Anisotropic Nanocellulose Aerogel Loaded with Modified UiO-66 as Efficient Adsorbent for Heavy Metal Ions Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CNF
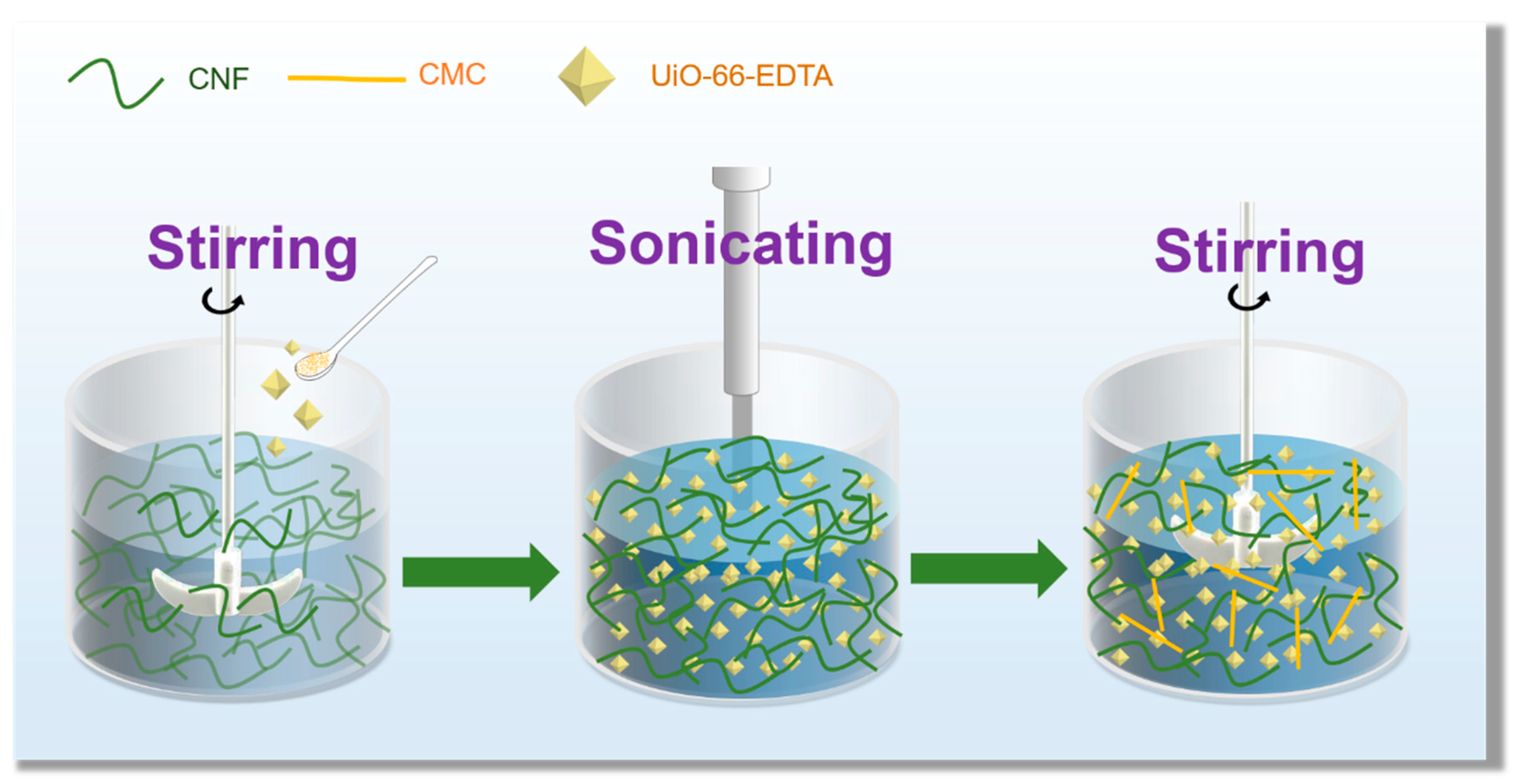
2.3. Preparation of Modified MOF (UiO-66-EDTA)
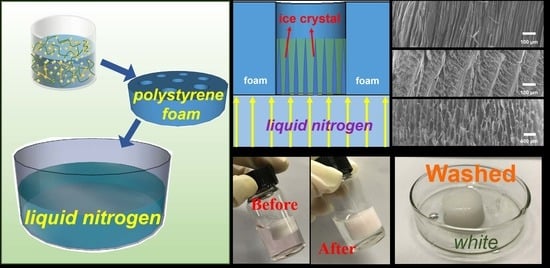
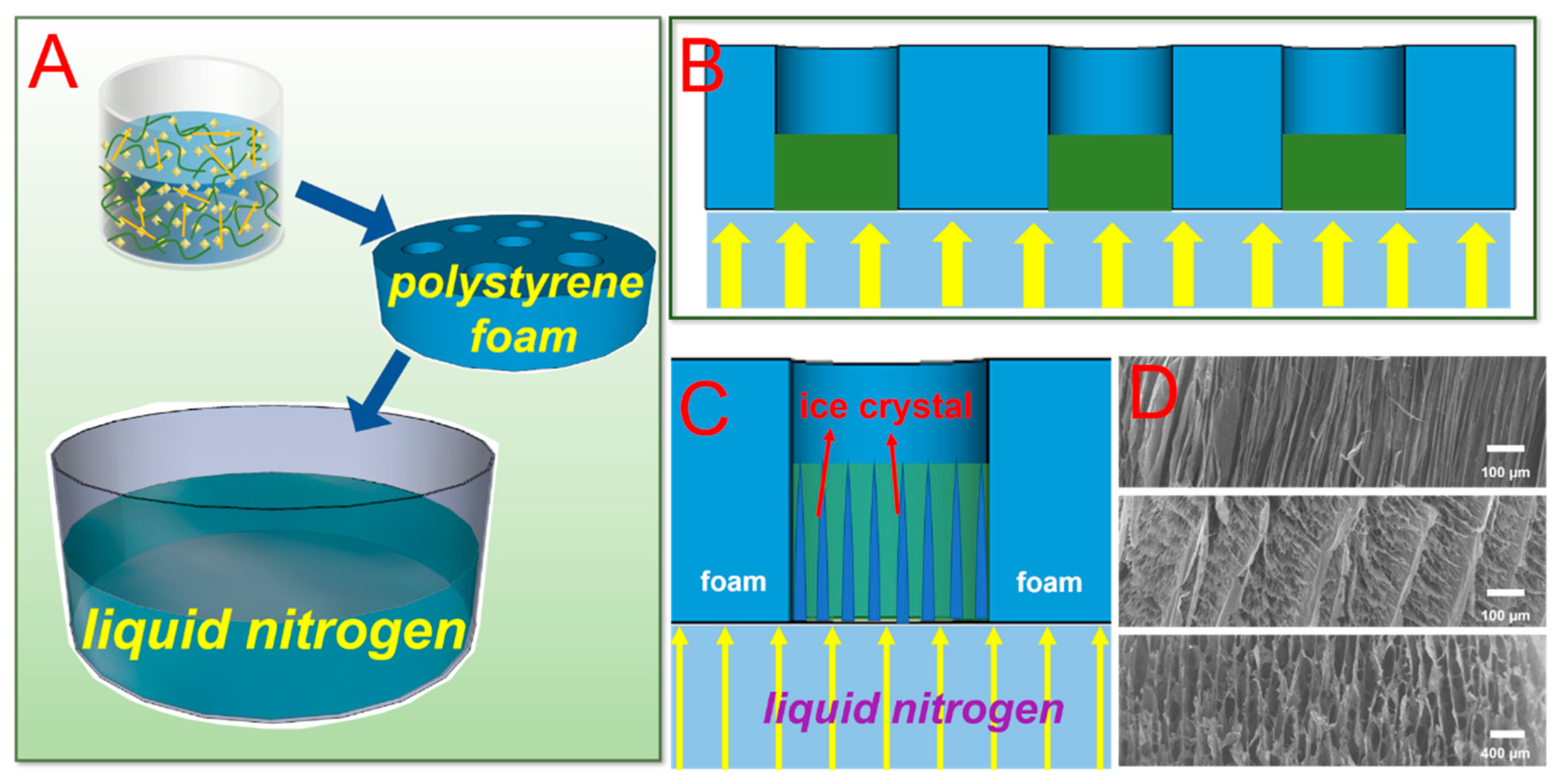
2.4. Synthesis of Anisotropic U-EDTACCA
2.5. Characterization
2.6. Density and Porosity of Hybrid Aerogels
2.7. Heavy Metal Ions Adsorption Experiments
3. Results and Discussion
3.1. Preparation of U-EDTACCA
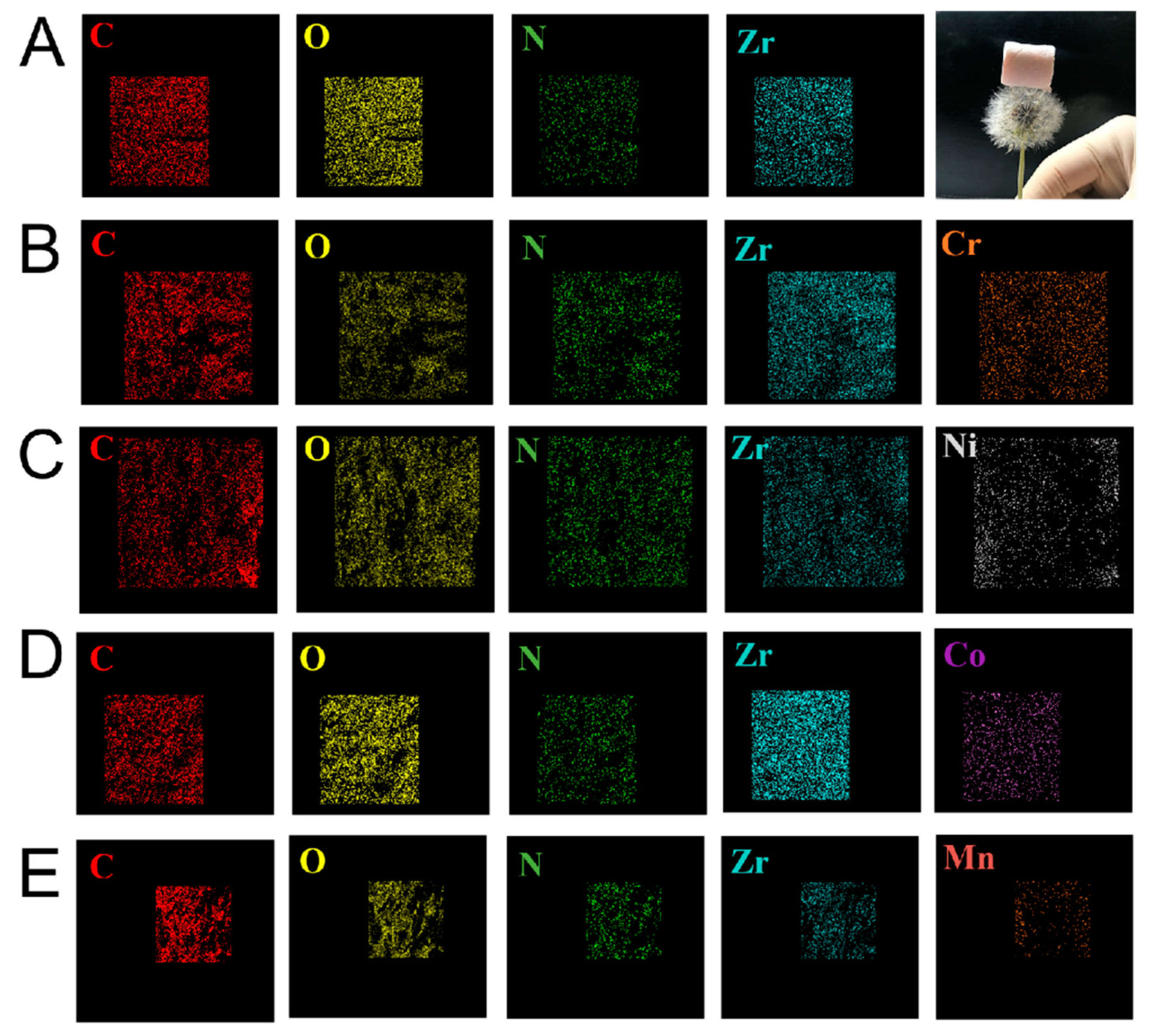
3.2. Surface Morphology Analysis
3.3. Chemical Composition
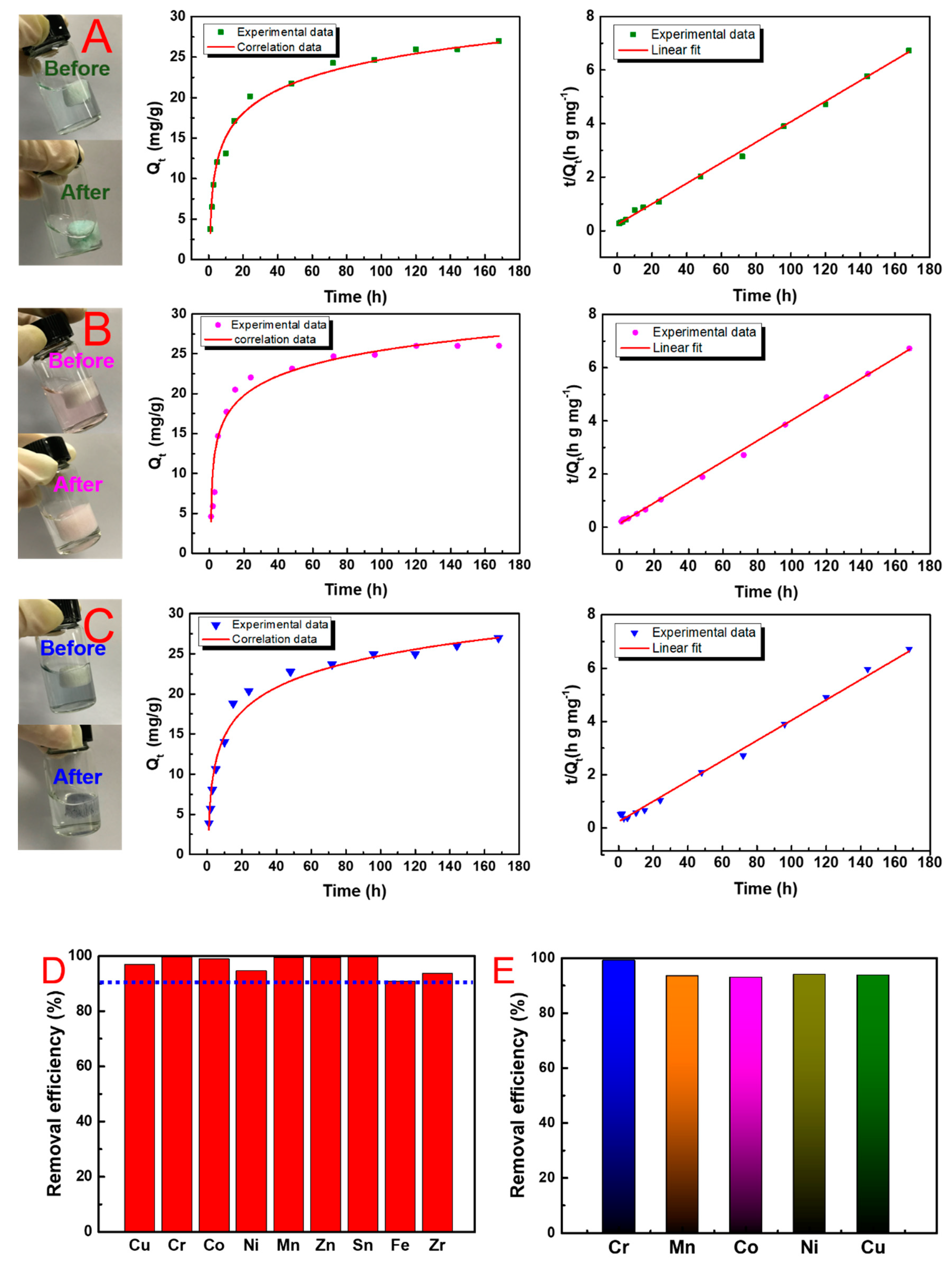
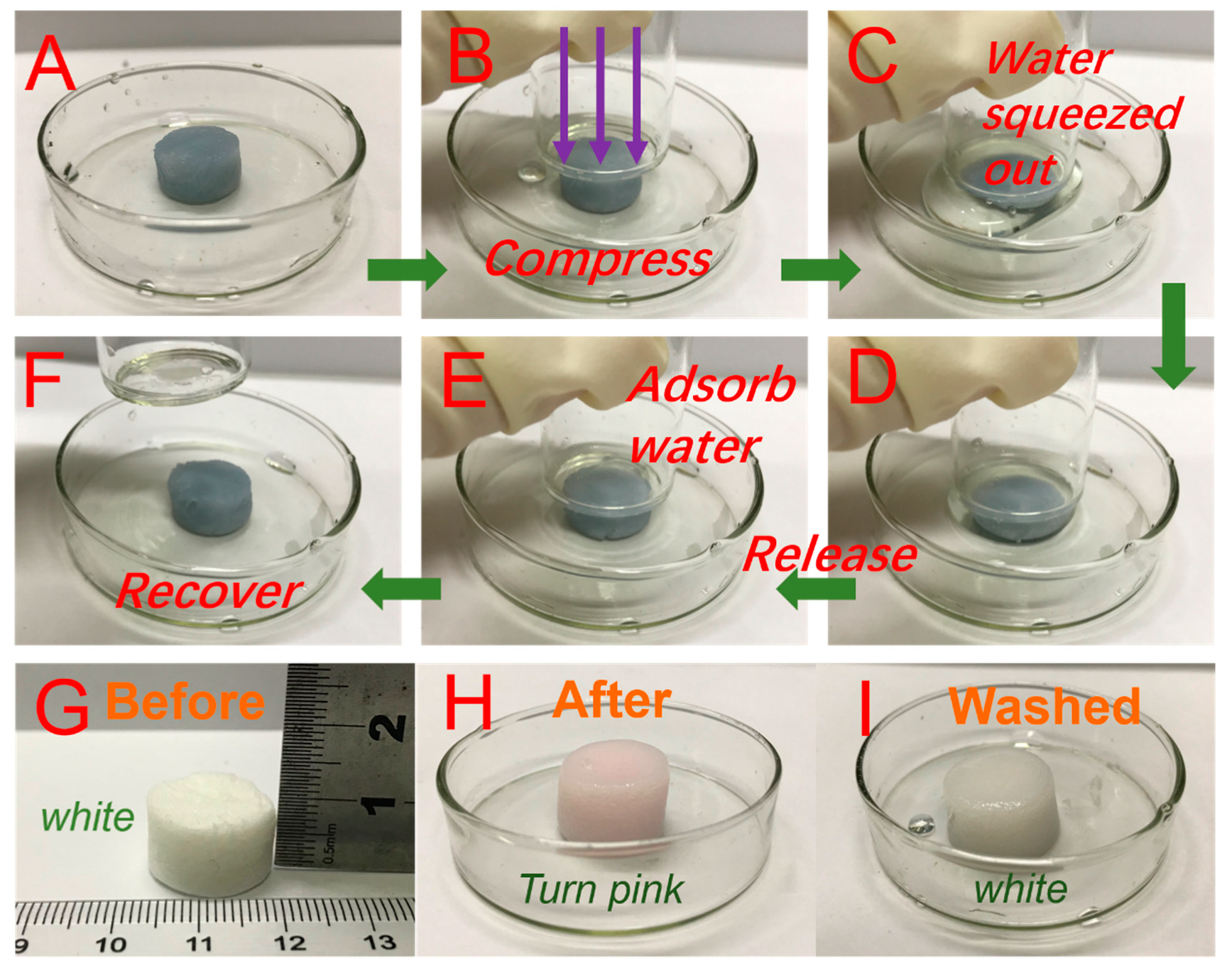
3.4. Adsorption Performance Analysis
3.5. Recycling of Adsorbents
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wadhawan, S.; Jain, A.; Nayyar, J.; Mehta, S.K. Role of nanomaterials as adsorbents in heavy metal ion removal from waste water: A review. J. Water Process. Eng. 2020, 33, 101038. [Google Scholar] [CrossRef]
- Lin, S.H.; Lin, C.M. Treatment of textile waste effluents by ozonation and chemical coagulation. Water Res. 1993, 27, 1743–1748. [Google Scholar] [CrossRef]
- Melnyk, I.; Pogorilyi, R.P.; Zub, Y.L.; Vaclavikova, M.; Gdula, K.; Dabrowski, A.; Seisenbaeva, G.A.; Kessler, V.G. Protection of Thiol Groups on the Surface of Magnetic Adsorbents and Their Application for Wastewater Treatment. Sci. Rep. 2018, 8, 8592. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, L.; Yu, C.; Li, K.; Tian, Y.; Jiang, L. In Situ Separation of Chemical Reaction Systems Based on a Special Wettable PTFE Membrane. Adv. Funct. Mater. 2017, 28, 1703970. [Google Scholar] [CrossRef]
- Kwak, S.; Yoo, J.-C.; Moon, D.H.; Baek, K. Role of clay minerals on reduction of Cr(VI). Geoderma 2018, 312, 1–5. [Google Scholar] [CrossRef]
- Wang, J.; Liu, M.; Duan, C.; Sun, J.; Xu, Y. Preparation and characterization of cellulose-based adsorbent and its application in heavy metal ions removal. Carbohydr. Polym. 2019, 206, 837–843. [Google Scholar] [CrossRef]
- Matlock, M.M.; Howerton, B.S.; Atwood, D.A. Chemical precipitation of heavy metals from acid mine drainage. Water Res. 2002, 36, 4757–4764. [Google Scholar] [CrossRef]
- Zuo, K.; Wu, J.; Chen, S.; Ji, X.; Wu, W. Superamphiphobic nanocellulose aerogels loaded with silica nanoparticles. Cellulose 2019, 26, 9661–9671. [Google Scholar] [CrossRef]
- Ngah, W.W.; Hanafiah, M.A.K.M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef] [PubMed]
- Parale, V.G.; Kim, T.; Phadtare, V.D.; Yadav, H.M.; Park, H.-H. Enhanced photocatalytic activity of a mesoporous TiO2 aerogel decorated onto three-dimensional carbon foam. J. Mol. Liq. 2019, 277, 424–433. [Google Scholar] [CrossRef]
- Kim, T.; Parale, V.G.; Jung, H.-N.-R.; Kim, Y.; Driss, Z.; Driss, D.; Bouabidi, A.; Euchy, S.; Park, H.-H. Facile Synthesis of SnO₂ Aerogel/Reduced Graphene Oxide Nanocomposites via in Situ Annealing for the Photocatalytic Degradation of Methyl Orange. Nanomaterials 2019, 9, 358. [Google Scholar] [CrossRef]
- Parale, V.G.; Kim, T.; Lee, K.-Y.; Phadtare, V.D.; Dhavale, R.P.; Jung, H.-N.-R.; Park, H.-H. Hydrophobic TiO2–SiO2 composite aerogels synthesized via in situ epoxy-ring opening polymerization and sol-gel process for enhanced degradation activity. Ceram. Int. 2020, 46, 4939–4946. [Google Scholar] [CrossRef]
- Yu, Z.; Qin, B.; Ma, Z.; Huang, J.; Li, S.; Zhao, H.; Li, H.; Zhu, Y.-B.; Wu, H.; Yu, S.-H. Superelastic Hard Carbon Nanofiber Aerogels. Adv. Mater. 2019, 31, e1900651. [Google Scholar] [CrossRef]
- Geng, S.; Wei, J.; Jonasson, S.; Hedlund, J.; Oksman, K. Multifunctional Carbon Aerogels with Hierarchical Anisotropic Structure Derived from Lignin and Cellulose Nanofibers for CO2 Capture and Energy Storage. ACS Appl. Mater. Interfaces 2020, 12, 7432–7441. [Google Scholar] [CrossRef] [PubMed]
- Köse, K.; Mavlan, M.; Youngblood, J.P. Applications and impact of nanocellulose based adsorbents. Cellulose 2020, 27, 2967–2990. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, X.; Zhou, H.; Li, J. Preparation of magnetic hydrophobic polyvinyl alcohol (PVA)–cellulose nanofiber (CNF) aerogels as effective oil absorbents. Cellulose 2017, 25, 1217–1227. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Super water absorbing and shape memory nanocellulose aerogels from TEMPO-oxidized cellulose nanofibrils via cyclic freezing–thawing. J. Mater. Chem. A 2014, 2, 350–359. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, L.; Wu, W.; Xiao, H. Methods and applications of nanocellulose loaded with inorganic nanomaterials: A review. Carbohydr. Polym. 2020, 229, 115454. [Google Scholar] [CrossRef]
- Li, T.; Song, J.; Zhao, X.; Yang, Z.; Pastel, G.; Xu, S.; Jia, C.; Dai, J.; Chen, C.; Gong, A.; et al. Anisotropic, lightweight, strong, and super thermally insulating nanowood with naturally aligned nanocellulose. Sci. Adv. 2018, 4, eaar3724. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Z.; Wu, X.; Wang, J.; Yang, S. Poly(vinyl alcohol)-Mediated Graphene Aerogels with Tailorable Architectures and Advanced Properties for Anisotropic Sensing. J. Phys. Chem. C 2019, 123, 3781–3789. [Google Scholar] [CrossRef]
- Wu, B.; Zhu, G.; Dufresne, A.; Lin, N. Fluorescent Aerogels Based on Chemical Crosslinking between Nanocellulose and Carbon Dots for Optical Sensor. ACS Appl. Mater. Interfaces 2019, 11, 16048–16058. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cranston, E.D. Chemically Cross-Linked Cellulose Nanocrystal Aerogels with Shape Recovery and Superabsorbent Properties. Chem. Mater. 2014, 26, 6016–6025. [Google Scholar] [CrossRef]
- Hwa, Y.; Yi, E.; Shen, H.; Sung, Y.; Kou, J.; Chen, K.; Parkinson, D.Y.; Doeff, M.M.; Cairns, E.J.; Sung, Y. Three-Dimensionally Aligned Sulfur Electrodes by Directional Freeze Tape Casting. Nano Lett. 2019, 19, 4731–4737. [Google Scholar] [CrossRef]
- Han, X.; Yang, S.; Schröder, M. Porous metal–organic frameworks as emerging sorbents for clean air. Nat. Rev. Chem. 2019, 3, 108–118. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Nuli, Y.; Zhou, J.; Yang, J.; Wang, J. Metal Organic Framework (MOF)-Derived carbon-encapsulated cuprous sulfide cathode based on displacement reaction for Hybrid Mg2+/Li+ batteries. J. Power Sources 2020, 445, 227325. [Google Scholar] [CrossRef]
- Wang, R.; He, M.; Zhou, Y.; Nie, S.; Wang, Y.; Liu, W.; He, Q.; Wu, W.; Bu, X.; Yang, X. Metal−organic frameworks self-templated cubic hollow Co/N/C@MnO2 composites for electromagnetic wave absorption. Carbon 2020, 156, 378–388. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2011, 112, 1105–1125. [Google Scholar] [CrossRef]
- Hou, X.; Zhou, H.; Zhang, J.; Cai, Y.; Huang, F.; Wei, Q. High Adsorption Pearl-Necklace-Like Composite Membrane Based on Metal-Organic Framework for Heavy Metal Ion Removal. Part. Part. Syst. Charact. 2018, 35, 1–8. [Google Scholar] [CrossRef]
- Aghajanzadeh, M.; Zamani, M.; Molavi, H.; Manjili, H.K.; Danafar, H.; Shojaei, A. Preparation of Metal–Organic Frameworks UiO-66 for Adsorptive Removal of Methotrexate from Aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2017, 28, 177–186. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, H.; Zhang, Y.; Kang, C.; Chen, S.; Song, L.; Liu, D.; Zhong, C. A versatile MOF-based trap for heavy metal ion capture and dispersion. Nat. Commun. 2018, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Van De Voorde, B.; Bueken, B.; Denayer, J.; De Vos, D. Adsorptive separation on metal–organic frameworks in the liquid phase. Chem. Soc. Rev. 2014, 43, 5766–5788. [Google Scholar] [CrossRef]
- Liu, F.; Xiong, W.; Feng, X.; Shi, L.; Chen, D.; Zhang, Y. A novel monolith ZnS-ZIF-8 adsorption material for ultraeffective Hg (II) capture from wastewater. J. Hazard. Mater. 2019, 367, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and Stability of Tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Lei, C.; Gao, J.; Ren, W.; Xie, Y.; Abdalkarim, S.Y.H.; Wang, S.; Ni, Q.; Yao, J. Fabrication of metal-organic frameworks@cellulose aerogels composite materials for removal of heavy metal ions in water. Carbohydr. Polym. 2019, 205, 35–41. [Google Scholar] [CrossRef]
- Saliba, R.; Gauthier, H.; Gauthier, R. Adsorption of Heavy Metal Ions on Virgin and Chemically-modified Lignocellulosic Materials. Adsorpt. Sci. Technol. 2005, 23, 313–322. [Google Scholar] [CrossRef]
- El-Khouly, A.S.; Takahashi, Y.; Saafan, A.A.; Kenawy, E.-R.; Hafiz, Y.A. Study of heavy metal ion absorbance by amidoxime group introduced to cellulose-graft-polyacrylonitrile. J. Appl. Polym. Sci. 2010, 120, 866–873. [Google Scholar] [CrossRef]
- Astrini, N.; Anah, L.; Haryadi, H.R. Adsorption of Heavy Metal Ion from Aqueous Solution by Using Cellulose Based Hydrogel Composite. Macromol. Symp. 2015, 353, 191–197. [Google Scholar] [CrossRef]
- Liu, P.; Borrell, P.F.; Božič, M.; Kokol, V.; Oksman, K.; Mathew, A.P. Nanocelluloses and their phosphorylated derivatives for selective adsorption of Ag+, Cu2+ and Fe3+ from industrial effluents. J. Hazard. Mater. 2015, 294, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Omri, A.; Benzina, M. Removal of manganese(II) ions from aqueous solutions by adsorption on activated carbon derived a new precursor: Ziziphus spina-christi seeds. Alex. Eng. J. 2012, 51, 343–350. [Google Scholar] [CrossRef]



| Adsorbents | Qmax (mg g−1) | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cu2+ | Cr3+ | Co2+ | Ni2+ | Mn2+ | Zn2+ | Fe3+ | Zr4+ | ||
| UiO-66-NH2@CA | 39 | [37] | |||||||
| Am-WS | 136 | [38] | |||||||
| 2C-g-PAN | 100 | 49 | [39] | ||||||
| 3CMC-g-PAA/MT | 286 | [40] | |||||||
| 4Phos-CNCSL | 115 | [41] | |||||||
| Carboxymethylated cellulose fiber | 17 | 12 | [7] | ||||||
| Activated carbon | 172 | [42] | |||||||
| U-EDTACCA | 104 | 396 | 307 | 120 | 251 | 215 | 115 | 165 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Tan, S.; Xu, Z. Anisotropic Nanocellulose Aerogel Loaded with Modified UiO-66 as Efficient Adsorbent for Heavy Metal Ions Removal. Nanomaterials 2020, 10, 1114. https://doi.org/10.3390/nano10061114
Li J, Tan S, Xu Z. Anisotropic Nanocellulose Aerogel Loaded with Modified UiO-66 as Efficient Adsorbent for Heavy Metal Ions Removal. Nanomaterials. 2020; 10(6):1114. https://doi.org/10.3390/nano10061114
Chicago/Turabian StyleLi, Jiajia, Sicong Tan, and Zhaoyang Xu. 2020. "Anisotropic Nanocellulose Aerogel Loaded with Modified UiO-66 as Efficient Adsorbent for Heavy Metal Ions Removal" Nanomaterials 10, no. 6: 1114. https://doi.org/10.3390/nano10061114
APA StyleLi, J., Tan, S., & Xu, Z. (2020). Anisotropic Nanocellulose Aerogel Loaded with Modified UiO-66 as Efficient Adsorbent for Heavy Metal Ions Removal. Nanomaterials, 10(6), 1114. https://doi.org/10.3390/nano10061114