Co3V2O8 Nanoparticles Supported on Reduced Graphene Oxide for Efficient Lithium Storage
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Samples
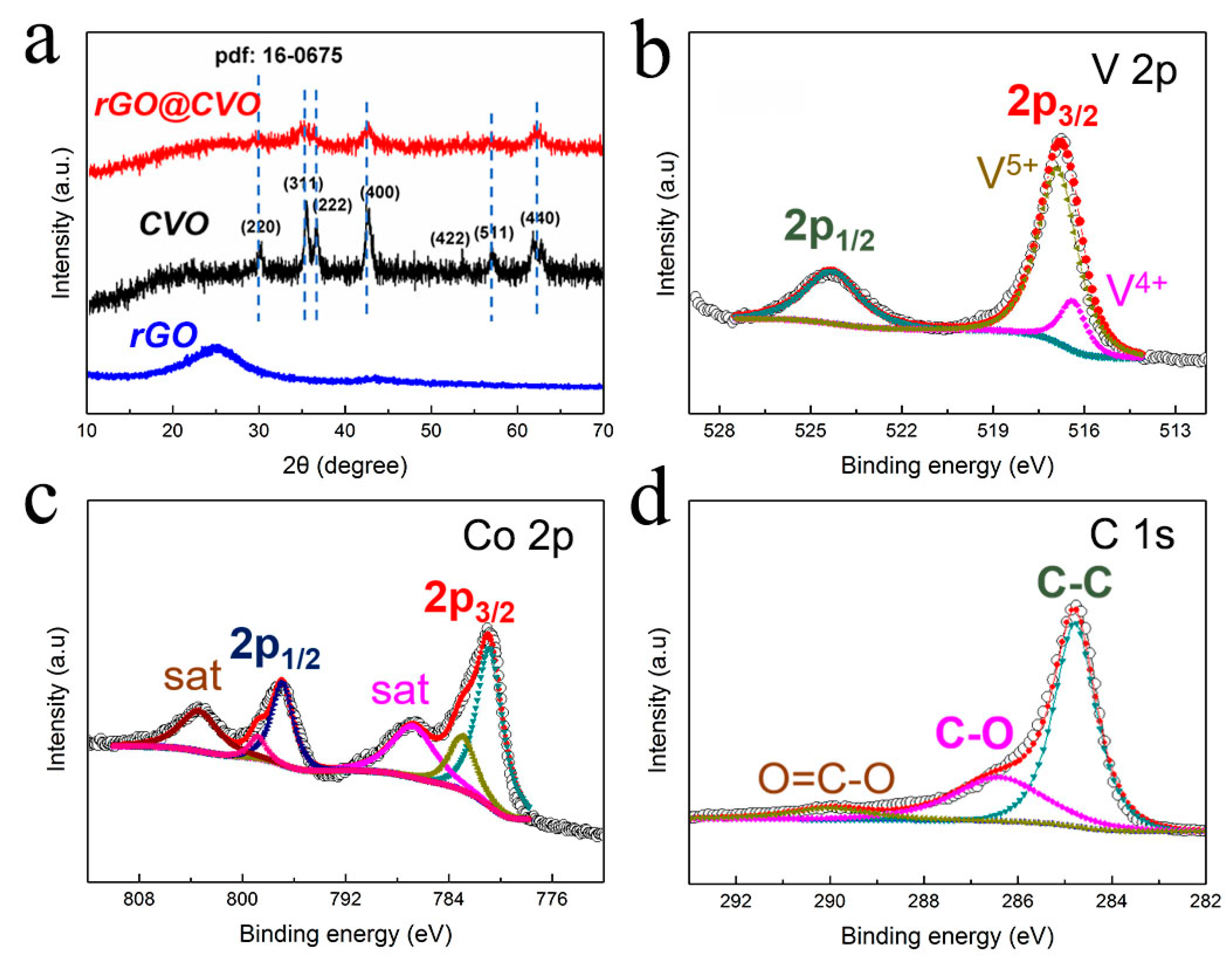
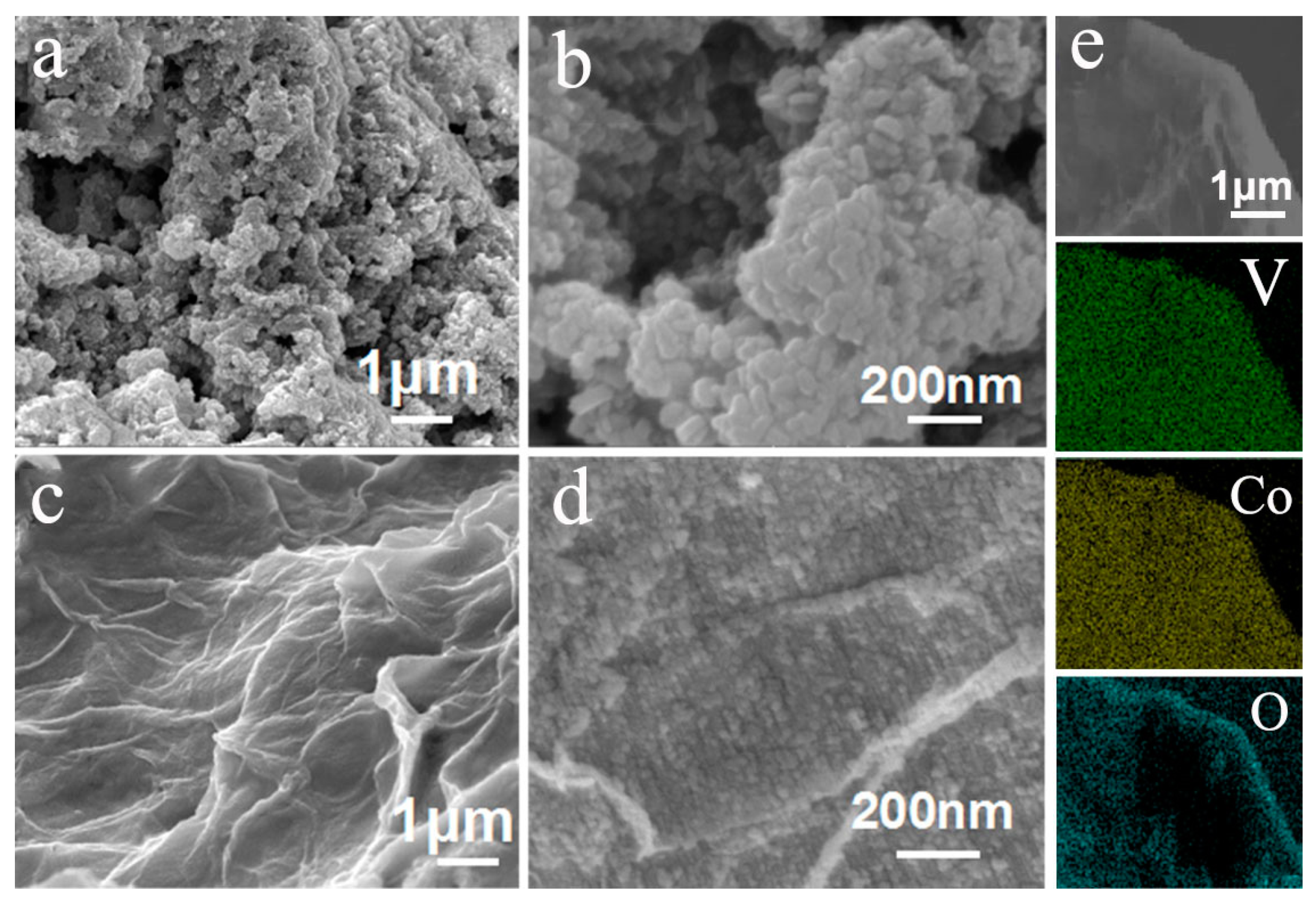
2.3. Material Characterization
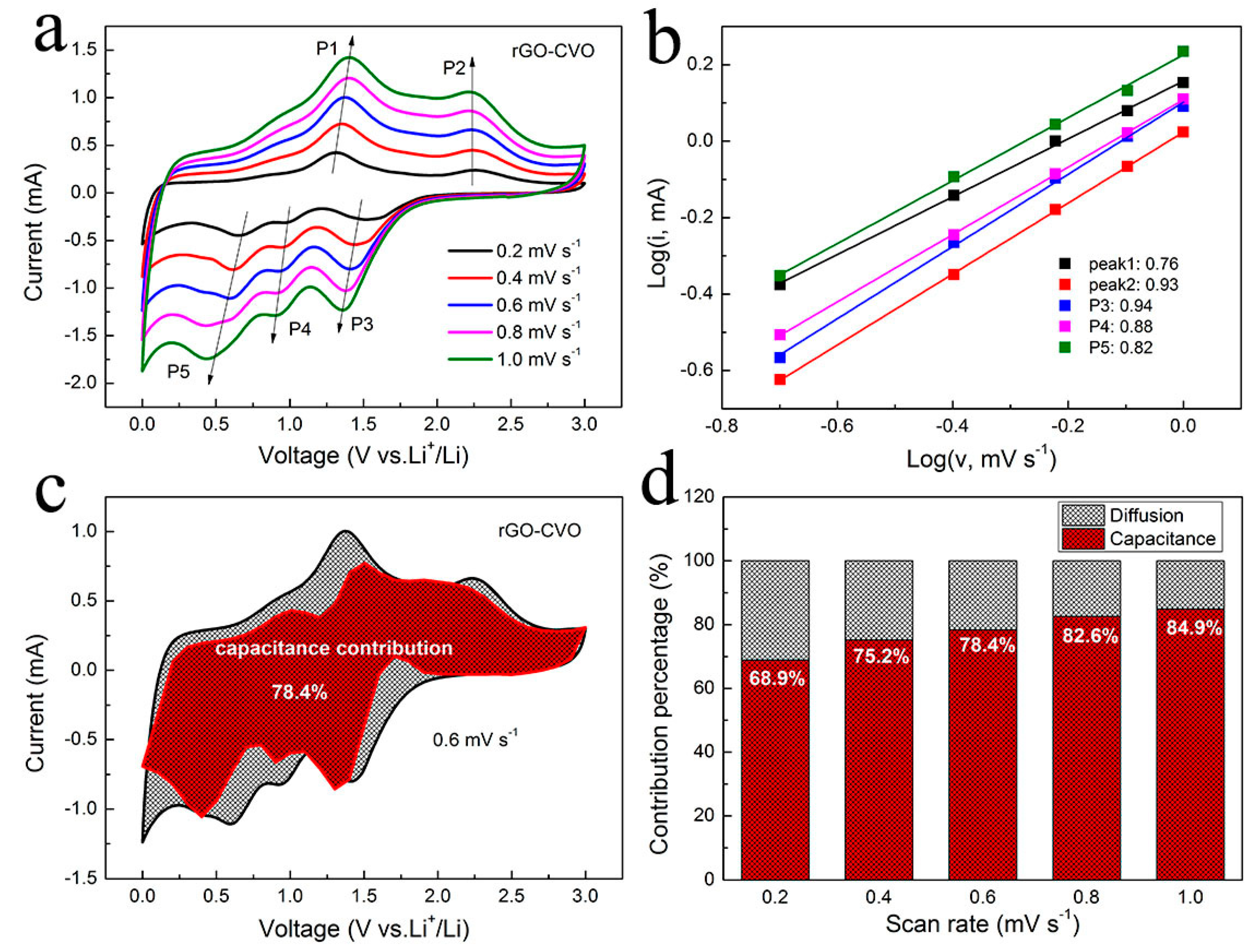
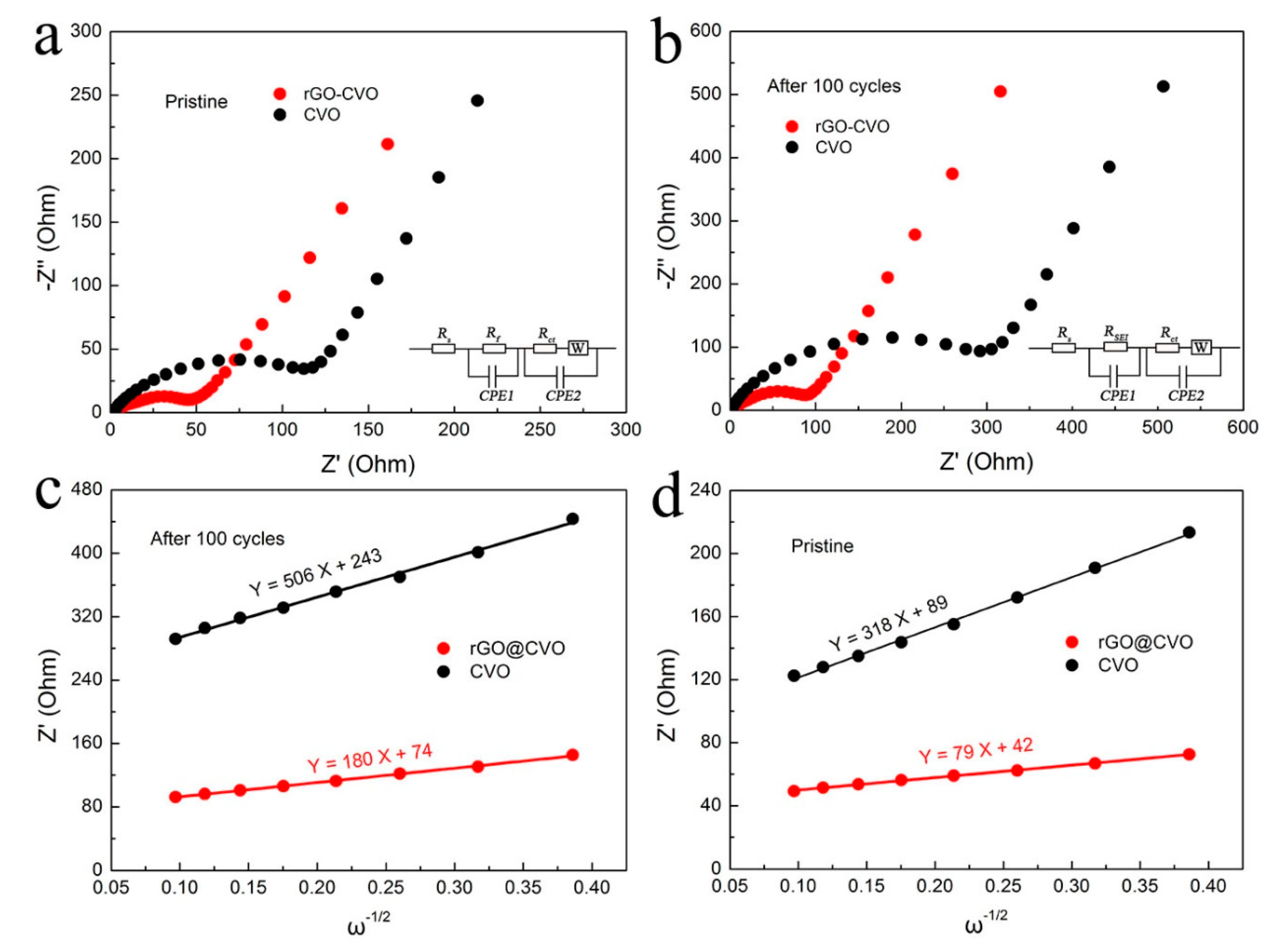
2.4. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fu, L.; Wang, X.; Ma, J.; Zhang, C.; He, J.; Xu, H.; Chai, J.; Li, S.; Chai, F.; Cui, G. Graphene-encapsulated copper tin sulfide submicron spheres as high-capacity binder-free anode for lithium-ion batteries. ChemElectroChem 2017, 4, 1124–1129. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, H.H.; Chen, H.; Cheng, Y.; Zhang, Q.; Xie, Q.; Wang, L.; Zhang, K.; Wang, M.S.; Peng, D.L.; et al. Fabrication and understanding of Cu3Si-Si@carbon@graphene nanocomposites as high-performance anodes for lithium-ion batteries. Nanoscale 2018, 10, 22203–22214. [Google Scholar] [CrossRef]
- Chen, G.; An, J.; Meng, Y.; Yuan, C.; Matthews, B.; Dou, F.; Shi, L.; Zhou, Y.; Song, P.; Wu, G. Cation and anion Co-doping synergy to improve structural stability of Li-and Mn-rich layered cathode materials for lithium-ion batteries. Nano Energy 2019, 57, 157–165. [Google Scholar] [CrossRef]
- Ma, K.; Jiang, H.; Hu, Y.J.; Li, C.Z. 2D nanospace confined synthesis of pseudocapacitance-dominated MoS2-in-Ti3C2 superstructure for ultrafast and stable Li/Na-Ion batteries. Adv. Funct. Mater. 2018, 28, 1804306. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, C.; Chen, B.; Zhang, Z.; Wang, X.; Zhao, J.; He, J.; Du, H.; Cui, G. Graphene boosted Cu2GeS3 for advanced lithium-ion batteries. Inorg. Chem. Front. 2017, 4, 541–546. [Google Scholar] [CrossRef]
- Ye, X.; Lin, Z.; Liang, S.; Huang, X.; Qiu, X.; Qiu, Y.; Liu, X.; Xie, D.; Deng, H.; Xiong, X.; et al. Upcycling of electroplating sludge into ultrafine Sn@C nanorods with highly stable lithium storage performance. Nano Lett. 2019, 19, 1860–1866. [Google Scholar] [CrossRef]
- Yin, H.; Li, Q.; Cao, M.; Zhang, W.; Zhao, H.; Li, C.; Huo, K.; Zhu, M. Nanosized-bismuth-embedded 1D carbon nanofibers as high-performance anodes for lithium-ion and sodium-ion batteries. Nano Res. 2017, 10, 2156–2167. [Google Scholar] [CrossRef]
- Shang, C.; Hu, L.; Fu, L.; Huang, L.; Xue, B.; Wang, X.; Shui, L.; Zhou, G. Improving lithium storage capability of ternary Sn-based sulfides by enhancing inactive/active element ratio. Solid State Ion. 2019, 337, 47–55. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Zhu, J.; Meng, T.; Ma, L.; Zhang, H.; Xu, M.; Jiang, J.; Li, C.M. One-dimensional integrated MnS@Carbon nanoreactors hybrid: An alternative anode for full-cell Li-Ion and Na-Ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 27911–27919. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, D.; Wu, J.; Wang, Z.; Huang, S.; Xu, Y.; Chen, Z.; Zhao, B.; Zhang, J. Sandwich-like SnS2/Graphene/SnS2 with expanded interlayer distance as high-rate lithium/sodium-Ion battery anode materials. ACS Nano 2019, 13, 9100–9111. [Google Scholar] [CrossRef]
- Natarajan, S.; Bajaj, H.C.; Aravindan, V. Template-free synthesis of carbon hollow spheres and reduced graphene oxide from spent lithium-ion batteries towards efficient gas storage. J. Mater. Chem. A 2019, 7, 3244–3252. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sultana, I.; Yang, T.; Chen, Z.; Sharma, N.; Glushenkov, A.M.; Chen, Y. Lithium germanate (Li2GeO3): A high-performance anode material for lithium-ion batteries. Angew. Chem. Int. Ed. 2016, 55, 16059–16063. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.T.; Le, H.T.T.; Kim, C.; Lee, J.-Y.; Fisher, J.G.; Kim, I.-D.; Park, C.-J. Mass-scalable synthesis of 3D porous germanium—Carbon composite particles as an ultra-high rate anode for lithium ion batteries. Energy Environ. Sci. 2015, 8, 3577–3588. [Google Scholar] [CrossRef]
- Sathiya, M.; Prakash, A.S.; Ramesha, K.; Tarascon, J.M.; Shukla, A.K. V2O5-anchored carbon nanotubes for enhanced electrochemical energy storage. J. Am. Chem. Soc. 2011, 133, 16291–16299. [Google Scholar] [CrossRef]
- Gong, F.; Xia, D.; Bi, C.; Yang, J.; Zeng, W.; Chen, C.; Ding, Y.; Xu, Z.; Liao, J.; Wu, M. Systematic comparison of hollow and solid Co3V2O8 micro-pencils as advanced anode materials for lithium ion batteries. Electrochim. Acta 2018, 264, 358–366. [Google Scholar] [CrossRef]
- Zhang, Q.; Pei, J.; Chen, G.; Bie, C.; Sun, J.; Liu, J. Porous Co3V2O8 nanosheets with ultrahigh performance as anode materials for lithium Ion batteries. Adv. Mater. Interfaces 2017, 4, 1700054. [Google Scholar] [CrossRef]
- Gao, G.; Lu, S.; Dong, B.; Xiang, Y.; Xi, K.; Ding, S. Mesoporous Co3V2O8 nanoparticles grown on reduced graphene oxide as a high-rate and long-life anode material for lithium-ion batteries. J. Mater. Chem. A 2016, 4, 6264–6270. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, J.H.; Kang, Y.C. Synthesis of carbonaceous/carbon-free nanofibers consisted of Co3V2O8 nanocrystals for lithium-ion battery anode with ultralong cycle life. Electrochim. Acta 2019, 313, 48–58. [Google Scholar] [CrossRef]
- Soundharrajan, V.; Sambandam, B.; Song, J.; Kim, S.; Jo, J.; Duong, P.T.; Kim, S.; Mathew, V.; Kim, J. Facile green synthesis of a Co3V2O8 nanoparticle electrode for high energy lithium-ion battery applications. J. Colloid Interface Sci. 2017, 501, 133–141. [Google Scholar] [CrossRef]
- Chai, H.; Wang, Y.; Fang, Y.; Lv, Y.; Dong, H.; Jia, D.; Zhou, W. Low-cost synthesis of hierarchical Co3V2O8 microspheres as high-performance anode materials for lithium-ion batteries. Chem. Eng. J. 2017, 326, 587–593. [Google Scholar] [CrossRef]
- Zhang, C.; Chai, F.; Fu, L.; Hu, P.; Pang, S.; Cui, G. Lithium storage in a highly conductive Cu3Ge boosted Ge/graphene aerogel. J Mater. Chem. A 2015, 3, 22552–22556. [Google Scholar] [CrossRef]
- Feng, J.; Li, Q.; Wang, H.; Zhang, M.; Yang, X.; Yuan, R.; Chai, Y. Hexagonal prism structured MnSe stabilized by nitrogen-doped carbon for high performance lithium ion batteries. J. Alloys Compd. 2019, 789, 451–459. [Google Scholar] [CrossRef]
- Tian, X.; Du, L.; Yan, Y.; Wu, S. An Investigation into the charge-storage mechanism of MnO@Graphite as anode for lithium-Ion batteries at low temperature. ChemElectroChem 2019, 6, 2248–2253. [Google Scholar] [CrossRef]
- Yin, J.; Sun, P.; Qu, G.; Xiang, G.; Hou, P.; Xu, X. A new CoO/Co2B/rGO nanocomposite anode with large capacitive contribution for high-efficiency and durable lithium storage. Appl. Surf. Sci. 2020, 508, 144698. [Google Scholar] [CrossRef]
- Yin, X.; Zhi, C.; Sun, W.; Lv, L.-P.; Wang, Y. Multilayer NiO@Co3O4@graphene quantum dots hollow spheres for high-performance lithium-ion batteries and supercapacitors. J. Mater. Chem. A 2019, 7, 7800–7814. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, A.; Shen, C.; Liu, Q.; Cao, X.; Ma, Y.; Chen, L.; Lau, C.; Chen, T.C.; Wei, F.; et al. Red phosphorus nanodots on reduced graphene oxide as a flexible and ultra-fast anode for sodium-ion batteries. ACS Nano 2017, 11, 5530–5537. [Google Scholar] [CrossRef]
- Guo, A.; Chen, E.; Wygant, B.R.; Heller, A.; Mullins, C.B. Lead oxide microparticles coated by ethylenediamine-cross-linked graphene oxide for lithium ion battery anodes. ACS Appl. Energy Mater. 2019, 2, 3017–3020. [Google Scholar] [CrossRef]
- Kumar, H.V.; Woltornist, S.J.; Adamson, D.H. Fractionation and characterization of graphene oxide by oxidation extent through emulsion stabilization. Carbon 2016, 98, 491–495. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, S.; Wang, G.; Niu, Z.; Yang, J.; Wu, W. Effect of oxidation debris on spectroscopic and macroscopic properties of graphene oxide. Carbon 2014, 76, 203–211. [Google Scholar] [CrossRef]
- Chandra, M.; Khan, T.S.; Shukla, R.; Ahamad, S.; Gupta, A.; Basu, S.; Haider, M.A.; Dhaka, R.S. Diffusion coefficient and electrochemical performance of NaVO3 anode in Li/Na batteries. Electrochim. Acta 2020, 331, 135293. [Google Scholar] [CrossRef]
- Zhang, W.; Yue, Z.; Wang, Q.; Zeng, X.; Fu, C.; Li, Q.; Li, X.; Fang, L.; Li, L. Carbon-encapsulated CoS2 nanoparticles anchored on N-doped carbon nanofibers derived from ZIF-8/ZIF-67 as anode for sodium-ion batteries. Chem. Eng. J. 2020, 380, 122548. [Google Scholar] [CrossRef]
- Hu, L.; Shang, C.; Huang, L.; Wang, X.; Zhou, G. Cu3Ge coated by nitrogen-doped carbon nanorods as advanced sodium-ion battery anodes. Ionics 2019, 26, 719–726. [Google Scholar] [CrossRef]
- Hong, W.; Ge, P.; Jiang, Y.; Yang, L.; Tian, Y.; Zou, G.; Cao, X.; Hou, H.; Ji, X. Yolk-shell-structured bismuth@N-Doped carbon anode for lithium-ion battery with high volumetric capacity. ACS Appl. Mater. Interfaces 2019, 11, 10829–10840. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yin, Z.; Yan, G.; Wang, Z.; Guo, H.; Li, G.; Liu, Y.; Li, L.; Wang, J. Synergy of interlayer expansion and capacitive contribution promoting sodium ion storage in S, N-Doped mesoporous carbon nanofiber. J. Power Sources 2020, 449, 227514. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Luo, Z.; Zhang, C.; Zuo, Y.; Zhang, T.; Tang, P.; Infante-Carrió, M.F.; Arbiol, J.; Llorca, J. Co–Sn nanocrystalline solid solutions as anode materials in lithium-Ion batteries with high pseudocapacitive contribution. ChemSusChem 2019, 12, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Gao, H.; Hao, J.; Zhang, S.; Li, P.; Liu, Y.; Chen, J.; Guo, Z. Yolk–Shell structured FeP@C Nanoboxes as advanced anode materials for rechargeable lithium-/potassium-Ion batteries. Adv. Funct. Mater. 2019, 29, 1808291. [Google Scholar] [CrossRef]
- Cheng, M.; Jiang, Y.; Yao, W.; Yuan, Y.; Deivanayagam, R.; Foroozan, T.; Huang, Z.; Song, B.; Rojaee, R.; Shokuhfar, T.; et al. Elevated-temperature 3D printing of hybrid solid-state electrolyte for Li-Ion batteries. Adv. Mater. 2018, 30, 1800615. [Google Scholar] [CrossRef]
- Chen, W.; Qi, S.; Yu, M.; Feng, X.; Cui, S.; Zhang, J.; Mi, L. Design of FeS2@rGO composite with enhanced rate and cyclic performances for sodium ion batteries. Electrochim. Acta 2017, 230, 1–9. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.; Wei, W.; Ke, S.; Zeng, X.; Chen, D.; Lin, P. Synthesis of Ni@NiSn composite with high lithium-Ion diffusion coefficient for fast-charging lithium-Ion batteries. Glob. Chall. 2019, 1900073. [Google Scholar] [CrossRef]
- Xiao, Z.; Lei, C.; Yu, C.; Chen, X.; Zhu, Z.; Jiang, H.; Wei, F. Si@Si3N4@C composite with egg-like structure as high-performance anode material for lithium ion batteries. Energy Storage Mater. 2020, 24, 565–573. [Google Scholar] [CrossRef]
- Hu, L.; Shang, C.; Akinoglu, E.M.; Wang, X.; Zhou, G. Cu2Se nanoparticles encapsulated by nitrogen-doped carbon nanofibers for efficient sodium storage. Nanomaterials 2020, 10, 302. [Google Scholar] [CrossRef] [PubMed]


| Samples | Lithium Ion Batteries | |
|---|---|---|
| Cycling Performance (Reversible Capacity) | Rate Performance | |
| rGO@CVO (This work) | 738 mAh g−1 at 0.5 A g−1 | 482 mAh g−1 at 10 A g−1 |
| rGO@Co3V2O8 NP (Ref. [17]) | 1050 mAh g−1 at 0.05 A g−1 | 161 mAh g−1 at 10 A g−1, 120 mAh g−1 at 20 A g−1 |
| C–CVO/400 (Ref. [18]) | 735 mAh g−1 at 1 A g−1 | 422 mAh g−1 at 10 A g−1 |
| Co3V2O8 HMMSs (Ref. [20]) | 984 mAh g−1 at 0.5 A g−1 | 545 mAh g−1 at 2 A g−1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Shang, C. Co3V2O8 Nanoparticles Supported on Reduced Graphene Oxide for Efficient Lithium Storage. Nanomaterials 2020, 10, 740. https://doi.org/10.3390/nano10040740
Hu L, Shang C. Co3V2O8 Nanoparticles Supported on Reduced Graphene Oxide for Efficient Lithium Storage. Nanomaterials. 2020; 10(4):740. https://doi.org/10.3390/nano10040740
Chicago/Turabian StyleHu, Le, and Chaoqun Shang. 2020. "Co3V2O8 Nanoparticles Supported on Reduced Graphene Oxide for Efficient Lithium Storage" Nanomaterials 10, no. 4: 740. https://doi.org/10.3390/nano10040740
APA StyleHu, L., & Shang, C. (2020). Co3V2O8 Nanoparticles Supported on Reduced Graphene Oxide for Efficient Lithium Storage. Nanomaterials, 10(4), 740. https://doi.org/10.3390/nano10040740





