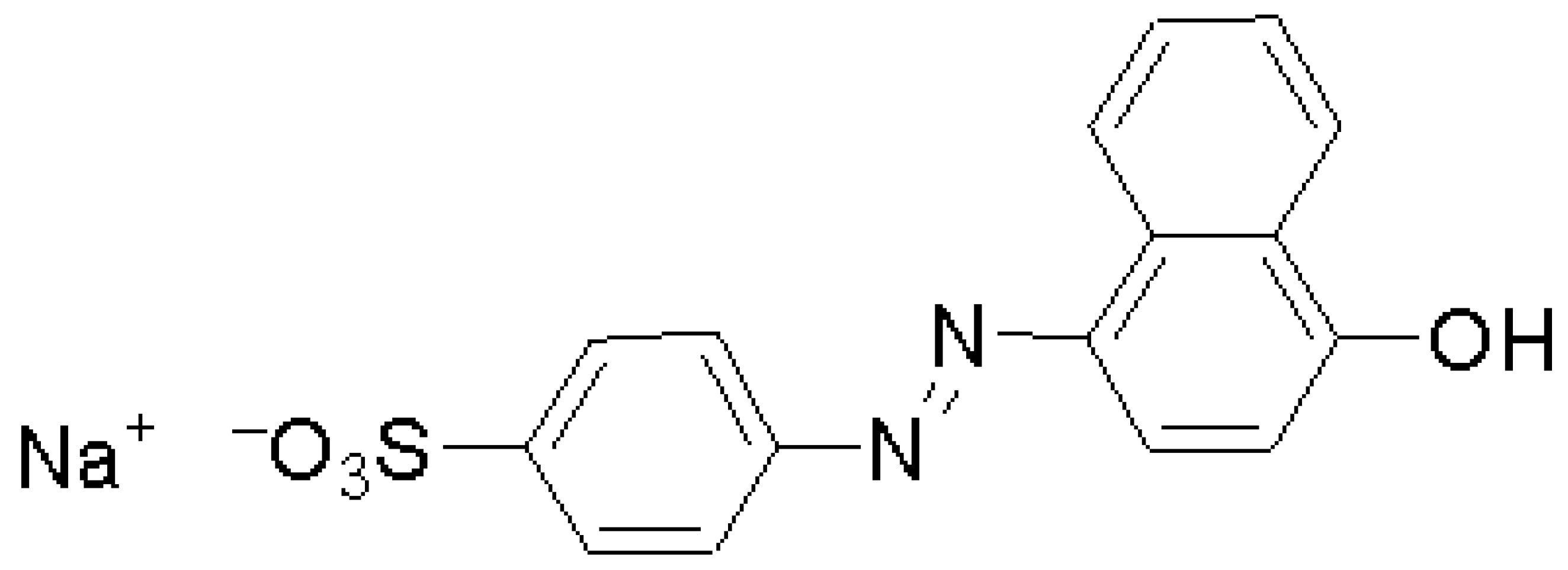
Surface Modification of Bentonite with Polymer Brushes and Its Application as an Efficient Adsorbent for the Removal of Hazardous Dye Orange I
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Aminosilane Functionalized Bentonite (Bent-APTES)
2.3. Preparation of Bent-Br
2.4. Preparation of Bent-PDMAEMA
2.5. Characterizations
2.6. Batch Adsorption Experiments
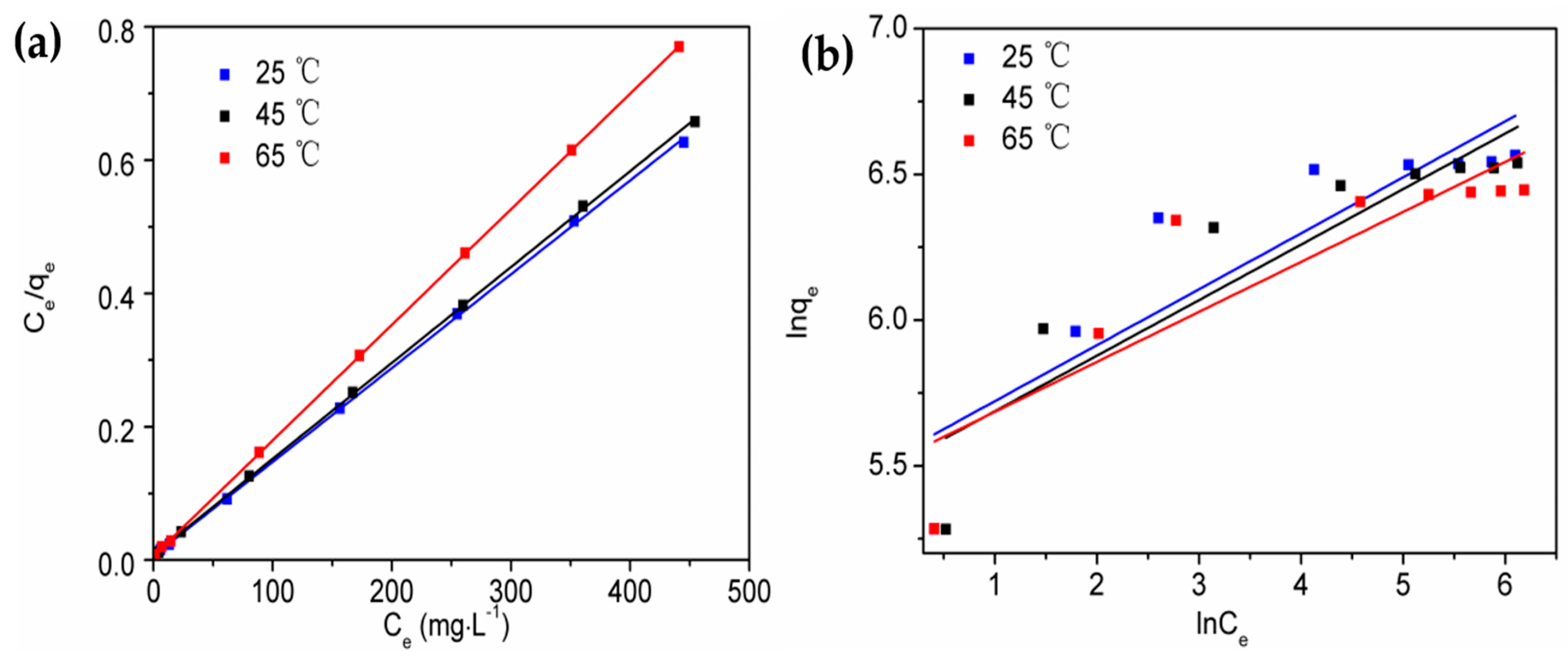
2.7. Adsorption Isotherms
2.7.1. Langmuir Isotherm
2.7.2. Freundlich Isotherm
2.8. Adsorption Kinetics
3. Results and Discussion
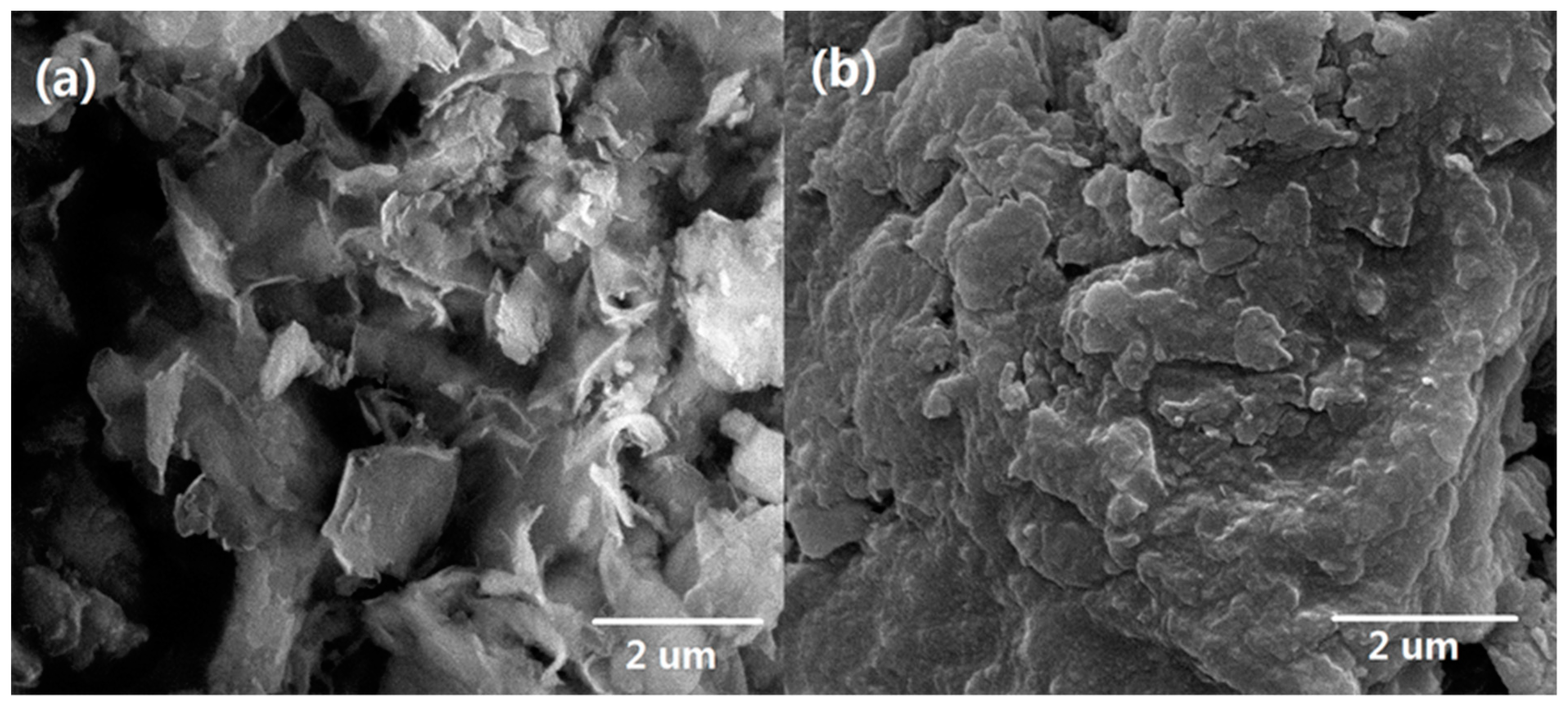
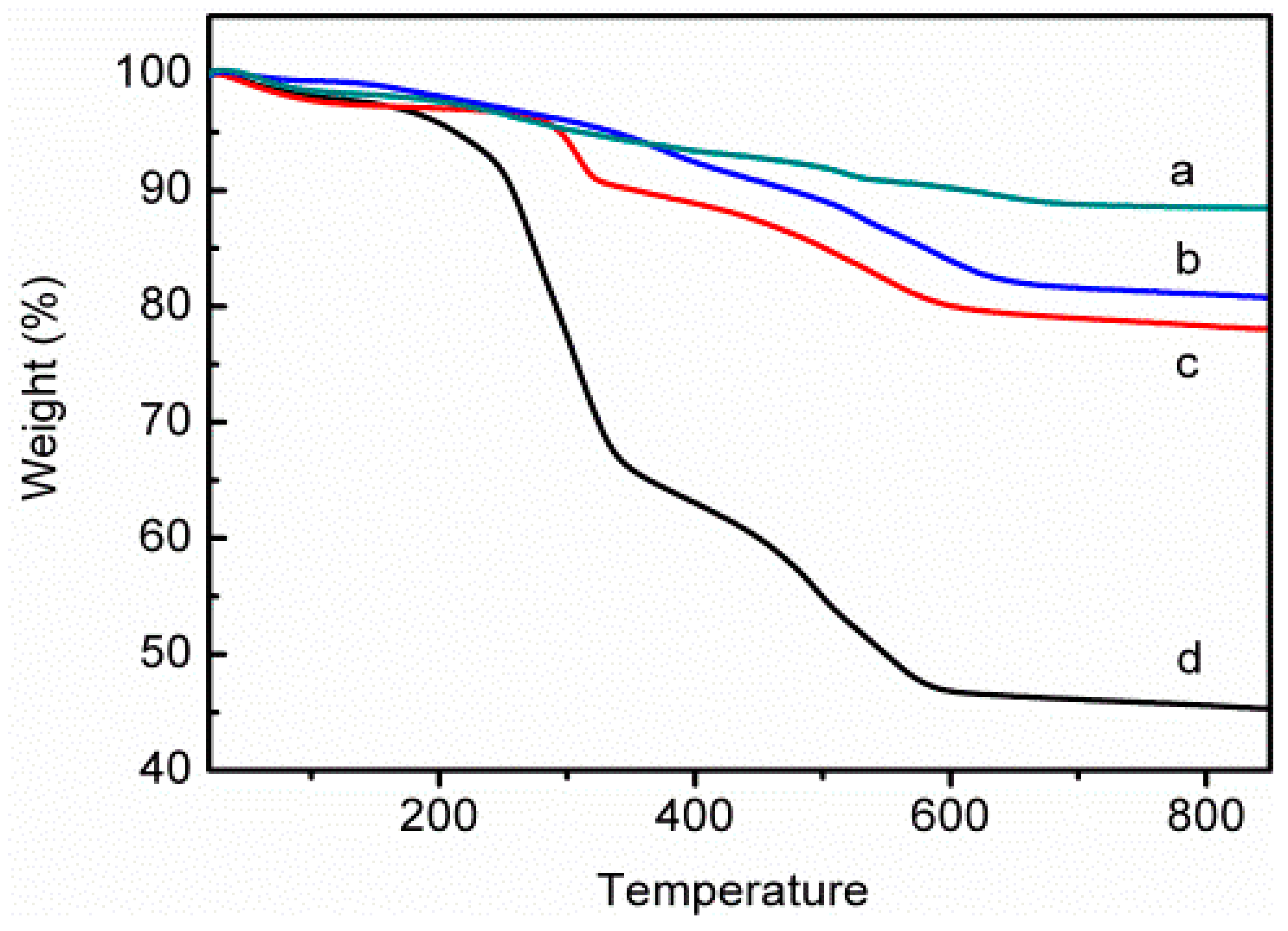
3.1. Characterizations of Bent-PDMAEMA
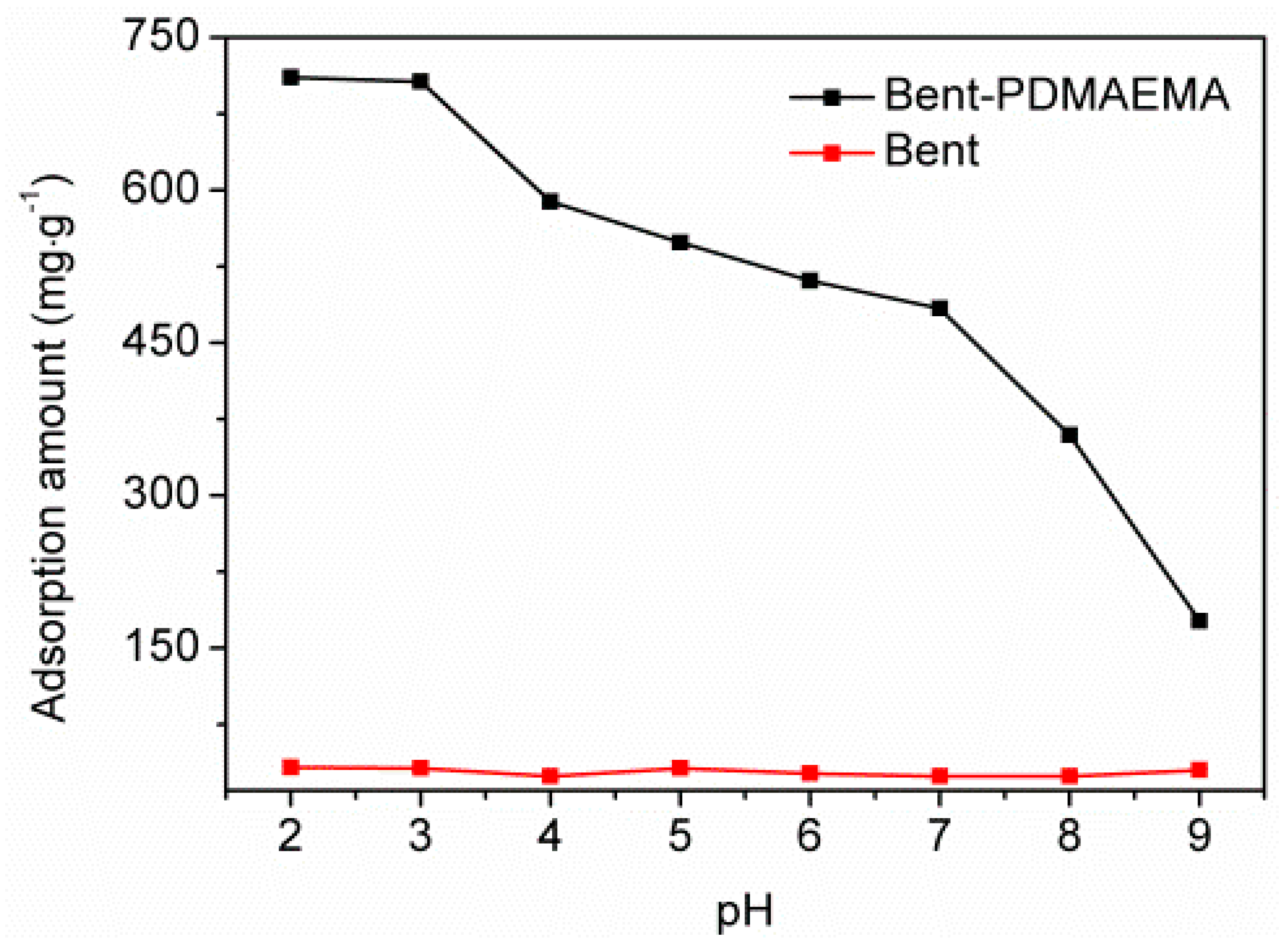
3.2. Effect of pH
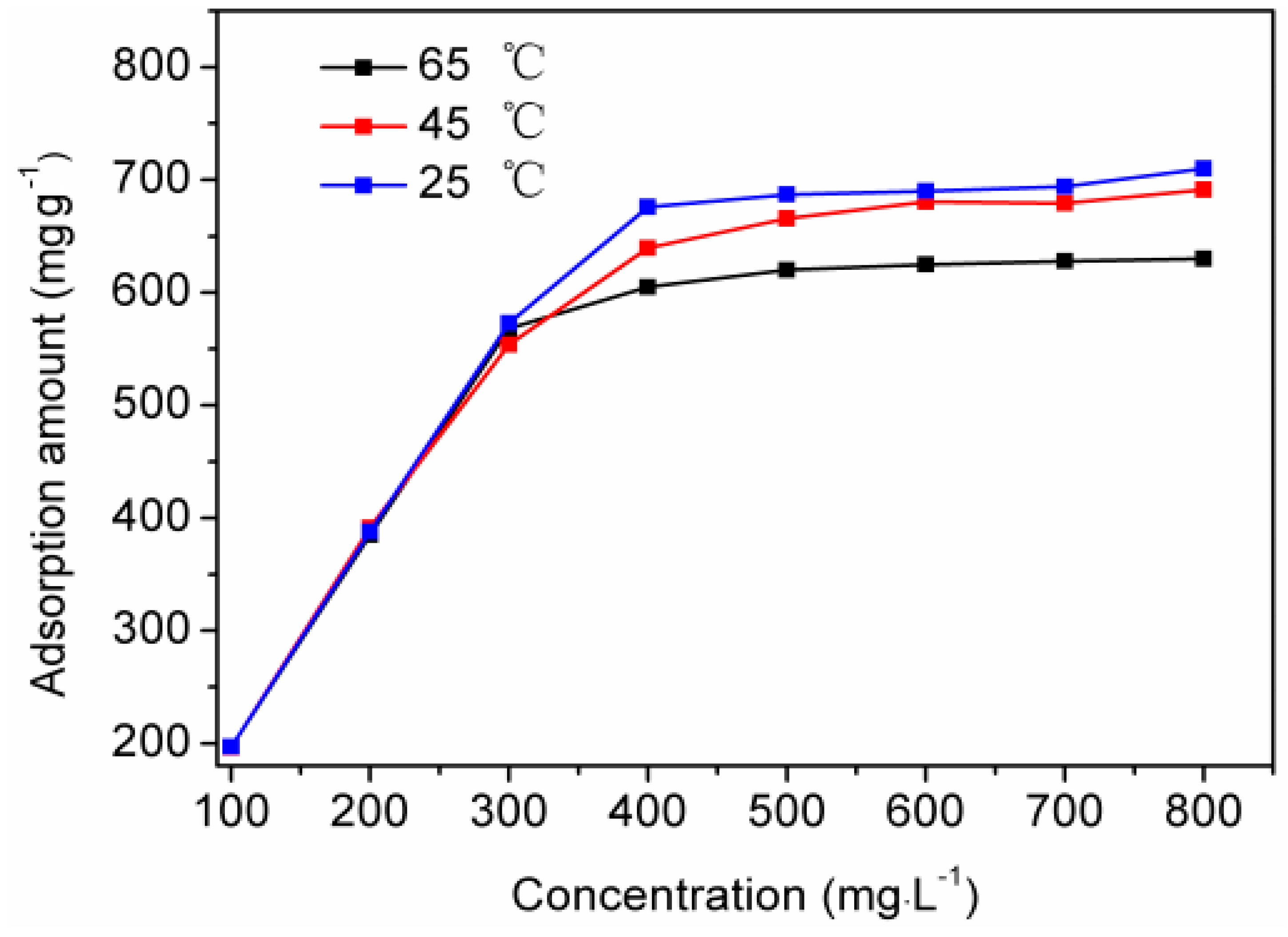
3.3. Effect of Initial Concentration of Orange I
3.4. Effect of the Contact Time
3.5. Comparison of Adsorption Ability of Bent-PDMAEMA with other Materials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mousally, S.M.; Al-Zaydi, K.M.; Petrier, C.; Arab, S.T.; Refat, M.S. Study of sonochemical effect on dibenzothiophene in deionized water, natural water and sea water. Sci. Adv. Mater. 2019, 11, 1684–1691. [Google Scholar] [CrossRef]
- Dehgan-Reyhan, S.; Asbaghi, N.S.; Soleimani, M.; Samuei, S.; Razmi, H. Designing and development of nanosorbent graphene oxide/eggshell membrane for removing heavy metals from water pollution. Sens. Lett. 2019, 17, 546–554. [Google Scholar] [CrossRef]
- Li, J.; Chang, Q.G.; Ma, W.W.; Lu, J.J.; Tong, Y.B.; Zhou, L. Preparation and characterization of fluoride adsorption efficiency from water of mesoporous hollow CaO spheres. Sci. Adv. Mater. 2019, 11, 1189–1197. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kaur, Y.; Umar, A.; Chaudhary, G.R. Ionic liquid and surfactant functionalized ZnO nanoadsorbent for recyclable proficient adsorption of toxic dyes from waste water. J. Mol. Liq. 2016, 224, 1294–1304. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, X.; Qu, F.; Umar, A.; Wu, X. Hierarchical WO3 nanostructures assembled by nanosheets and their applications in wastewater purification. J. Alloy. Compd. 2016, 689, 570–574. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, Z.; Zhang, Y.; Umar, A.; Guo, Z.; O’Hare, D.; Wang, Q. Hierarchical Fe3O4 core–shell layered double hydroxide composites as magnetic adsorbents for anionic dye removal from wastewater. Eur. J. Inorg. Chem. 2015, 2015, 4182–4191. [Google Scholar] [CrossRef]
- Chaudhary, G.R.; Saharan, P.; Kumar, A.; Mehta, S.K.; Mor, S.; Umar, A. Adsorption studies of cationic, anionic and azo-dyes via monodispersed Fe3O4 nanoparticles. J. Nanosci. Nanotechnol. 2013, 13, 3240–3245. [Google Scholar] [CrossRef]
- Almeida, E.J.R.; Corso, C.R. Decolorization and removal of toxicity of textile azo dyes using fungal biomass pelletized. Int. J. Environ. Sci. Technol. 2019, 16, 1319–1328. [Google Scholar] [CrossRef]
- Aliouche, S.; Djebbar, K.; Sehili, T. Removal of an azo dye (Alizarin yellow) in homogeneous medium using direct photolysis, acetone/UV, H2O2/UV, S2O82−/UV, H2O2/ S2O82−/UV, and S2O82−/heat. Desalin. Water Treat. 2016, 57, 18182–18193. [Google Scholar] [CrossRef]
- Xue, T.; Gao, Y.; Zhang, Z.; Umar, A.; Yan, X.; Zhang, X.; Guo, Z.; Wang, Q. Adsorption of acid red from dye wastewater by Zn2Al-NO3 LDHs and the resource of adsorbent sludge as nanofiller for polypropylene. J. Alloy. Compd. 2014, 587, 99–104. [Google Scholar] [CrossRef]
- Xu, P.; Li, B.; Yu, J.; Liu, L.; Xu, J.; Wang, Z.; Fan, Y. Enhancing the adsorption of methylene blue onto hemp hurd powder by tailoring its surface properties. Sci. Adv. Mater. 2019, 11, 661–671. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, L.-C.; Wang, R.; Wang, L.; Zhang, W.; Cheng, X.; Liu, C.; Ni, S.-J.; Wang, J.-J. The experiments and speciation simulation of vanadium adsorption on nano-goethite. Sci. Adv. Mater. 2019, 11, 569–579. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, S.; Umar, A.; Jha, M.; Mehta, S.K.; Kansal, S.K. Nanocuboidal-shaped zirconium based metal organic framework for the enhanced adsorptive removal of nonsteroidal anti-inflammatory drug, ketorolac tromethamine, from aqueous phase. New J. Chem. 2018, 42, 1921–1930. [Google Scholar] [CrossRef]
- Shi, M.; Zhao, Z.; Song, Y.; Wang, Z.; Fan, T.; Li, J. Competitive adsorption of Pb and Cd on ferrihydrite modified by humic acid. Sci. Adv. Mater. 2019, 11, 1180–1188. [Google Scholar] [CrossRef]
- Chaturvedi, G.; Kaur, A.; Umar, A.; Khan, M.A.; Algarni, H.; Kansal, S.K. Removal of fluoroquinolone drug, levofloxacin, from aqueous phase over iron based MOFs, MIL-100 (Fe). J. Solid State Chem. 2020, 281, 121029. [Google Scholar] [CrossRef]
- Yan, T.; Li, N.; Qiao, Z.; Li, W.; Wang, H.; Jing, Z.; Yu, Y.; Jiang, Z. Ultrathin sodium ferric silicate 2D nanosheets: A novel and robust adsorbent for selective removal of cationic dyes in wastewater. J. Alloy. Compd. 2019, 784, 256–265. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, W.; Tian, Z.; Zhao, Y.; Shen, Z. Facile fabrication of Bi2WO6/BiOCl hierarchical structure as adsorbents for methylene blue dye removal. Mater. Res. Express 2019, 6, 055034. [Google Scholar] [CrossRef]
- Zhang, P.; Ouyang, S.; Li, P.; Huang, Y.; Frost, R.L. Enhanced removal of ionic dyes by hierarchical organic three-dimensional layered double hydroxide prepared via soft-template synthesis with mechanism study. Chem. Eng. J. 2019, 360, 1137–1149. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Paliychuk, N.; Bitra, R.B.; Shyichuk, A.; Naushad, M.; Mironyuk, I.; Ziółkowska, D. Adsorptive removal of toxic methylene blue and acid orange 7 dyes from aqueous medium using cobalt-zinc ferrite nanoadsorbents. Desalin. Water Treat. 2019, 150, 374–385. [Google Scholar] [CrossRef]
- Vatanpour, V.; Shahsavarifar, S.; Khorshidi, S.; Masteri-Farahani, M. A novel antifouling ultrafiltration membranes prepared from percarboxylic acid functionalized SiO2 bound Fe3O4 nanoparticle (SCMNP-COOOH)/polyethersulfone nanocomposite for BSA separation and dye removal. J. Chem. Technol. Biotechnol. 2019, 94, 1341–1353. [Google Scholar] [CrossRef]
- Blachnio, M.; Budnyak, T.M.; Derylo-Marczewska, A.; Marczewski, A.W.; Tertykh, V.A. Chitosan–silica hybrid composites for removal of sulfonated azo dyes from aqueous solutions. Langmuir 2018, 34, 2258–2273. [Google Scholar] [CrossRef] [PubMed]
- Volikov, A.B.; Ponomarenko, S.A.; Konstantinov, A.I.; Hatfield, K.; Perminova, I.V. Nature-like solution for removal of direct brown 1 azo dye from aqueous phase using humics-modified silica gel. Chemosphere 2016, 145, 83–88. [Google Scholar] [CrossRef]
- Barbusinski, K.; Klis, S.; Thomas, M.; Kudlek, E. Application of fenton reagent modified with nano zero-valent iron to removal of azo dyes (AR27 and RB5) from aqueous solutions. OCHRONA SRODOWISKA 2018, 40, 35–39. [Google Scholar]
- Dalvand, A.; Nabizadeh, R.; Ganjali, M.R.; Khoobi, M.; Nazmara, S.; Mahvi, A.H. Modeling of reactive blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: Optimization, reusability, kinetic and equilibrium studies. J. Magn. Magn. Mater. 2016, 404, 179–189. [Google Scholar] [CrossRef]
- Duman, O.; Tunç, S.; Bozoğlan, B.K.; Polat, T.G. Removal of triphenylmethane and reactive azo dyes from aqueous solution by magnetic carbon nanotube-κ-carrageenan-Fe3O4 nanocomposite. J. Alloy. Compd. 2016, 687, 370–383. [Google Scholar] [CrossRef]
- Es’ haghzade, Z.; Pajootan, E.; Bahrami, H.; Arami, M. Facile synthesis of Fe3O4 nanoparticles via aqueous based electro chemical route for heterogeneous electro-Fenton removal of azo dyes. J. Taiwan Inst. Chem. Eng. 2017, 71, 91–105. [Google Scholar] [CrossRef]
- Jung, K.W.; Choi, B.H.; Ahn, K.H.; Lee, S.H. Synthesis of a novel magnetic Fe3O4/γ-Al2O3 hybrid composite using electrode-alternation technique for the removal of an azo dye. Appl. Surf. Sci. 2017, 423, 383–393. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.I.; Kamoun, E.A.; El-Shanshory, A.A.; Soliman, H.M.; Aly, H.F. Evaluation of graphene oxide-activated carbon as effective composite adsorbent toward the removal of cationic dyes: Composite preparation, characterization and adsorption parameters. J. Mol. Liq. 2019, 279, 530–539. [Google Scholar] [CrossRef]
- Chen, S.; Chen, G.; Chen, H.; Sun, Y.; Yu, X.; Su, Y.; Tang, S. Preparation of porous carbon-based material from corn straw via mixed alkali and its application for removal of dye. Colloids Surf. A 2019, 568, 173–183. [Google Scholar] [CrossRef]
- Arsalani, N.; Nasiri, R.; Zarei, M. Synthesis of magnetic 3D graphene decorated with CaCO3 for anionic azo dye removal from aqueous solution: Kinetic and RSM modeling approach. Chem. Eng. Res. Des. 2018, 136, 795–805. [Google Scholar] [CrossRef]
- Kong, Y.; Zhuang, Y.; Han, Z.; Yu, J.; Shi, B.; Han, K.; Hao, H. Dye removal by eco-friendly physically cross-linked double network polymer hydrogel beads and their functionalized composites. J. Environ. Sci. 2019, 78, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, F.; Liu, T.; Peng, N.; Gai, C. Removal of azo dye by a highly graphitized and heteroatom doped carbon derived from fish waste: Adsorption equilibrium and kinetics. J. Environ. Manag. 2016, 182, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, Y.; Deng, B.; Zhu, K.; Zhang, H. Activated carbon adsorptive removal of azo dye and peroxydisulfate regeneration: From a batch study to continuous column operation. Environ. Sci. Pollut. Res. 2017, 24, 4932–4941. [Google Scholar] [CrossRef] [PubMed]
- Saavedra-Labastida, E.; Díaz-Nava, M.C.; Illescas, J.; Muro, C. Comparison of the removal of an anionic dye from aqueous solutions by adsorption with organically modified clays and their composites. Water Air Soil Pollut. 2019, 230, 88. [Google Scholar] [CrossRef]
- Ngulube, T.; Gumbo, J.R.; Masindi, V.; Maity, A. Preparation and characterisation of high performing magnesite-halloysite nanocomposite and its application in the removal of methylene blue dye. J. Mol. Struct. 2019, 1184, 389–399. [Google Scholar] [CrossRef]
- Berez, A.; Schäfer, G.; Ayari, F.; Trabelsi-Ayadi, M. Adsorptive removal of azo dyes from aqueous solutions by natural bentonite under static and dynamic flow conditions. Int. J. Environ. Sci. Technol. 2016, 13, 1625–1640. [Google Scholar] [CrossRef]
- Tie, J.; Fang, X.; Wang, X.; Zhang, Y.; Gu, T.; Deng, S.; Li, G.; Tang, D. Adsorptive removal of a reactive azo dye using polyaniline-intercalated bentonite. Pol. J. Environ. Stud. 2017, 26, 1259–1268. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Q.; You, Q.; Liao, G.; Xia, H.; Wang, D. Porous polyimide framework: A novel versatile adsorbent for highly efficient removals of azo dye and antibiotic. React. Funct. Polym. 2016, 103, 9–16. [Google Scholar] [CrossRef]
- Sánchez-Duarte, R.G.; López-Cervantes, J.; Sánchez-Machado, D.I.; Correa-Murrieta, M.A.; Núñez-Gastélum, J.A.; Rodríguez-Núñez, J.R. Chitosan-based adsorbents gels for the removal of tris-AZO dye isotherms and kinetics studies. Environ. Eng. Manag. J. 2016, 15, 2469–2478. [Google Scholar]
- Ou, H.; You, Q.; Li, J.; Liao, G.; Xia, H.; Wang, D. A rich-amine porous organic polymer: An efficient and recyclable adsorbent for removal of azo dye and chlorophenol. RSC Adv. 2016, 6, 98487–98497. [Google Scholar] [CrossRef]
- Lin, J.; Jiang, B.; Zhan, Y. Effect of pre-treatment of bentonite with sodium and calcium ions on phosphate adsorption onto zirconium-modified bentonite. J. Environ. Manag. 2018, 217, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Jiao, S.; Ma, Z.; Lin, H.; Gao, W.; Chen, J. Humic acid-modified bentonite composite material enhances urea-nitrogen use efficiency. Chemosphere 2020, 255, 126976. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Pei, M.; He, Y.; Yu, F.; Guo, W.; Wang, L. Preparation, characterization and application of magnetic Fe3O4-CS for the adsorption of orange I from aqueous solutions. PLoS ONE 2014, 9, e108647. [Google Scholar] [CrossRef]
- García, E.R.; Medina, R.L.; Lozano, M.M.; Hernández Pérez, I.; Valero, M.J.; Franco, A.M.M. Adsorption of azo-dye orange II from aqueous solutions using a metal-organic framework material: Iron-benzenetricarboxylate. Materials 2014, 7, 8037–8057. [Google Scholar] [CrossRef] [PubMed]
- Extremera, R.; Pavlovic, I.; Pérez, M.R.; Barriga, C. Removal of acid orange 10 by calcined Mg/Al layered double hydroxides from water and recovery of the adsorbed dye. Chem. Eng. J. 2012, 213, 392–400. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, X.; Wang, X. Adsorption of acid orange 10 on cross-linked porous polyimide. SN Appl. Sci. 2019, 1, 239. [Google Scholar] [CrossRef]




| Adsorbent Materials | Maximum Dye Adsorption | Reference |
|---|---|---|
| Fe3O4-CS for Orange I | 183.2 mg·g−1 | [43] |
| Iron-benzenetricarboxylate for Orange II | 435 mg·g−1 | [44] |
| MgAl-layered double hydroxides for acid Orange 10 | 1.47 mmol·g−1 | [45] |
| Cross-linked porous polyimide for Orange 10 | 500 mg/L | [46] |
| Bent-PDMAEMA for Orange I | 700 mg·L−1 | Present work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Umar, A.; Du, Y.; Wang, L.; Pei, M. Surface Modification of Bentonite with Polymer Brushes and Its Application as an Efficient Adsorbent for the Removal of Hazardous Dye Orange I. Nanomaterials 2020, 10, 1112. https://doi.org/10.3390/nano10061112
Guo W, Umar A, Du Y, Wang L, Pei M. Surface Modification of Bentonite with Polymer Brushes and Its Application as an Efficient Adsorbent for the Removal of Hazardous Dye Orange I. Nanomaterials. 2020; 10(6):1112. https://doi.org/10.3390/nano10061112
Chicago/Turabian StyleGuo, Wenjuan, Ahmad Umar, Yankai Du, Luyan Wang, and Meishan Pei. 2020. "Surface Modification of Bentonite with Polymer Brushes and Its Application as an Efficient Adsorbent for the Removal of Hazardous Dye Orange I" Nanomaterials 10, no. 6: 1112. https://doi.org/10.3390/nano10061112
APA StyleGuo, W., Umar, A., Du, Y., Wang, L., & Pei, M. (2020). Surface Modification of Bentonite with Polymer Brushes and Its Application as an Efficient Adsorbent for the Removal of Hazardous Dye Orange I. Nanomaterials, 10(6), 1112. https://doi.org/10.3390/nano10061112






