Densification-Induced Structure Changes in Basolite MOFs: Effect on Low-Pressure CH4 Adsorption
Abstract
:1. Introduction
2. Materials and Methods
3. Results
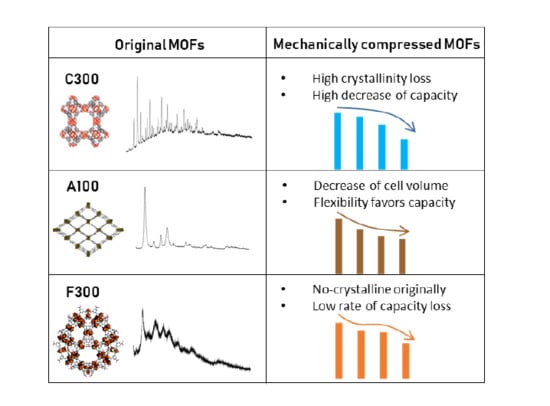
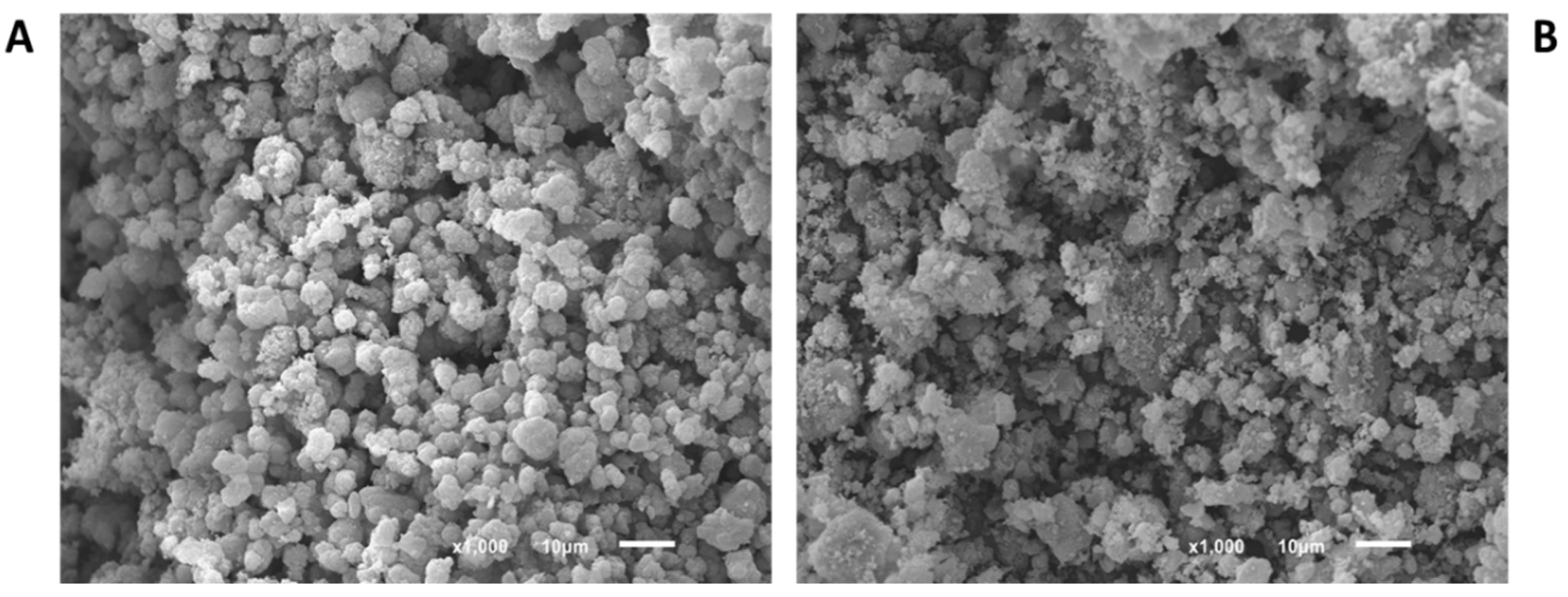
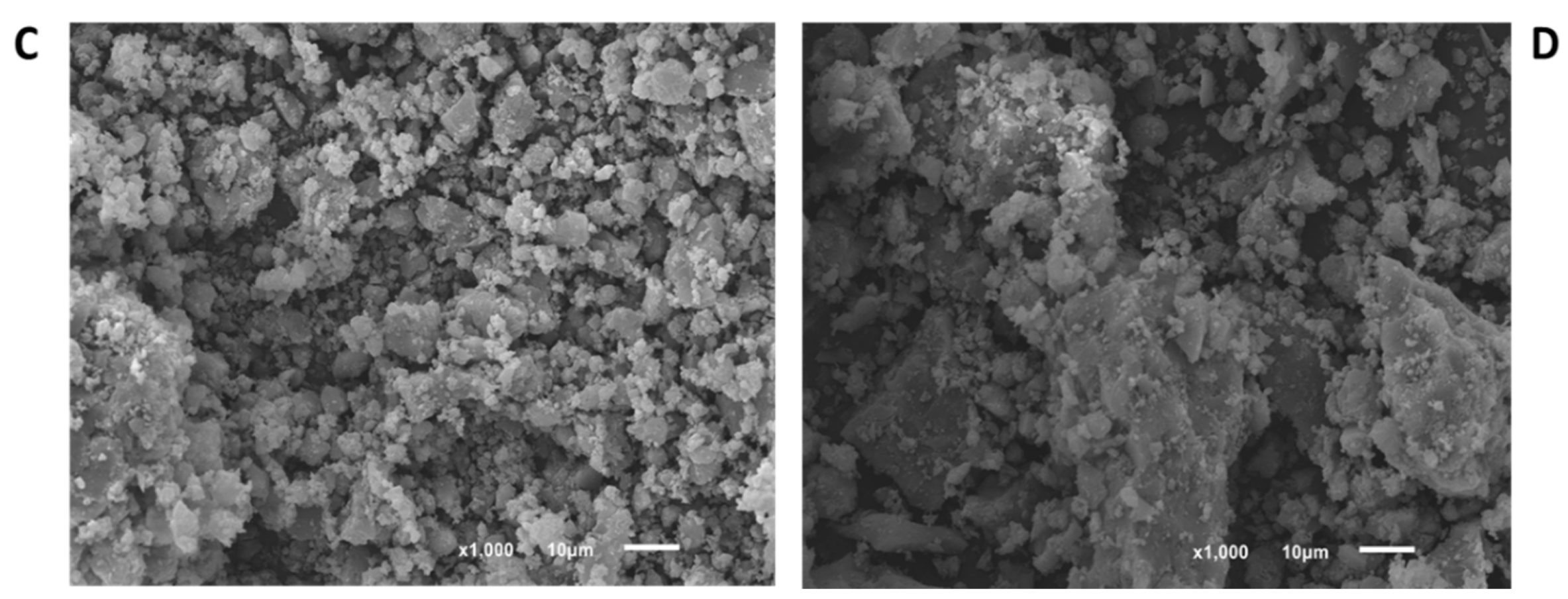
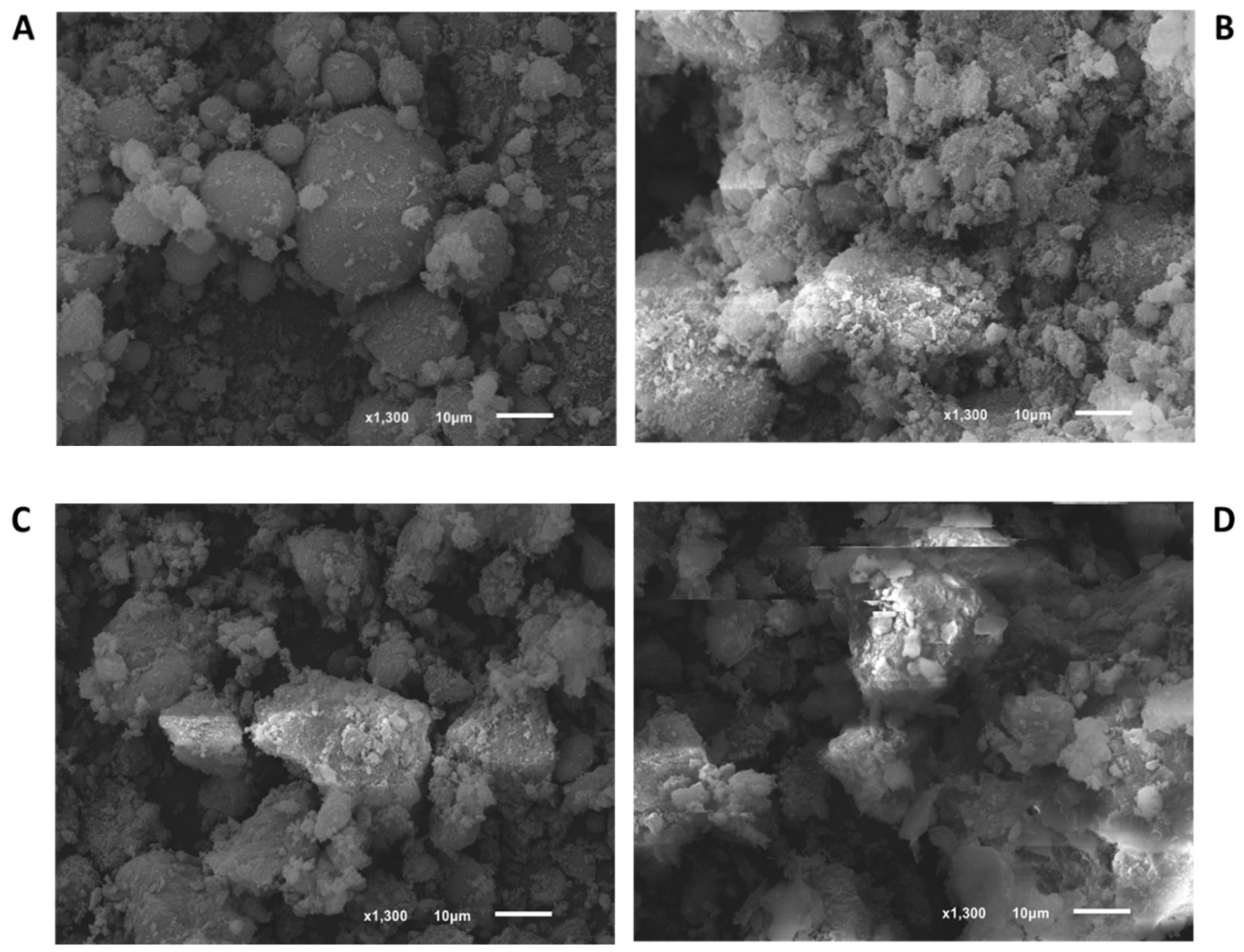
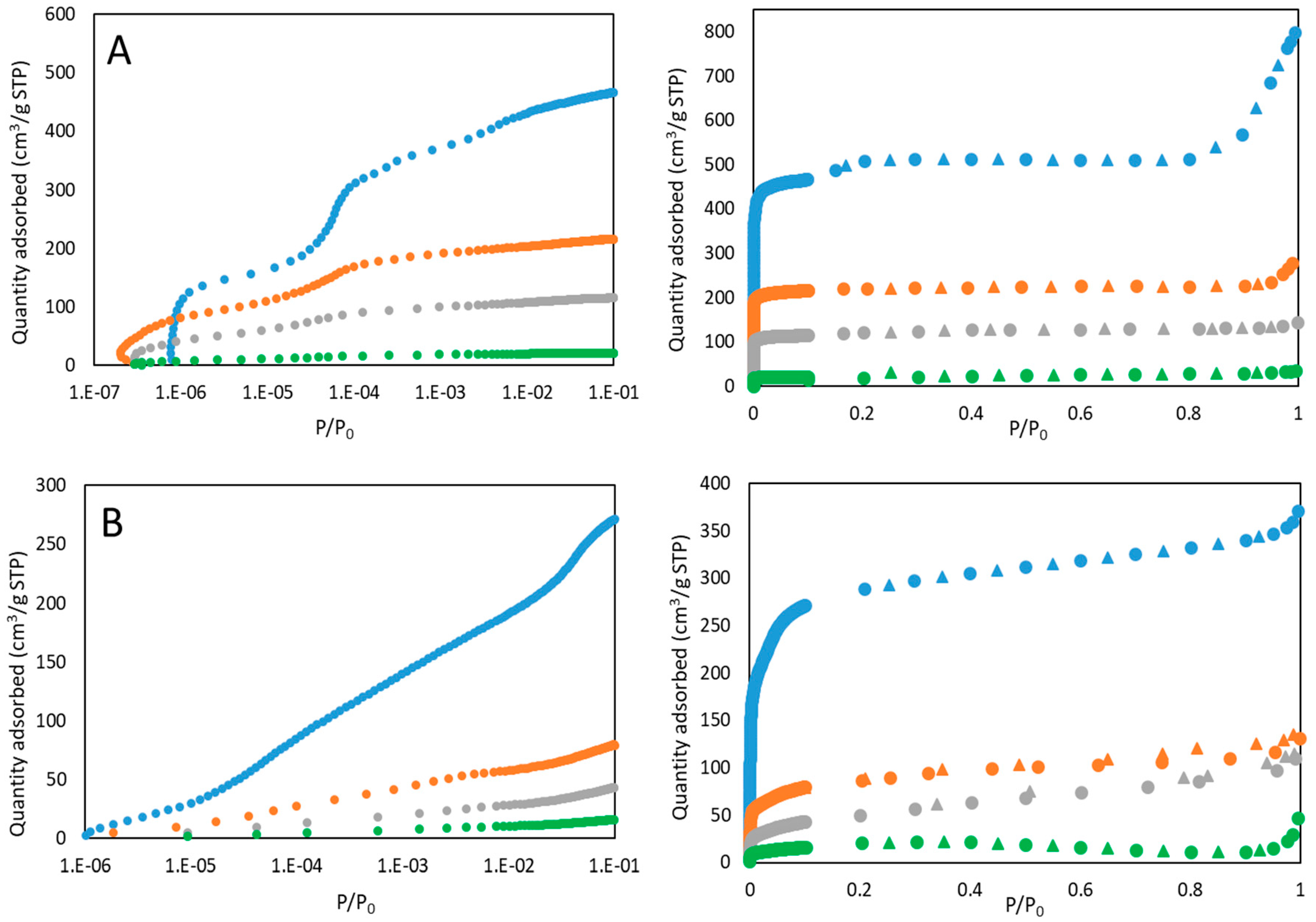
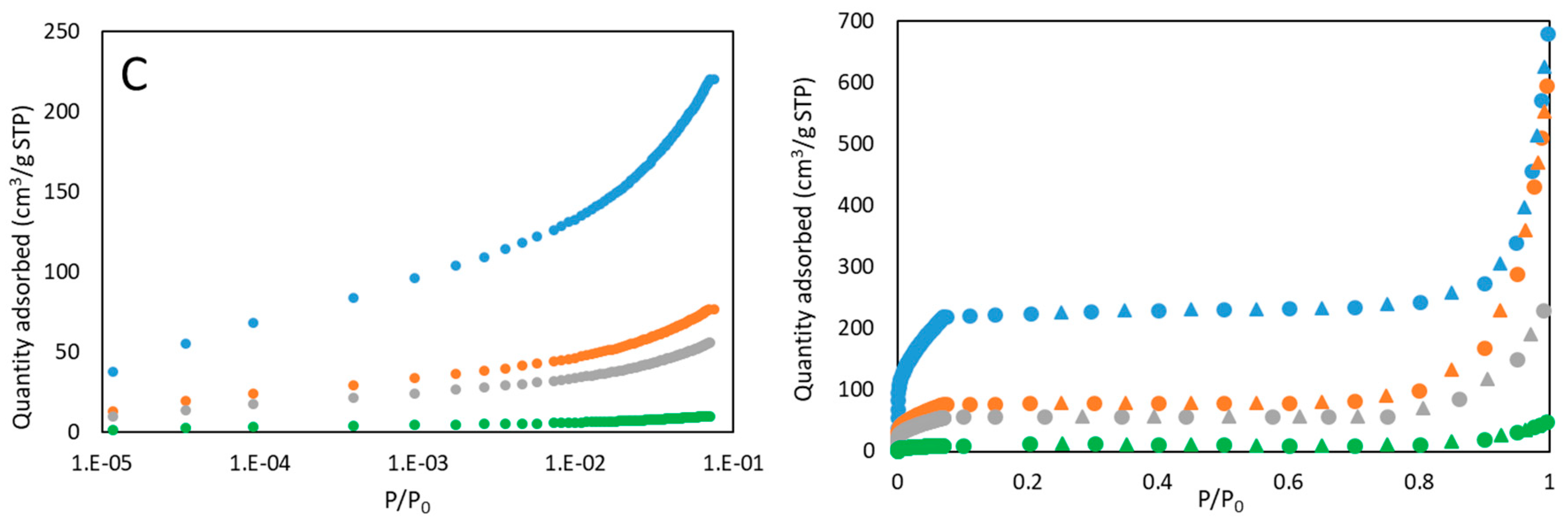
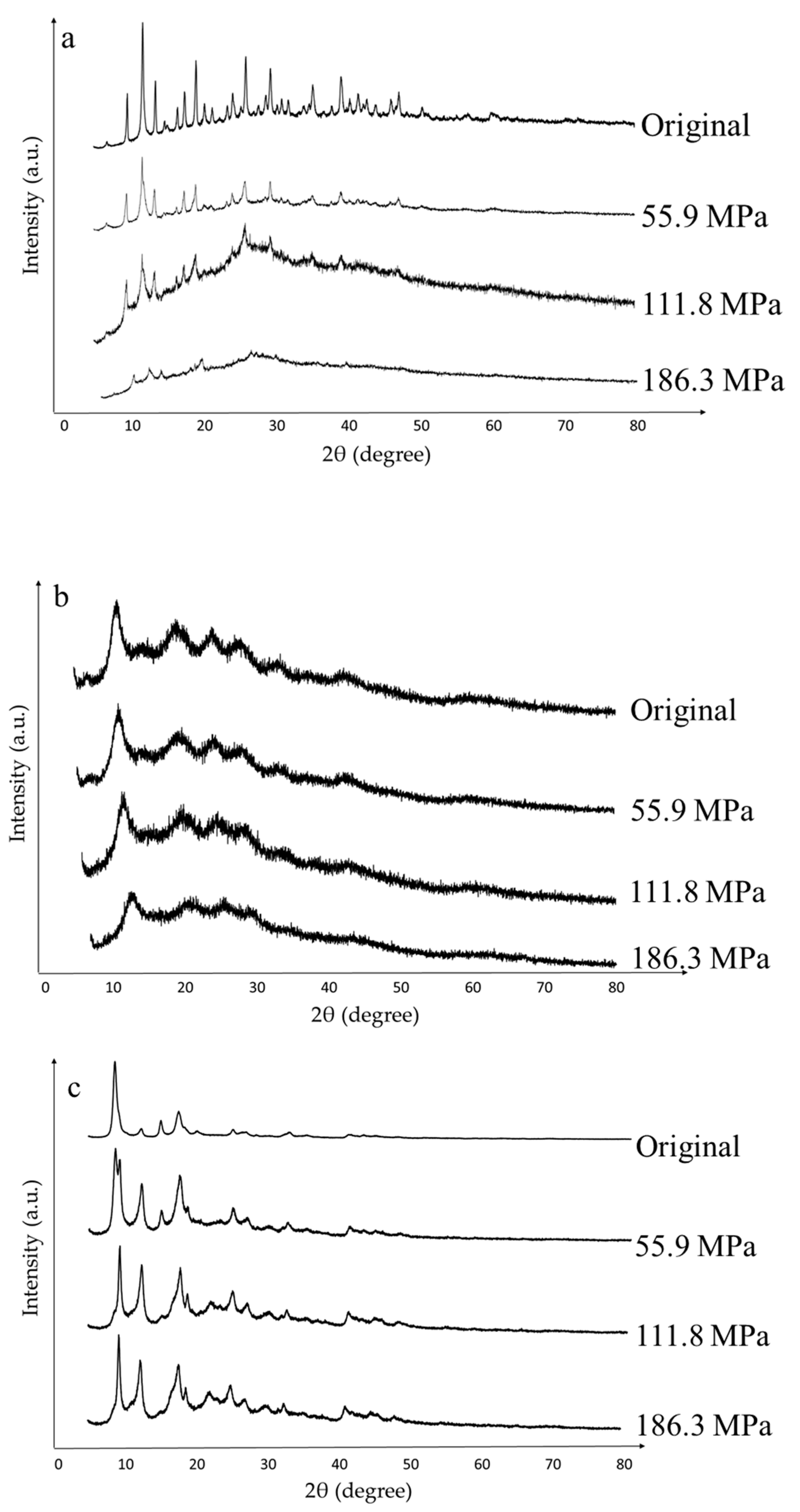
3.1. Materials Characterization
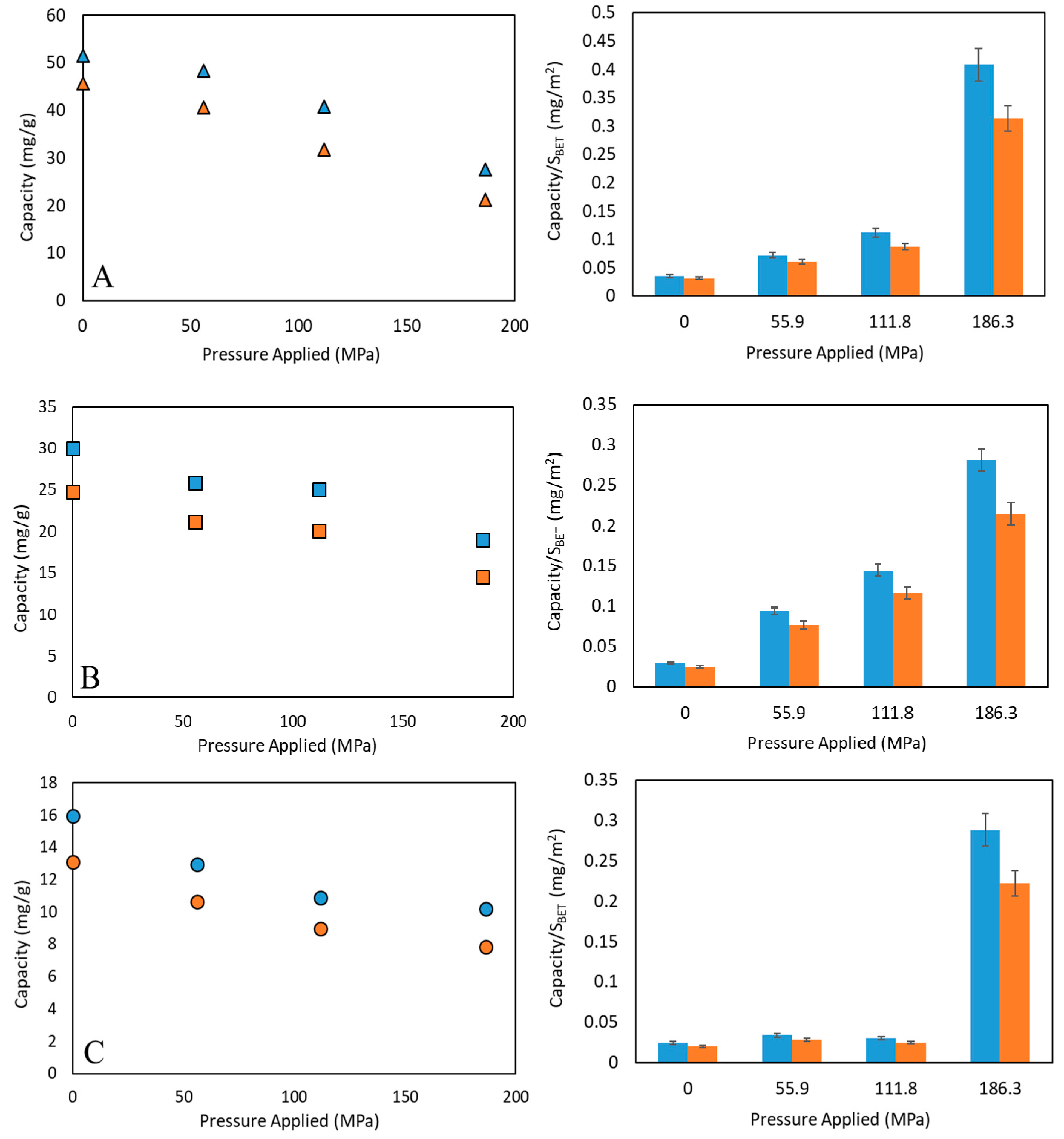
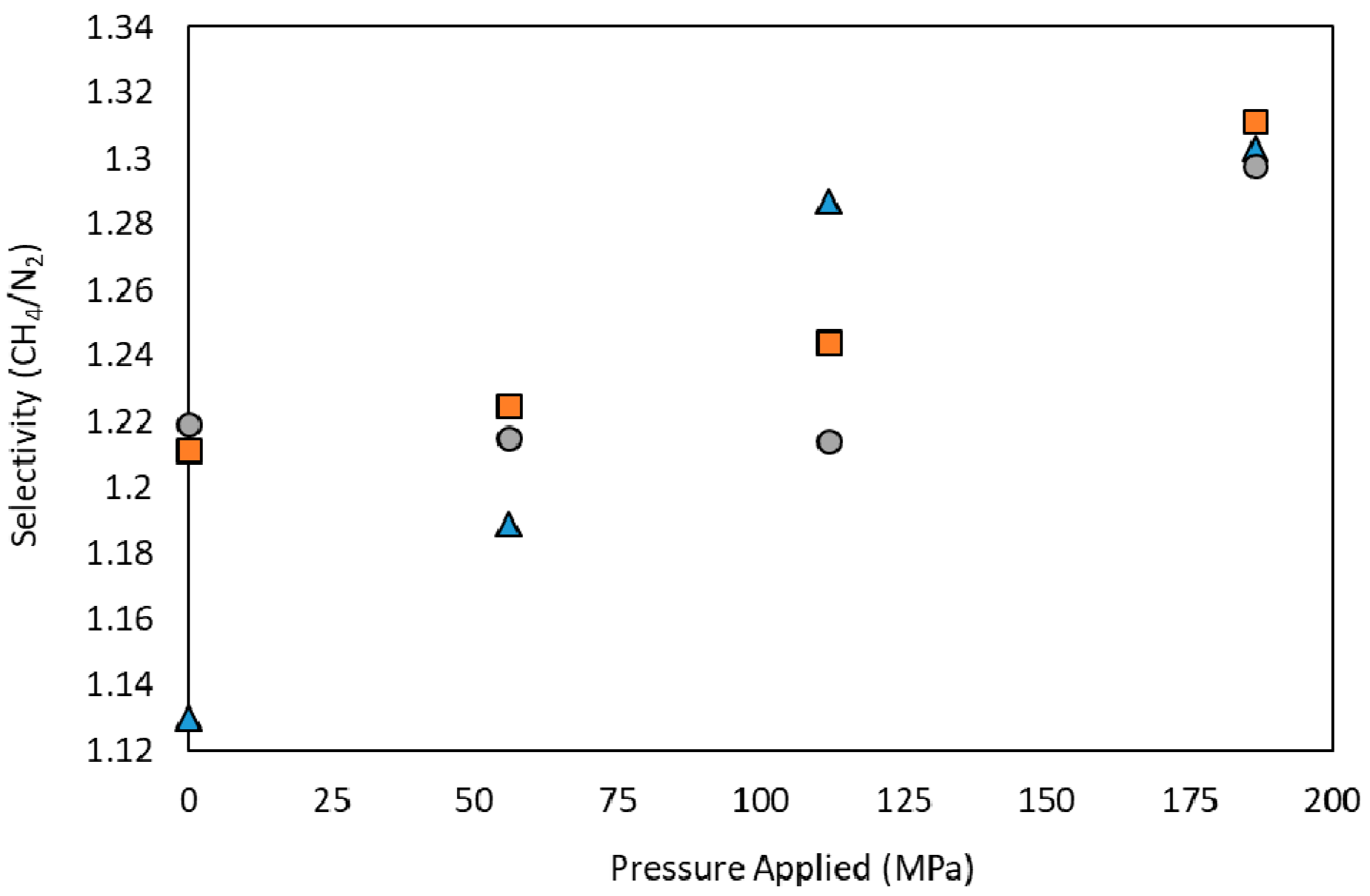
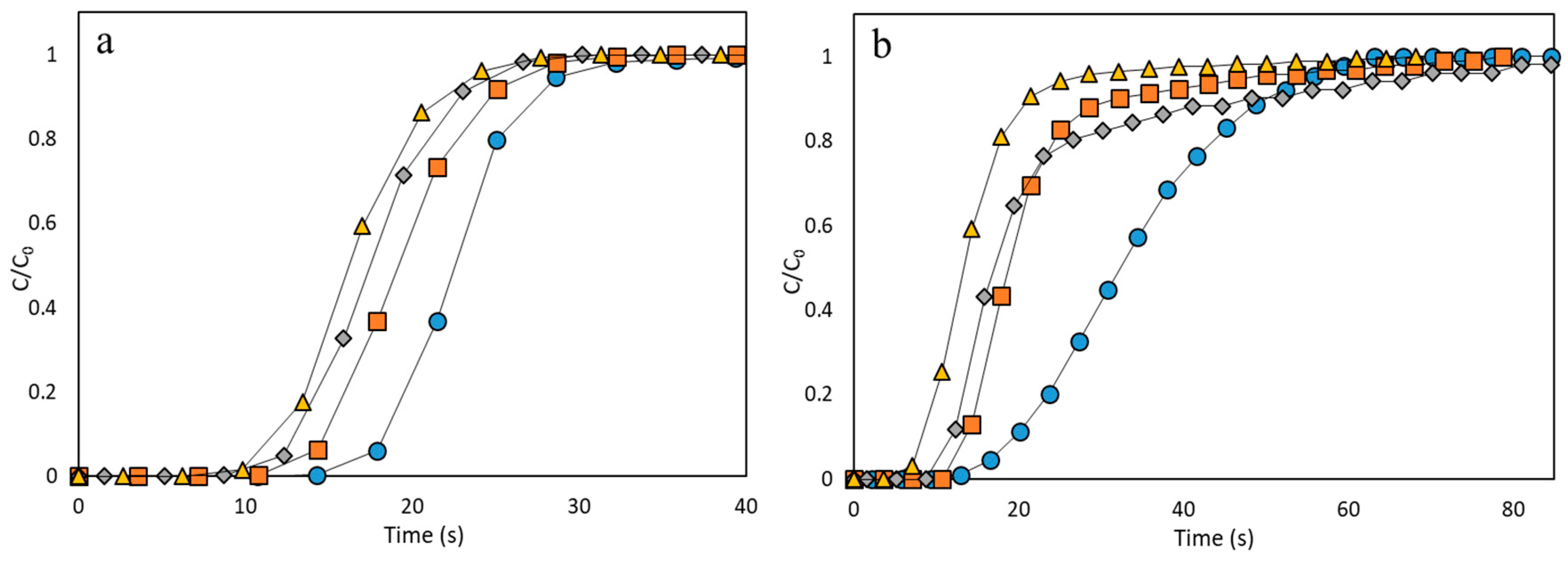
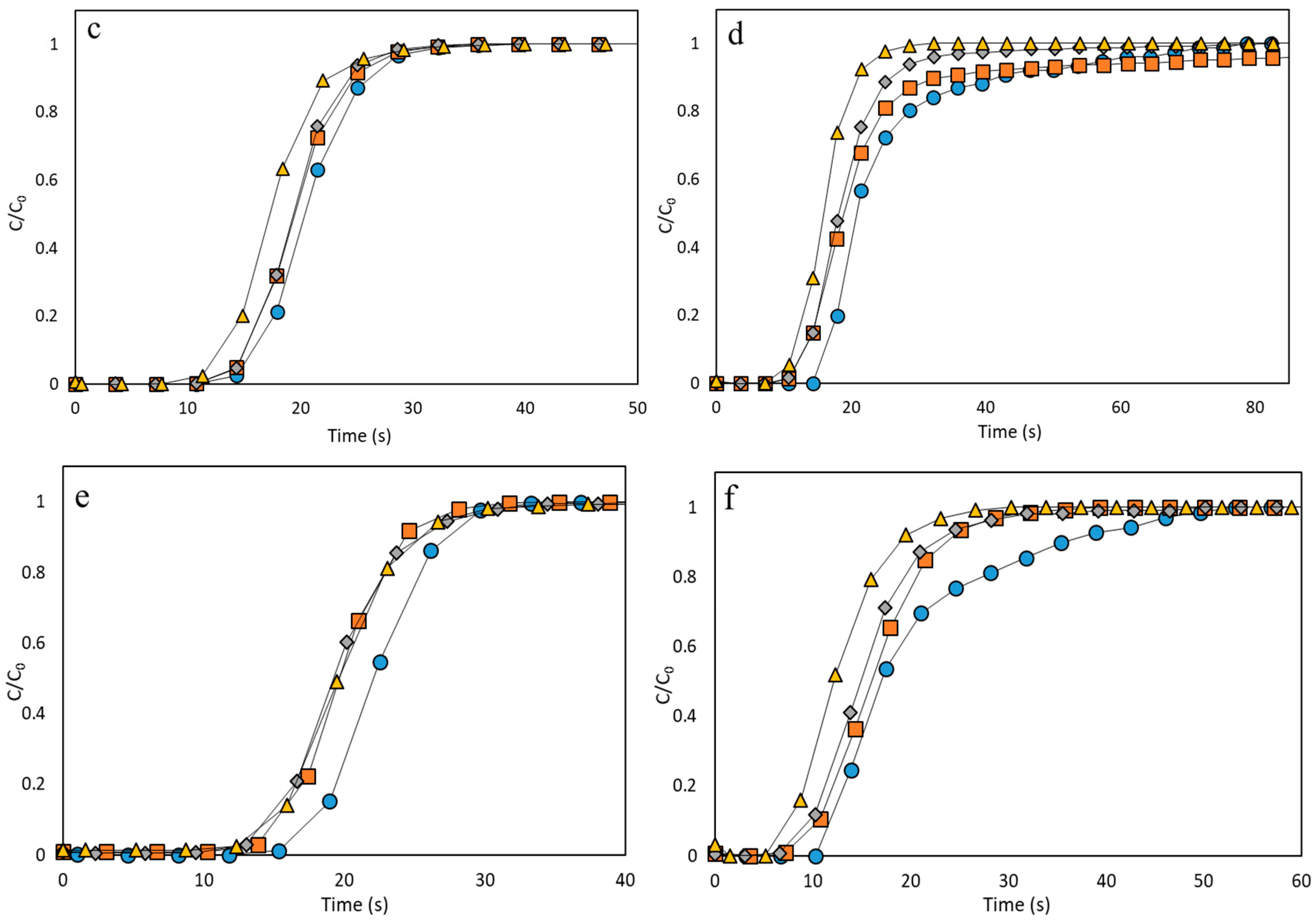
3.2. Performance Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newell, R.; Raimi, D.; Aldana, G. Global Energy Outlook 2019: The Next Generation of Energy; Report 8–19; Resources for the Future: Washington, DC, USA, 2019. [Google Scholar]
- Coronavirus Has Reminded Us How Much We Depend on Electricity. Available online: www.weforum.org/agenda/2020/03/coronavirus-crisis-future-of-energy (accessed on 29 March 2020).
- Ferreira, A.; Ribeiro, A.; Kulaç, S.; Rodrigues, A. Methane purification by adsorptive processes on MIL-53(Al). Chem. Eng. Sci. 2015, 124, 79–95. [Google Scholar] [CrossRef]
- Cavenati, S.; Grande, C.; Rodrigues, A. Separation of CH4/CO2/N2 mixtures by layered pressure swing adsorption for upgrade of natural gas. Chem. Eng. Sci. 2006, 61, 3893–3906. [Google Scholar] [CrossRef]
- Moreira, M.; Ribeiro, A.; Ferreira, A.; Rodrigues, A. Cryogenic pressure temperature swing adsorption process for natural gas upgrade. Sep. Purif. Technol. 2017, 173, 339–356. [Google Scholar] [CrossRef]
- Campo, M.; Ribeiro, A.; Ferreira, A.; Santos, J.; Lutz, C.; Loureiro, J.; Rodrigues, A. Carbon dioxide removal for methane upgrade by a VSA process using an improved 13X zeolite. Fuel Process. Technol. 2016, 143, 185–194. [Google Scholar] [CrossRef]
- Alonso-Vicario, A.; Ochoa-Gómez, J.; Gil-Río, S.; Gómez-Jiménez, O.; Ramírez-López, C.; Torrecilla-Soria, J.; Domínguez, A. Purification and upgrading of biogas by pressure swing adsorption on synthetic and natural zeolites. Microporous Mesoporous Mater. 2010, 134, 100–107. [Google Scholar] [CrossRef]
- Li, P.; Tezel, H. Adsorption separation of N2, O2, CO2 and CH4 gases by β-zeolite. Microporous Mesoporous Mater. 2007, 98, 94–101. [Google Scholar] [CrossRef]
- Yi, H.; Li, F.; Ning, P.; Tang, X.; Peng, J.; Li, Y.; Deng, H. Adsorption separation of CO2, CH4 and N2 on microwave activated carbon. Chem. Eng. J. 2013, 215, 635–642. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, J. Fugitive methane emissions from Indian coal mining and handling activities: Estimates, mitigation and opportunities for its utilization to generate clean energy. Energy Procedia 2016, 90, 336–348. [Google Scholar] [CrossRef]
- Cluff, D.; Kennedy, G.; Bennett, J.; Foster, P. Capturing energy from ventilation air methane a preliminary design for a new approach. Appl. Therm. Eng. 2015, 90, 1151–1163. [Google Scholar] [CrossRef]
- Karakurt, I.; Aydin, G.; Aydiner, K. Mine ventilation air methane as a sustainable energy source. Renew. Sustain. Energy Rev. 2011, 15, 1042–1049. [Google Scholar] [CrossRef]
- Li, Q.; Ruan, M.; Zheng, Y.; Mei, X.; Lin, B. Investigation on the selective adsorption and separation properties of coal mine methane in ZIF-68 by molecular simulations. Adsorption 2017, 23, 163–174. [Google Scholar] [CrossRef]
- Norouzi, A. Modeling of adsorption in a packed bed tower, the case study of methane removal and parametric calculation. J. Environ. Treat. Tech. 2019, 7, 324–333. [Google Scholar]
- Grünker, R.; Bon, V.; Müller, P.; Stoeck, U.; Krause, S.; Mueller, U.; Senkovska, I.; Kaskel, S. A new metal-organic framework with ultra-high surface area. Chem. Commun. 2014, 50, 3450–3452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senkovska, I.; Kaskel, S. High pressure methane adsorption in the metal-organic frameworks Cu3(btc)2, Zn2(bdc)2dabco, and Cr3F(H2O)2O(bdc)3. Microporous Mesoporous Mater. 2008, 112, 108–115. [Google Scholar] [CrossRef]
- Rowsell, J.; Yaghi, O. Metal-organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Liang, Z.; Marshall, M.; Chaffee, A. CO2 adsorption-based separation by metal organic framework (Cu-BTC) versus zeolite (13X). Energy Fuels 2009, 23, 2785–2789. [Google Scholar] [CrossRef]
- Blanco-Brieva, G.; Campos-Martin, J.M.; Al-Zahrani, S.M.; Fierro, J.L.G. Effectiveness of metal-organic frameworks for removal of refractory organo-sulfur compound present in liquid fuels. Fuel 2011, 90, 190–197. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.; Skinner, W.; Wang, Z.; Jiang, H. Metal-organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Arami-Niya, A.; Birkett, G.; Zhu, Z.; Rufford, T. Gate opening effect of zeolitic imidazolate framework ZIF-7 for adsorption of CH4 and CO2 from N2. J. Mater. Chem. A 2017, 5, 21389–21399. [Google Scholar] [CrossRef] [Green Version]
- Eyer, S.; Stadie, N.; Borgschulte, A.; Emmenegger, L.; Mohn, J. Methane preconcentration by adsorption: A methodology for materials and conditions selection. Adsorption 2014, 20, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Bastin, L.; Bárcia, P.; Hurtado, E.; Silva, J.; Rodrigues, A.; Chen, B. A microporous metal-organic framework for separation of CO2/N2 and CO2/CH4 by fixed-bed adsorption. J. Phys. Chem. C 2008, 112, 1575–1581. [Google Scholar] [CrossRef]
- Bloch, E.; Queen, W.; Krishna, R.; Zadrozny, J.; Brown, C.; Long, J. Hydrocarbon separations in a metal-organic framework with open iron(II) coordination sites. Science 2012, 335, 1606–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.; Garai, B.; Reinsch, H.; Li, W.; Dissegna, S.; Bon, V.; Senkovska, I.; Fischer, R.; Kaskel, S.; Janiak, C.; et al. Metal-organic frameworks in Germany: From synthesis to function. Coord. Chem. Rev. 2019, 380, 378–418. [Google Scholar] [CrossRef] [Green Version]
- Koekemoer, A.; Luckos, A. Effect of material type and particle size distribution on pressure drop in packed beds of large particles: Extending the Ergun equation. Fuel 2015, 158, 232–238. [Google Scholar] [CrossRef]
- Malkoc, E.; Nuhoglu, Y.; Abali, Y. Cr(VI) adsorption by waste acorn of Quercus Ithaburensis in fixed beds: Prediction of breakthrough curves. Chem. Eng. J. 2006, 119, 61–68. [Google Scholar] [CrossRef]
- Edubilli, S.; Gumma, S. A systematic evaluation of UiO-66 metal organic framework for CO2/N2 separation. Sep. Purif. Technol. 2019, 224, 85–94. [Google Scholar] [CrossRef]
- Hou, P.; Orikasa, H.; Itoi, H.; Nishihara, H.; Kyotani, T. Densification of ordered microporous carbons and controlling their micropore size by hot-pressing. Carbon 2007, 45, 2011–2016. [Google Scholar] [CrossRef]
- Tian, T.; Zeng, Z.; Vulpe, D.; Casco, M.; Divitini, G.; Midgley, P.; Silvestre-Albero, J.; Tan, J.; Moghadam, P.; Fairen-Jimenez, D. A sol-gel monolithic metal-organic framework with enhanced methane uptake. Nat. Mater. 2018, 17, 174–179. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Shah, B.; Zhao, D. CO2 capture in metal-organic framework adsorbents: An engineering perspective. Adv. Sustain. Syst. 2018, 3, 1800080. [Google Scholar] [CrossRef] [Green Version]
- Beurroies, I.; Boulhout, M.; Llewellyn, P.; Kuchta, B.; Férey, G.; Serre, C.; Denoyel, R. Using pressure to provoke the structural transition of metal-organic frameworks. Angew. Chem. Int. Ed. 2010, 49, 7526–7529. [Google Scholar] [CrossRef] [Green Version]
- Manos, G.; Dunne, L. Predicting the features of methane adsorption in large pore metal-organic frameworks for energy storage. Nanomaterials 2018, 8, 818. [Google Scholar] [CrossRef] [Green Version]
- Purewall, J.J.; Liu, D.; Yang, J.; Sudik, A.; Siegel, D.J.; Maurer, S.; Müller, U. Increased volumetric hydrogen uptake of MOF-5 by powder densification. Int. J. Hydrogen Energy 2012, 37, 2723–2727. [Google Scholar] [CrossRef]
- Nandasiri, M.; Jambovane, S.; McGrail, B.; Schaef, H.; Nune, S. Adsorption, separation, and catalytic properties of densified metal-organic frameworks. Coord. Chem. Rev. 2016, 311, 38–52. [Google Scholar] [CrossRef] [Green Version]
- Anderlean, O.; Blanita, G.; Borodi, G.; Lazar, M.; Misan, I.; Coldea, I.; Lupu, D. Volumetric hydrogen adsorption capacity of densified MIL-101 monoliths. Int. J. Hydrogen Energy 2013, 38, 7046–7055. [Google Scholar]
- Majchrzak-Kuçeba, I.; Sciubidlo, A. Shaping metal-organic framework (MOF) powder materials for CO2 capture applications—A thermogravimetric study. J. Therm. Anal. Calorim. 2019, 138, 4139–4144. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Sun, X.; Pang, J.; Sun, D.; Liu, D.; Zhou, H. PCN-250 under pressure: Sequential phase transformation and the implications for MOF densification. Joule 2017, 1, 806–815. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.; Olivier, J.; Rodríguez-Reinoso, F.; Rouquerol, J.; Sing, K. Physisorption of gases, with special reference to the evaluation of surface and pore size distribution (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Sing, K.; Everett, D.; Haul, R.; Moscou, L.; Pierotti, R.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Muttakin, M.; Mitra, S.; Thu, K.; Ito, K.; Saha, B. Theoretical framework to evaluate minimum desorption temperature for IUPAC classified adsorption isotherms. Int. J. Heat Mass Trans. 2018, 122, 795–805. [Google Scholar] [CrossRef]
- Casco, M.; Fernández-Catalá, J.; Martínez-Escandell, M.; Rodríguez-Reinoso, F.; Ramos-Férnandez, E.; Silvestre-Albero, J. Improved mechanical stability of HKUST-1 in confined nanospace. Chem. Commun. 2015, 51, 14191–14194. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, R.; Antunes, C.; Garate, A.; Portela, A.; Plaza, M.; Mota, J.; Esteves, A. Binderless shaped metal-organic framework particles: Impact on carbon dioxide adsorption. Microporous Mesoporous Mater. 2019, 275, 111–121. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Nakata, K. Effects of crystallinity, {001}/{101} ratio, and Au decoration on the photocatalytic activity of anatase TiO2 crystals. Chin. J. Catal. 2019, 40, 403–412. [Google Scholar] [CrossRef]
- Nobar, S. Cu-BTC synthesis, characterization and preparation for adsorption studies. Mater. Chem. Phys. 2018, 213, 343–351. [Google Scholar] [CrossRef]
- Schlichte, K.; Kratzke, T.; Kaskel, S. Improved synthesis, thermal stability and catalytic properties of the metal-organic framework compound Cu3(BTC)2. Microporous Mesoporous Mater. 2004, 73, 81–88. [Google Scholar] [CrossRef]
- Prestipino, C.; Regli, L.; Vitillo, J.G.; Bonino, F.; Damin, A.; Lamberti, C.; Zecchina, A.; Solari, P.; Kongshaug, K.; Bordiga, S. Local structure of framework Cu(II) in HKUST-1 metallorganic framework: Spectroscopic characterization upon activation and interaction with adsorbates. Chem. Mater. 2006, 18, 1337–1346. [Google Scholar] [CrossRef]
- Yang, A.; Li, P.; Zhong, J. Facile preparation of low-cost HKUST-1 with lattice vacancies and high-efficiency adsorption for uranium. RCS Adv. 2019, 9, 10320–10325. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Zhou, G.; Xu, Q. The elasticity of MOFs under mechanical pressure. RCS Adv. 2016, 44, 37506–37514. [Google Scholar] [CrossRef]
- McKellar, S.; Moggach, S. Structural studies of metal-organic frameworks under high pressure. Acta Cryst. 2015, B71, 587–607. [Google Scholar] [CrossRef]
- Terracina, A.; Todaro, M.; Mazaj, M.; Agnello, S.; Gelardi, F.; Buscarino, G. Unveiled the source of the structural instability of HKUST-1 powders upon mechanical compaction: Definition of a fully preserving tableting method. J. Phys. Chem. C 2018, 123, 1730–1741. [Google Scholar] [CrossRef]
- Wu, H.; Yildirim, T.; Zhou, W. Exceptional mechanical stability of highly porous zirconium metal-organic framework UiO-66 and its important implications. J. Phys. Chem. Lett. 2013, 4, 925–930. [Google Scholar] [CrossRef]
- Peng, Y.; Krungleviciute, V.; Eryazici, I.; Hupp, J.; Farha, O.; Yildirim, T. Methane storage in metal-organic frameworks: Current records, surprise findings, and challenges. J. Am. Chem. Soc. 2013, 135, 11887–11894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Sánchez, M.; Asua, I.; Ruano, D.; Díaz, K. Direct synthesis, structural features, and enhanced catalytic activity of the Basolite F300-like semiamorphous Fe-BTC framework. Cryst. Growth Des. 2015, 15, 4498–4506. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Horcajada, P.; Gibson, E.; Vishnuvarthan, M.; Vimont, A.; Greneche, J.; Serre, C.; Daturi, M.; Garcia, H. Comparison of porous iron trimesates Basolite F300 and MIL-100(Fe) as heterogeneous catalysts for Lewis acid and oxidation reactions: Roles of structural defects and stability. ACS Catal. 2012, 2, 2060–2065. [Google Scholar] [CrossRef]
- Seo, Y.; Yoon, J.; Lee, J.; Lee, U.; Hwang, Y.; Jun, C.; Horcajada, P.; Serre, C.; Chang, J. Large scale fluorine-free synthesis of hierarchically porous iron(III) trimesate MIL-100(Fe) with a zeolite MTN topology. Microporous Mesoporous Mater. 2012, 157, 137–145. [Google Scholar] [CrossRef]
- Bennet, T.; Cheetham, A. Amorphous Metal-Organic Frameworks. Acc. Chem. Res. 2014, 47, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.; Zhang, L.; Zhang, J.; Aggarwal, S. Removal of arsenic(III) from aqueous solution using metal organic framework-graphene oxide nanocomposite. Nanomaterials 2018, 8, 1062. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.; Uppara, H.; Mandal, B.; Gumma, S. Adsorption and separation of carbon dioxide using MIL-53(Al) metal-organic framework. Ind. Eng. Chem. Res. 2014, 53, 19747–19753. [Google Scholar] [CrossRef]
- Llewellyn, P.; Horcajada, P.; Maurin, G.; Devic, T.; Rosenbach, N.; Bourrelly, S.; Serre, C.; Vincent, D.; Loera-Serna, S.; Filinchuk, Y.; et al. Complex adsorption of short linear alkanes in the flexible metal-organic-framework MIL-53(Fe). J. Am. Chem. Soc. 2009, 131, 13002–13008. [Google Scholar] [CrossRef]
- Alaerts, L.; Kirschhock, C.; Maes, M.; Veen, M.; Finsy, V.; Depla, A.; Martens, J.; Baron, G.; Jacobs, P.; Denayer, J.; et al. Selective adsorption and separation of xylene isomers and ethylbenzene with the microporous vanadium(IV) terephthalate MIL-47. Angew. Chem. 2007, 119, 4371–4375. [Google Scholar] [CrossRef]
- Neimark, A.; Coudert, F.; Triguero, C.; Boutin, A.; Fuchs, A.; Beurroies, I.; Denoye, R. Structural transitions in MIL-53(Cr): View from outside and inside. Langmuir 2011, 27, 4734–4741. [Google Scholar] [CrossRef] [Green Version]
- Serra-Crespo, P.; Dikhtiarenko, A.; Stavitski, E.; Juan-Alcañiz, J.; Kapteijn, F.; Coudert, F.; Gascon, J. Experimental evidence of negative linear compressibility in the MIL-53 metal-organic framework family. CrystEngComm 2015, 17, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Ghoufi, A.; Subercaze, A.; Ma, Q.; Yot, P.; Ke, Y.; Puente-Orench, I.; Devic, T.; Guillerm, V.; Zhong, C.; Serre, C.; et al. Comparative guest, thermal, and mechanical breathing of the porous metal organic framework MIL-53(Cr): A computational exploration supported by experiments. J. Phys. Chem. C 2012, 116, 13289–13295. [Google Scholar] [CrossRef]
- Reinsch, H.; Pillai, R.; Siegel, R.; Senker, J.; Lieb, A.; Maurin, G.; Stock, N. Structure and properties of Al-MIL-53-ADP, a breathing MOF based on the aliphatic linker molecule adipic acid. Dalton Trans. 2016, 45, 4179–4186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghysels, A.; Vanduyfhys, L.; Vandichel, M.; Waroquier, M.; Speybroeck, V.; Smit, B. On the thermodynamics of framework breathing: A free energy model for gas adsorption in MIL-53. J. Phys. Chem. C 2013, 117, 11540–11554. [Google Scholar] [CrossRef] [Green Version]
- Boutin, A.; Couck, S.; Coudert, F.; Serra-Crespo, P.; Gascon, J.; Kapteijn, F.; Fuchs, A.; Denayer, J. Thermodynamic analysis of the breathing of amino-functionalized MIL-53(Al) upon CO2 adsorption. Microporous Mesoporous Mater. 2011, 140, 108–113. [Google Scholar] [CrossRef] [Green Version]
- Finsy, V.; Ma, L.; Alaerts, L.; Vos, D.; Baron, G.; Denayer, J. Separation of CO2/CH4 mixtures with the MIL-53(Al) metal-organic framework. Microporous Mesoporous Mater. 2009, 120, 221–227. [Google Scholar] [CrossRef]
- Valekar, A.; Cho, K.; Lee, U.; Lee, J.; Yoon, J.; Hwang, Y.; Lee, S.; Cho, S.; Chang, J. Shaping of porous metal-organic framework granules using mesoporous p-alumina as a binder. RSC Adv. 2017, 7, 55767–55777. [Google Scholar] [CrossRef] [Green Version]
- Ursueguía, D.; Díaz, E.; Ordóñez, S. Adsorption of methane and nitrogen on Basolite MOFs: Equilibrium and kinetic studies. Microporous Mesoporous Mater. 2020, 298, 110048. [Google Scholar] [CrossRef]
- Kosuge, K.; Kubo, S.; Kikukawa, N.; Takemori, M. Effect of pore structure in mesoporous silicas on VOC dynamic adsorption/desorption performance. Langmuir 2007, 23, 3095–3102. [Google Scholar] [CrossRef]
- Murillo, R.; García, T.; Aylón, E.; Callén, M.; Navarro, M.; López, J.; Mastral, A. Adsorption of phenanthrene on activated carbons: Breakthrough curve modeling. Carbon 2004, 42, 2009–2017. [Google Scholar] [CrossRef]
- Howarth, A.; Liu, Y.; Li, P.; Li, Z.; Wang, T.; Hupp, J.; Farha, O. Chemical, thermal and mechanical stabilities of metal-organic frameworks. Nat. Rev. Mater. 2016, 1, 1–15. [Google Scholar] [CrossRef]
- Mehlhorn, D.; Valiullin, R.; Kärger, J.; Cho, K.; Ryoo, R. Intracrystalline diffusion in mesoporous zeolites. ChemPhysChem 2012, 13, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Alvaro, M.; Hwang, Y.; Seo, Y.; Corma, A.; García, H. Intracrystalline diffusion in Metal Organic Framework during heterogeneous catalysis: Influence of particle size on the activity of MIL-100 (Fe) for oxidation reactions. Dalton Trans. 2011, 40, 10719–10724. [Google Scholar] [CrossRef]
- Lyubchyk, A.; Esteves, I.; Cruz, F.; Mota, J. Experimental and theoretical studies of supercritical methane adsorption in the MIL-53(Al) metal organic framework. J. Phys. Chem. C 2011, 115, 20628–20638. [Google Scholar] [CrossRef]

| Material | Applied Pressure (MPa) | BET Specific Surface Area (m2/g) | BJH Mesopore Volume (cm3/g) | t-Plot Micropore Volume (cm3/g) | Average Pore Size (Å) |
|---|---|---|---|---|---|
| C300 | 0 | 1466.8 | 0.53 | 0.71 | 33.7 |
| 55.9 | 671.2 | 0.09 | 0.29 | 22.9 | |
| 111.8 | 364.8 | 0.07 | 0.14 | 23.2 | |
| 186.3 | 67.6 | 0.06 | 0.02 | 47.3 | |
| F300 | 0 | 1015.4 | 0.15 | 0.27 | 16.5 |
| 55.9 | 276.2 | 0.09 | 0.06 | 22.3 | |
| 111.8 | 173.4 | 0.11 | 0.02 | 28.8 | |
| 186.3 | 67.8 | 0.06 | 0.01 | 41.2 | |
| A100 | 0 | 655.9 | 0.77 | 0.28 | 64.0 |
| 55.9 | 380.2 | 0.57 | 0.06 | 65.8 | |
| 111.8 | 362.1 | 0.54 | 0.05 | 65.1 | |
| 186.3 | 35.4 | 0.06 | 0.01 | 70.1 |
| Material | Pressure (MPa) | Crystallinity Loss (%) |
|---|---|---|
| C300 | 0 | 0 |
| 55.9 | 19.51 | |
| 111.8 | 62.07 | |
| 186.3 | 69.93 | |
| F300 | 0 | 0 |
| 55.9 | 0 | |
| 111.8 | 4.31 | |
| 186.3 | 14.08 | |
| A100 | 0 | 0 |
| 55.9 | 69.35 | |
| 111.8 | 68.52 | |
| 186.3 | 68.38 |
| Material | Pressure (MPa) | a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | Volume (Å3) |
|---|---|---|---|---|---|---|---|---|
| C300 | 0 | 25.9 | 25.9 | 25.9 | 90 | 90 | 90 | 17,452.2 |
| 55.9 | 26.2 | 26.2 | 26.2 | 90 | 90 | 90 | 17,992.8 | |
| 111.8 | 26.2 | 26.2 | 26.2 | 90 | 90 | 90 | 17,992.8 | |
| 186.3 | 26.1 | 26.1 | 26.1 | 90 | 90 | 90 | 17,901.2 | |
| A100 | 0 | 16.1 | 6.56 | 13.2 | 90 | 90 | 90 | 1397.4 |
| 55.9 | 6.56 | 14.3 | 14.8 | 90 | 105 | 90 | 1351.7 | |
| 111.8 | 6.43 | 12.9 | 16.2 | 90 | 108 | 90 | 1280.5 | |
| 186.3 | 5.83 | 13.7 | 16.1 | 90 | 110 | 90 | 1205.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ursueguía, D.; Díaz, E.; Ordóñez, S. Densification-Induced Structure Changes in Basolite MOFs: Effect on Low-Pressure CH4 Adsorption. Nanomaterials 2020, 10, 1089. https://doi.org/10.3390/nano10061089
Ursueguía D, Díaz E, Ordóñez S. Densification-Induced Structure Changes in Basolite MOFs: Effect on Low-Pressure CH4 Adsorption. Nanomaterials. 2020; 10(6):1089. https://doi.org/10.3390/nano10061089
Chicago/Turabian StyleUrsueguía, David, Eva Díaz, and Salvador Ordóñez. 2020. "Densification-Induced Structure Changes in Basolite MOFs: Effect on Low-Pressure CH4 Adsorption" Nanomaterials 10, no. 6: 1089. https://doi.org/10.3390/nano10061089
APA StyleUrsueguía, D., Díaz, E., & Ordóñez, S. (2020). Densification-Induced Structure Changes in Basolite MOFs: Effect on Low-Pressure CH4 Adsorption. Nanomaterials, 10(6), 1089. https://doi.org/10.3390/nano10061089