Influence of Carbyne Content on the Mechanical Performance of Nanothick Amorphous Carbon Coatings
Abstract
1. Introduction
2. Experimental Section
2.1. Thin Film Deposition
2.2. Thin Film Characterization
3. Results and Discussion
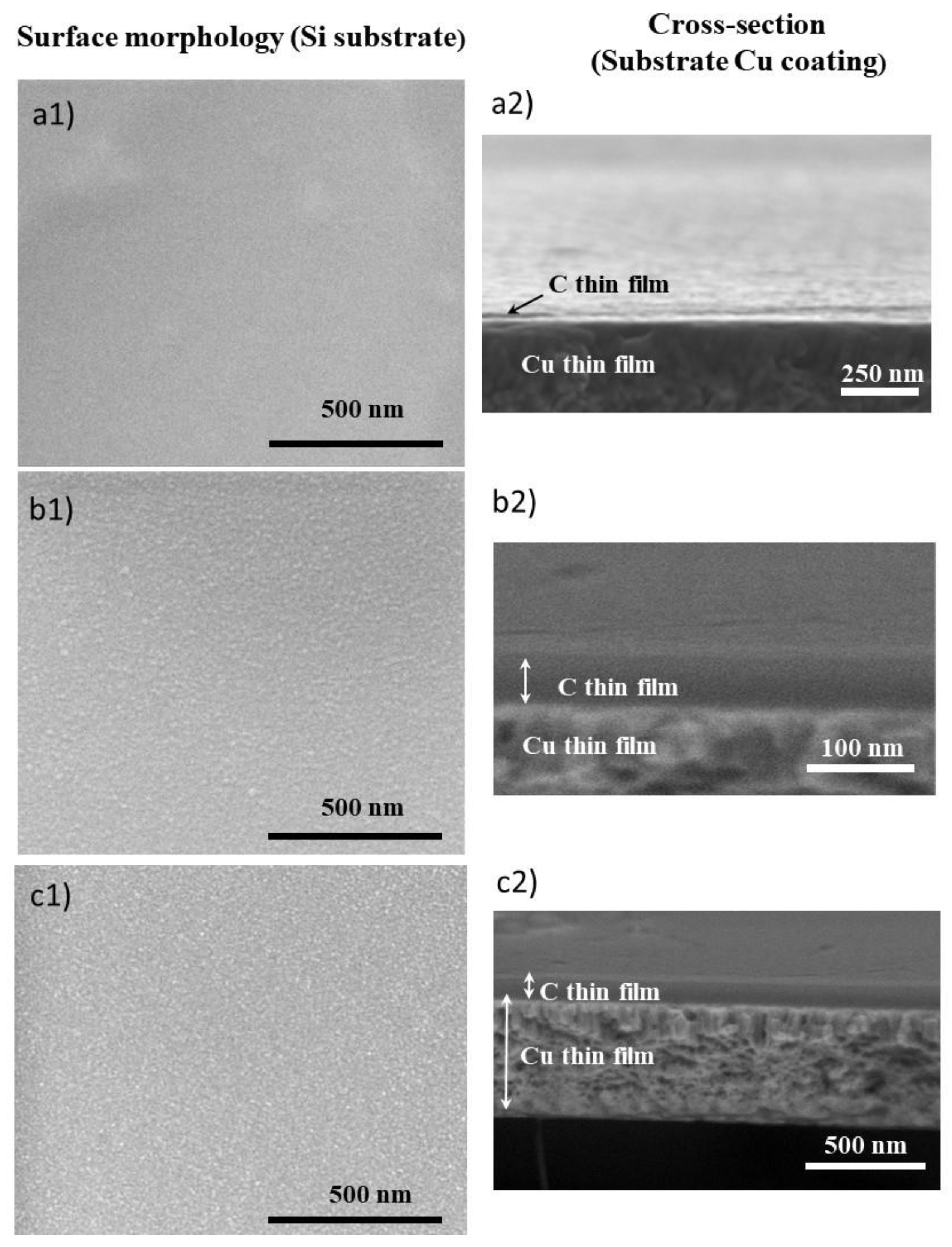
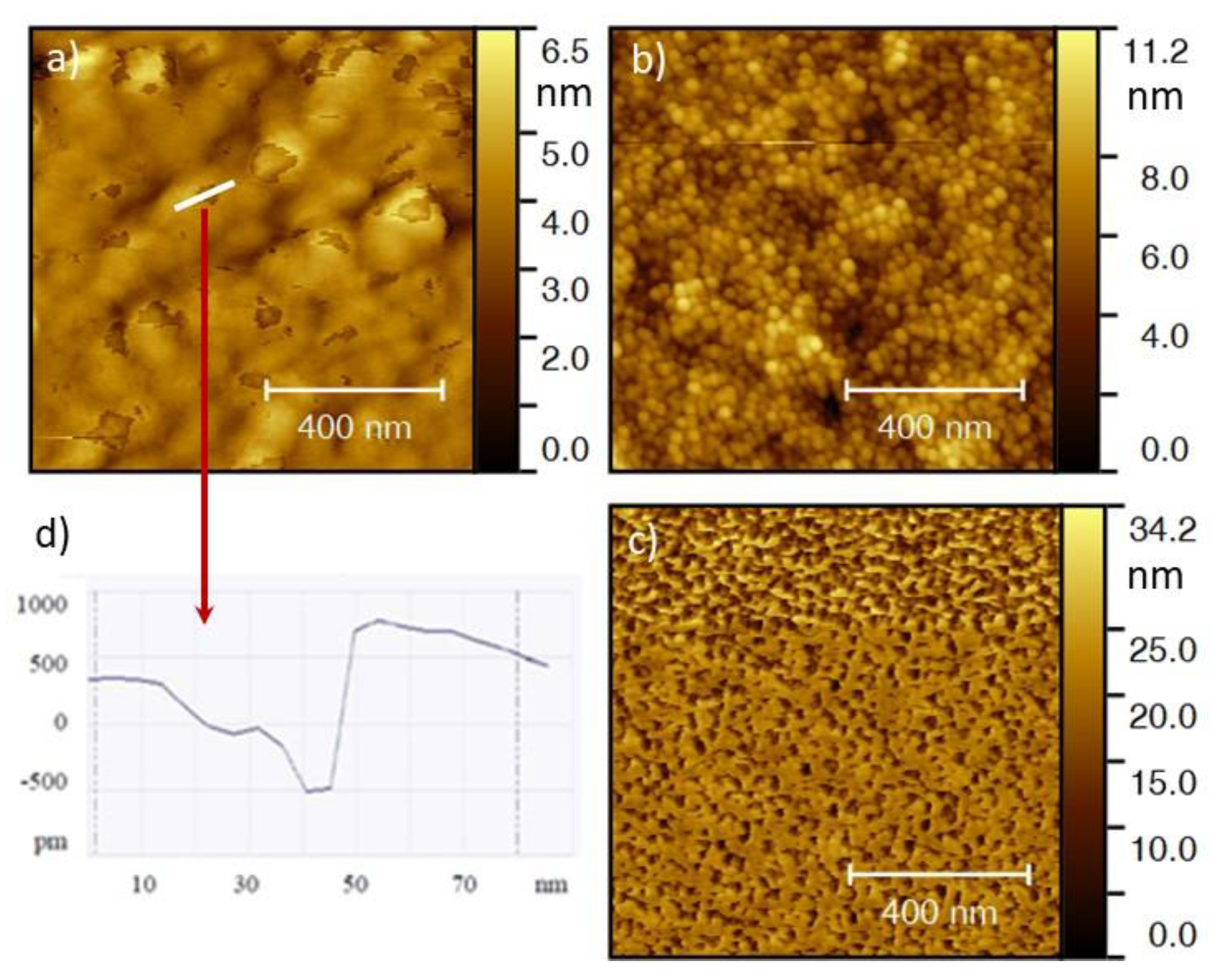
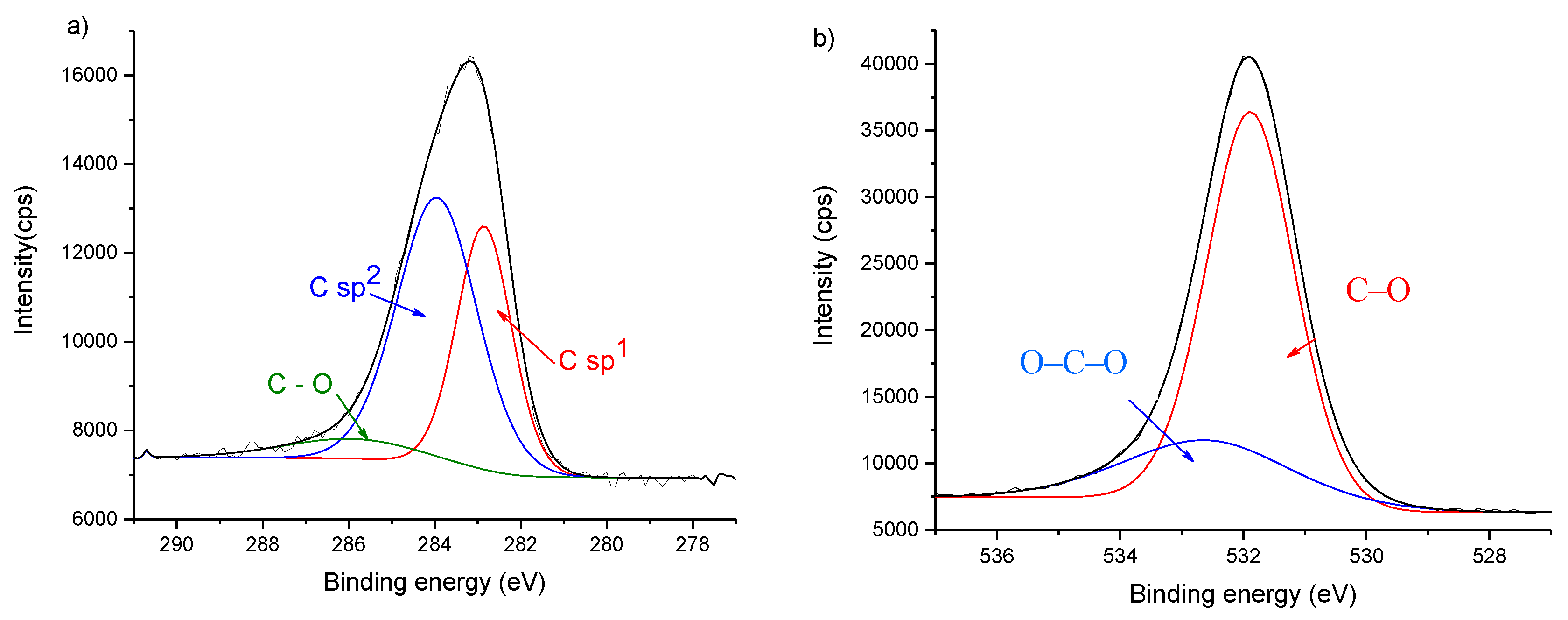
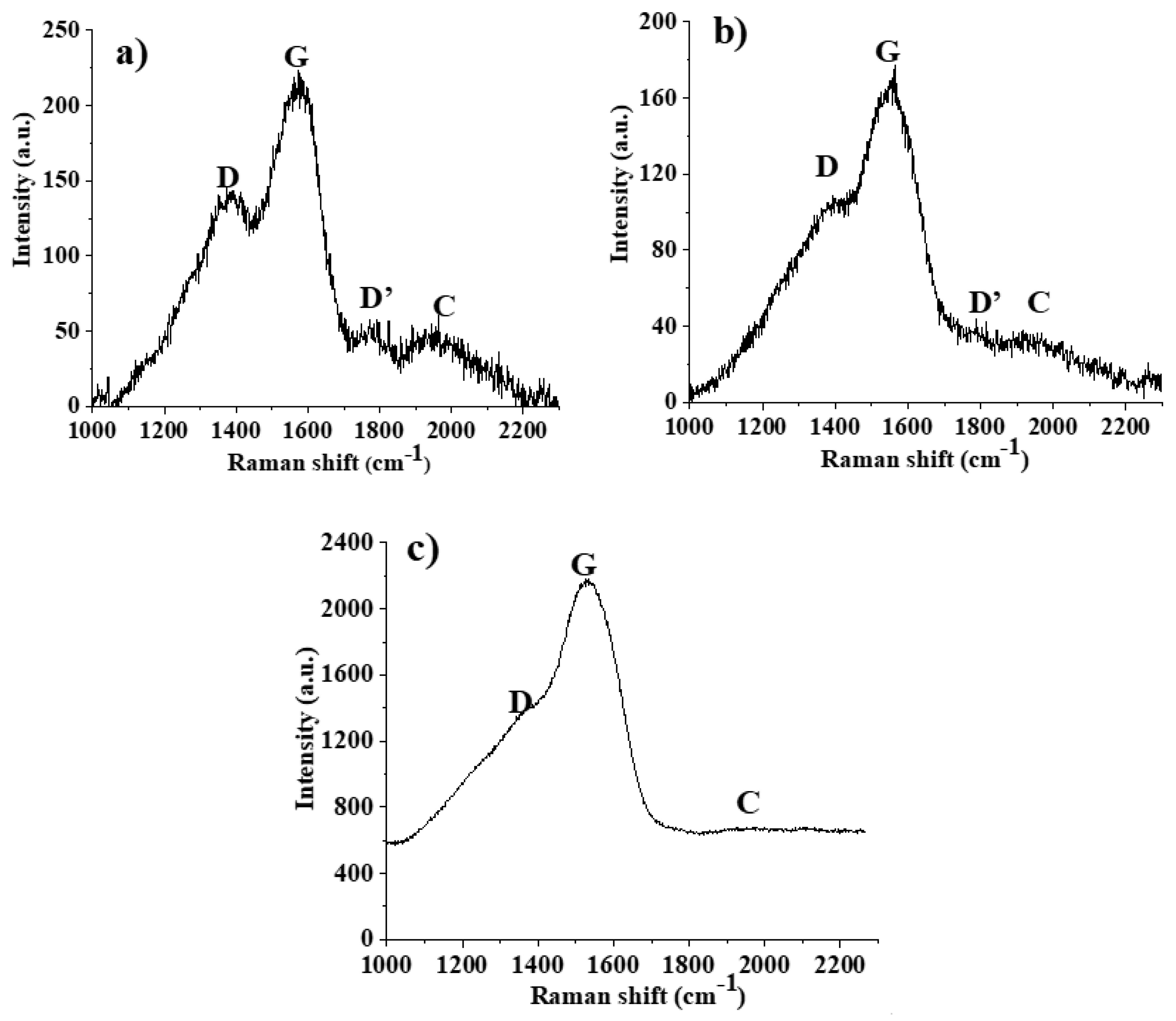
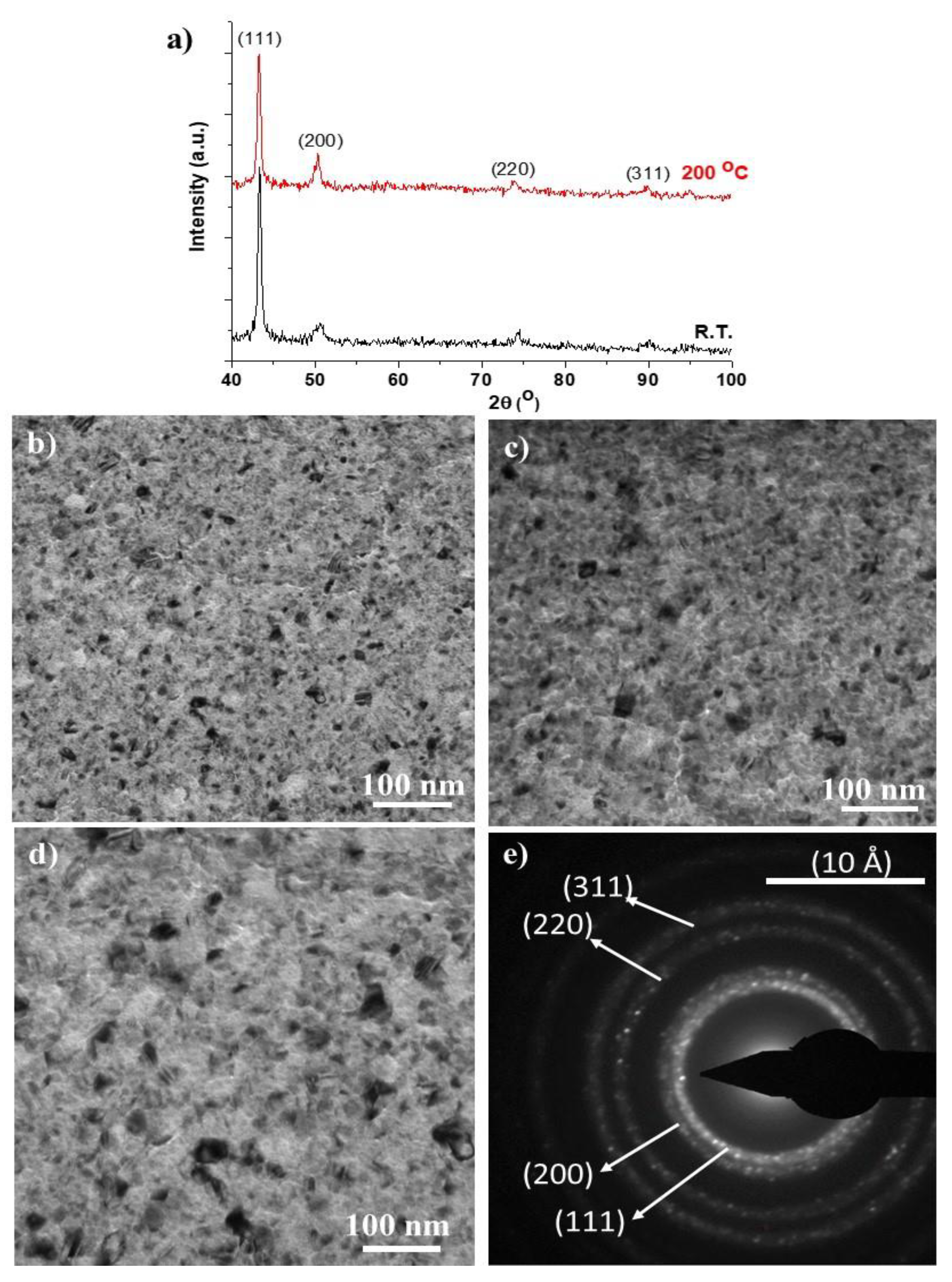
3.1. Characterization of the Thin Films
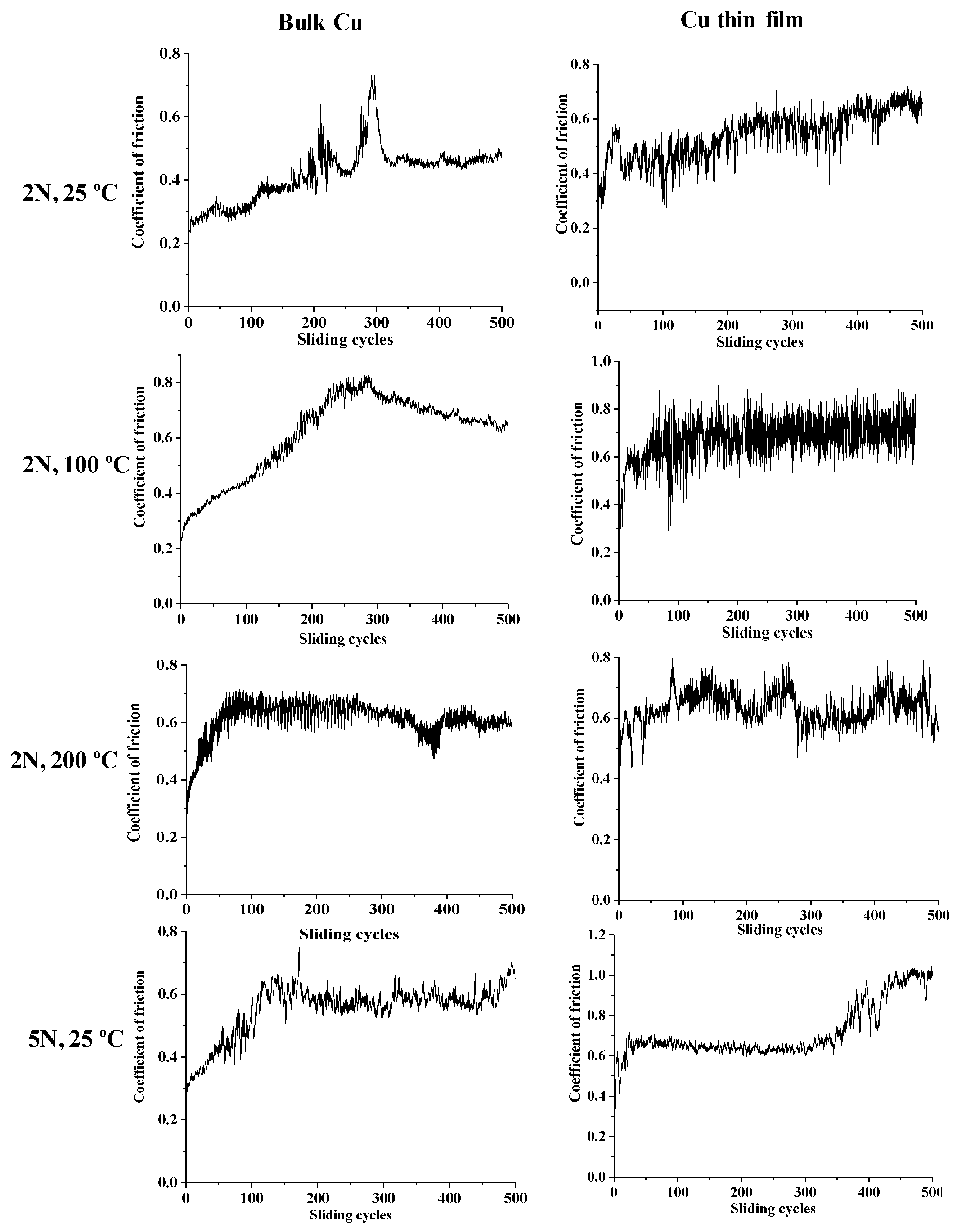
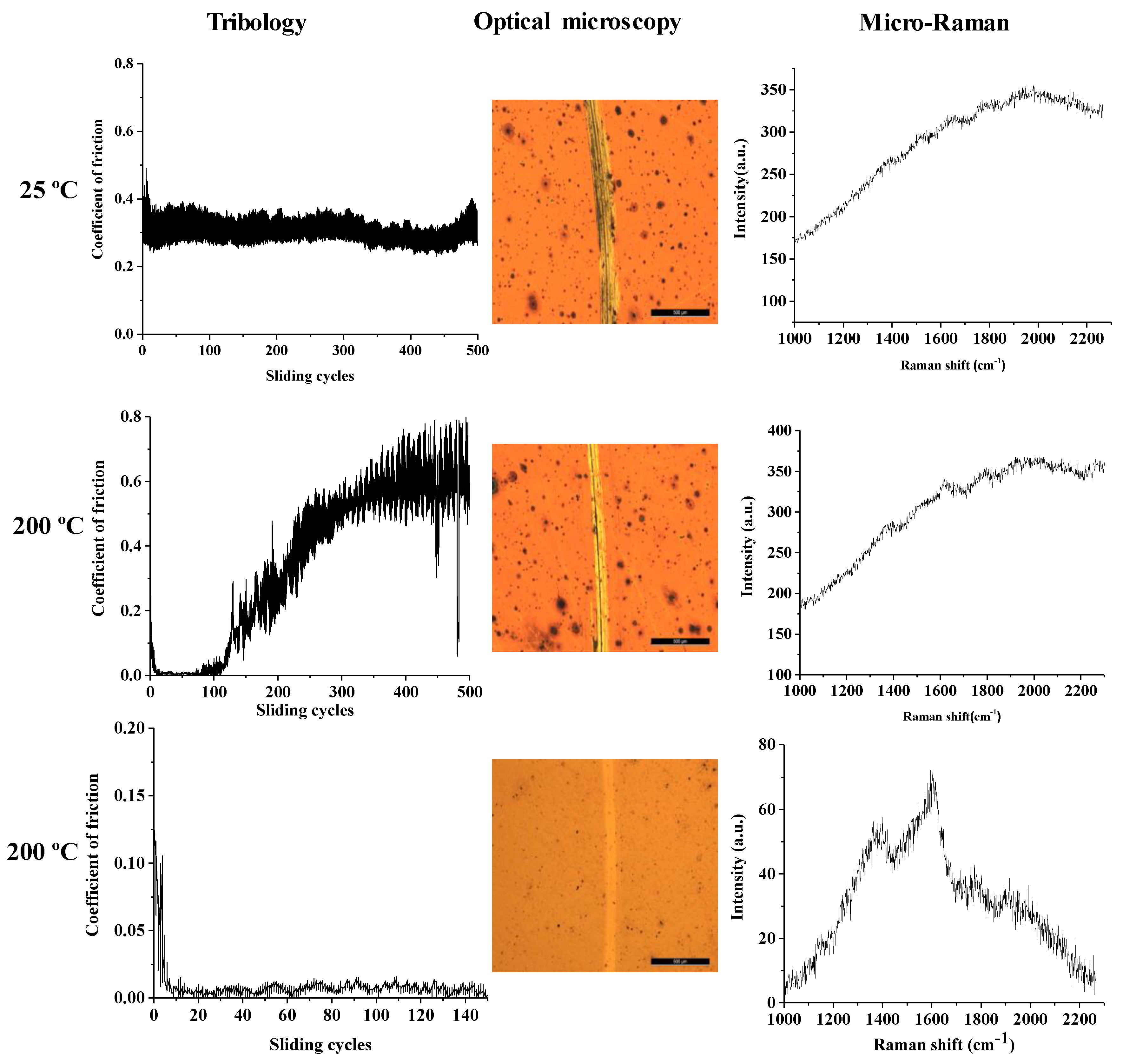
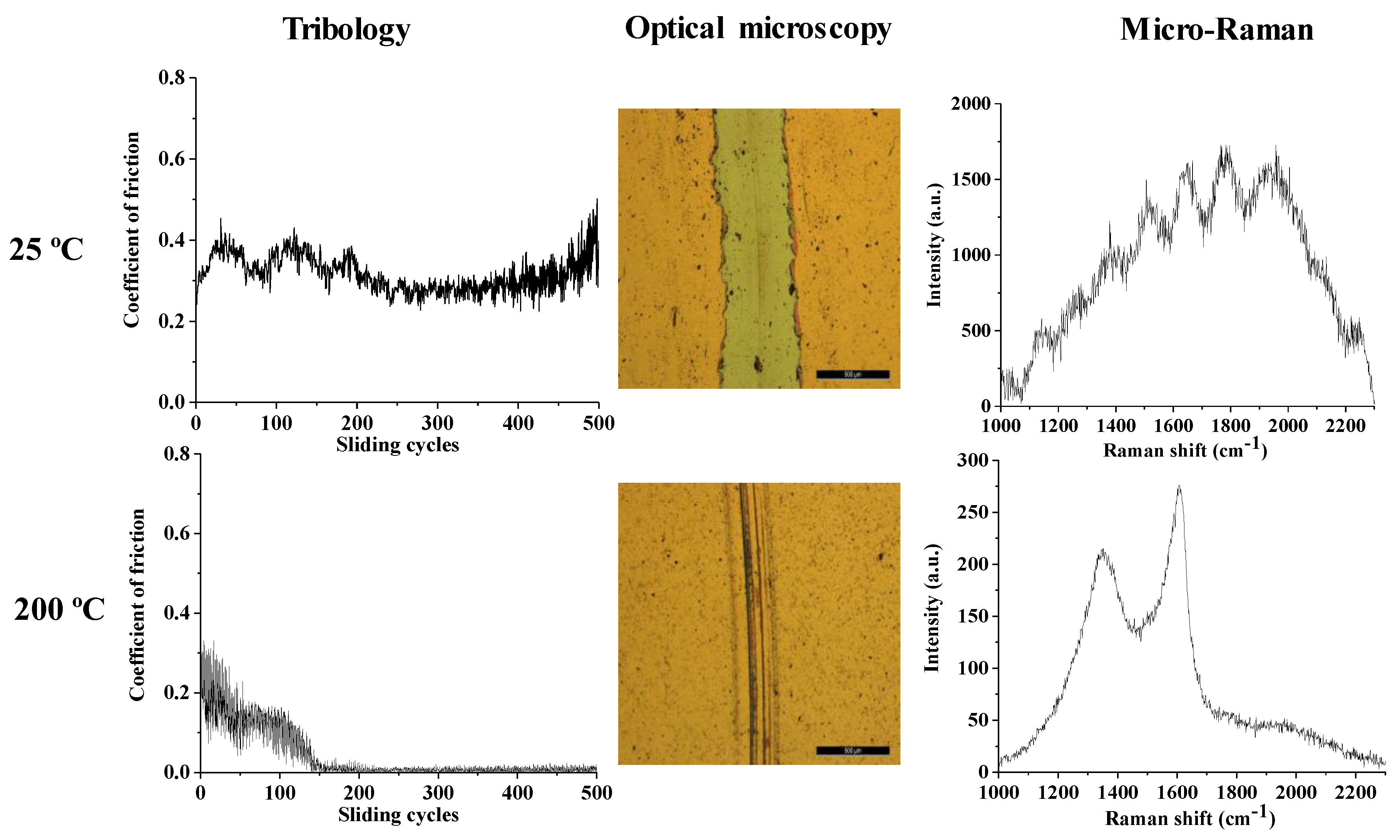
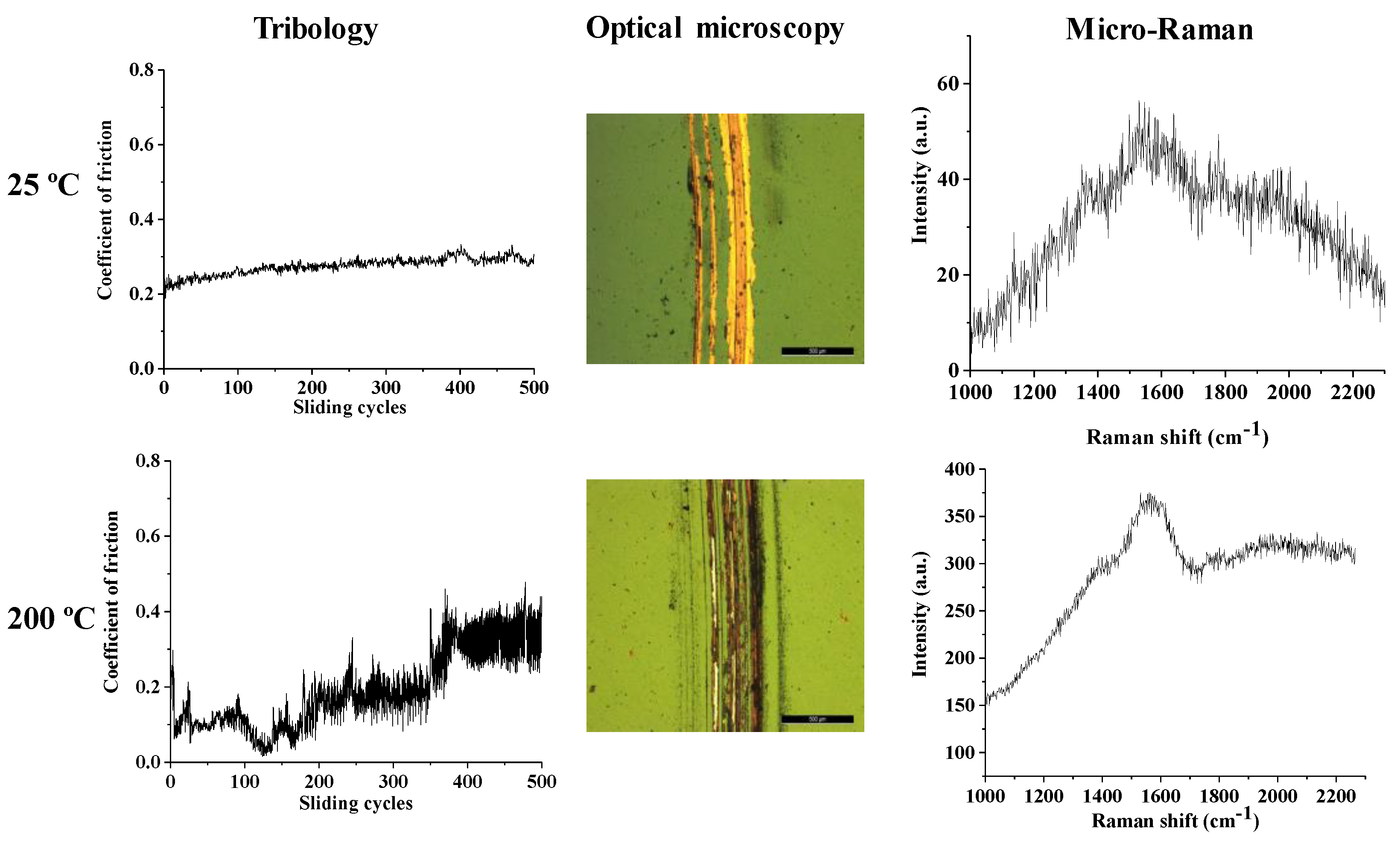
3.2. Mechanical Properties
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pellicer, E.; Varea, A.; Pané, S.; Nelson, B.J.; Menéndez, E.; Estrader, M.; Suriñach, S.; Baró, M.B.; Nogués, J.; Sort, J. Nanocrystalline electroplated Cu–Ni: Metallic thin films with enhanced mechanical properties and tunable magnetic behavior. Adv. Funct. Mater. 2010, 20, 983–991. [Google Scholar] [CrossRef]
- Hsu, K.S.; Lin, M.T.; Tong, C.J. The measurement of cyclic creep behavior in copper thin film using microtensile testing. In MEMS and Nanotechnology; Springer: London, UK, 2011; Volume 4, pp. 31–38. [Google Scholar]
- Torazawa, N.; Hirao, S.; Kanayama, S.; Korogib, H.; Matsumoto, S. The development of Cu filling and reliability performance with Ru-Ta alloy barrier for Cu interconnects. J. Electrochem. Soc. 2016, 163, 173–178. [Google Scholar] [CrossRef]
- Gambino, J.; Chen, F.; He, J. Copper interconnect technology for the 32 nm node and beyond. In Proceedings of the IEEE Custom Integrated Circuits Conference, Rome, Italy, 13–16 September 2009; pp. 141–148. [Google Scholar]
- Josell, D.; Brongersma, S.H.; Tőkei, Z. Size-dependent resistivity in nanoscale interconnects. Annu. Rev. Mater. Res. 2009, 39, 231–254. [Google Scholar] [CrossRef]
- Lazarus, N.; Meyer, C.D.; Bedair, S.S.; Song, X.; Boteler, L.M.; Kierzewski, I.M. Thick film oxidation of copper in an electroplated MEMS process. J. Micromech. Microeng. 2013, 23, 065017. [Google Scholar] [CrossRef]
- Chen, H.; Filleter, T. Effect of structure on the tribology of ultrathin graphene and graphene oxide films. Nanotechnology 2015, 26, 135702. [Google Scholar] [CrossRef] [PubMed]
- Savage, N. Materials science: Super carbon. Nature 2012, 483, S30–S31. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kim, D.-E. Water lubrication of stainless steel using reduced graphene oxide coating. Sci. Rep. 2015, 5, 17034. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.; Erdemir, A.; Sumant, A.V. Graphene: A new emerging lubricant. Mater. Today 2014, 17, 31–32. [Google Scholar] [CrossRef]
- Khan, Z.H.; Kermany, A.R.; Öchsner, A.; Iacopi, F. Mechanical and electromechanical properties of graphene and their potential applications in MEMS. J. Phys. D: Appl. Phys. 2017, 50, 053003. [Google Scholar] [CrossRef]
- Yuanhong, X.; Jingquan, L. Graphene as transparent electrodes: Fabrication and new emerging applications. Small 2016, 12, 1400–1419. [Google Scholar]
- Kim, K.-S.; Lee, H.-J.; Lee, C.; Lee, S.-K.; Jang, H.; Ahn, J.-H.; Kim, J.-H.; Lee, H.-J. Chemical vapor deposition-grown graphene: The thinnest solid lubricant. ACS Nano 2011, 5, 5107–5114. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Li, Q.; Carpick, R.; Kysar, J.W.; Hone, J. Elastic and frictional properties of graphene. Phys. Status Solidi B 2009, 246, 2562–2567. [Google Scholar] [CrossRef]
- Ko, J.-H.; Kwon, S.; Byun, I.-S.; Choi, J.S.; Park, B.H.; Kim, Y.-H.; Park, J.Y. Nanotribological properties of fluorinated, hydrogenated, and oxidized graphenes. Tribol. Lett. 2013, 50, 137–144. [Google Scholar] [CrossRef]
- Li, Q.; Lee, C.; Carpick, R.W.; Hone, J. Substrate effect on thickness-dependent friction on graphene. Phys. Status Solidi B 2010, 247, 2909–2914. [Google Scholar] [CrossRef]
- Wenjuan, Z.; Low, T.; Perebeinos, V.; Bol, A.A.; Zhu, Y.; Yan, H.; Tersof, J.; Avouris, P. Structure and electronic transport in graphene wrinkles. Nano Lett. 2012, 12, 3431–3436. [Google Scholar]
- Marchena, M.; Wagner, F.; Arliguie, T.; Zhu, B.; Johnson, B.; Fernández, M.; Chen, T.L.; Chang, T.; Lee, R.; Pruneri, V.; et al. Dry transfer of graphene to dielectrics and flexible substrates using polyimide as a transparent and stable intermediate layer. 2D Mater. 2018, 5, 035022. [Google Scholar] [CrossRef]
- Banhart, F.; Kotakoski, J.; Krasheninnikov, A.V. Structural defects in graphene. ACS Nano 2011, 5, 26–41. [Google Scholar] [CrossRef]
- Parveen, K.; Wani, M.F. Synthesis and tribological properties of graphene: A review. Jurnal Tribologi 2017, 13, 36–71. [Google Scholar]
- Peng, Y.; Wang, Z.; Zou, K. Friction and wear properties of different types of graphene nanosheets as effective solid lubricants. Langmuir 2015, 31, 7782–7791. [Google Scholar] [CrossRef]
- Kinoshita, H.; Kume, I.; Tagawa, M.; Ohmae, N. High friction of a vertically aligned carbon-nanotube film in microtribology. Appl. Phys. Lett. 2004, 85, 2780–2788. [Google Scholar] [CrossRef]
- Berman, D.; Deshmukh, S.A.; Sankaranarayanan, S.K.R.S.; Erdemir, A.; Sumant, A.V. Extraordinary macroscale wear resistance of one atom thick graphene layer. Adv. Funct. Mater. 2014, 24, 6640–6646. [Google Scholar] [CrossRef]
- Strmčnik, E.; Majdič, F.; Kalin, M. Water-lubricated behaviour of AISI 440C stainless steel and a DLC coating for an orbital hydraulic motor application. Tribol. Int. 2019, 131, 128–136. [Google Scholar] [CrossRef]
- Su, F.; Chen, G.; Sun, J. Synthesis of hydrogenated DLC film by PECVD and its tribocorrosion behaviors under the lubricating condition of graphene oxide dispersed in water. Tribol. Int. 2019, 130, 1–8. [Google Scholar] [CrossRef]
- Tyagi, A.; Walia, R.S.; Murtaza, Q.; Pandey, S.M.; Tyagi, P.K.; Bajaj, B. A critical review of diamond like carbon coating for wear resistance applications. Int. J. Refract. Met. Hard Mater. 2019, 78, 107–122. [Google Scholar] [CrossRef]
- Nißen, S.; Heeg, J.; Wienecke, M.; Behrend, D.; Warkentin, M. Enhancing adhesion strength of a-C:H:Cu composite coatings on Ti6Al4V by graded copper deposition in a rf PVD/PECVD hybrid process. Surf. Coat. Technol. 2018, 350, 659–671. [Google Scholar] [CrossRef]
- Kang, K.-N.; Lee, J.; Lee, K.Y.; Kang, Y.; Park, Y.S. Tribological and electrical properties of chromium-doped carbon films fabricated by unbalanced magnetron sputtering for medical stents. J. Nanosci. Nanotechnol. 2019, 19, 1415–1419. [Google Scholar] [CrossRef]
- Chen, Z.; He, H.; Xiao, C.; Kim, S.H. Effect of humidity on friction and wear—A critical review. Lubricants 2018, 6, 74. [Google Scholar] [CrossRef]
- Pinho, A.C.; Piedade, A.P. Zeta potential, contact angles, and AFM imaging of protein conformation adsorbed on hybrid nanocomposite surfaces. ACS Appl. Mater. Interfaces 2013, 5, 8187–8194. [Google Scholar] [CrossRef]
- Piedade, A.P.; Nunes, J.; Vieira, M.T. Thin films with chemically graded functionality based on fluorine polymers and stainless steel. Acta Biomat. 2008, 4, 1073–1080. [Google Scholar] [CrossRef]
- Piedade, A.P.; Vieira, M.T.; Martins, A.; Silva, F. In vitro behaviour of nanocrystalline silver-sputtered thin films. Nanotechnology 2008, 18, 105103. [Google Scholar] [CrossRef]
- Carvalho, D.; Sousa, T.; Morais, P.V.; Piedade, A.P. Polymer/metal nanocomposite coating with antimicrobial activity against hospital isolated pathogen. Appl. Surf. Sci. 2016, 379, 489–496. [Google Scholar] [CrossRef]
- Reichelt, K.; Jiang, X. The preparation of thin films by physical vapour depositions methods. Thin Solid Films 1990, 191, 91–126. [Google Scholar] [CrossRef]
- Greene, J.E. Tracing the recorded history of thin-film sputter deposition: From the 1800s to 2017. J. Vac. Sci. Technol. A 2017, 35, 05C204. [Google Scholar] [CrossRef]
- Martins, L.G.P.; Song, Y.; Zeng, T.; Dresselhaus, M.S.; Kong, J.; Araujo, P.T. Direct transfer of graphene onto flexible substrates. PNAS 2013, 110, 17762–17767. [Google Scholar] [CrossRef] [PubMed]
- Lide, D.R. Handbook of Chemistry and Physics, 85th ed.; CRC Press: New York, NY, USA, 2004. [Google Scholar]
- Danno, T.; Okada, Y.; Kawaguchi, J. XPS study of carbyne-like carbon films. AIP Conf. Proceed. 2004, 723, 431–434. [Google Scholar]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Hong, J.; Park, M.K.; Lee, E.J.; Lee, D.; Hwang, D.S.; Ryu, S. Origin of new broad Raman D and G peaks in annealed graphene. Sci. Rep. 2013, 3, 2700. [Google Scholar] [CrossRef]
- Casari, C.S.; Milani, A. Carbyne: From the elusive allotrope to stable carbon atom wires. MRS Commun. 2018, 8, 207–219. [Google Scholar] [CrossRef]
- Zabel, J.; Nair, R.R.; Ott, A.; Georgiou, T.; Geim, A.K.; Novoselov, K.S.; Casiraghi, C. Raman spectroscopy of graphene and bilayer under biaxial strain: Bubbles and balloons. Nano Lett. 2012, 12, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, J. Wetting transparency of graphene. Nat. Mat. 2012, 11, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Palaniselvam, T.; Aiyappa, H.B.; Kurungot, S. An efficient oxygen reduction electrocatalyst from graphene by simultaneously generating pores and nitrogen doped active sites. J. Mater. Chem. 2012, 22, 23799–23805. [Google Scholar] [CrossRef]
- Lv, Q.; Si, W.; He, J.; Sun, L.; Zhang, C.; Wang, N.; Yang, Z.; Li, X.; Wang, X.; Deng, W.; et al. Selectively nitrogen-doped carbon materials as superior metal-free catalysts for oxygen reduction. Nat. Commun. 2018, 9, 3376–3386. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Li, Y.; Liu, X.; Liu, Q.; Shang, J.; Duan, H.; Dai, L.; Shui, J. Zigzag carbon as efficient and stable oxygen reduction electrocatalyst for proton exchange membrane fuel cells. Nat. Commun. 2018, 9, 3819–3826. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.; Casiraghi, C. Probing the nature of defects in graphene by Raman spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Tao, N.R.; Wang, L.B.; Ding, B.Z.; Lu, K. Grain growth and strain release in nanocrystalline copper. J. Appl. Phys. 2001, 89, 6408–6414. [Google Scholar] [CrossRef]
- Vaz, D.; Piedade, A.P. Structure and mechanical properties of a copper combustion chamber throughout its life cycle. Metals 2018, 8, 362. [Google Scholar] [CrossRef]
- Zhang, S. Nanostructured Thin Films and Coatings: Mechanical Properties; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Kotrechko, S.; Mikhailovskij, I.; Mazilova, T.; Sadanov, E.; Timoshevskii, A.; Stetsenko, N.; Matviychuk, Y. Mechanical properties of carbine: Experiment and simulations. Nanoscale Res. Lett. 2015, 10, 24. [Google Scholar] [CrossRef]
- Mirzaeifar, R.; Qin, Z.; Buehler, M.J. Tensile strength of carbine chains in varied chemical environments and structural length. Nanotechnology 2014, 25, 371001. [Google Scholar] [CrossRef]
- Timoshevskii, A.; Kotrechko, S.; Matviychuk, Y. Atomic structure and mechanical properties of carbyne. Phys. Rev. B 2015, 91, 245434. [Google Scholar] [CrossRef]
- Bryant, P.J.; Gutshall, P.L.; Taylor, L.H. A Study of mechanisms of graphitic friction and wear. Wear 1964, 7, 118–126. [Google Scholar] [CrossRef]

| Designation | Deposition Parameters | Thickness (nm) | ||
|---|---|---|---|---|
| Ddep Cu (W·mm−2) | Ddep C (W·mm−2) | Time (s) | ||
| Cu | 3.2 × 10−2 | 0 | 600 | 600 |
| C15 | 0 | 2.5 × 10−2 | 300 | 15 |
| C60 | 0 | 2.5 × 10−2 | 1800 | 60 |
| C100 | 0 | 2.5 × 10−2 | 3600 | 100 |
| C Bonds | Surface | |||
|---|---|---|---|---|
| C15 | C60 | C100 | ||
| sp1 | Peak Position (eV) | 282.6 | 282.4 | n.d. |
| Concentration (%) | 35.7 | 5.2 | n.d. | |
| FWHM (eV) | 1.4 | 1.9 | n.d. | |
| sp2 | Peak Position (eV) | 284.1 | 284.2 | 284.2 |
| Concentration (%) | 57.2 | 60.3 | 47.4 | |
| FWHM (eV) | 1.9 | 1.3 | 1.3 | |
| sp3 | Peak Position (eV) | n.d. | 284.9 | 285.0 |
| Concentration (%) | n.d. | 18.0 | 34.0 | |
| FWHM (eV) | n.d. | 1.7 | 1.4 | |
| C–O | Peak Position (eV) | 286.1 | 286.3 | 286.2 |
| Concentration (%) | 7.1 | 9.3 | 15.2 | |
| FWHM (eV) | 2.0 | 1.7 | 2.2 | |
| O–C–O | Peak Position (eV) | 287.6 | 287.5 | n.d. |
| Concentration (%) | 3.4 | 7.2 | n.d. | |
| FWHM (eV) | 2.0 | 1.7 | n.d. | |
| Surface | G Peak Position (cm−1) | Intensity Ratios | ||
|---|---|---|---|---|
| ID/IG | IC/IG | ID/ID’ | ||
| C15 | 1574 | 0.57 | 0.30 | 3.0 |
| C60 | 1551 | 0.62 | 0.25 | 3.3 |
| C100 | 1531 | 0.37 | 0.04 | 5.2 |
| Surface | H (GPa) | E (GPa) |
|---|---|---|
| Cu | 2.64 ± 0.07 | 168 ± 10 |
| C15 | 2.75 ± 0.26 | 164 ± 13 |
| C60 | 2.86 ± 0.07 | 126 ± 5 |
| C100 | 2.95 ± 0.10 | 101 ± 2 |
| Bulk Copper | 1.91 ± 0.09 | 144 ± 19 |
| Bulk Copper (1 N) | 0.99 ± 0.02 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piedade, A.P.; Cangueiro, L. Influence of Carbyne Content on the Mechanical Performance of Nanothick Amorphous Carbon Coatings. Nanomaterials 2020, 10, 780. https://doi.org/10.3390/nano10040780
Piedade AP, Cangueiro L. Influence of Carbyne Content on the Mechanical Performance of Nanothick Amorphous Carbon Coatings. Nanomaterials. 2020; 10(4):780. https://doi.org/10.3390/nano10040780
Chicago/Turabian StylePiedade, Ana P., and Liliana Cangueiro. 2020. "Influence of Carbyne Content on the Mechanical Performance of Nanothick Amorphous Carbon Coatings" Nanomaterials 10, no. 4: 780. https://doi.org/10.3390/nano10040780
APA StylePiedade, A. P., & Cangueiro, L. (2020). Influence of Carbyne Content on the Mechanical Performance of Nanothick Amorphous Carbon Coatings. Nanomaterials, 10(4), 780. https://doi.org/10.3390/nano10040780






