing Intestinal Digestion and Absorption of Fat Using a Nature-Derived Biopolymer: Interference of Triglyceride Hydrolysis by Nanocellulose. ACS Nano 2018, 12, 6469–6479. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kong, F. In vitro investigation of the influence of nano-fibrillated cellulose on lipid digestion and absorption. Int. J. Biol. Macromol. 2019, 139, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Kong, F.B. In vitro investigation of the influence of nano-cellulose on starch and milk digestion and mineral adsorption. Int. J. Biol. Macromol. 2019, 137, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kerr, W.L.; Kong, F.; Dee, D.R.; Lin, M. Influence of nano-fibrillated cellulose (NFC) on starch digestion and glucose absorption. Carbohydr. Polym. 2018, 196, 146–153. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Cao, X.Q.; Molina, R.M.; Silva, D.I.; Bhattacharya, K.; Ng, K.W.; Loo, S.C.J.; Brain, J.D.; Demokritou, P. Toxicological effects of ingested nanocellulose in in vitro intestinal epithelium and in vivo rat models. Environ. Sci.-Nano 2019, 6, 2105–2115. [Google Scholar] [CrossRef]
- Chen, Y.J.; Lin, Y.J.; Nagy, T.; Kong, F.B.; Guo, T.L. Subchronic exposure to cellulose nanofibrils induces nutritional risk by non-specifically reducing the intestinal absorption. Carbohyd. Polym. 2020, 229, 115536. [Google Scholar] [CrossRef] [PubMed]
- Park, M.V.D.Z.; Catalan, J.; Ferraz, N.; Cabellos, J.; Vanhauten, R.; Vazquez-Campos, S.; Janer, G. Development of a systematic method to assess similarity between nanomaterials for human hazard evaluation purposes—Lessons learnt. Nanotoxicology 2018, 12, 652–676. [Google Scholar] [CrossRef]
- Tomic, S.; Kokol, V.; Mihajlovic, D.; Mircic, A.; Colic, M. Native cellulose nanofibrills induce immune tolerance in vitro by acting on dendritic cells. Sci. Rep.-UK 2016, 6, 1–14. [Google Scholar]
- Stadler, M.; Dersch, P.; Heinz, D. How to Overcome the Antibiotic Crisis Facts, Challenges, Technologies and Future Perspectives Preface. Curr. Top. Microbiol. 2016, 398, V–IX. [Google Scholar]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.M.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep.-UK 2016, 6, 38610. [Google Scholar] [CrossRef]
- Chen, H.Q.; Wang, B.; Gao, D.; Guan, M.; Zheng, L.N.; Ouyang, H.; Chai, Z.F.; Zhao, Y.L.; Feng, W.Y. Broad-Spectrum Antibacterial Activity of Carbon Nanotubes to Human Gut Bacteria. Small 2013, 9, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Falco, C.Y.; Belgacem, M.N.; Bras, J. Surface cationized cellulose nanofibrils for the production of contact active antimicrobial surfaces. Carbohyd. Polym. 2016, 135, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Powell, L.C.; Khan, S.; Chinga-Carrasco, G.; Wright, C.J.; Hill, K.E.; Thomas, D.W. An investigation of Pseudomonas aeruginosa biofilm growth on novel nanocellulose fibre dressings. Carbohyd. Polym. 2016, 137, 191–197. [Google Scholar] [CrossRef]
- Jack, A.A.; Nordli, H.R.; Powell, L.C.; Powell, K.A.; Kishnani, H.; Johnsen, P.O.; Pukstad, B.; Thomas, D.W.; Chinga-Carrasco, G.; Hill, K.E. The interaction of wood nanocellulose dressings and the wound pathogen P. aeruginosa. Carbohyd. Polym. 2017, 157, 1955–1962. [Google Scholar] [CrossRef]
- Mou, K.W.; Li, J.J.; Wang, Y.Y.; Cha, R.T.; Jiang, X.Y. 2,3-Dialdehyde nanofibrillated cellulose as a potential material for the treatment of MRSA infection. J. Mater. Chem. B 2017, 5, 7876–7884. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Heitz, K.; Strømme, M.; Welch, K.; Ferraz, N. Ion-crosslinked wood-derived nanocellulose hydrogels with tunable antibacterial properties: Candidate materials for advanced wound care applications. Carbohydr. Polym. 2018, 181, 345–350. [Google Scholar] [CrossRef] [PubMed]
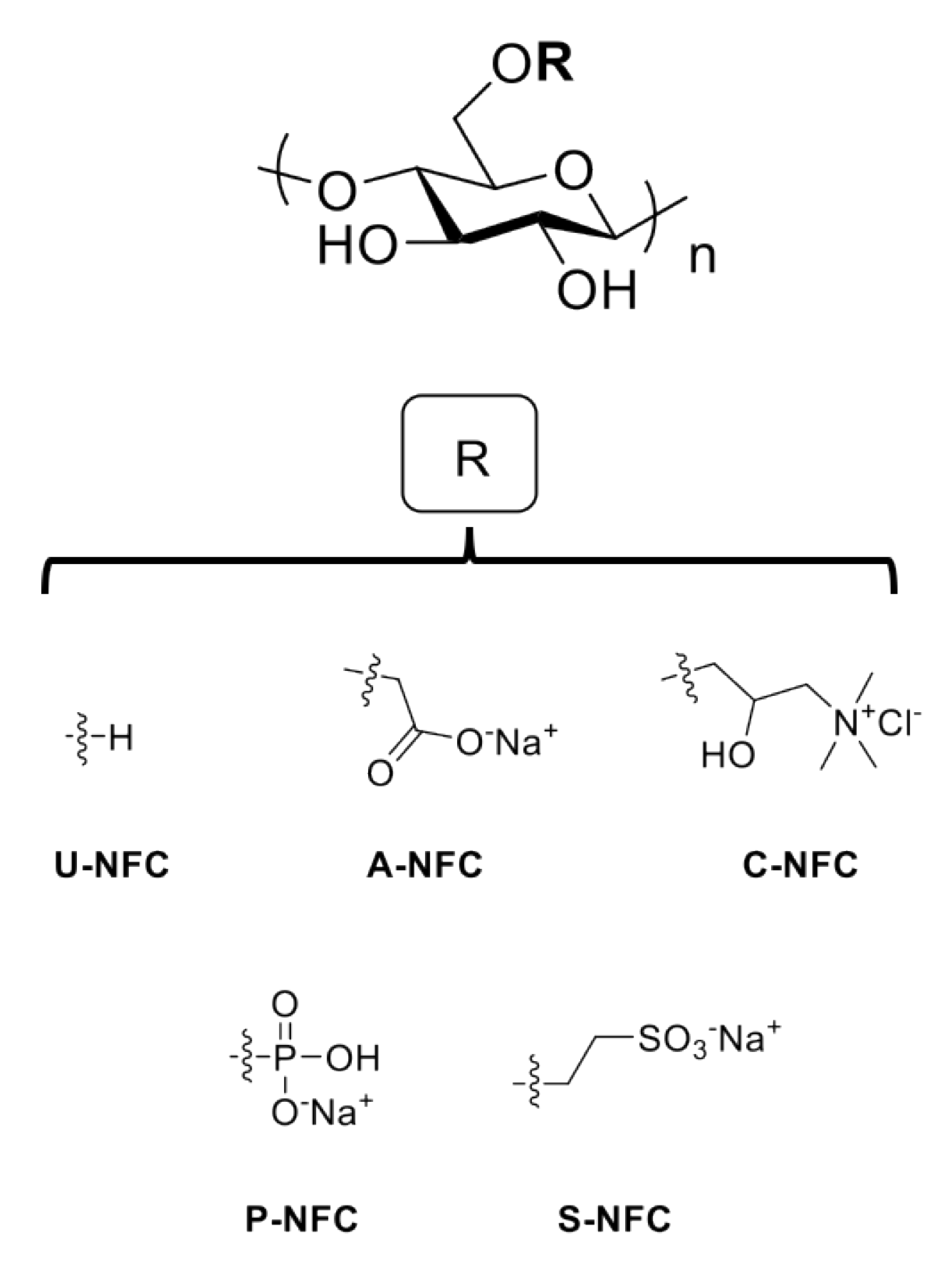

| Z-Potential (mV) | |||||||
|---|---|---|---|---|---|---|---|
| NFC Sample | Surface Modification | Content Functional Groups (mol/g) | Degree of Crystallinity (%) | NaCl b | MEM c | LB d | MRS e |
| U-NFC | None | 30 a | 61 | −10.9 ± 2.5 | −8.7 ± 1.3 | −8.9± 0.7 | −6.6± 0.8 |
| A-NFC | Carboxymethylation | 570 | 52 | −24.6 ± 2.2 | −9.2 ± 1.1 | −13.0 ± 0.8 | −6.6 ± 0.7 |
| C-NFC | Hydroxypropyl-trimethylammonium substitution | 634 | 61 | 17.4 ± 2.2 | −9.6 ± 0.7 | −11.7 ± 0.9 | −4.5 ± 0.2 |
| P-NFC | Phosphorylation | 1109 | 45 | −31.1 ± 1.2 | −9.5 ± 0.4 | −16.3 ± 1.6 | −14.5 ± 0.6 |
| S-NFC | Sulfoethylation | 444 | 56 | −23.8 ± 1.6 | −9.8 ± 1.1 | −11.8 ± 0 .6 | −6.2 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, V.R.; Strømme, M.; Ferraz, N. In Vitro Biological Impact of Nanocellulose Fibers on Human Gut Bacteria and Gastrointestinal Cells. Nanomaterials 2020, 10, 1159. https://doi.org/10.3390/nano10061159
Lopes VR, Strømme M, Ferraz N. In Vitro Biological Impact of Nanocellulose Fibers on Human Gut Bacteria and Gastrointestinal Cells. Nanomaterials. 2020; 10(6):1159. https://doi.org/10.3390/nano10061159
Chicago/Turabian StyleLopes, Viviana R., Maria Strømme, and Natalia Ferraz. 2020. "In Vitro Biological Impact of Nanocellulose Fibers on Human Gut Bacteria and Gastrointestinal Cells" Nanomaterials 10, no. 6: 1159. https://doi.org/10.3390/nano10061159
APA StyleLopes, V. R., Strømme, M., & Ferraz, N. (2020). In Vitro Biological Impact of Nanocellulose Fibers on Human Gut Bacteria and Gastrointestinal Cells. Nanomaterials, 10(6), 1159. https://doi.org/10.3390/nano10061159







