Surface Interactions and Mechanisms Study on the Removal of Iodide from Water by Use of Natural Zeolite-Based Silver Nanocomposites
Abstract
:1. Introduction
2. Materials & Methods
2.1. Materials and Modification
Natural Zeolite Pre-Treatment and Modification
2.2. Materials Characterization
2.3. Adsorption Kinetics and Equilibrium
3. Results and Discussion
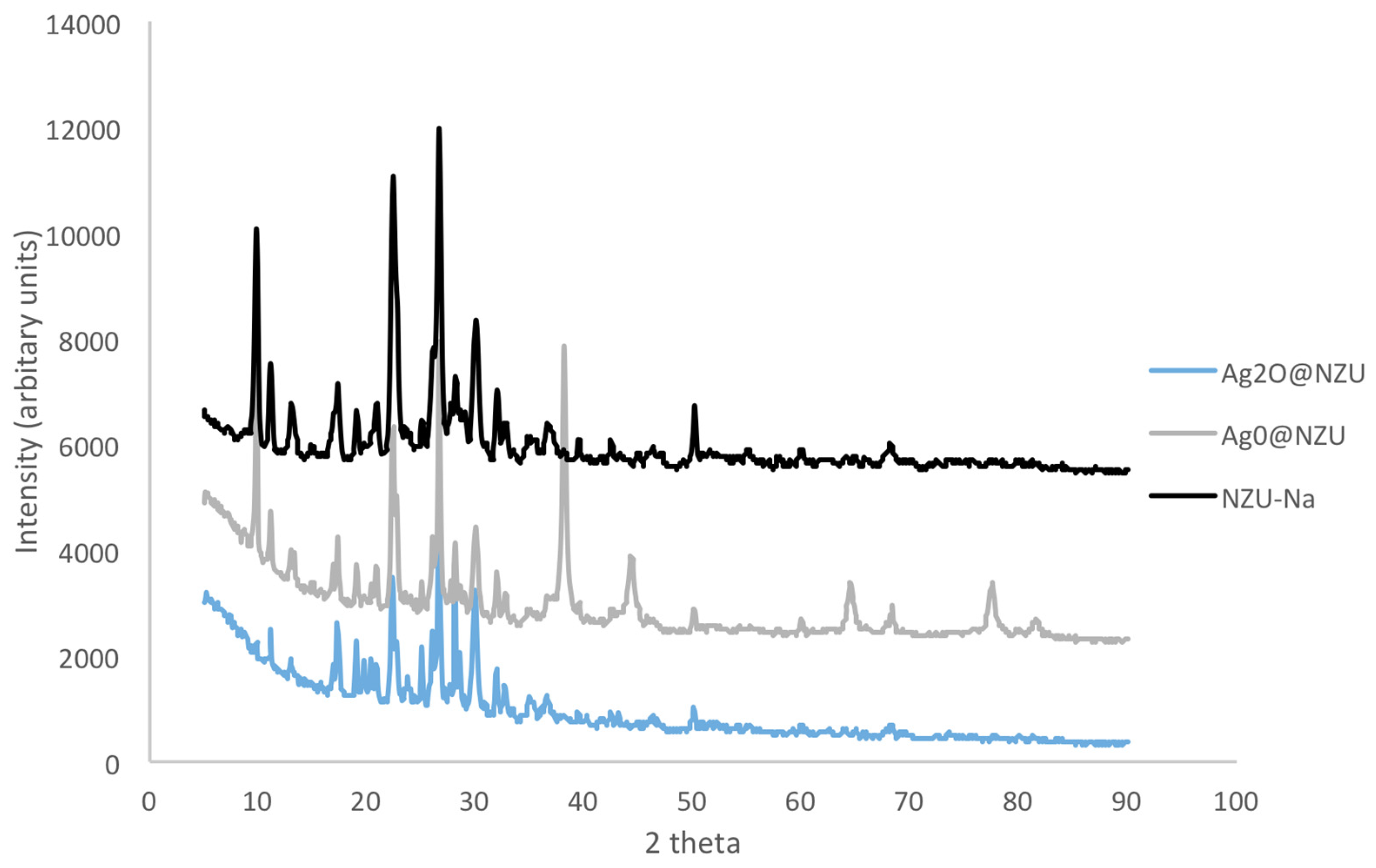
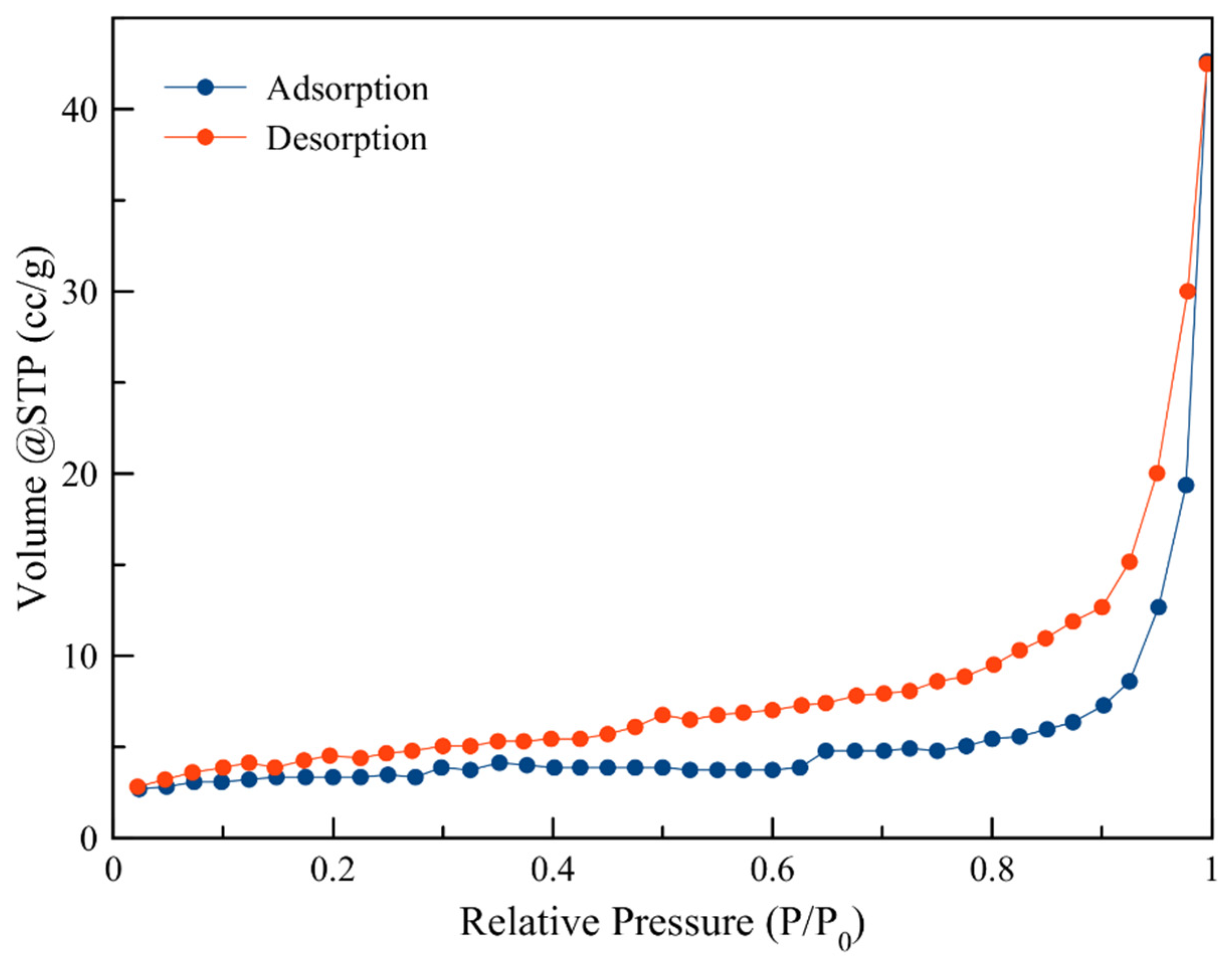
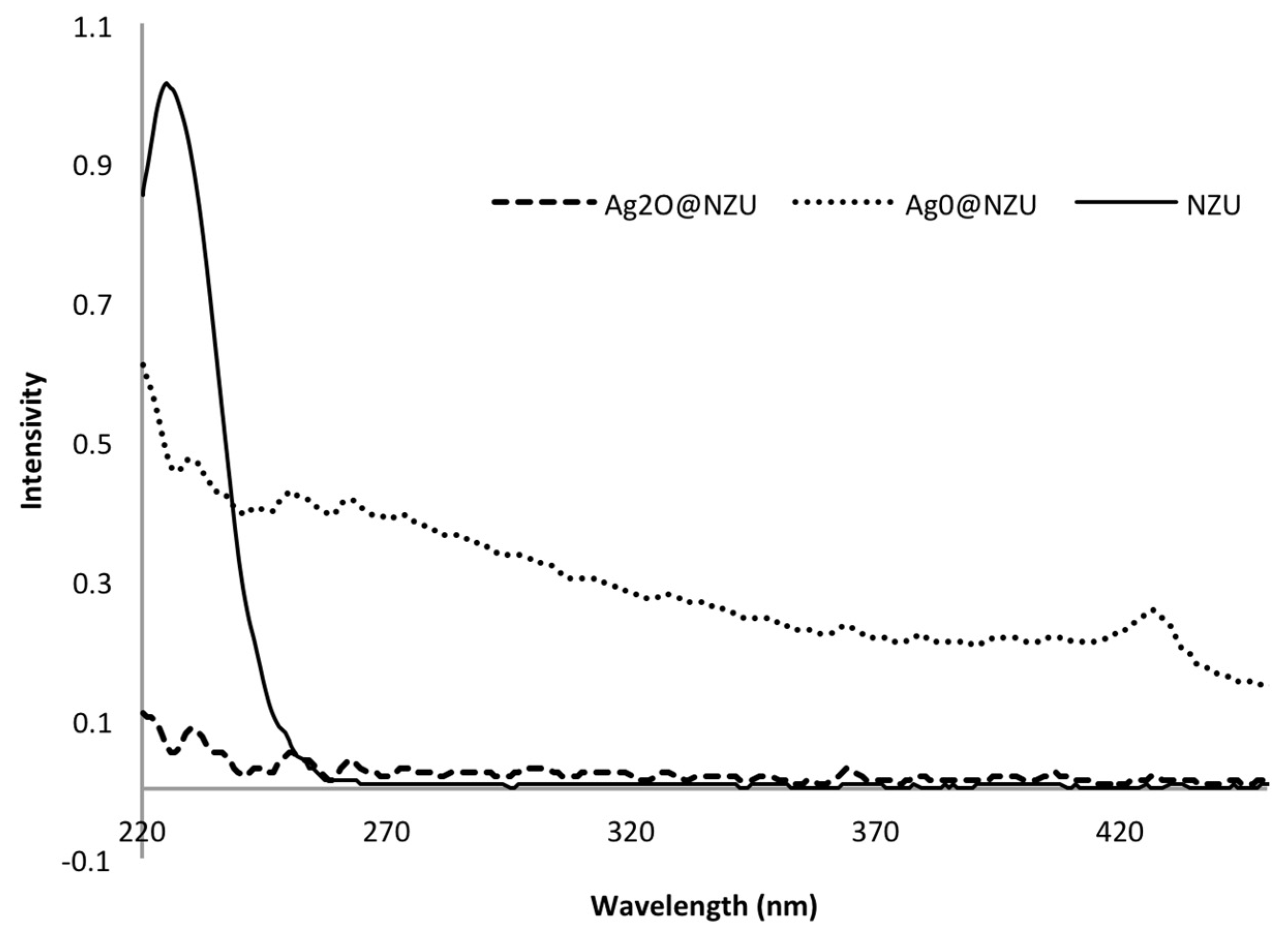
3.1. Characterization of Modified Zeolites
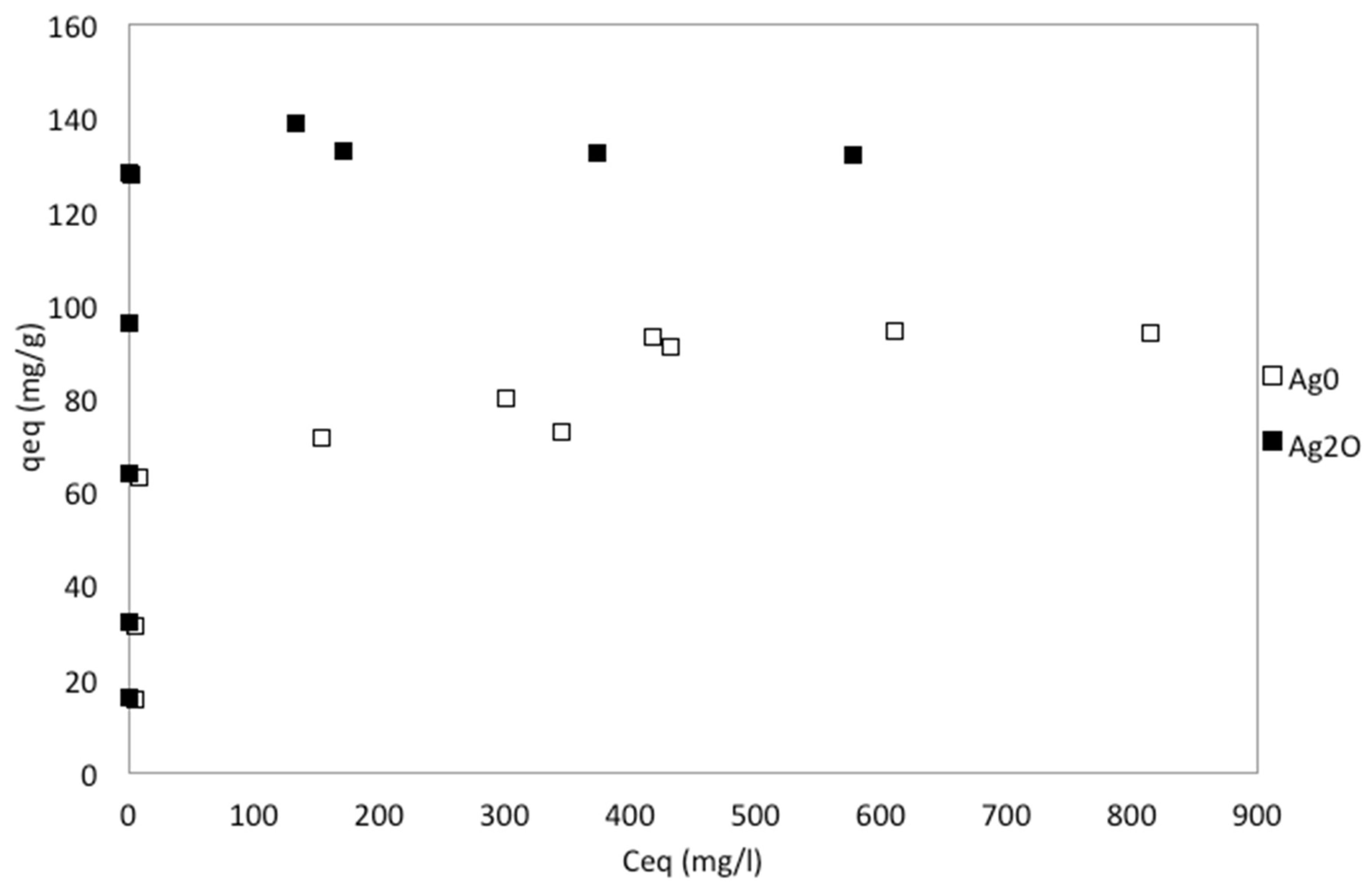
3.2. The Adsorption Kinetics and Equilibrium
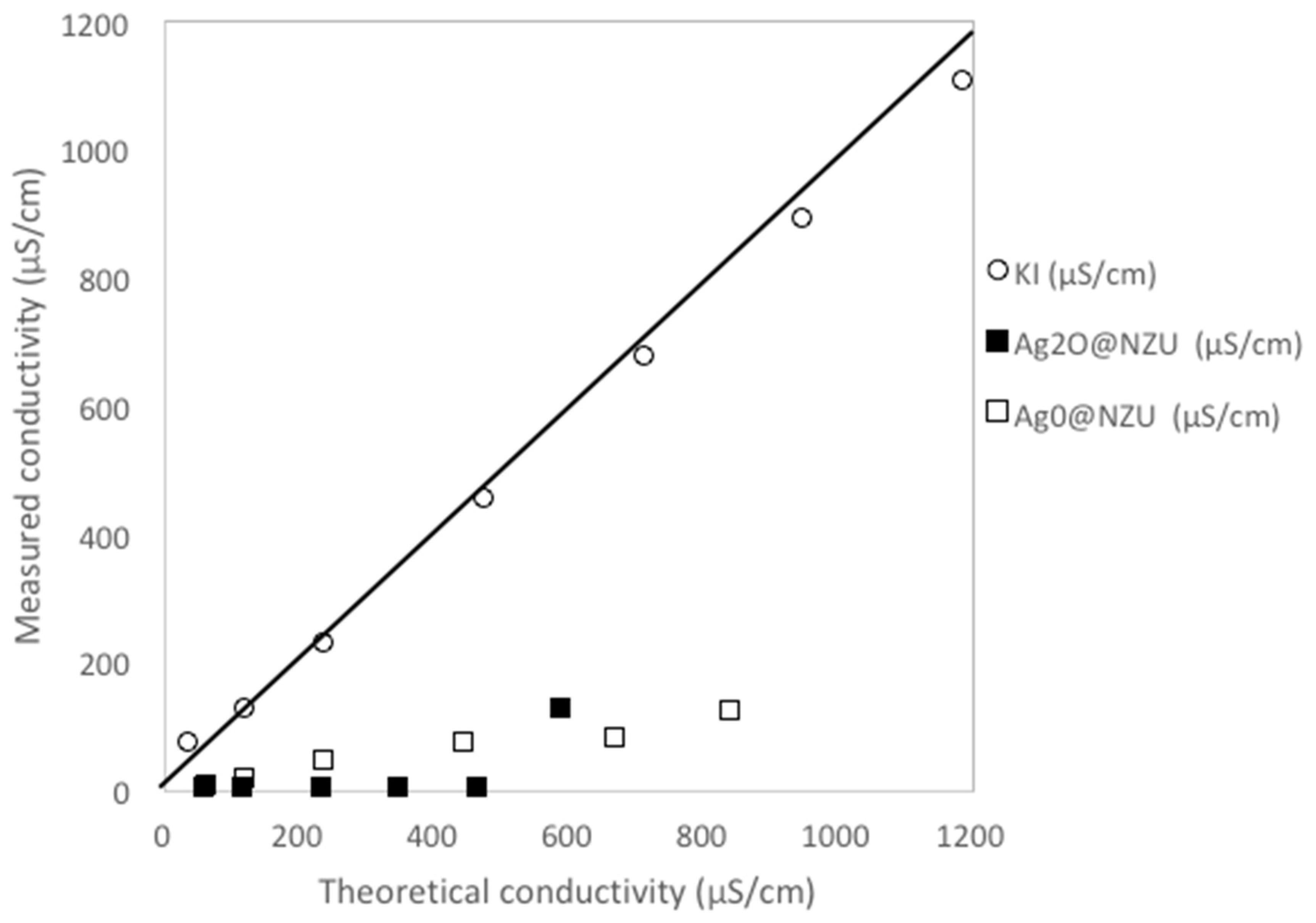
3.3. Removal Mechanism and Surface Interactions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jang, J.; Lee, D.S. Magnetite nanoparticles supported on organically modified montmorillonite for adsorptive removal of iodide from aqueous solution: Optimization using response surface methodology. Sci. Total Environ. 2018, 615, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Qi, L.; Liu, X.; Liu, Y.; Jiao, Y.; Chen, S.; Yang, Y. Synthesis of Cu/Cu2O hydrides for enhanced removal of iodide from water. J. Hazard. Mater. 2017, 328, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Huve, J.; Ryzhikov, A.; Nouali, H.; Lalia, V.; Augé, G.; Daou, T.J. Porous sorbents for the capture of radioactive iodine compounds: A review. RSC Adv. 2018, 8, 29248–29273. [Google Scholar] [CrossRef] [Green Version]
- Mao, P.; Liu, Y.; Liu, X.; Wang, Y.; Liang, J.; Zhou, Q.; Dai, Y.; Jiao, Y.; Chen, S.; Yang, Y. Bimetallic AgCu/Cu2O hybrid for the synergetic adsorption of iodide from solution. Chemosphere 2017, 180, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Tauanov, Z.; Inglezakis, V.J. Removal of iodide from water using silver nanoparticles-impregnated synthetic zeolites. Sci. Total Environ. 2019, 682, 259–270. [Google Scholar] [CrossRef]
- Yoshida, S.; Ojino, M.; Ozaki, T.; Hatanaka, T.; Nomura, K.; Ishii, M.; Koriyama, K.; Akashi, M. Guidelines for iodine prophylaxis as a protective measure: Information for physicians. Japan Med. Assoc. J. 2014, 57, 113–123. [Google Scholar]
- Kireev, S.V.; Shnyrev, S.L. Study of molecular iodine, iodate ions, iodide ions, and triiodide ions solutions absorption in the UV and visible light spectral bands. Laser Phys. 2015, 25, 75602. [Google Scholar] [CrossRef]
- Xing, C.; Yang, G.; Lu, P.; Shen, W.; Gai, X.; Tan, L.; Mao, J.; Wang, T.; Yang, R.; Tsubaki, N. A hierarchically spherical Co-based zeolite catalyst with aggregated nanorods structure for improved Fischer-Tropsch synthesis reaction activity and isoparaffin selectivity. Microporous Mesoporous Mater. 2016, 233, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Andersen, S.; Guan, H.; Teng, W.; Laurberg, P. Speciation of iodine in high iodine groundwater in China associated with goitre and hypothyroidism. Biol. Trace Elem. Res. 2009, 128, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.L.; Trzebicka, B.; Kostova, B.; Petrov, P.D. Super-macroporous dextran cryogels via UV-induced crosslinking: Synthesis and characterization. Polym. Int. 2017, 66, 1306–1311. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Z.; Maberly, G. Iodine-rich drinking water of natural origin in China. Lancet 1998, 352, 2024. [Google Scholar] [CrossRef]
- Hamasaki, T.; Nakamichi, N.; Teruya, K.; Shirahata, S. Removal efficiency of radioactive cesium and iodine ions by a flow-type apparatus designed for electrochemically reduced water production. PLoS ONE 2014, 9, e102218. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Kagoshima, M.; Yamasaki, M.; Honda, Y. Radioactive iodine waste treatment using electrodialysis with an anion exchange paper membrane. Appl. Radiat. Isot. 2004, 61, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Yu, S.H.; Yao, Q.Z.; Fu, S.Q.; Zhou, G.T. One-step synthesis of Ag2O@Mg(OH)2 nanocomposite as an efficient scavenger for iodine and uranium. J. Colloid Interface Sci. 2018, 510, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Polo, M.; Rivera-Utrilla, J.; Salhi, E.; von Gunten, U. Ag-doped carbon aerogels for removing halide ions in water treatment. Water Res. 2007, 41, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, Q.; Zhang, X.; Liu, Y.; Wang, P.; Jiao, Y.; Yang, Y. Nanometer mixed-valence silver oxide enhancing adsorption of ZIF-8 for removal of iodide in solution. Sci. Total Environ. 2019, 646, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Asmussen, R.M.; Neeway, J.J.; Lawter, A.R.; Wilson, A.; Qafoku, N.P. Silver-based getters for 129I removal from low-activity waste. Radiochim. Acta 2016, 104, 905–913. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Wang, Y.; Liu, C.; Ma, W. Enhanced removal of iodide from aqueous solution by ozonation and subsequent adsorption on Ag-Ag2O modified on Carbon Spheres. Appl. Surf. Sci. 2018, 427, 753–762. [Google Scholar] [CrossRef]
- Golubeva, O.Y.; Ul’yanova, N.Y. Stabilization of silver nanoparticles and clusters in porous zeolite matrices with Rho, Beta, and paulingite structures. Glas. Phys. Chem. 2015, 41, 537–544. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Loizidou, M.D.; Grigoropoulou, H.P. Equilibrium and kinetic ion exchange studies of Pb2+, Cr3+, Fe3+ and Cu2+ on natural clinoptilolite. Water Res. 2002, 36, 2784–2792. [Google Scholar] [CrossRef]
- Kuntubek, A.; Kinayat, N.; Meiramkulova, K.; Poulopoulos, S.; Bear, J.C.; Inglezakis, V.J. Catalytic Oxidation of Methylene Blue by Use of Natural Zeolite-Based Silver and Magnetite Nanocomposites. Processes 2020, 8, 471. [Google Scholar] [CrossRef] [Green Version]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Naidu, R. Defluoridation of drinking water using adsorption processes. J. Hazard. Mater. 2013, 248–249, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chebbi, M.; Azambre, B.; Cantrel, L.; Huvé, M.; Albiol, T. Influence of structural, textural and chemical parameters of silver zeolites on the retention of methyl iodide. Microporous Mesoporous Mater. 2017, 244, 137–150. [Google Scholar] [CrossRef]
- Warchoł, J.; Misaelides, P.; Petrus, R.; Zamboulis, D. Preparation and application of organo-modified zeolitic material in the removal of chromates and iodides. J. Hazard. Mater. 2006, 137, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Faghihian, H.; Malekpour, A.; Maragheh, M.G. Removal of radioactive iodide by surfactant-modified zeolites. Adsorpt. Sci. Technol. 2003, 21, 373–381. [Google Scholar] [CrossRef]
- Chmielewská-Horváthová, E.; Lesný, J. Iodide adsorption on the surface of chemically pretreated clinoptilolite. J. Radioanal. Nucl. Chem. Lett. 1995, 200, 351–363. [Google Scholar] [CrossRef]
- Han, S.; Um, W.; Kim, W.S. Development of bismuth-functionalized graphene oxide to remove radioactive iodine. Dalt. Trans. 2019, 48, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Faghihian, H.; Maragheh, M.G.; Malekpour, A. Adsorption of radioactive iodide by natural zeolites. J. Radioanal. Nucl. Chem. 2002, 254, 545–550. [Google Scholar] [CrossRef]
- Gradev, G.D. Sorption of iodide ions on cationic forms of clinoptilolite. J. Radioanal. Nucl. Chem. Artic. 1987, 116, 341–346. [Google Scholar] [CrossRef]
- Park, S.; An, H.; Park, M.B.; Lee, J. Adsorption behavior of methyl iodide on a silver ion-exchanged ZSM-5. Microporous Mesoporous Mater. 2020, 294, 109842. [Google Scholar] [CrossRef]
- Azambre, B.; Chebbi, M.; Hijazi, A. Effects of the cation and Si/Al ratio on CH3I adsorption by faujasite zeolites. Chem. Eng. J. 2020, 379, 122308. [Google Scholar] [CrossRef]
- Chapman, K.W.; Chupas, P.J.; Nenoff, T.M. Radioactive iodine capture in silver-containing mordenites through nanoscale silver iodide formation. J. Am. Chem. Soc. 2010, 132, 8897–8899. [Google Scholar] [CrossRef] [PubMed]
- Baimenov, A.Z.; Berillo, D.A.; Inglezakis, V.J. Cryogel-based Ag°/Ag2O nanocomposites for iodide removal from water. J. Mol. Liq. 2019, 299, 112134. [Google Scholar] [CrossRef]
- Lihareva, N.; Dimova, L.; Petrov, O.; Tzvetanova, Y. Ag+ sorption on natural and Na-exchanged clinoptilolite from Eastern Rhodopes, Bulgaria. Microporous Mesoporous Mater. 2010, 130, 32–37. [Google Scholar] [CrossRef]
- Bo, A.; Sarina, S.; Zheng, Z.; Yang, D.; Liu, H.; Zhu, H. Removal of radioactive iodine from water using Ag2O grafted titanate nanolamina as efficient adsorbent. J. Hazard. Mater. 2013, 246–247, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Tauanov, Z.; Tsakiridis, P.E.; Shah, D.; Inglezakis, V.J. Synthetic sodalite doped with silver nanoparticles: Characterization and mercury (II) removal from aqueous solutions. J. Environ. Sci. Heal. Part A Toxic/Hazardous Subst. Environ. Eng. 2019, 54, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Tauanov, Z.; Tsakiridis, P.E.; Mikhalovsky, S.V.; Inglezakis, V.J. Synthetic coal fly ash-derived zeolites doped with silver nanoparticles for mercury (II) removal from water. J. Environ. Manage. 2018, 224, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Mulfinger, L.; Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and Study of Silver Nanoparticles. J. Chem. Educ. 2007, 84, 322. [Google Scholar] [CrossRef]
- Kassim, I.A.R.; Moloney, G.; Busili, A.; Nur, A.Y.; Paron, P.; Jooste, P.; Gadain, H.; Seal, A.J. Iodine Intake in Somalia Is Excessive and Associated with the Source of Household Drinking Water. J. Nutr. 2014, 144, 375–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henjum, S.; Barikmo, I.; Gjerlaug, A.K.; Mohamed-Lehabib, A.; Oshaug, A.; Arne Strand, T.; Torheim, L.E. Endemic goitre and excessive iodine in urine and drinking water among Saharawi refugee children. Proc. Public Health Nutr. 2010, 13, 1472–1477. [Google Scholar]
- Moritz, M.; Geszke-Moritz, M. The newest achievements in synthesis, immobilization and practical applications of antibacterial nanoparticles. Chem. Eng. J. 2013, 228, 596–613. [Google Scholar] [CrossRef]
- Stylianou, M.; Inglezakis, V.; Agapiou, A.; Itskos, G.; Jetybayeva, A.; Loizidou, M. A comparative study on phyllosilicate and tectosillicate mineral structural properties. Desalin. Water Treat. 2018, 112, 119–146. [Google Scholar] [CrossRef] [Green Version]
- Tarach, K.; Góra-Marek, K.; Chrzan, M.; Walas, S. Quantification of silver sites in zeolites: Carbon monoxide sorption monitored by IR spectroscopy. J. Phys. Chem. C 2014, 118, 23751–23760. [Google Scholar] [CrossRef]
- Kubota, T.; Fukutani, S.; Ohta, T.; Mahara, Y. Removal of radioactive cesium, strontium, and iodine from natural waters using bentonite, zeolite, and activated carbon. J. Radioanal. Nucl. Chem. 2013, 296, 981–984. [Google Scholar] [CrossRef] [Green Version]
- Reháková, M.; Sopková, A.; Casciola, M.; Bastl, Z. Ac and dc conductivity study of natural zeolitic material of the clinoptilolite type and its iodine forms. Solid State Ionics 1993, 66, 189–194. [Google Scholar] [CrossRef]
- Reháková, M.; Sopková, A.; Lokaj, J. Natural zeolitic material of the clinoptilolite type doped with iodine and its ions. J. Incl. Phenom. Mol. Recognit. Chem. 1992, 14, 47–54. [Google Scholar] [CrossRef]
- Burgess, A.E.; Davidson, J.C. A kinetic-equilibrium study of a triiodide concentration maximum formed by the persulfate-iodide reaction. J. Chem. Educ. 2012, 89, 814–816. [Google Scholar] [CrossRef]
- Mao, P.; Liu, Y.; Jiao, Y.; Chen, S.; Yang, Y. Enhanced uptake of iodide on Ag@Cu2O nanoparticles. Chemosphere 2016, 164, 396–403. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Li, B.; Liu, D.; Zhou, Y. Highly selective anchoring silver nanoclusters on MOF/SOF heterostructured framework for efficient adsorption of radioactive iodine from aqueous solution. Chemosphere 2020, 252, 126448. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Qi, B.; Liu, Y.; Zhao, L.; Jiao, Y.; Zhang, Y.; Jiang, Z.; Li, Q.; Wang, J.; Chen, S.; et al. AgII doped MIL-101 and its adsorption of iodine with high speed in solution. J. Solid State Chem. 2016, 237, 274–283. [Google Scholar] [CrossRef]
- Zhao, X.; Han, X.; Li, Z.; Huang, H.; Liu, D.; Zhong, C. Enhanced removal of iodide from water induced by a metal-incorporated porous metal-organic framework. Appl. Surf. Sci. 2015, 351, 760–764. [Google Scholar] [CrossRef]
- Lauridsen, J.; Eklund, P.; Lu, J.; Knutsson, A.; Odén, M.; Mannerbro, R.; Andersson, A.M.; Hultman, L. Microstructural and chemical analysis of AgI coatings used as a solid lubricant in electrical sliding contacts. Tribol. Lett. 2012, 46, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Malm, J.O.; Schmid, G.; Morun, B. A high-resolution electron microscopy study of silver particles and surfaces. Philos. Mag. A Phys. Condens. Matter Struct. Defects Mech. Prop. 1991, 63, 487–502. [Google Scholar] [CrossRef]
- Ng, C.H.B.; Fan, W.Y. Controlled synthesis of β-AgI nanoplatelets from selective nucleation of twinned Ag seeds in a tandem reaction. J. Phys. Chem. C 2007, 111, 2953–2958. [Google Scholar] [CrossRef]
- NIST NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database 20, Version 4.1.
- Zhai, Q.Z.; Qiu, S.; Xiao, F.S.; Zhang, Z.T.; Shao, C.L.; Han, Y. Preparation, characterization, and optical properties of the host-guest nanocomposite material zeolite-silver iodide. Mater. Res. Bull. 2000, 35, 59–73. [Google Scholar] [CrossRef]
- Bashouti, M.Y.; Talebi, R.; Kassar, T.; Nahal, A.; Ristein, J.; Unruh, T.; Christiansen, S.H. Systematic Surface Phase Transition of Ag Thin Films by Iodine Functionalization at Room Temperature: Evolution of Optoelectronic and Texture Properties. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastl, Z.; Gehlmann, H. X-ray photoelectron spectroscopic (XPS) studies of iodine oxocompounds. Collect. Czechoslov. Chem. Commun. 1988, 53, 425–432. [Google Scholar] [CrossRef]
- Taucher, T.C.; Hehn, I.; Hofmann, O.T.; Zharnikov, M.; Zojer, E. Understanding Chemical versus Electrostatic Shifts in X-ray Photoelectron Spectra of Organic Self-Assembled Monolayers. J. Phys. Chem. C 2016, 120, 3428–3437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarasola, A.; Abadía, M.; Rogero, C.; Garcia-Lekue, A. Theoretical Insights into Unexpected Molecular Core Level Shifts: Chemical and Surface Effects. J. Phys. Chem. Lett. 2017, 8, 5718–5724. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, M.; Genet, M.J.; Gaigneaux, E.M.; Debecker, D.P. Calibration of the X-ray photoelectron spectroscopy binding energy scale for the characterization of heterogeneous catalysts: Is everything really under control? ChemPhysChem 2013, 14, 3618–3626. [Google Scholar] [CrossRef] [PubMed]
- Zojer, E.; Taucher, T.C.; Hofmann, O.T. The Impact of Dipolar Layers on the Electronic Properties of Organic/Inorganic Hybrid Interfaces. Adv. Mater. Interfaces 2019, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Grünert, W.; Schlogl, R. Photoelectron Spectroscopy of Zeolites. Mol. Sieves 2004, 4, 467–515. [Google Scholar]
- Günter, T.; Casapu, M.; Doronkin, D.; Mangold, S.; Trouillet, V.; Augenstein, T.; Grunwaldt, J.D. Potential and limitations of natural chabazite for selective catalytic reduction of NOx with NH3. Chemie-Ingenieur-Technik 2013, 85, 632–641. [Google Scholar] [CrossRef]
- Ruiz-Serrano, D.; Flores-Acosta, M.; Conde-Barajas, E.; Ramírez-Rosales, D.; Yáñez-Limón, J.M.; Ramírez-Bon, R. Study by XPS of different conditioning processes to improve the cation exchange in clinoptilolite. J. Mol. Struct. 2010, 980, 149–155. [Google Scholar] [CrossRef]
- Chmielewská, E.; Tylus, W.; Drábik, M.; Majzlan, J.; Kravčak, J.; Williams, C.; Čaplovičová, M. Čaplovič Structure investigation of nano-FeO(OH) modified clinoptilolite tuff for antimony removal. Microporous Mesoporous Mater. 2017, 248, 222–233. [Google Scholar] [CrossRef]
- Panayotova, M.I.; Mintcheva, N.N.; Gemishev, O.T.; Tyuliev, G.T.; Gicheva, G.D.; Djerahov, L.P. Preparation and antimicrobial properties of silver nanoparticles supported by natural zeolite clinoptilolite. Bulg. Chem. Commun. 2018, 50, 211–218. [Google Scholar]
- Borkó, L.; Nagy, I.; Schay, Z.; Guczi, L. Low and high activity states in the oxidation of m-xylene on palladium catalysts. Appl. Catal. A Gen. 1996, 147, 95–108. [Google Scholar] [CrossRef]
- Wei, Y.X.; Ye, Z.F.; Wang, Y.L.; Ma, M.G.; Li, Y.F. Enhanced ammonia nitrogen removal using consistent ammonium exchange of modified zeolite and biological regeneration in a sequencing batch reactor process. Environ. Technol. 2011, 32, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Greczynski, G.; Hultman, L. Compromising Science by Ignorant Instrument Calibration—Need to Revisit Half a Century of Published XPS Data. Angew. Chemie Int. Ed. 2020, 59, 5002–5006. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, M.; Liu, G.; Zhang, L.; He, Y.; Xing, X.; Qian, Z.; Zheng, J.; Xu, C. Enhanced Iodide Removal from Water by Nano-Silver Modified Anion Exchanger. Ind. Eng. Chem. Res. 2018, 57, 17401–17408. [Google Scholar] [CrossRef]
- Liu, S.; Wang, N.; Zhang, Y.; Li, Y.; Han, Z.; Na, P. Efficient removal of radioactive iodide ions from water by three-dimensional Ag2O-Ag/TiO2composites under visible light irradiation. J. Hazard. Mater. 2015, 284, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Stewart, S.; Shoesmith, D.W.; Wren, J.C. Interaction of Aqueous Iodine Species with Ag2O∕Ag Surfaces. J. Electrochem. Soc. 2007, 154, F70. [Google Scholar] [CrossRef] [Green Version]
- Buffle, J.; Zhang, Z.; Startchev, K. Metal flux and dynamic speciation at (Bio)interfaces. Part I: Critical evaluation and compilation of physicochemical parameters for complexes with simple ligands and fulvic/humic substances. Environ. Sci. Technol. 2007, 41, 7609–7620. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Polo, M.; Rivera-Utrilla, J.; Salhi, E.; von Gunten, U. Removal of bromide and iodide anions from drinking water by silver-activated carbon aerogels. J. Colloid Interface Sci. 2006, 300, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Polo, A.M.; Velo-Gala, I.; Sánchez-Polo, M.; von Gunten, U.; López-Peñalver, J.J.; Rivera-Utrilla, J. Halide removal from aqueous solution by novel silver-polymeric materials. Sci. Total Environ. 2016, 573, 1125–1131. [Google Scholar]
- Hoskins, J.S.; Karanfil, T.; Serkiz, S.M. Removal and sequestration of iodide using silver-impregnated activated carbon. Environ. Sci. Technol. 2002, 36, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Krausmann, E.; Drossinos, Y. A model of silver ± iodine reactions in a light water reactor containment sump under severe accident conditions. J. Nucl. Mater. 1999, 264, 113–121. [Google Scholar] [CrossRef]
- MATSUOKA, S.; NAKAMURA, H.; TAMURA, T.; Takano, T.; ITO, Y. Stability and Chemical Form of Iodine Sorbed on Silver-Exchanged Zeolite X. J. Nucl. Sci. Technol. 1984, 22, 862–870. [Google Scholar] [CrossRef]
- Wu, L.; Sawada, J.A.; Kuznicki, D.B.; Kuznicki, T.; Kuznicki, S.M. Iodine adsorption on silver-exchanged titania-derived adsorbents. J. Radioanal. Nucl. Chem. 2014, 302, 527–532. [Google Scholar] [CrossRef]
- Validzic, I.L.; Kegel, W.K. Influence of adsorbing species on properties of equilibrium silver iodide clusters. J. Colloid Interface Sci. 2004, 275, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Mladenovic, I.L.; Kegel, W.K.; Bomans, P.; Frederik, P.M. Observation of equilibrium, nanometer-sized clusters of silver iodide in aqueous solutions. J. Phys. Chem. B 2003, 107, 5717–5722. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.L.; Smith, B.A.; Ball, W.P.; Fairbrother, D.H. Assessing the colloidal properties of engineered nanoparticles in water: Case studies from fullerene C60 nanoparticles and carbon nanotubes. Environ. Chem. 2010, 7, 10–27. [Google Scholar] [CrossRef] [Green Version]
- Tauanov, Z.; Lee, J.; Inglezakis, V.J. Mercury reduction and chemisorption on the surface of synthetic zeolite silver nanocomposites: Equilibrium studies and mechanisms. J. Mol. Liq. 2020, 305, 112825. [Google Scholar] [CrossRef]




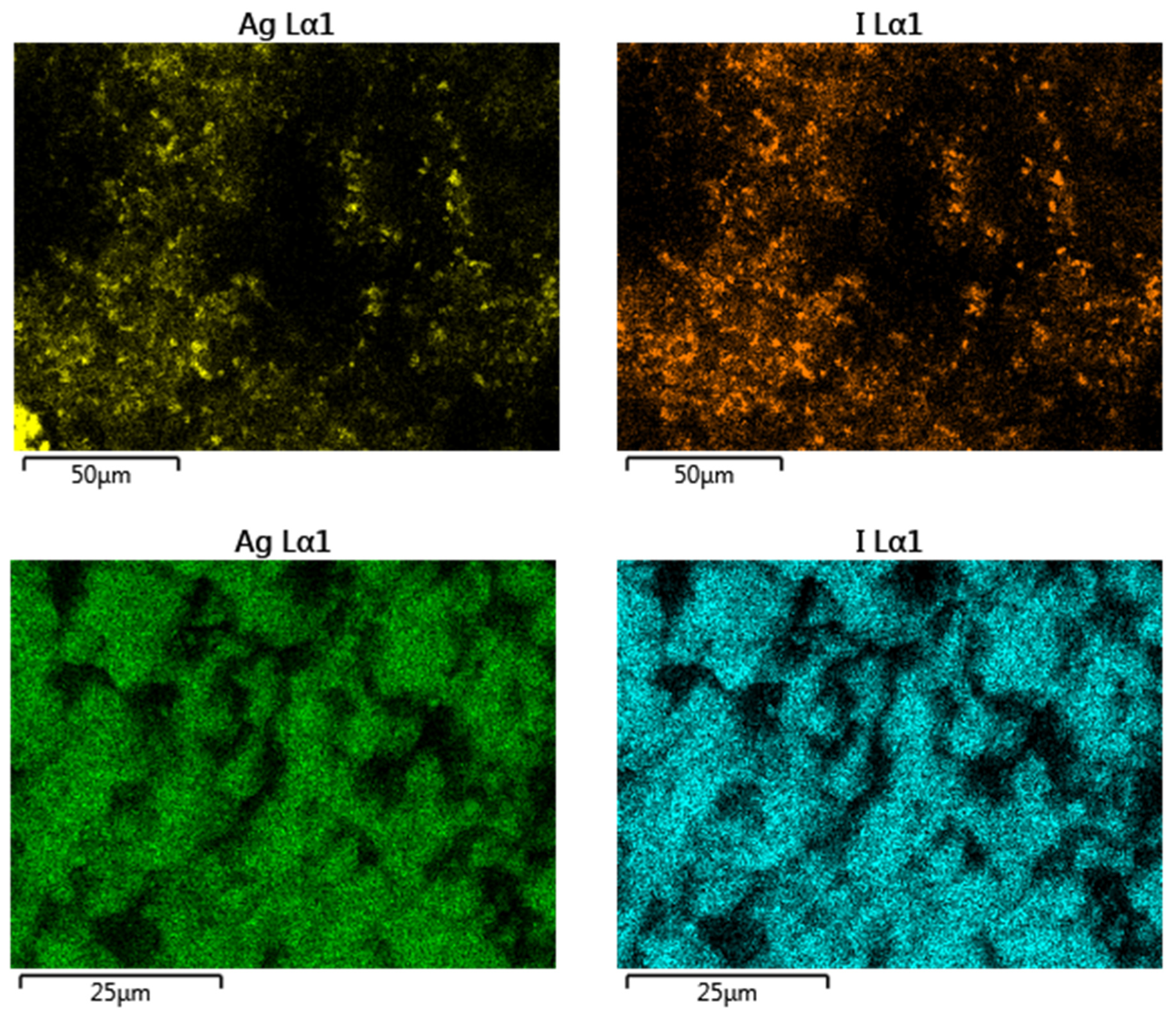
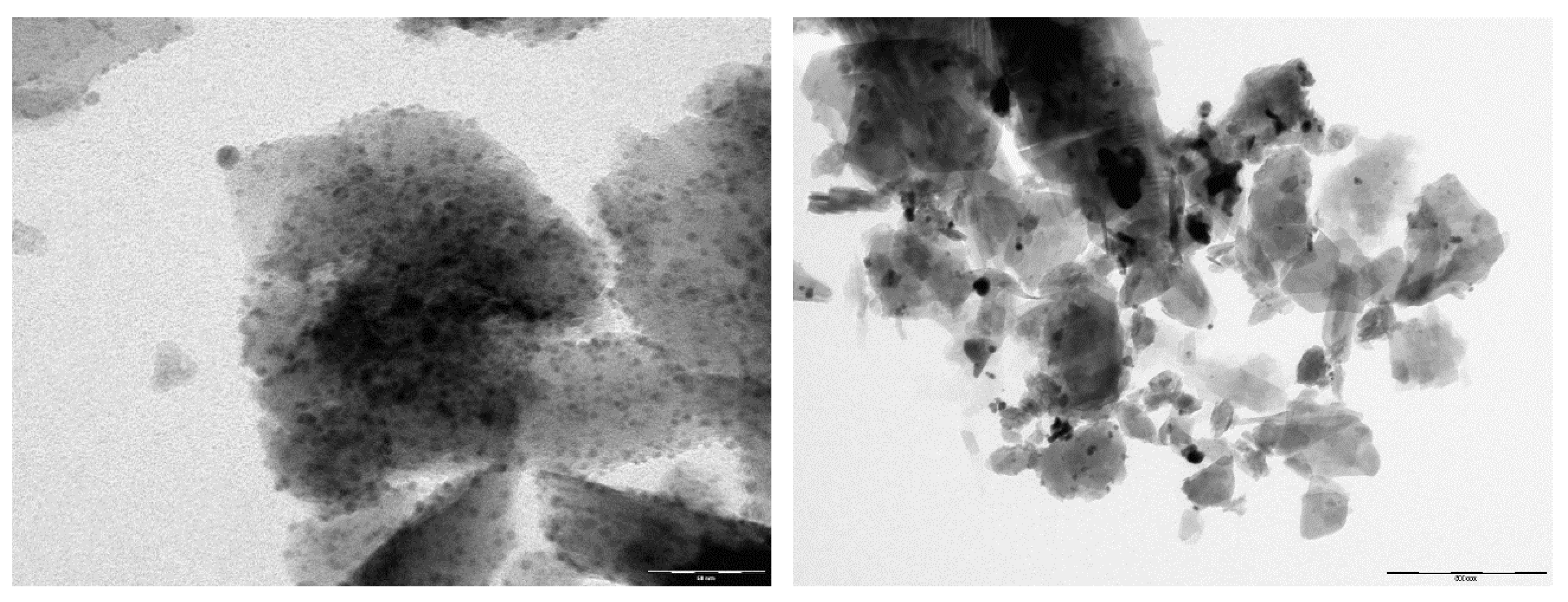
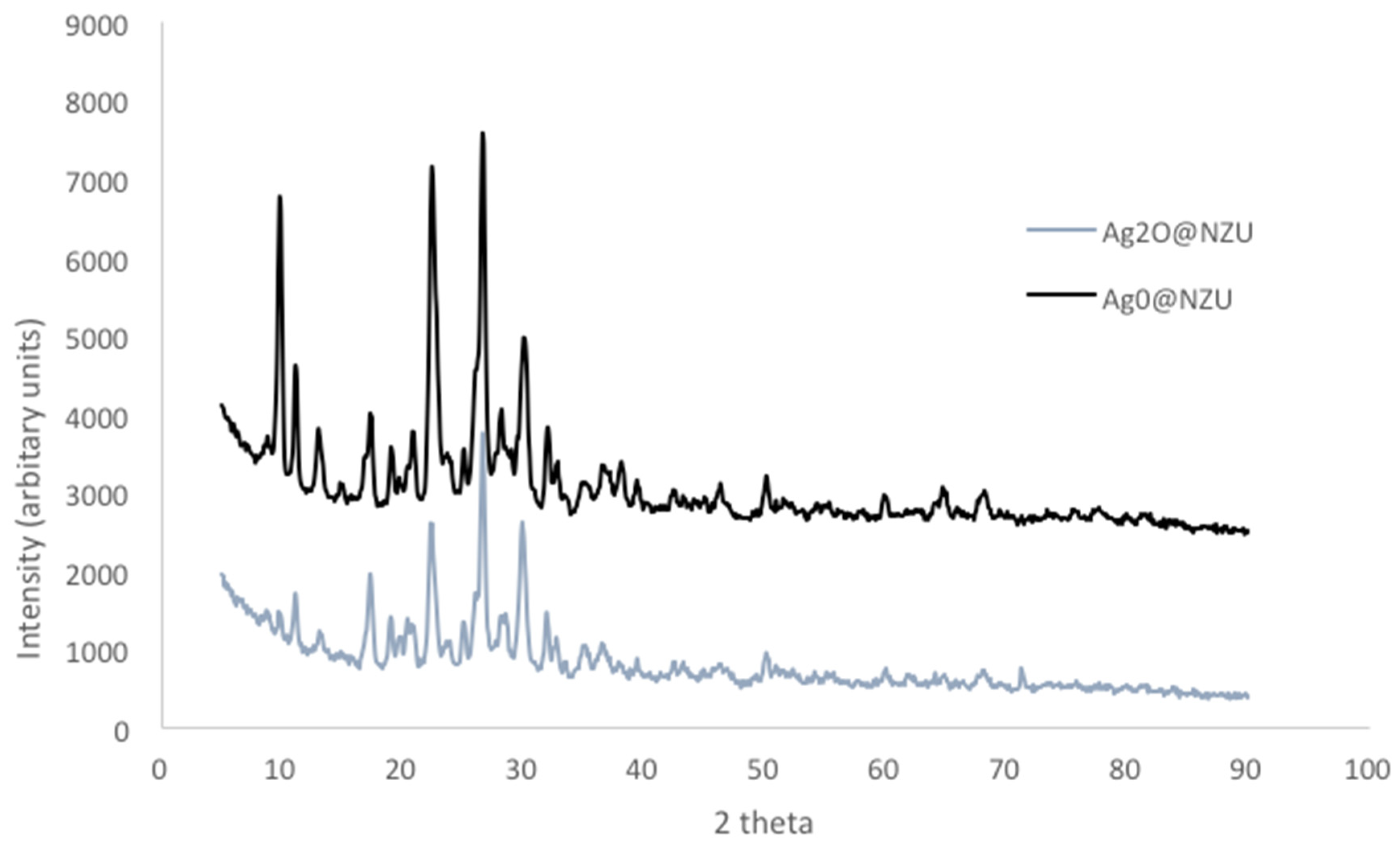

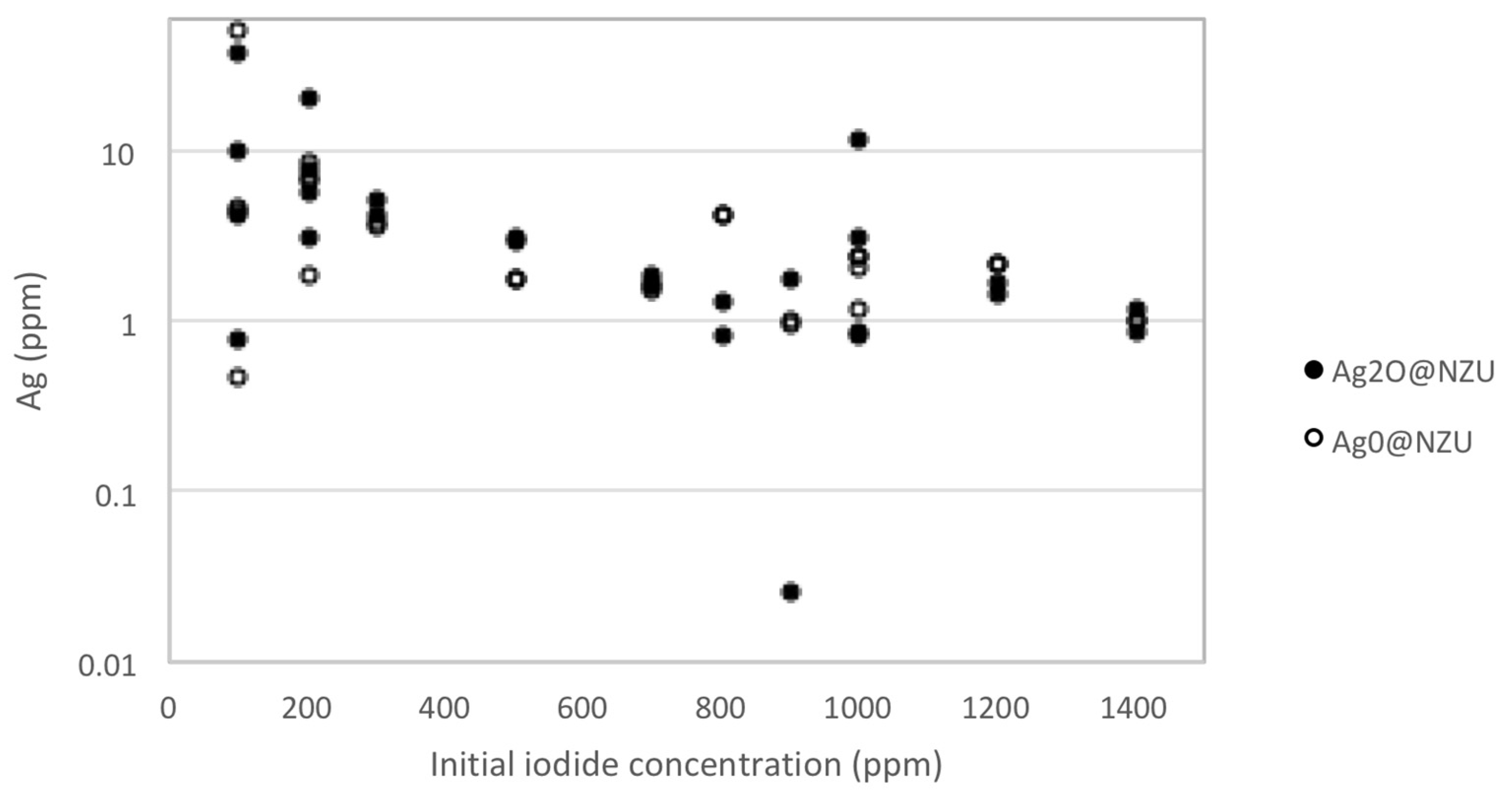
| Major Phase 1 | Silver form | I−/I2, (ppm) | Initial pH | m/V (g/L) | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|---|---|---|---|
| Water phase (I−) | ||||||
| Analcime/ Sodalite (S) | Ag0 | 450 | 2.5 | 5 | 20.44 | [5] |
| Ag-Z (S) | Ag+ | 10 | 6.2 | 5 | 1.9 | [27] |
| Clinoptilolite/ natrolite | Ag+ | 1270–12,700 | 3–10 | 5 | 146/52 | [28] |
| Clinoptilolite | Ag+ | 381–762 | 6–7 | - | 89 | [26] |
| Clinoptilolite | Ag+ | 10−5 Ci/l 2 | - | - | 178 | [29] |
| Gas phase (I2) | ||||||
| ZSM 5 (S) 3 | Ag+ | 2000 | - | - | 0.05 | [30] |
| Faujasite 3 | Ag0 | 1333 | - | - | 223 | [31] |
| Modernite | Ag+/Ag0 | - | - | - | - | [32] |
| Faujasite 3 Modenite 3 Ferrierite 3 ZSM 5 (S) 3 | ||||||
| BETA zeolite (S) 3 | Ag+ | 1333 | - | - | 46–267 | [23] |
| Sample | Surface Area (BET, m2/g) | Total Pore Volume (BJH, cm3/g) | Average Pore Size (BJH, nm) |
|---|---|---|---|
| NZU | 12.6 | 0.027 | 5.82 |
| NZU-Na | 12.5 | 0.032 | 2.98 |
| Ag2O@NZU | 8.70 | 0.061 | 5.42 |
| Ag0@NZU | 14.0 | 0.034 | 2.97 |
| NZU | NZU-Na | Ag2O@NZU | Ag0@NZU | |
|---|---|---|---|---|
| Na2O | 1.13 ± 0.01 | 1.94 | 1.08 ± 1.32 | 2.42 ± 1.34 |
| MgO | 0.33 ± 0.06 | 0.27 | 0.41 ± 0.18 | 0.41 ± 0.15 |
| Al2O3 | 12.72 ± 0.33 | 12.70 | 11.67 ± 0.37 | 12.06 ± 0.38 |
| SiO2 | 76.55 ± 1.10 | 78.52 | 70.97 ± 1.74 | 72.84 ± 2.08 |
| K2O | 3.22 ± 0.33 | 3.16 | 2.20 ± 0.18 | 2.45 ± 0.26 |
| CaO | 2.20 ± 0.29 | 0.82 | 0.44 ± 0.06 | 0.52 ± 0.03 |
| Fe2O3 | 1.60 ± 0.05 | 1.49 | 1.40 ± 0.26 | 1.41 ± 0.20 |
| Ag2O | - | - | 9.58 ± 1.40 | 5.92 ± 3.55 |
| Ag2O@NZU | Ag0@NZU | |
|---|---|---|
| Na2O | 0.4 | 1.37 |
| MgO | 0.69 | 0.63 |
| Al2O3 | 11.60 | 12.00 |
| SiO2 | 65.60 | 68.50 |
| K2O | 6.94 | 7.18 |
| CaO | 0.35 | 0.41 |
| Fe2O3 | 1.29 | 1.21 |
| Ag2O | 8.73 | 5.78 |
| I | 3.97 | 2.27 |
| Sample | Surface Area (BET, m2/g) | Total Pore Volume (BJH, cm3/g) | Average Pore Size (BJH, nm) |
|---|---|---|---|
| Ag2O@NZU | 11.9 | 0.035 | 3.73 |
| Ag0@NZU | 10.5 | 0.028 | 9.02 |
| Ag2O | Ag0 | Ag2O (200 ppm I−) | Ag0 (200 ppm I−) | Ag2O (800 ppm I−) | Ag0 (800 ppm I−) | |
|---|---|---|---|---|---|---|
| C1s Scan A | 284.6 | 284.6 | 284.6 | 284.6 | 284.6 | 284.6 |
| Ag3d5/2 Env. A | 369.2 | 368.5 | 370.4 | 368.6 | 369.1 | 368.5 |
| Ag3d5/2 Env. B | 370.4 | 369.7 | - | - | - | - |
| I3d5/2 Scan A | - | - | 620.6 | 620.0 | 620.6 | 619.9 |
| Na1s Scan A | 1073.2 | 1073.1 | 1072.7 | 1072.9 | 1072.7 | 1072.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inglezakis, V.J.; Satayeva, A.; Yagofarova, A.; Tauanov, Z.; Meiramkulova, K.; Farrando-Pérez, J.; Bear, J.C. Surface Interactions and Mechanisms Study on the Removal of Iodide from Water by Use of Natural Zeolite-Based Silver Nanocomposites. Nanomaterials 2020, 10, 1156. https://doi.org/10.3390/nano10061156
Inglezakis VJ, Satayeva A, Yagofarova A, Tauanov Z, Meiramkulova K, Farrando-Pérez J, Bear JC. Surface Interactions and Mechanisms Study on the Removal of Iodide from Water by Use of Natural Zeolite-Based Silver Nanocomposites. Nanomaterials. 2020; 10(6):1156. https://doi.org/10.3390/nano10061156
Chicago/Turabian StyleInglezakis, Vassilis J., Aliya Satayeva, Almira Yagofarova, Zhandos Tauanov, Kulyash Meiramkulova, Judit Farrando-Pérez, and Joseph C. Bear. 2020. "Surface Interactions and Mechanisms Study on the Removal of Iodide from Water by Use of Natural Zeolite-Based Silver Nanocomposites" Nanomaterials 10, no. 6: 1156. https://doi.org/10.3390/nano10061156
APA StyleInglezakis, V. J., Satayeva, A., Yagofarova, A., Tauanov, Z., Meiramkulova, K., Farrando-Pérez, J., & Bear, J. C. (2020). Surface Interactions and Mechanisms Study on the Removal of Iodide from Water by Use of Natural Zeolite-Based Silver Nanocomposites. Nanomaterials, 10(6), 1156. https://doi.org/10.3390/nano10061156






