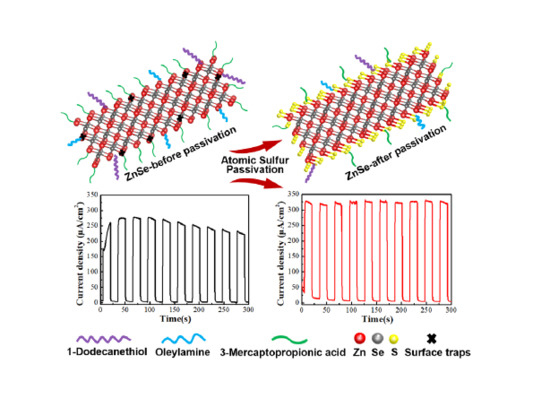
Atomic Sulfur Passivation Improves the Photoelectrochemical Performance of ZnSe Nanorods
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Synthesis of ZnSe NRs
2.3. Synthesis of ZnSe/ZnS Core/Shell NRs
2.4. Ligand Exchange on ZnSe NRs, and Fabrication of TiO2/ZnSe NR-Based Photoanodes
2.5. Atomic Sulfur Passivation of ZnSe NRs-Based Photoanodes
2.6. Depositing ZnS Layer on ZnSe NRs-Based Photoanodes by the Successive Ionic Layer Adsorption and Reaction (SILAR) Method
2.7. Characterization
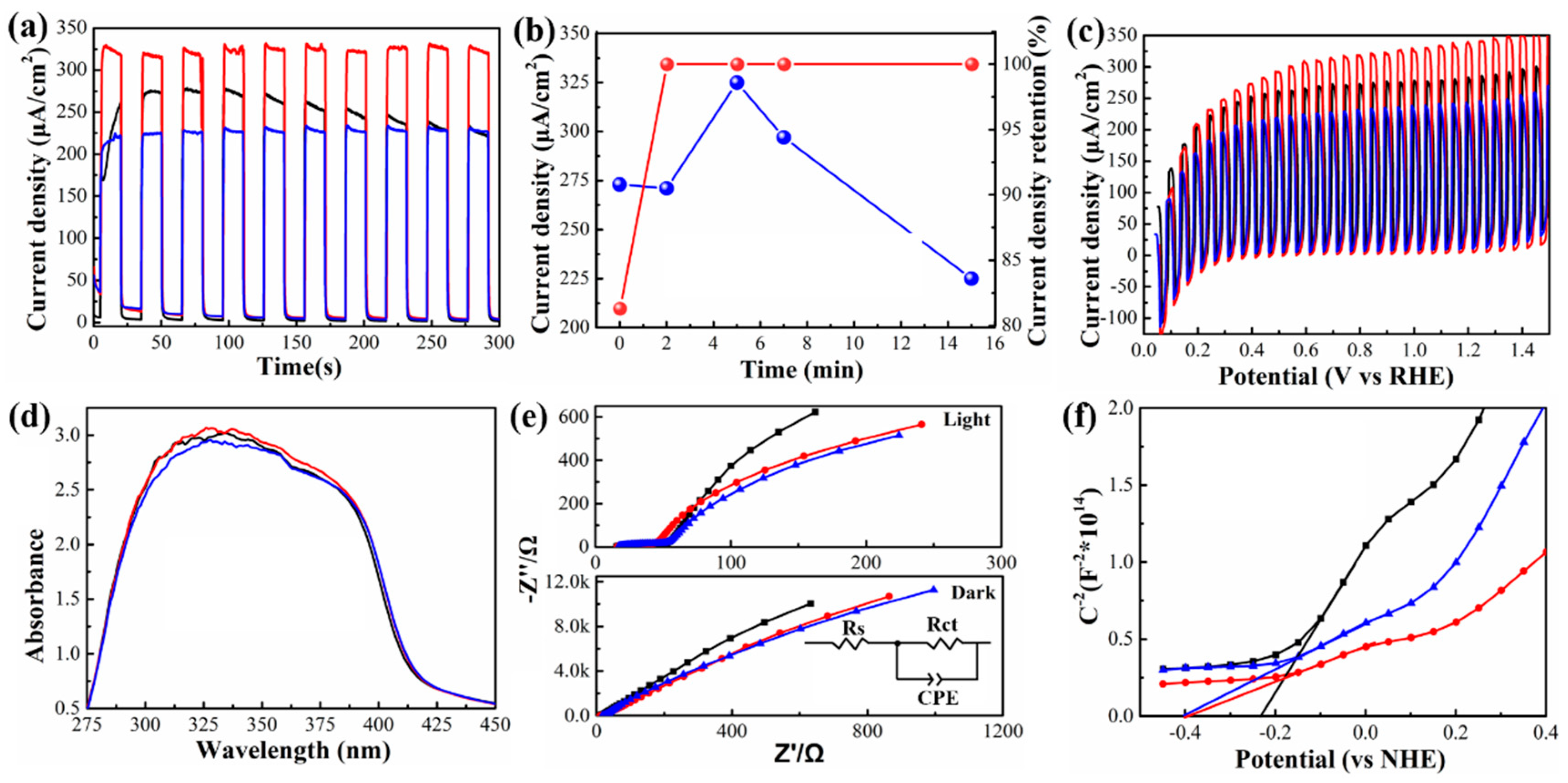
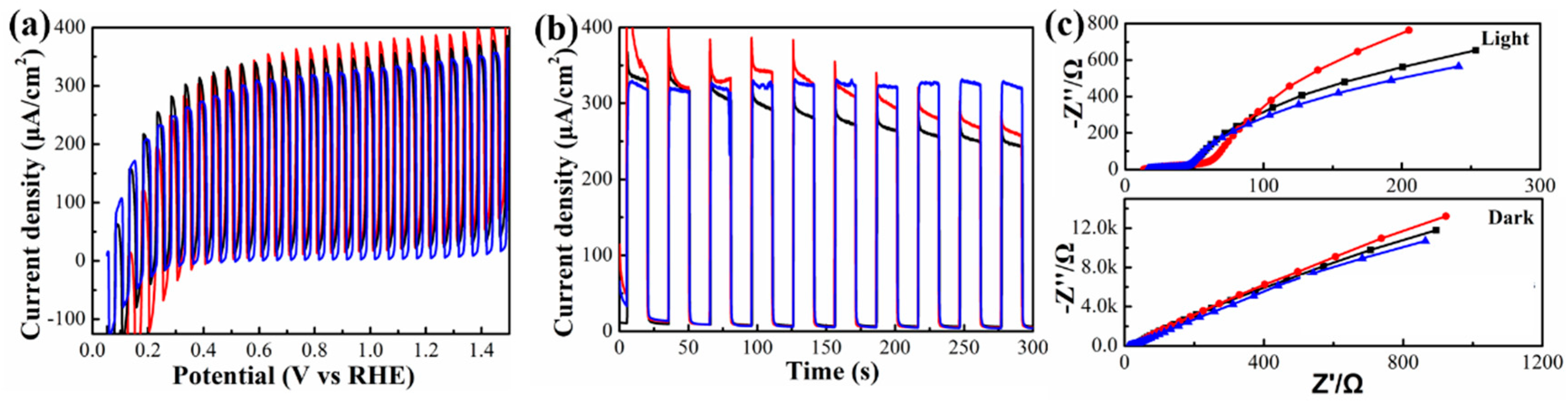
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.S.; Shen, S. Hydrogen production: Catalysing artificial photosynthesis. Nat. Photon. 2013, 7, 944. [Google Scholar] [CrossRef]
- Abe, R.; Sayama, K.; Arakawa, H. Significant effect of iodide addition on water splitting into H2 and O2 over Pt-loaded TiO2 photocatalyst: Suppression of backward reaction. Chem. Phys. Lett. 2003, 371, 360–364. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tan, H.L.; Toe, C.Y.; Scott, J.; Wang, L.; Amal, R.; Ng, Y.H. Photocatalytic and Photoelectrochemical Systems: Similarities and Differences. Adv. Mater. 2019, e1904717. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Wang, S.; Guan, B.Y.; Wang, X.; Lou, X.W.D. Formation of hierarchical Co9S8@ ZnIn2S4 heterostructured cages as an efficient photocatalyst for hydrogen evolution. J. Am. Chem. Soc. 2018, 140, 15145–15148. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, S.; Yu, Y.; Tian, H.; Zhao, Y.-L.; Ren, J.-C.; Huang, C.; Bian, H.; Huang, M.; An, L. Hydrogen-Location-Sensitive Modulation of the Redox Reactivity for Oxygen-Deficient TiO2. J. Am. Chem. Soc. 2019, 141, 8407–8411. [Google Scholar] [CrossRef]
- Park, J.; Yang, W.; Tan, J.; Lee, H.; Yun, J.W.; Shim, S.G.; Park, Y.S.; Moon, J. Hierarchal Nanorod-Derived Bilayer Strategy to Enhance the Photocurrent Density of Sb2Se3 Photocathodes for Photoelectrochemical Water Splitting. ACS Energy Lett. 2019, 5, 136–145. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Batmunkh, M.; Bat-Erdene, M.; Shapter, J.G.; Mullins, C.B. p-Type BP nanosheet photocatalyst with AQE of 3.9% in the absence of a noble metal cocatalyst: Investigation and elucidation of photophysical properties. J. Mater. Chem. A 2018, 6, 18403–18408. [Google Scholar] [CrossRef]
- Hao, L.; Kang, L.; Huang, H.; Ye, L.; Han, K.; Yang, S.; Yu, H.; Batmunkh, M.; Zhang, Y.; Ma, T. Surface-halogenation-induced atomic-site activation and local charge separation for superb CO2 photoreduction. Adv. Mater. 2019, 31, 1900546. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-H.; Nguyen, B.-S.; Hu, C.; Nguyen, C.C.; Nguyen, D.L.T.; Nguyen Dinh, M.T.; Vo, D.-V.N.; Trinh, Q.T.; Shokouhimehr, M.; Hasani, A. Novel Architecture Titanium Carbide (Ti3C2Tx) MXene Cocatalysts toward Photocatalytic Hydrogen Production: A Mini-Review. Nanomaterials 2020, 10, 602. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Chen, C.K.; Chang, Y.C.; Tsai, C.W.; Liu, R.S.; Hu, S.F.; Chang, W.S.; Chen, K.H. Quantum dot monolayer sensitized ZnO nanowire-array photoelectrodes: True efficiency for water splitting. Angew. Chem. Int. Ed. 2010, 49, 5966–5969. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Sun, B.; Shi, T.; Tan, X.; Peng, Z.; Liao, G. Quantum dot-sensitized hierarchical micro/nanowire architecture for photoelectrochemical water splitting. ACS Nano 2014, 8, 7163–7169. [Google Scholar] [CrossRef]
- Wu, H.L.; Li, X.B.; Tung, C.H.; Wu, L.Z. Sensitized Photocathodes: Recent Advances in Sensitized Photocathodes: From Molecular Dyes to Semiconducting Quantum Dots. Adv. Sci. 2018, 5, 1870023. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Liu, J.; Han, B.; Hu, X.; Yang, F.; Xu, Z.; Li, Y.; Jia, S.; Li, Z. Carbon quantum dot implanted graphite carbon nitride nanotubes: Excellent charge separation and enhanced photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2018, 57, 5765–5771. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, H.; Li, Y.; Pang, Y.; Yin, Z.; Sun, H.; Zhang, L.C.; Wang, S.; Saunders, M.; Barker, E.; et al. Spontaneous Formation of Noble- and Heavy-Metal-Free Alloyed Semiconductor Quantum Rods for Efficient Photocatalysis. Adv. Mater. 2018, 30, e1803351. [Google Scholar] [CrossRef]
- Moreels, I.; Lambert, K.; De Muynck, D.; Vanhaecke, F.; Poelman, D.; Martins, J.C.; Allan, G.; Hens, Z. Composition and size-dependent extinction coefficient of colloidal PbSe quantum dots. Chem. Mater. 2007, 19, 6101–6106. [Google Scholar] [CrossRef]
- Morris-Cohen, A.J.; Frederick, M.T.; Lilly, G.D.; McArthur, E.A.; Weiss, E.A. Organic surfactant-controlled composition of the surfaces of CdSe quantum dots. J. Phys. Chem. Lett. 2010, 1, 1078–1081. [Google Scholar] [CrossRef]
- Wei, H.H.; Evans, C.M.; Swartz, B.D.; Neukirch, A.J.; Young, J.; Prezhdo, O.V.; Krauss, T.D. Colloidal semiconductor quantum dots with tunable surface composition. Nano Lett. 2012, 12, 4465–4471. [Google Scholar] [CrossRef] [PubMed]
- Reeves, K.G.; Schleife, A.; Correa, A.A.; Kanai, Y. Role of surface termination on hot electron relaxation in silicon quantum dots: A first-principles dynamics simulation study. Nano Lett. 2015, 15, 6429–6433. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Fernando, G.; Li, R.; Papadimitrakopoulos, F.; Shi, N.; Ramprasad, R. First principles study of CdSe quantum dots: Stability, surface unsaturations, and experimental validation. Appl. Phys. Lett. 2006, 88, 231910. [Google Scholar] [CrossRef]
- Hines, D.A.; Kamat, P.V. Recent advances in quantum dot surface chemistry. ACS Appl. Mater. Interfaces 2014, 6, 3041–3057. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, N.; Bozyigit, D.; Vuttivorakulchai, K.; Luisier, M.; Infante, I.; Wood, V. Tuning Electron-Phonon Interactions in Nanocrystals through Surface Termination. Nano Lett. 2018, 18, 2233–2242. [Google Scholar] [CrossRef]
- Subila, K.B.; Kishore Kumar, G.; Shivaprasad, S.M.; George Thomas, K. Luminescence Properties of CdSe Quantum Dots: Role of Crystal Structure and Surface Composition. J. Phys. Chem. Lett. 2013, 4, 2774–2779. [Google Scholar] [CrossRef]
- Giansante, C.; Infante, I. Surface Traps in Colloidal Quantum Dots: A Combined Experimental and Theoretical Perspective. J. Phys. Chem. Lett. 2017, 8, 5209–5215. [Google Scholar] [CrossRef]
- Li, W.; Zhong, X. Capping Ligand-Induced Self-Assembly for Quantum Dot Sensitized Solar Cells. J. Phys. Chem. Lett. 2015, 6, 796–806. [Google Scholar] [CrossRef]
- Baker, D.R.; Kamat, P.V. Tuning the emission of CdSe quantum dots by controlled trap enhancement. Langmuir 2010, 26, 11272–11276. [Google Scholar] [CrossRef]
- Zhu, H.; Song, N.; Lian, T. Controlling charge separation and recombination rates in CdSe/ZnS type I core− shell quantum dots by shell thicknesses. J. Am. Chem. Soc. 2010, 132, 15038–15045. [Google Scholar] [CrossRef]
- Jeong, S.; Achermann, M.; Nanda, J.; Ivanov, S.; Klimov, V.I.; Hollingsworth, J.A. Effect of the thiol− thiolate equilibrium on the photophysical properties of aqueous CdSe/ZnS nanocrystal quantum dots. J. Am. Chem. Soc. 2005, 127, 10126–10127. [Google Scholar] [CrossRef]
- Luan, C.; Vaneski, A.; Susha, A.S.; Xu, X.; Wang, H.-E.; Chen, X.; Xu, J.; Zhang, W.; Lee, C.-S.; Rogach, A.L. Facile solution growth of vertically aligned ZnO nanorods sensitized with aqueous CdS and CdSe quantum dots for photovoltaic applications. Nanoscale Res. Lett. 2011, 6, 340. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Luan, C.; Xu, X.; Kershaw, S.V.; Rogach, A.L. In situ versus ex situ assembly of aqueous-based thioacid capped CdSe nanocrystals within mesoporous TiO2 films for quantum dot sensitized solar cells. J. Phys. Chem. C 2012, 116, 484–489. [Google Scholar] [CrossRef]
- Hetsch, F.; Xu, X.; Wang, H.; Kershaw, S.V.; Rogach, A.L. Semiconductor nanocrystal quantum dots as solar cell components and photosensitizers: Material, charge transfer, and separation aspects of some device topologies. J. Phys. Chem. Lett. 2011, 2, 1879–1887. [Google Scholar] [CrossRef]
- Sambur, J.B.; Parkinson, B.A. CdSe/ZnS core/shell quantum dot sensitization of low index TiO2 single crystal surfaces. J. Am. Chem. Soc. 2010, 132, 2130–2131. [Google Scholar] [CrossRef] [PubMed]
- Abdellah, M.; Zidek, K.; Zheng, K.; Chábera, P.; Messing, M.E.; Pullerits, T. Balancing electron transfer and surface passivation in gradient CdSe/ZnS core–shell quantum dots attached to ZnO. J. Phys. Chem. Lett. 2013, 4, 1760–1765. [Google Scholar] [CrossRef]
- Baranov, A.; Rakovich, Y.P.; Donegan, J.; Perova, T.; Moore, R.; Talapin, D.; Rogach, A.; Masumoto, Y.; Nabiev, I. Effect of ZnS shell thickness on the phonon spectra in CdSe quantum dots. Phy. Rev. B 2003, 68, 165306. [Google Scholar] [CrossRef]
- Huang, F.; Hou, J.; Zhang, Q.; Wang, Y.; Massé, R.C.; Peng, S.; Wang, H.; Liu, J.; Cao, G. Doubling the power conversion efficiency in CdS/CdSe quantum dot sensitized solar cells with a ZnSe passivation layer. Nano Energy 2016, 26, 114–122. [Google Scholar] [CrossRef]
- Huang, F.; Hou, J.; Wang, H.; Tang, H.; Liu, Z.; Zhang, L.; Zhang, Q.; Peng, S.; Liu, J.; Cao, G. Impacts of surface or interface chemistry of ZnSe passivation layer on the performance of CdS/CdSe quantum dot sensitized solar cells. Nano Energy 2017, 32, 433–440. [Google Scholar] [CrossRef]
- Liu, B.; Li, X.-B.; Gao, Y.-J.; Li, Z.-J.; Meng, Q.-Y.; Tung, C.-H.; Wu, L.-Z. A solution-processed, mercaptoacetic acid-engineered CdSe quantum dot photocathode for efficient hydrogen production under visible light irradiation. Energy Environ. Sci. 2015, 8, 1443–1449. [Google Scholar] [CrossRef]
- Trevisan, R.; Rodenas, P.; Gonzalez-Pedro, V.; Sima, C.; Sanchez, R.S.; Barea, E.M.; Mora-Sero, I.; Fabregat-Santiago, F.; Gimenez, S. Harnessing infrared photons for photoelectrochemical hydrogen generation. A PbS quantum dot based “quasi-artificial leaf”. J. Phys. Chem. Lett. 2012, 4, 141–146. [Google Scholar] [CrossRef]
- Kuehnel, M.F.; Creissen, C.E.; Sahm, C.D.; Wielend, D.; Schlosser, A.; Orchard, K.L.; Reisner, E. ZnSe Nanorods as Visible-Light Absorbers for Photocatalytic and Photoelectrochemical H2 Evolution in Water. Angew. Chem. Int. Ed. 2019, 58, 5059–5063. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Kershaw, S.V.; Rogach, A.L. Synthesis of Anisotropic ZnSe Nanorods with Zinc-Blende Crystal Structure. Angew. Chem. Int. Ed. 2020, 59, 5385–5391. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.F. Linker-assisted assembly and interfacial electron-transfer reactivity of quantum dot− substrate architectures. J. Phys. Chem. Lett. 2010, 1, 2299–2309. [Google Scholar] [CrossRef]
- Hines, M.A.; Guyot-Sionnest, P. Bright UV-blue luminescent colloidal ZnSe nanocrystals. J. Phys. Chem. B 1998, 102, 3655–3657. [Google Scholar] [CrossRef]
- Ning, J.; Liu, J.; Levi-Kalisman, Y.; Frenkel, A.I.; Banin, U. Controlling Anisotropic Growth of Colloidal ZnSe Nanostructures. J. Am. Chem. Soc. 2018, 140, 14627–14637. [Google Scholar] [CrossRef]
- Adachi, S.; Taguchi, T. Optical properties of ZnSe. Phys. Rev. B 1991, 43, 9569. [Google Scholar] [CrossRef]
- Koole, R.; Luigjes, B.; Tachiya, M.; Pool, R.; Vlugt, T.; de Mello Donegá, C.; Meijerink, A.; Vanmaekelbergh, D. Differences in cross-link chemistry between rigid and flexible dithiol molecules revealed by optical studies of CdTe quantum dots. J. Phys. Chem. C 2007, 111, 11208–11215. [Google Scholar] [CrossRef]
- Kalyuzhny, G.; Murray, R.W. Ligand effects on optical properties of CdSe nanocrystals. J. Phys. Chem. B 2005, 109, 7012–7021. [Google Scholar] [CrossRef]
- Gao, Y.; Peng, X. Photogenerated excitons in plain core CdSe nanocrystals with unity radiative decay in single channel: The effects of surface and ligands. J. Am. Chem. Soc. 2015, 137, 4230–4235. [Google Scholar] [CrossRef]
- Fu, H.; Zunger, A. InP quantum dots: Electronic structure, surface effects, and the redshifted emission. Phys. Rev. B 1997, 56, 1496. [Google Scholar] [CrossRef]
- Ji, X.; Copenhaver, D.; Sichmeller, C.; Peng, X. Ligand bonding and dynamics on colloidal nanocrystals at room temperature: The case of alkylamines on CdSe nanocrystals. J. Am. Chem. Soc. 2008, 130, 5726–5735. [Google Scholar] [CrossRef]
- Anderson, N.C.; Hendricks, M.P.; Choi, J.J.; Owen, J.S. Ligand exchange and the stoichiometry of metal chalcogenide nanocrystals: Spectroscopic observation of facile metal-carboxylate displacement and binding. J. Am. Chem. Soc. 2013, 135, 18536–18548. [Google Scholar] [CrossRef]
- Ip, A.H.; Thon, S.M.; Hoogland, S.; Voznyy, O.; Zhitomirsky, D.; Debnath, R.; Levina, L.; Rollny, L.R.; Carey, G.H.; Fischer, A.; et al. Hybrid passivated colloidal quantum dot solids. Nat. Nanotechnol. 2012, 7, 577–582. [Google Scholar] [CrossRef]
- Perry, D.; Waiskopf, N.; Verbitsky, L.; Remennik, S.; Banin, U. Shell Stabilization of Photocatalytic ZnSe Nanorods. ChemCatChem 2019, 11, 6208–6212. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.; Wu, M.; Wu, H.; Zhang, J.; Li, W.; Cao, C. Photoelectrocatalytic degradation of sulfosalicylic acid and its electrochemical impedance spectroscopy investigation. J. Phys. Chem. A 2000, 104, 7016–7020. [Google Scholar] [CrossRef]
- Deng, Z.; Lie, F.L.; Shen, S.; Ghosh, I.; Mansuripur, M.; Muscat, A.J. Water-based route to ligand-selective synthesis of ZnSe and Cd-doped ZnSe quantum dots with tunable ultraviolet A to blue photoluminescence. Langmuir 2008, 25, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Huang, M.; Zhao, Y.-L.; Xiong, W.; Kershaw, S.V.; Yu, Y.; Li, W.; Dudka, T.; Zhang, R.-Q. Collaborative enhancement of photon harvesting and charge carrier dynamics in carbon nitride photoelectrode. Appl. Cata. B Environ. 2018, 237, 783–790. [Google Scholar] [CrossRef]
- Lad, A.D.; Mahamuni, S. Effect of ZnS shell formation on the confined energy levels of ZnSe quantum dots. Phys. Rev. B 2008, 78, 125421. [Google Scholar] [CrossRef]
- Tamang, S.; Lincheneau, C.; Hermans, Y.; Jeong, S.; Reiss, P. Chemistry of InP nanocrystal syntheses. Chem. Mater. 2016, 28, 2491–2506. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, Q.; Xu, B.; Hou, J.; Wang, Y.; Massé, R.C.; Peng, S.; Liu, J.; Cao, G. A comparison of ZnS and ZnSe passivation layers on CdS/CdSe co-sensitized quantum dot solar cells. J. Mater. Chem. A 2016, 4, 14773–14780. [Google Scholar] [CrossRef]
- Fan, X.B.; Yu, S.; Wang, X.; Li, Z.J.; Zhan, F.; Li, J.X.; Gao, Y.J.; Xia, A.D.; Tao, Y.; Li, X.B. Susceptible surface sulfide regulates catalytic activity of CdSe quantum dots for hydrogen photogeneration. Adv. Mater. 2019, 31, 1804872. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, F.; Ning, J.; Xiong, W.; Shen, T.; Zhao, Y.; Tian, J.; Zhang, R.; Rogach, A.L. Atomic Sulfur Passivation Improves the Photoelectrochemical Performance of ZnSe Nanorods. Nanomaterials 2020, 10, 1081. https://doi.org/10.3390/nano10061081
Huang F, Ning J, Xiong W, Shen T, Zhao Y, Tian J, Zhang R, Rogach AL. Atomic Sulfur Passivation Improves the Photoelectrochemical Performance of ZnSe Nanorods. Nanomaterials. 2020; 10(6):1081. https://doi.org/10.3390/nano10061081
Chicago/Turabian StyleHuang, Fei, Jiajia Ning, Wei Xiong, Ting Shen, Yanling Zhao, Jianjun Tian, Ruiqin Zhang, and Andrey L. Rogach. 2020. "Atomic Sulfur Passivation Improves the Photoelectrochemical Performance of ZnSe Nanorods" Nanomaterials 10, no. 6: 1081. https://doi.org/10.3390/nano10061081
APA StyleHuang, F., Ning, J., Xiong, W., Shen, T., Zhao, Y., Tian, J., Zhang, R., & Rogach, A. L. (2020). Atomic Sulfur Passivation Improves the Photoelectrochemical Performance of ZnSe Nanorods. Nanomaterials, 10(6), 1081. https://doi.org/10.3390/nano10061081